Synthesis and magnetooptic characterization of Cu-doped ZnO/MgO and ZnO/oleic acid core/shell nanoparticles
J. Mikulski*a,
B. Sikoraa,
K. Fronca,
P. Aleshkevycha,
S. Kreta,
J. Suffczyńskib,
J. Papierskab,
Ł. Kłopotowskia and
J. Kossuta
aInstitute of Physics Polish Academy of Science, Al. Lotników 32/46, 02-668 Warsaw, Poland. E-mail: mikulski@ifpan.edu.pl
bInstitute of Experimental Physics, Faculty of Physics, University of Warsaw, ul. Pasteura 5, 02-093 Warsaw, Poland
First published on 18th April 2016
Abstract
The effect of Cu ion doping on the photoluminescence (PL) and magnetic behavior of ZnO/MgO and ZnO/oleic acid core/shell nanoparticles is investigated. By means of transmission electron microscopy we determine the average size distribution of the synthesized nanoparticles, while by studying electron paramagnetic resonance we determine the oxidation state of the Cu ions to be Cu2+. The magnetic field only slightly shifts spectrally the emission of the ensemble of such quantum dots. On the other hand, the magnetic field induces a considerable strengthening of the PL intensity and a sizable circular polarization of the emission of ZnO/MgO nanocrystals.
1 Introduction
ZnO nanostructures have many potential applications, e.g., in photocatalysis,1 as solar cells,2 gas sensors,3 fuel cells,4 in photovoltaics,5 and are known for their antibacterial activity.6 Materials based on zinc oxide are inexpensive, non-toxic even in the form of nanoparticles (NP) and they are relatively easy to manufacture. In recent years the technology of doping of ZnO NP with various transition metal ions has been developed.7 Manganese,8 cobalt,9 and nickel ions10 on the second oxidation level are the most popular dopants, but ions in other oxidation states such as silver(I),11 iron(III)12,13 and chromium(III)14 were also used. The transition metal ions in ZnO NPs are expected to modify their electrical, optical and magnetic properties. Photoluminescence properties of ZnO NPs are also of great interest due to their application in the field of lasing devices, light emitting diodes, and optical sensing devices, etc. Predictions of ferromagnetic Curie temperatures15 exceeding the room temperature makes such dilute magnetic semiconductor (DMS) oxide NP very promising for implementation in future spintronics devices. Copper doped ZnO would be particularly attractive for device implementation since both ZnO and Cu are relatively inexpensive and abundant.16 The possibility of using copper ions as the dopants, bringing para- or ferromagnetic properties to ZnO, is however still being discussed. Recently, a very interesting behaviour of ZnSe/CdSe core/shell copper doped NPs was reported. Not only was a strong magnetic circular dichroism detected in those structures, but the magnetic properties were strongly enhanced by an additional illumination. As noted above, there exists a literature record related to the magnetic properties of ZnO crystallites doped with various transition metal ions, but their emission properties in the presence of an external magnetic field are rarely described. Here we report on the synthesis and advanced structural and magnetospectroscopic characterization of ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cu NPs coated either with MgO or with oleic acid. The concentration of Cu dopants ranged from 1 molar percent up to 10 mol%. Absorption measurements do not show any dependence of the NP band gap on Cu concentration. Electron paramagnetic resonance measurements reveal that the copper is introduced in the second oxidation level giving rise to localized magnetic moments from the incompletely filled Cu d-shell. Photoluminescence studies at 2 K demonstrate a strong increase of the circular polarization as the magnetic field is increased to 10 T – a possible signature of the enhanced Zeeman splitting due to sp–d exchange between the localized Cu ions and the charge carriers.
Cu NPs coated either with MgO or with oleic acid. The concentration of Cu dopants ranged from 1 molar percent up to 10 mol%. Absorption measurements do not show any dependence of the NP band gap on Cu concentration. Electron paramagnetic resonance measurements reveal that the copper is introduced in the second oxidation level giving rise to localized magnetic moments from the incompletely filled Cu d-shell. Photoluminescence studies at 2 K demonstrate a strong increase of the circular polarization as the magnetic field is increased to 10 T – a possible signature of the enhanced Zeeman splitting due to sp–d exchange between the localized Cu ions and the charge carriers.
2 Experimental
2.1 Synthesis of Zn1−xCuxO/MgO
The most common chemical method of fabrication of colloidal ZnO NPs containing copper ions is co-precipitation using various precursors. ZnO obtained by chemical methods have a well-defined wurtzite crystal lattice.17All chemicals we use to prepare undoped and Cu doped ZnO were purchased from Sigma-Aldrich and used without further purification. ZnO/MgO core/shell nanoparticles are prepared by the reaction presented in Fig. 1. First, 0.4 mmol of zinc acetate dihydrate and the appropriate amount of copper acetate monohydrate (1 mol%, 2 mol%, 5 mol%, 10 mol%) are added to 100 ml of ethanol and stirred at 40 °C until a clear solution is obtained. The reaction is initiated by the dropwise addition of a NaOH solution (15 mmol in 74 ml of ethanol) to a zinc acetate dihydrate solution at about 0 °C while stirring vigorously. The mixture is then heated to 45 °C and held for 3 h. 0.07 mmol of magnesium acetate in 50 ml of ethanol is added to the resulting solution of ZnO nanoparticles. After a few minutes, 0.4 mmol of NaOH in 30 ml of ethanol is added to this solution. The synthesis is carried out at 45 °C overnight. The synthesized ZnO/MgO core/shell nanoparticles are centrifuged (6000 rpm, 15 min, 20 °C) and then purified three times with ethanol to remove CH3COO− and Na+ ions. Subsequently, the nanoparticles are dissolved in ethanol.18
2.2 Synthesis of Zn1−xCuxO/oleic acid
Nanoparticles coated with oleic acid are also prepared by co-precipitation in a homogeneous solution according to Fig. 2. First, 10 mmol of zinc acetate dehydrate, the appropriate amount of copper acetate monohydrate (1 mol%, 2 mol%) and 3 mmol of oleic acid is added to 100 ml of ethanol. The reaction is initiated by the addition of a NaOH solution (30 mmol in 100 ml of ethanol) to a zinc acetate dihydrate solution at room temperature with vigorous stirring. The mixture is heated to 45 °C and held overnight. Oleic acid attaches to the particle surface by carboxyl group, blocking further growth. The synthesized oleic acid coated ZnO nanoparticles are centrifuged (3000 rpm, 15 min, 20 °C) and then purified three times with ethanol subsequently, the nanoparticles are dissolved in toluene.The above given molar fractions of Cu in the nanocrystals are only crude estimates based on the amount of copper containing reagents. To check the real composition of the nanocrystals (in particular Cu content) we attempted to perform SIMS (Secondary Ion Mass Spectrometry) and RBS (Rutherford Back Scattering). Unfortunately, nearly the same atomic masses of Zn and Cu made those determinations very imprecise. We can only deduce that in the sample with nominally 1% of Cu we indeed have smaller ratio of copper to zinc (by weight) by a factor of least 3 that in the sample which nominally contains 10% of Cu. We note however that if the charge state of a large percentage of Cu ions would differ from Cu(II) it would lead to a significant shift of the RBS signal from that of Zn(II). Since we do not observe such a shift we infer indirectly that a majority of copper ions is on the second oxidation level which is consistent with our EPR studies.
3 Results and discussion
3.1 Morphology and chemical composition
The transmission electron microscopy (TEM) images of single zinc oxide nanoparticles are shown in Fig. 3. High-resolution images of the ZnO NPs show crystalline planes and confirm the wurtzite ZnO structure without precipitation of copper oxide, even in the case of 10% dopant concentration. TEM images do not reveal any presence of the MgO shell, probably because it is relatively thin, less than 1 nm. Normally the MgO crystal structure is cubic, but we expect a hexagonal structure since the shell thickness is smaller than the critical value for the relaxation from the hexagonal to the cubic structure,18 as has been observed in ZnO/MgO wires19 and other II–VI semiconductor nanocrystals of the core/shell type.20 Smaller magnification TEM images show agglomerates containing randomly arranged ZnO NPs. To determine their size, we employ a Fourier transformation filter. After the inverse transformation, we obtain the image containing only NPs with a specific orientation of the crystal lattice. This analysis of TEM images allows to single out individual NPs and indicates that the particles have an oval shape with aspect ratio of about 1.5 and an average size along the longer axis of about 4.6 nm independent of the Cu concentration see Table 1.| Copper (%) | Abs/nm | Abs error/nm | TEM/nm | TEM error/nm |
|---|---|---|---|---|
| 0 | 4.5 | 4.0–5.1 | 4.5 | 0.7 |
| 1 | 4.5 | 4.0–5.1 | 4.6 | 0.6 |
| 2 | 4.5 | 4.0–5.1 | 4.6 | 0.5 |
| 10 | 4.5 | 4.0–5.1 | 5.4 | 0.9 |
The electron paramagnetic resonance (EPR) technique confirms the presence of copper ions inside the ZnO core and allows determination of their oxidation level. Specifically, EPR offers the possibility to distinguish between copper ions in the first (I) and second (II) oxidation states, as only copper(II) has a total spin different from zero. XPS measurements (spectra not shown here) give ambiguous results. Peaks visible in the spectra at the binding energies equal to 933 and 952 eV can correspond both to Cu(I) and Cu(II). The literature mentions those additional peaks between 933 and 952 eV and above 955 in the case of Cu(II), but only when the intensity of signal is sufficiently strong (like in pure, bulk CuO).21–25 Also the angle between the detector and the normal, which is different for each nanoparticle in the pellet, affects the intensity of the absence peak. The presence of ions in the second oxidation state is confirmed by EPR studies (see below), but the presence of copper ions in the first oxidation level cannot be excluded. The 3d9 electron configuration of the Cu2+ ions is characterized by spin S = 1/2 and leads to a single anisotropic resonance line with hyperfine splitting due to nuclear spin I = 3/2. In powdered samples, the observed EPR spectrum of Cu(II) is averaged due to a random orientation of [CuO4] complexes with respect to the magnetic field direction. EPR spectra measured for various levels of Cu doping are shown in Fig. 4. The spectra show a typical resonance line shape for Cu(II) ions under influence of a nearly axial crystal field. Two main features are visible in the experimental spectra of derivative absorption: there are four poorly resolved maxima in the low-field part (2600–3100 G) which are due to a hyperfine structure and a strong minimum at 3300 G due to an unresolved hyperfine structure. The resonance line position of a single Cu2+ ion is described by the spin Hamiltonian composed of the Zeeman and hyperfine structure terms:
| Ĥ = μ0BģŜ + ĝÂÎ | (1) |
By simulating the powder EPR spectra using Simfonia software, we find that copper ions built into the ZnO lattice are described by an axial spin-Hamiltonian with gperp = 2.055, gpar = 2.42, Aperp = 0.0025 cm−1 and Apar = 0.0080 cm−1. The obtained parameters of the EPR spectra confirm that Cu2+ ions are indeed incorporated into ZnO, without formation of precipitates of interstitial copper(II) oxide, since only one position of the EPR peak was visible. This remains true even for 10% of dopant concentration. The parameters above are in accord with the findings of ref. 26. Importantly, we note that the samples that are nominally free of Cu doping do not show any EPR signal.
3.2 Spectroscopic properties
The bandgap of ZnO nanoparticles is determined by measuring the absorption in solution at room temperature. Fig. 5 shows the absorption spectra of ZnO NPs with MgO and oleic acid shells. | ||
| Fig. 5 Absorption spectra of (A) Zn1−xCuxO/MgO, (B) Zn1−xCuxO/oleic acid at T = 298 K. Inserts shows the evolution of the absorption edge, measured as the deflection point of the spectrum. | ||
The spectra indicate the bandgap shifts toward the blue relative to the bulk crystals of ZnO (3.44 eV at T = 298 K). In principle, the energy of the absorption edge depends on three factors: (i) the particle size through the effect of quantum confinement (ii) the strain exerted by the shell on the core, and (iii) the bandgap shift due to the presence of copper. Since we do not see any clear dependence on the Cu concentration, we conclude that the impact of copper is smaller than the remaining two effects. We also rule out the influence of strain, since the shell thickness is rather small and in the case of the oleic acid, the organic compound is not expected to exert any strain. Therefore, we associate the blue shift of the band gap with the quantum confinement. This is expected in such small NPs with a diameter of less than 5 nm. For particles coated with oleic acid, the size is determined not by thermodynamic parameters but by kinetics of acid adsorption on the surface. As a result, we obtain a smaller dispersion in NP size, which results in a sharper band edge observed in absorption measurement. In order to determine the approximate sizes of ZnO NPs from the absorption spectra we use an effective mass model:27
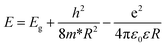 | (2) |
According to ref. 29, the NP diameter should decrease with the increasing concentration of the dopant. In our case, this relationship appears to be random. It is most likely connected with restrictions of chemical methods, which are ruled by many thermodynamic parameters, such as local temperature changes in the reaction mixture.
There exist a vast number of references dealing with photoluminescence studies in ZnO-based nanocrystals.30,31 These allow us to identify unambiguously the transitions – particularly those associated with excitinic transitions – which are observed in our samples. We examine photoluminescence spectra of our nanoparticles as a function of the concentration of Cu. For that purpose 20 μl of the nanoparticles suspended in ethanol at a concentration of 5 mg ml−1 are dropped on a silicon substrate with an area of one square centimeter and spin coated (3000 rpm/45 s). Generally, the photoluminescence spectra of ZnO:Cu nanoparticles suspended in solution show two emission bands, as shown in Fig. 6. The narrow band in the UV region of bandgap corresponds to the exciton recombination. The broad band with a maximum in the green range of the spectrum is most likely related to oxygen vacancies located on the NP surfaces. Intensity of the excitonic emission is greater in the case of the NPs coated with magnesium oxide. A smaller magnitude of blinking effects in the case of these NPs suggests that efficient exciton emission arises thanks to a more efficient passivation of the NPs surface in the case of the MgO coating. We note that regardless of the coverage type the emission is shifted towards higher energies, compared to the bulk material which – as in the case of the absorption spectra – we ascribe to the size confinement. Increasing the concentration of Cu2+ leads to a luminescence quenching both of the UV and VIS emissions with the defect-related emission being more sensitive to the amount of Cu introduced into NP. The content of copper ions in excess of 5% quenches luminescence entirely. We ascribe this effect to the efficient transfer of excitation to internal transitions of Cu2+ ions, similarly as observed previously for, e.g., Co or Mn doped ZnO layers or NPs.
 | ||
| Fig. 6 Photoluminescence spectra of Zn1−xCuxO NPs at T = 6 K (A) covered by MgO, and (B) covered by oleic acid. | ||
3.3 Magnetooptical properties
Generally, all the PL spectra exhibit a narrow peak in the UV related to the near bandgap emission and a broad peak in the green range related to deep crystal defects. In the PL spectra measured at room temperature on suspensions of ZnO NPs, the position of the UV PL peak corresponds to the onset of the absorption edge. At 6 K, this peak is blueshifted with respect to room temperature by about 100 meV. We interpret this peak as originating from exciton recombination, blueshifted with respect to the bulk ZnO bandgap due to quantum confinement. The intensity of this peak is larger for ZnO/MgO NPs than for ZnO NPs coated with oleic acid. Also, the former samples exhibit weaker PL blinking evidenced in the microphotoluminescence studies on small number of NPs cast from strongly diluted solutions. Both of these effects suggest that the MgO shell is more efficient in passivation of the nonradiative surface recombination pathways. The broad peak is interpreted by a vast majority of authors as an emission related to singly ionized oxygen vacancies (VO˙).11,32–34 Namely, it is due to a recombination of an electron from a level close to the conduction band edge with a deeply trapped hole, which tunnels into the ZnO core from the surface and forms VO˙. Crucially, we do not observe the transition between the conduction band electron and the hole from the Cu ion in the d9 electron configuration.29,35 For ZnO NPs with a radius of 4.5 nm, this transition was reported to appear at about 2.9 eV.29 The absence of this transition in our spectra shows that the electron configuration of the Cu ion changes under UV irradiation from d9 observed in EPR measurements performed without UV, to d10 with UV present in PL experiments. This observation is consistent with the UV-induced n-type photodoping,36,37 which shifts the Fermi level towards the conduction band. As a result, the d-shell of the Cu ion fills to the d10 configuration. The UV induced change of the electron configuration of the Cu ion has consequences for its magnetic behavior. As shown extensively for CdSe NPs doped with Mn ions38 and also for ZnO films doped with Mn2+, Co2+, or Ni2+ ions,39–41 the presence of the transition metal ions with a partially filled d-shell results in a significant enhancement of the Zeeman splitting of the conduction and valence band states. These effects can be probed in PL, magnetic circular dichroism of the absorption, or time-resolved Faraday rotation. Here, we present results of a PL measurement in magnetic fields up to 10 T. In Fig. 7, we show the PL spectra of ZnO/MgO NPs in 0 T and in 10 T, measured for two circular polarizations of the detected light. Within the spectral resolution of about 0.5 meV being due to the PL linewidth no splitting can be resolved. The same result is obtained on NPs coated with oleic acid. As the magnetic field is increased, the PL intensity in σ+ polarization becomes stronger that the PL signal in σ− polarization. We define the circular polarization degree as P = (I+ − I−)/(I+ + I−), where I± denotes the PL intensity in σ± polarizations. As can be seen in Fig. 8, P reaches about 40% at 10 T. Polarization degrees of a similar magnitude were also reported for CdSe NPs.42 This polarization occurs as a result of the increased population of the lower exciton level from the Zeeman split doublet. Since the splitting cannot be directly resolved, we assume that it is due to the intrinsic ZnO g-factor with a reported value of less than 0.3.43 In addition it has been shown that in the case of ZnO:mn the speed enhancement of Zeeman splitting is strongly reduced as compared to expectations based on the true values of alpha and beta exchange constant.44 We therefore conclude that under UV excitation our NPs do not reveal any enhancement of the Zeeman effect consistent with the UV-induced photodoping effect. Here we show the date only for the sample containing nominally 1% of Cu since the higher percentage samples gave a very weak and inaccurate signal with even greater error bars than in Fig. 8. Therefore the analysis of those lines would be very speculative. | ||
| Fig. 8 Degree of circular polarization (DCP) of the luminescence of Zn0.99Cu0.01O/MgO (measured for two identical samples). | ||
4 Conclusion
In summary, pure and Cu (1%, 2%, 5% and 10% mol) doped ZnO nanoparticles coated by magnesium oxide or oleic acid samples are prepared by a co-precipitation method at room temperature. The samples are studied by electron paramagnetic resonance, transmission electron microscopy, optical absorption and magnetphotoluminescence. All these methods indicate that copper is incorporated into the nanocrystals in the second oxidation level Cu2+ and with no contribution from other precipitates of copper or copper oxide. The studies in the presence of the magnetic field indicate that the Zn1−xCuxO nanocrystals show behaviour characteristic for other ZnO-based dilute magnetic semiconductors. More studies are required to confirm this conclusion in an unambiguous way. In particular, studies of magnetic circular dichroism would be helpful in this respect.Acknowledgements
The research was partially supported by the grant of Polish National Science Center 2013/08/A/ST3/00297. We acknowledge Laurent Nittler for XPS measurement and R. Jakiela and Adam Barcz for SIMS and RBS experiments.References
- S. Xiao, L. Zhao, X. Leng, X. Lang and J. Lian, Appl. Surf. Sci., 2014, 299, 97 CrossRef CAS.
- J. Huang, Z. Yin and Q. Zheng, Energy Environ. Sci., 2011, 4, 3861 CAS.
- P. P. Wang, Q. Qi, R. F. Xuan, J. Zhao, L. J. Zhou and G. D. Li, RSC Adv., 2013, 3, 19853 RSC.
- Y. Qinand, X. D. Wang and Z. L. Wang, Nature, 2008, 451, 809 CrossRef PubMed.
- H. S. Song, W. J. Zhang, C. Cheng, Y. B. Tang, L. B. Luo, X. Chen, C. Y. Luan, X. M. Meng, J. A. Zapien, N. Wang, C. S. Lee, I. Bello and S. T. Lee, Cryst. Growth Des., 2011, 11, 147 CAS.
- Y. Xie, Y. He, P. L. Irwin, T. Jin and X. M. Shi, Appl. Environ. Microbiol., 2011, 77, 2325 CrossRef CAS PubMed.
- W. Pacuski, Introduction to the Physics of Diluted Magnetic Semiconductors, Springer Series in Materials Science, Springer, Heidelberg, 2010, vol. 144, p. 37 Search PubMed.
- D. W. Wu, Z. B. Huang, G. G. Yin, Y. D. Ya, X. M. Lia, D. Han and X. Huang, CrystEngComm, 2010, 12, 192 RSC.
- S. Kuriakose, B. Satpati and S. Mohapatra, Phys. Chem. Chem. Phys., 2014, 16, 12741 RSC.
- J. K. Salem, T. M. Hammad and R. R. Harrios, J. Mater. Sci.: Mater. Electron., 2012, 5, 24 Search PubMed.
- P. Fageria, S. Gangopadhyay and S. Pande, RSC Adv., 2014, 4, 24962 RSC.
- R. Heitz, A. Hoffmann and I. Broser, Phys. Rev. B: Condens. Matter, 1992, 45, 8977 CrossRef CAS.
- J. Kaur, R. Kotnala, V. Gupta and K. C. Verma, Curr. Appl. Phys., 2014, 14, 749 CrossRef.
- L. Li, W. Wang, H. Liu, X. Liu, Q. Song and S. Ren, J. Phys. Chem. C, 2009, 113, 8460 CAS.
- T. Dietl, H. Ohno, F. Matsukura, J. Cibert and D. Ferrand, Science, 2000, 287, 1019 CrossRef CAS PubMed.
- F. F. Ca, S. Xin, Y. G. Guo and L. J. Wan, Phys. Chem. Chem. Phys., 2011, 13, 2014 RSC.
- J. P. Sahare and V. Kumar, Int. J. Innovative Technol. Exploring Eng., 2012, 3(6), 15 Search PubMed.
- B. Sikora, K. Fronc, I. Kamińska, K. Koper, M. Chwastyk, P. Stępień, W. Paszkowicz, T. Wojciechowski, K. Sobczak and D. Elbaum, RSC Adv., 2015, 5, 1323 RSC.
- X. Q. Meng, H. Peng, Y. Q. Gai and J. Li, J. Phys. Chem. C, 2010, 114(3), 1467 CAS.
- D. Bera, P. H. Holloway and H. Yang, ECS Trans., 2006, 3, 5 CAS.
- J. K. Cooper, S. Gul, S. A. Lindley, J. Yano and J. Z. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 10055 CAS.
- M. Biesinger, L. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2010, 257, 887 CrossRef CAS.
- P. Lazcano, M. Batzill, U. Diebold and P. Häberle, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 035435 CrossRef.
- S. Karamat, R. Rawat, T. Tan, P. Lee, S. Springham, A. Chen and H. Sun, J. Supercond. Novel Magn., 2013, 26, 187 CrossRef CAS.
- L. Chowa, O. Lupana, G. Chaia, H. Khallafa, L. Onoa, B. R. Cuenyaa, I. Tiginyanuf, V. Ursakif, V. Sonteac and A. Schultea, J. Supercond. Novel Magn., 2013, 189, 399 Search PubMed.
- B. Babu, T. Aswani, G. T. Rao, R. J. Stella, B. Jayaraja and R. Ravikumar, J. Magn. Magn. Mater., 2014, 355, 76 CrossRef CAS.
- S. Kumar, F. Singh and A. Kapoor, Int. J. Recent Tech. Eng., 2014, 4(1), 25 Search PubMed.
- L. E. Brus, J. Phys. Chem., 1986, 90, 2555 CrossRef CAS.
- R. Viswanatha, S. Chakraborty, S. Basu and D. D. Sarma, J. Phys. Chem. B, 2006, 110, 22310 CrossRef CAS PubMed.
- M. Willanderv, O. Nur, J. R. Sadaf, M. I. Qadir, S. Zaman, A. Zainelabdin, N. Ban and I. Hussain, Materials, 2010, 3, 2643 CrossRef.
- G. Kiliani, R. Schneider, D. Litvinov, D. Gerthsen, M. Fonin, U. Rudiger, A. Leitenstorfer and R. Bratschitsch, Opt. Express, 2011, 19, 1641–1647 CrossRef CAS PubMed.
- A. van Dijken, E. A. Meulenkamp, D. Vanmaekelbergh and A. Meijerink, J. Phys. Chem. B, 2000, 104(8), 1715 CrossRef CAS.
- N. S. Norberg and D. R. Gamelin, J. Phys. Chem. B, 2005, 109(44), 20810 CrossRef CAS PubMed.
- M. Ghosh and A. K. Raychaudhuri, Nanotechnology, 2008, 19, 445704 CrossRef PubMed.
- S. Brovelli, C. Galland, R. Viswanatha and V. I. Klimov, Nano Lett., 2012, 12(8), 4372 CrossRef CAS PubMed.
- W. K. Liu, K. M. Whitaker, K. R. Kittilstved and D. R. Gamelin, J. Am. Chem. Soc., 2006, 128, 3910 CrossRef CAS PubMed.
- A. W. Cohn, N. Janßen, J. M. Mayer and D. R. Gamelin, J. Phys. Chem. C, 2012, 116, 20633 CAS.
- R. Beaulac, P. I. Archer, S. T. Ochsenbein and D. R. Gamelin, Adv. Funct. Mater., 2008, 18, 3873 CrossRef CAS.
- N. S. Norberg, K. R. Kittilstved, J. E. Amonette, R. K. Kukkadapu, D. A. Schwartz and D. R. Gamelin, J. Am. Chem. Soc., 2004, 126, 9387 CrossRef CAS PubMed.
- D. A. Schwartz, N. S. Norberg, Q. P. Nguyen, J. M. Parker and D. R. Gamelin, J. Am. Chem. Soc., 2003, 125, 13205 CrossRef CAS PubMed.
- K. M. Whitaker, M. Raskin, G. Kiliani, K. Beha, S. T. Ochsenbein, N. Janssen, M. Fonin, W. Rüdiger, A. Leitenstorfer, D. R. Gamelin and R. Bratschitsch, Nano Lett., 2011, 11, 3355 CrossRef CAS PubMed.
- Y. Liu, S. Hasdemir, M. Shayegan, L. N. Pfeiffer, K. W. West and K. W. Baldwin, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 035302 CrossRef.
- W. Pacuski, D. Ferrand, J. Cibert, C. Deparis, A. Gaj, P. Kossacki and C. Morhain, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 035214 CrossRef.
- W. Pacuski, J. Suffczyński, P. Osewski, P. Kossacki, A. Golnik, J. A. Gaj, C. Deparis, C. Morhain, E. Chikoidze, Y. Dumont, D. Ferrand, J. Cibert and T. Dietl, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 1 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |





