Mechanochemically synthesized m-BiVO4 nanoparticles for visible light photocatalysis
Q. Luo†
ab,
L. Zhang†*a,
X. Chena,
O. K. Tan*a and
K. C. Leongb
aNanomaterials Lab, School of Electrical and Electronic Engineering, Nanyang Technological University, 50 Nanyang Avenue, 639798, Singapore. E-mail: zh0012li@e.ntu.edu.sg; EOKTAN@ntu.edu.sg
bGLOBALFOUNDRIES Singapore Pte Ltd, 738406, Singapore
First published on 1st February 2016
Abstract
In this work, a mechanochemical high energy ball milling approach was used to synthesize monoclinic BiVO4 (m-BiVO4) nanoparticles in an attempt to simultaneously reduce the particle size and improve the throughput for practical photocatalytic applications. The effect of annealing to eliminate the induced defects and thus enhance the reactivity was studied on the mechanochemically synthesized BiVO4 nanoparticles. Besides using the conventional characterization tools of XRD, Raman, FE-SEM, HRTEM, XPS and UV-vis diffuse reflectance to examine the crystalline structure, morphology, chemical states and visible light absorption, a customized Kelvin probe coupled with an LED light source was developed as a non-contact tool to study the surface photovoltage (SPV) response for understanding charge generation and separation. The photocatalytic performance was finally evaluated for the degradation of Rhodamine B (RhB) under visible light irradiation to correlate with these physicochemical properties.
1. Introduction
Semiconductor photocatalysis has gained a lot of research interest in the past few decades for applications in artificial photosynthesis1–3 and environmental remediation.4 TiO2, as the most practical and prevalent photocatalyst, has been the target for extensive research. However, UV light occupying only less than 5% of the solar spectrum is required for the activation of TiO2 due to its large bandgap of 3.0–3.2 eV, placing fundamental limits on efficient utilization of the solar energy. A lot of effort has therefore been devoted to exploiting alternative semiconductors in an attempt to harness the abundant visible light portion (ca. 43%) in the solar spectrum or the relatively weak room light energy for more practical applications.Among a number of non-titania based visible light driven photocatalysts under consideration, bismuth vanadate (BiVO4) emerges as one of the most promising candidates since its first demonstration for O2 evolution under visible light irradiation by Kudo et al.5 In nature, BiVO4 exists in three crystalline phase forms: tetragonal zircon (z-t), tetragonal scheelite (s-t) and monoclinic sheetlite (m).6 Under different thermal or preparation conditions, these phases can undergo phase transformations.7 As compared to the tetragonal counterparts, m-BiVO4 with a narrow bandgap of ca. 2.4 eV demonstrates superior photocatalytic effect under visible light irradiation7 and has been therefore investigated and synthesized via various approaches, including solid state reaction,8 co-precipitation process,9 hydrothermal route,10,11 chemical bath deposition,12 flame spray pyrolysis,13 sonochemical route14 and microwave-assisted methods.15,16 It is well known that the photocatalytic activity intimately relates to the particle size and surface area of the powders. However, many of the abovementioned methods usually lead to the formation of gross particles with small surface area, either due to the high calcination temperature in solid state reaction or because of the tiny solubility product of BiVO4 in the aqueous solution.17 In this regard, it is essential to develop methods to synthesize nano-sized m-BiVO4 with high surface area so as to improve the photocatalytic efficiency.
There have been some reported works on preparation of nano-sized m-BiVO4 in literature.10,11,15,18,19 Our group has previously synthesized m-BiVO4 octahedral nano-crystals using hydrothermal route in the presence of sodium dodecyl benzene sulfonate (SDBS).10 Controllable morphologies and uniform sizes could be obtained. Yet, the BET surface area remains unsatisfactory only up to 4 m2 g−1. Sun et al. has reported modified hydrothermal method to synthesize nano-BiVO4 particles in the presence of ethylene diamine tetraacetic acid (EDTA).11 Instead of using EDTA as a chelating agent as in the conventional hydrothermal process,18 EDTA was introduced prior to the precipitation process so the growth of BiVO4 crystallite was well controlled to achieve a large surface area up to 10 m2 g−1. Reverse-microemulsion process has been reported by Chung and Lu to produce nano-scaled m-BiVO4 at a heating temperature of 400 °C to obtain average particle size down to 35 nm.18 More recently, a novel microwave-assisted approach based on in situ twin polymerization has been reported by Hofmann et al. for the preparation of nano-sized m-BiVO4 with BET surface areas of 7–16 m2 g−1.15 Conventional high energy ball milling process has also been explored by Venkatesan et al. to obtain BiVO4 nanoparticles with spherical-like morphology and average sizes about 20 nm by optimizing milling times and ball to powder ratio (BPR).19
The last mechanochemical process represents a simple and effective mean with relative high yield to obtain fine particles with large surface area as compared to other complex approaches involving many steps or with a low throughput, and was thus investigated in the present work for the synthesis of m-BiVO4 nanoparticles for photocatalytic degradation of organic pollutants. It is noteworthy that along with decreasing the particle size, intensive milling often produces defects and microstress due to the meta-stable high energy state. Thus, post-annealing to eliminate the induced defects and enhance the photo-reactivity20,21 of the mechanochemically synthesized m-BiVO4 nanoparticles was also of our particular interests and was investigated and optimized in our present study. The physicochemical properties of the mechanochemically synthesized m-BiVO4 annealed at different temperatures were characterized, and their photocatalytic performance was evaluated and compared for the degradation of Rhodamine B (RhB) dye under visible light irradiation. In particular, surface photovoltage (SPV) in dark and upon irradiation were recorded for the first time to correlate with the photocatalytic activities, which is also a surface phenomenon.
2. Experimental
2.1 Materials preparation
BiVO4 powders were synthesized by mechanochemical ball milling approach using 23.298 g of Bi2O3 (99.9%, Sinopharm) and 9.094 g of V2O5 (99.8%, Alfa Aesar) powders as the starting materials. A 250 ml tungsten carbide jar and 10 pcs of 20 mm tungsten carbide balls were used as milling medium; the milling process was performed using Retsch PM100 planetary high energy ball milling system in air at room temperature for up to 8 h at 200 rpm. The milling was stopped for 5 min after every 25 min milling to cool down the system. The collected powders were then carried on with wet-milling process to reduce the particle size with the weight ratio of 1 mm YSZ (Yttria-Stabilized Zirconia) ball![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BiVO4 powder
BiVO4 powder![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol = 20
ethanol = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 in an YSZ bowl, and a milling speed of 550 rpm for 8 h. The powders were finally separated from YSZ balls using 325# sieve and subject to annealing at different temperatures from 300 to 600 °C in dry air for 1 h to eliminate the defects induced during the ball milling process.
0.5 in an YSZ bowl, and a milling speed of 550 rpm for 8 h. The powders were finally separated from YSZ balls using 325# sieve and subject to annealing at different temperatures from 300 to 600 °C in dry air for 1 h to eliminate the defects induced during the ball milling process.
2.2 Materials characterization
The crystallographic structures were studied using a X-ray diffractometer (D5000, Simens) with an X-ray source of 1.5406 Å Cu Kα in the range of 2θ = 10–70° and a Raman spectroscopy (alpha 300R, Witec) equipped with a green laser (λ = 532 nm) for excitation. The surface morphologies were studied with field emission scanning electron microscopy (FE-SEM, Jeol JSM-6340F) and a transmission electron microscopy (TEM, JEM-2100F, JEOL) at 200 kV. The TEM samples were prepared by sonication of the powders in ethanol for 15 min and subsequently dropping the dispersion onto carbon copper grids until drying. The BET surface areas were measured on an adsorption analyzer (ASAP 2020, Micromeritics). UV-Vis spectrometer (Shimadzu UV-2450) operating in diffuse reflectance mode using BaSO4 as the reference was used to record the absorbance spectra after Kubelka–Munk transformation. The XPS is recorded with Thermo Fisher Scientific XPS, using Al Kα monochromatic X-ray source. All XPS spectra were referenced to the C 1s peak of adventitious hydrocarbon contamination located at 285.0 eV to correct the charging effect.The surface photovoltage spectroscopy (SPS) of the powders was characterized in vacuum by measuring the change of contact potential difference (ΔCPD) in dark and under illumination using a commercial UHV Kelvin probe unit (KP Technology Ltd) incorporated with high-power LED sources (Mightex LED) with switchable wavelength from UV (365 nm) to near infrared (850 nm) through a quartz window. The maximum power at its output for λ = 455 nm is 350 mW. The particulate films for KP measurements were prepared by dispersing the powders in propylene carbonate binder (QPAC@40) and coated onto FTO glass by doctor-blade method. 1.5 g of QPAC@40 was dissolved in 10 g acetone. After overnight stirring, 0.5 ml of the mixture solvent was extracted and added in 0.1 g powder to prepare the paste that was used in doctor blade.
In SPS measurement, the contact potential difference (CPD) is defined as:
| eCPD = ∅TIP − ∅S | (1) |
| eSPV = eΔ∅S = eΔCPD | (2) |
| SPV = ΔCPD = CPDdark − CPDlight | (3) |
2.3 Evaluation of photocatalytic performance
Photocatalytic activities of synthesized BiVO4 were evaluated by the degradation of RhB in aqueous solution under visible light irradiation. The light source used was a white LED (λ > 420 nm, Prizmatix optical devices) having an output powder density of 50 mW cm−2, as calibrated and measured by broadband energy/power meter (Melles Griot). The resultant visible light irradiance onto the sample surface was adjusted to 1.5 mW cm−2 to simulate the indoor irradiation conditions. In each experiment, 0.1 g of BiVO4 powder was added into 50 ml RhB solution with initial concentrate of 15 μM and was kept in dark condition for 3 h to establish absorption/desorption equilibrium. At each time point, the solution was sampled, centrifuged to remove photocatalyst particles and subsequently measured using UV-Vis spectrometer (Shimadzu UV-2450) to monitor the change in absorbance of RhB at 554 nm upon irradiation.3. Results and discussion
3.1 Crystalline structure and morphology
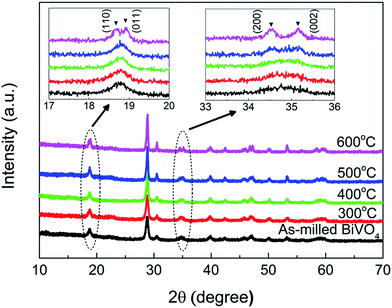
The XRD patterns of the mechanochemically synthesized BiVO4 annealed at different temperatures (Fig. 1) were indexed well with the pure phase of monoclinic sheetlite BiVO4 (JCPDS no. 04-1688). Noticeably, the characteristic peak splitting at 19° and 35° differentiating monoclinic phase from tetragonal phase (JCPDC no. 48-0744)22 could be identified clearly with increasing annealing temperatures as magnified in the inset, signifying the crystallinity improvement. The mean crystallite size of the as-milled BiVO4 powder was estimated to be 20.0 nm, consistent with that of 18–21.6 nm as reported by Venkatesan et al. for mechanochemically synthesized BiVO4 with milling time of 6–11 h.19 The average size gradually increased with annealing temperature in the order of 21–36 nm as summarized in Table 1. Therefore, elevated annealing temperature led to enhanced crystallinity yet contributed to grain growth as expected.| Annealing T (°C) | Crystallite sizea (nm) | BET SSA (m2 g−1) | Particle sizeb (nm) | Bandgap (eV) | SPV (mV) | Reaction constant k (h−1) |
|---|---|---|---|---|---|---|
a Estimated with Scherrer formula D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the wavelength of the X-ray (1.54 Å), and β is the full width at half maximum (FWHM) in radians for peak centered at 2θ = 28.8° and θ is the Bragg angle in the diffraction pattern.b dBET = 6/SBETρ assuming the particles are roughly spherical, ρBiVO4 = 6.95 g cm−3. θ, where λ is the wavelength of the X-ray (1.54 Å), and β is the full width at half maximum (FWHM) in radians for peak centered at 2θ = 28.8° and θ is the Bragg angle in the diffraction pattern.b dBET = 6/SBETρ assuming the particles are roughly spherical, ρBiVO4 = 6.95 g cm−3. |
||||||
| — | 20 | 17 | 51 | 2.66 | — | — |
| 300 | 21 | 16 | 53 | 2.64 | 160 | 0.056 |
| 400 | 26 | 10 | 91 | 2.61 | 210 | 0.107 |
| 500 | 32 | 3 | 276 | 2.60 | 100 | 0.046 |
| 600 | 36 | 1 | 639 | 2.59 | 80 | 0.031 |
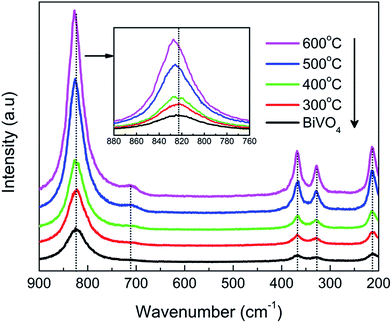
The local crystal structure was further examined by Raman study (Fig. 2). The five characteristic bands of monoclinic BiVO4 were clearly identified in all the samples, specifically, at 210 cm−1 for external mode, 327 and 368 cm−1 for asymmetric and symmetric bending of VO43− tetrahedron, 710 and 827 cm−1 for stretching mode of V–O bond and shorter V–O bond in VO43− tetrahedron.23 Raman band positions are very sensitive to the short range order.24 No obvious shift in band positions was noticeable at the first glance. However, close inspection of the strongest and sharpest peak (inset) revealed a blue shift towards higher frequency with annealing. The band position of the as-milled BiVO4 at 823 cm−1 was lower than the commonly reported value of 826–827 cm−1 as of typical BiVO4 particles25 as well as those with annealing in the present study. This could imply longer bond lengths of VO43− tetrahedron based on the established functional relationships between the Raman stretching frequencies and the metal–oxygen bond length.24,25 It could also be attributed to its nanoscale crystal grains in analogy to quantum confinement effect, which was theoretically predicted by Richter et al.26 whereby natural Lorentzian phonon band characteristics of infinite bulk solids evolve into an asymmetric Raman band when the physical size decreases and experimentally observed by Li et al. on mesoporous BiVO4 with particle size of ca. 8 nm.27 With elevated annealing temperature, the peak intensity increased significantly, confirming the improved crystallinity with post-annealing.
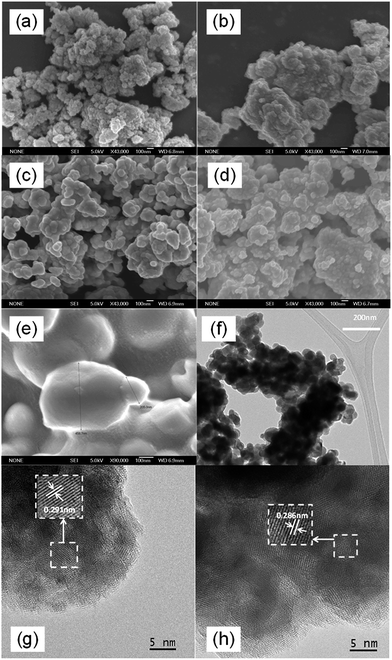
Fig. 3(a)–(e) presents the FESEM images of the as-milled BiVO4 and those with post-annealing at 300–600 °C. The as-milled BiVO4 sample was composed of spherical particles with average size of 50 nm. Annealing at 300 °C did not change the morphology and size distribution significantly. At an annealing temperature of 400 °C, despite with a significant segregation, the average particle size was still well below 100 nm. A significant increase in particle size was observed at elevated annealing temperatures above 500 °C as expected. The observation was in good agreement with the change of particle sizes estimated from the measured BET surface area (Table 1). It was also noteworthy that BiVO4 samples annealed up to 400 °C exhibited much higher BET surface area (10–17 m2 g−1) than most of the reported works in literature, affirming the effectiveness of mechanochemical high-energy ball milling process in producing nano-sized particles. The obtained surface areas were also in accordance with those of the nanosized BiVO4 reported by Sun et al. (10 m2 g−1)11 and Hofmann et al. (7–16 m2 g−1)15 or the nanoplatelets reported by Ressnig et al. (7 m2 g−1).28 TEM image in Fig. 3(f) re-confirmed the nano-crystalline nature of BiVO4 annealed at 400 °C. Corresponding HRTEM images in Fig. 3(g) and (h) exhibited well-crystallized grains with lattice spacing of 0.291 nm and 0.286 nm, which can be identified as (040) plane of monoclinic BiVO4,8 in good agreement with XRD analysis. In short, BiVO4 nanoparticles with monoclinic sheetlite structure were successfully synthesized by mechanochemical ball milling approach followed by post-annealing process. Good crystallinity and small particle size need to be compromised at optimum annealing temperature to achieve the desirable photoactivity.
3.2 Chemical states study
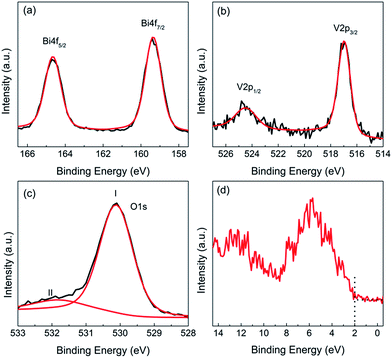
Fig. 4 shows the high-resolution XPS spectra of the three elements and total density of states (DOS) for the valence band (VB) in mechanochemically synthesized BiVO4 annealed at 400 °C. The well-deconvoluted spin–orbit split positioned at 164.6 eV and 159.3 eV corresponded to Bi 4f5/2 and Bi 4f7/2 orbits of the Bi3+ in the sample while the doublets centered at 524.5 eV and 517.0 eV were assigned to V 2p1/2 and V 2p3/2 orbits respectively, which is attributed to V5+ of BiVO4 particles.29 The intense O 1s peak at ca. 530.1 eV was due to the lattice oxygen in crystalline BiVO4 cell, while another relatively lower peak at ca. 532 eV was usually assigned to surface adventitious species such as hydrocarbon (C–H), carbonate species (C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and adsorbed water (OHads) bonds due to contamination from environment during characterization.30 Overall, the binding energies of the different elements were originated from the typical monoclinic scheelite BiVO4, consistent with the XRD analysis.
O) and adsorbed water (OHads) bonds due to contamination from environment during characterization.30 Overall, the binding energies of the different elements were originated from the typical monoclinic scheelite BiVO4, consistent with the XRD analysis.
 | ||
| Fig. 4 XPS spectra of Bi 4f (a), V 2p (b), O 1s (c) and VB scan (d) of mechanochemically synthesized BiVO4 annealed at 400 °C. | ||
The VB scan as shown in Fig. 4(d) allows us to understand the VB electronic structure of the m-BiVO4. It was noted that the VB edge was 2.0 eV below the Fermi energy level from the photoemission onset, suggesting it was an n-type semiconductor considering the overall bandgap energy of ca. 2.4 eV. The peak positions were consistent with those calculated and demonstrated by Cooper et al.31 Specifically, an isolated photoemission peak centered at ca. 12 eV was assigned to the partial DOS of Bi 6s states. The broad photoemission between 9 and 2 eV was ascribed to a combined DOS of unhybridized O 2pπ mixed with Bi 6s states at ca. 2.65 eV, O 2pπ state at ca. 3.67 eV, the hybridized O sp2/V 3d state at ca. 5.63 eV, and the hybridized O sp2/Bi 6p state near 7.45 eV with respect to the Fermi level in the VB.
3.3 Optical properties
The UV-vis diffuse reflectance absorption spectra of BiVO4 annealed at different temperatures are presented in Fig. 5(a) in comparison to the as-milled BiVO4. The main absorption band of all the samples was expanded on the wavelength range of 350–550 nm. With increasing annealing temperature, the fundamental absorption edge exhibited obvious red shift towards the visible light range while the absorption shoulder from 510 nm onwards diminished.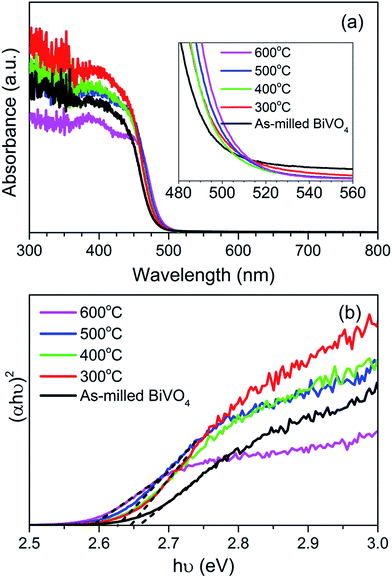 | ||
| Fig. 5 UV-vis absorption spectra of mechanochemically synthesized BiVO4 annealed at different temperatures (a) and their extrapolated bandgap (b). | ||
BiVO4 was commonly reported as a direct bandgap semiconductor material,32 with the conduction band minimum and valence band maximum comprised primarily of unoccupied V 3d orbital and hybrid orbitals of Bi 6s and O 2p, respectively.31 Thus, the bandgap of BiVO4 can be extrapolated from (αhν)2 versus photon energy plot (Tauc plot) based on the relation:
| α(hν) ∝ (hν − Eg)1/2 | (4) |
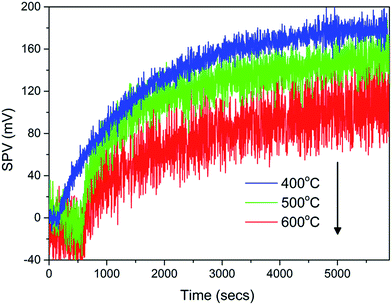
Kelvin probe based SPV measures the contact potential change as a result of illumination. Different from diffuse reflectance absorption spectra, the magnitude of the SPV does not only reply on the number of electron–hole pairs generated by photon absorption, but also the diffusion of these carriers to surface states which in turn, also depends on the charge separation efficiency.30 A larger surface photovoltage signal therefore indicates that more charge carriers can diffuse to the particle surface to cause a larger contact potential difference.33 As shown in Fig. 6, BiVO4 annealed at 400–600 °C showed a typical n-type semiconductor behavior with positive SPV values upon illumination, consistent with the conclusion drawn from XPS VB scan. The sample annealed at 400 °C had the largest SPV value of 210 mV as compared to those annealed at 500–600 °C as illustrated in Table 1, implying a better charge separation. With post-annealing, the good crystallization with fewer structural defects effectively reduced the recombination and promoted charge transportation. Simultaneously, the relative small size shortened the carrier transportation path to increase the charge separation efficiency, which might lead to a faster photodegradation rate.
3.4 Photocatalytic activities
RhB as an important organic dye pollute difficult to be degraded using visible light was used as a model dye contaminant to evaluate the photocatalytic activities of the mechanochemically synthesized BiVO4 powders annealed at different temperatures as shown in Fig. 7. The control with RhB in the absence of any photocatalysts did not show any degradation over time, excluding the possibility of photolysis. Under the dark condition, adsorption equilibrium was established within 2 h for all the samples. Upon light irradiation, the as-milled BiVO4 exhibited minimal photocatalytic effect, which could be attributed to the presence of distortion induced during milling process due to its ferroelastic properties.34 Such lattice distortion could be eliminated by re-crystallizing annealing above the transition temperature between m-BiVO4 and s-t-BiVO4. Photodegradation of RhB occurred only in the presence of annealed BiVO4 powders, reaffirming the necessity of post-annealing to enhance the crystallinity and thus the photocatalytic reactivity of mechanochemically synthesized BiVO4. The reaction constants were calculated based on first-order kinetic ln(C/Co) ∝ kt shown in the inset and was recorded in Table 1. In general, the degradation rate followed the same trend in SPV response: k400 °C > k300 °C > k500 °C > k600 °C. BiVO4 annealed at 400 °C showed the fastest degradation rate due to the optimization between crystallinity enhancement and particle size growth induced by post-annealing treatment. Further increase in annealing temperature resulted in deteriorated PCO efficiency, possibly attributed to significant growth of particle size and reduction of surface area that provided fewer active sites for PCO to take place.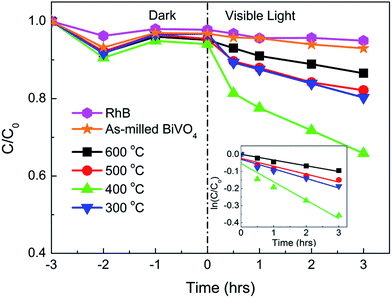 | ||
| Fig. 7 RhB degradation in the presence of annealed BiVO4 under white LED irradiation of 1.5 mW cm−2 with the inset showing the first order reaction kinetics. | ||
4. Conclusions
In summary, nano-sized monoclinic BiVO4 powders have been successfully synthesized using mechanochemical high energy ball milling approach. The obtained particle size was well below 100 nm and the BET surface area was close to or larger than 10 m2 g−1 when the post-annealing temperature did not exceed 400 °C. The good balance between crystallization and particle size distribution for BiVO4 annealed at 400 °C led to its superior photodegradation rate of RhB under visible light irradiation and the highest SPV response among all the annealed samples, rendering it as a promising visible light active photocatalyst suitable for environmental remediation. On top of that, its potential applications are foreseeable to be extended and implemented in other fields such as water splitting and charge storage. Further study to utilize it in thin film form is under investigation and will be published in a separate paper.References
- S. J. A. Moniz, S. A. Shevlin, D. J. Martin, Z.-X. Guo and J. Tang, Energy Environ. Sci., 2015, 8, 731–759 CAS.
- S. Das and W. M. A. Wan Daud, RSC Adv., 2014, 4, 20856–20893 RSC.
- R. L. House, N. Y. M. Iha, R. L. Coppo, L. Alibabaei, B. D. Sherman, P. Kang, M. K. Brennaman, P. G. Hoertz and T. J. Meyer, J. Photochem. Photobiol., C, 2015, 25, 32–45 CrossRef CAS.
- H. Xu, S. Ouyang, L. Liu, P. Reunchan, N. Umezawa and J. Ye, J. Mater. Chem. A, 2014, 2, 12642–12661 CAS.
- A. Kudo, K. Ueda, H. Kato and I. Mikami, Catal. Lett., 1998, 53, 229–230 CrossRef CAS.
- S. Tokunaga, H. Kato and A. Kudo, Chem. Mater., 2001, 13, 4624–4628 CrossRef CAS.
- A. Kudo, K. Omori and H. Kato, J. Am. Chem. Soc., 1999, 121, 11459–11467 CrossRef CAS.
- R. Roth and J. Waring, Am. Mineral., 1963, 48, 1348 CAS.
- J. Yu, Y. Zhang and A. Kudo, J. Solid State Chem., 2009, 182, 223–228 CrossRef CAS.
- M. Han, X. Chen, T. Sun, O. K. Tan and M. S. Tse, CrystEngComm, 2011, 13, 6674–6679 RSC.
- W. Sun, M. Xie, L. Jing, Y. Luan and H. Fu, J. Solid State Chem., 2011, 184, 3050–3054 CrossRef CAS.
- M. C. Neves and T. Trindade, Thin Solid Films, 2002, 406, 93–97 CrossRef CAS.
- N. C. Castillo, A. Heel, T. Graule and C. Pulgarin, Appl. Catal., B, 2010, 95, 335–347 CrossRef CAS.
- L. Zhou, W. Wang, S. Liu, L. Zhang, H. Xu and W. Zhu, J. Mol. Catal. A: Chem., 2006, 252, 120–124 CrossRef CAS.
- M. Hofmann, M. Rainer, S. Schulze, M. Hietschold and M. Mehring, ChemCatChem, 2015, 7, 1357–1365 CrossRef CAS.
- Y. Zhang, G. Li, X. Yang, H. Yang, Z. Lu and R. Chen, J. Alloys Compd., 2013, 551, 544–550 CrossRef CAS.
- M.-L. Guan, D.-K. Ma, S.-W. Hu, Y.-J. Chen and S.-M. Huang, Inorg. Chem., 2011, 50, 800–805 CrossRef CAS PubMed.
- C.-Y. Chung and C.-H. Lu, J. Alloys Compd., 2010, 502, L1–L5 CrossRef CAS.
- R. Venkatesan, S. Velumani and A. Kassiba, Mater. Chem. Phys., 2012, 135, 842–848 CrossRef CAS.
- A. Ebrahimi-Purkani and S. F. Kashani-Bozorg, J. Alloys Compd., 2008, 456, 211–215 CrossRef CAS.
- C. C. Koch and Y. S. Cho, Nanostruct. Mater., 1992, 1, 207–212 CrossRef CAS.
- W. Yin, W. Wang, L. Zhou, S. Sun and L. Zhang, J. Hazard. Mater., 2010, 173, 194–199 CrossRef CAS PubMed.
- A. Zhang and J. Zhang, Mater. Lett., 2009, 63, 1939–1942 CrossRef CAS.
- M. Gotić, S. Musić, M. Ivanda, M. Šoufek and S. Popović, J. Mol. Struct., 2005, 744–747, 535–540 CrossRef.
- J. Yu and A. Kudo, Adv. Funct. Mater., 2006, 16, 2163–2169 CrossRef CAS.
- H. Richter, Z. P. Wang and L. Ley, Solid State Commun., 1981, 39, 625–629 CrossRef CAS.
- G. Li, D. Zhang and J. C. Yu, Chem. Mater., 2008, 20, 3983–3992 CrossRef CAS.
- D. Ressnig, R. Kontic and G. R. Patzke, Mater. Chem. Phys., 2012, 135, 457–466 CrossRef CAS.
- X. Wu, H. Zhou, S. Gu, F. Wang, J. Liu and W. Li, RSC Adv., 2015, 5, 92769–92777 RSC.
- L. Zhang, P. Y. Tan, C. K. Lim, X. Guo, M. S. Tse, O. K. Tan and V. W. C. Chang, J. Environ. Chem. Eng., 2016, 4, 357–364 CrossRef CAS.
- J. K. Cooper, S. Gul, F. M. Toma, L. Chen, P.-A. Glans, J. Guo, J. W. Ager, J. Yano and I. D. Sharp, Chem. Mater., 2014, 26, 5365–5373 CrossRef CAS.
- A. Walsh, Y. Yan, M. N. Huda, M. M. Al-Jassim and S.-H. Wei, Chem. Mater., 2009, 21, 547–551 CrossRef CAS.
- X. Chen, Q. Luo, M. Han, O. K. Tan, M. S. Tse and H. Huang, J. Solid State Chem., 2012, 189, 80–84 CrossRef CAS.
- A. Pinczuk, B. Welber and F. H. Dacol, Solid State Commun., 1979, 29, 515–518 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |