A stable aggregate system of silyl ether substituted quinacridone and its aggregation-state changes induced by fluoride-ions: inspiration for a dual guaranteed strategy for probe design†
Peng Chenab,
Guo-Jie Liua,
Yuyang Wanga and
Sean Xiao-An Zhang*a
aState Key Laboratory of Supramolecular Structure and Materials, College of Chemistry, Jilin University, Changchun 130012, China. E-mail: seanzhang@jlu.edu.cn; Tel: +86-431-85153812
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China
First published on 1st March 2016
Abstract
A sterically hindered silyl ether substituted quinacridone was successfully synthesized and its aggregation behaviors in different solvents were studied. It was demonstrated that the aggregate system of t-butyldiphenylsilyl ether substituted quinacridone in tetrahydrofuran was very stable and its aggregation-state could be tunable by fluoride-ion induced intermolecular force (e.g. hydrogen bonding and π–π stacking interactions) destructions and a chemical bond cleavage. The two aggregation-state changes of the system could be applied for a new dual guaranteed strategy for real-time naked-eye detection of fluoride-ions, which could provide assurance for both rapid responsive time and extraordinary selectivity.
Introduction
In recent years, the rapid development of molecular probes for recognizing anions has attracted intensive interest from researchers in diverse fields. However, their sensing mechanisms are limited to resonance energy transfer, intramolecular charge transfer, photo-induced electron transfer, and aggregation-induced emission (AIE), etc.1 In the past several years, a novel sensing mechanism based on disaggregation induced signal changes, which features highly sensitive turn-on fluorescence and synthetic simplicity, has been explored in the probe design for protein sensing2–9 or metal ions detection.10–13 However, the related research is only in its infancy and the anion probes based on disaggregation have remained exceptionally rare.1,14 Especially, the design of anion probes based on disaggregation of organic molecules is still a challenge. Here we make an attempt to use organic dye molecules in this research field.Quinacridone, as a well-known red-violet pigment, is difficult to dissolve in most of the solvents due to forming infinite networks by strong intermolecular hydrogen bonding and π–π stacking interactions.15–17 Its derivatives with proper dispersity are good candidates for designing probes based on disaggregation, but there are no relevant reports so far. According to analysis of the intermolecular forces of some aggregated quinacridone derivatives, it was a good choice to change their aggregation-state by adding ions with high ability to destroy hydrogen bonding and π–π stacking interactions. Considering the high ability of fluoride ion (F−) to form hydrogen bond and F−–π interaction,18 in theory, some aggregates of organic molecules such as quinacridone derivatives, which were aggregated via hydrogen bonding and π–π stacking interactions, should be disaggregated upon addition of F−.
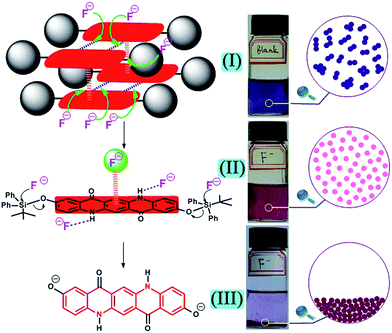
Once the desired F− induced disaggregation of quinacridone derivative was achieved, it might be used to detect F−. Then two problems must be solved. First, the destruction of intermolecular forces by F− has the advantages of rapid response time, but is more likely to have low selectivity over other strongly basic anions (e.g. AcO−, H2PO4−).18 Second, the designed quinacridone derivative must have appropriate dispersity (neither too easy nor too difficult to disperse) in the selected solvent and the resulted aggregate system must have good stability for detection process. Inspired by the above problems, a dual guaranteed strategy to detect F− was proposed and quinacridone derivative 2 was designed. As shown in Fig. 1, in the target molecule 2, t-butyldiphenylsilyl (TBDPS) group with large steric hindrance was introduced to the benzene ring through oxygen atom for the following reasons: on the one hand, the strong steric effect of TBDPS played a significant role to modulate the dispersity of the molecule by suppressing π–π stacking interactions, and the remaining intermolecular forces could be destroyed by F− for the first guarantee to detect F−; on the other hand, silicon–oxygen (Si–O) bond could be characteristically cleaved by F− to provide a second guarantee to detect F−. Furthermore, F− triggered intermolecular force changes were very rapid and the cleavage of Si–O bond needed more response time, which could result in two different phenomena respectively and ensure the unprecedented dual guaranteed design strategy for detection of F−.
 | ||
| Fig. 1 Design diagrams for F− induced aggregation-state changes of quinacridone derivatives and its potential application for a dual guaranteed strategy for probe design. | ||
Results and discussion
In order to examine our new design concept, the designed compound 2 was synthesized from 2,9-dihydroxy quinacridone 1,19 as shown in Scheme 1 (the detail was given in Experimental section). | ||
| Scheme 1 Synthesis of TBDPS substituted quinacridone 2. TBDPSCl = tert-butylchlorodiphenylsilane, DMAP = 4-dimethylaminopyridine. | ||
With 2 in hand, its aggregation-state in solvents was then studied. First, dimethyl sulfoxide (DMSO), in which 2 has good dispersity for 1H NMR analysis, was selected as the solvent to investigate the aggregation-state of 2. The 1H NMR spectra of 2 in DMSO-d6 at different concentrations were recorded in Fig. 2. As the concentration of 2 in DMSO-d6 increased from 1.42 mM to 60.9 mM at 25 °C, the N–H peak was shifted downfield which was attributed to that the formation of hydrogen bonds decreased the electron density around the Ha proton, and the aromatic Hb peak was shifted upfield as a result of the formation of the face-to-face stacking aggregates with gradually increased size.20–24 The above changes in the chemical shifts indicated that compound 2 in DMSO displayed obvious aggregation behavior when the concentration increased from 1.42 mM to 60.9 mM.
To further investigate the aggregation behaviors of 2, UV-vis absorption of 2 at a lower concentration in various solvents was then studied. As anticipated, unlike compound 1 with very poor solubility, 2 attached with sterically hindered silyl groups showed a higher solubility in some common organic solvents. As shown in Fig. 3a, the characteristic UV-vis absorption spectra of 2 (10 μM) in the region from 420 nm to 650 nm in dimethyl formamide (DMF), DMSO, N-methyl pyrrolidone (NMP), methanol (MeOH) and ethanol (EtOH) were all similar and had no maximum absorption wavelength shifts induced by different polarities of solvents, indicating that the interactions between molecules were completely eliminated and therefore 2 was in monomer state in these solvents at the concentration of 10 μM.19,25 In contrast, compared with the absorption region from 450 nm to 580 nm with two maximum wavelengths around 512 nm and 545 nm in the aforementioned solvents, the absorption spectrum of 2 in tetrahydrofuran (THF) had a lower and broader absorption region from 420 nm to 650 nm. The particular phenomena in THF was induced by intermolecular interactions, demonstrating that at the concentration of 10 μM 2 formed aggregates in THF while it was in monomer state in other used solvents. In addition, when 2 (0.1 mM) in various solvents were placed under natural light (Fig. 3b) and irradiated by UV light (Fig. 3c), it was remarkable that both the color and the fluorescence quenching observed with naked eye for the system of 2 in THF were unique, which could further demonstrate that 2 more easily formed aggregates in THF than in other used solvents.
 | ||
| Fig. 3 UV-vis spectra of 2 (10 μM) in various solvents (a) and photo images of 2 (0.1 mM) in various solvents under natural light (b) and irradiated by UV light (λ = 365 nm) (c). | ||
Then, MeOH and THF were selected as the representative solvents for further study. The normalized UV-vis absorption spectra of 2 in MeOH showed two bands at 512 nm and 545 nm (Fig. 4a). The ratio of the intensities of the bands at 512 nm to 545 nm increased obviously when the concentration was above 0.50 mM (Fig. 4a), which was due to monomers of 2 aggregating into higher oligomers as the concentration increased.21,23 As shown in the fluorescence spectra of 2 in MeOH (Fig. 4b), increasing concentration of 2 from 1.0 μM to 0.08 mM, the emission intensity around 575 nm increased gradually and reached a maximum value at a concentration of 0.08 mM. With even higher concentration, this emission intensity began to decrease and an obvious red shift of the emission band could be observed, which was owing to the aggregation-caused quenching effect. The decrease became obvious when the concentration was above 0.50 mM. This turning point further indicated the transition from monomer to aggregate in MeOH while the concentration increased from 1.0 μM to 0.50 mM.21,23 Unlike the phenomena in MeOH, 2 in THF already formed an obvious precipitate at the concentration of 0.20 mM, and the turning points in UV-vis and fluorescence spectra of 2 in THF did not appear at concentrations ranging from 1.5 μM to 0.05 mM. Analysis of the fluorescence spectra (Fig. 4c) demonstrated that the emission intensities both at 545 nm assignable to monomer emission and 625 nm assignable to aggregate emission26 increased gradually in the above concentration range. The above spectra suggested that 2 more easily formed aggregates in THF even at as low concentration as 1.5 μM. Noteworthily, in concentration ranges from 1.5 μM to 0.05 mM, the aggregates were uniformly dispersed in THF, and their UV-vis and fluorescence spectra had no obvious change after standing for 5 days at 25 °C, demonstrating the high stability of this system. Thus, the THF system of 2 in proper concentration ranges could be extremely useful to study its disaggregation behaviors induced by F− as designed originally.
 | ||
| Fig. 4 Normalized UV-vis spectra of 2 in MeOH (a), fluorescence emission spectra of 2 in MeOH (b), fluorescence excitation and emission spectra of 2 in THF (c) at different concentrations. | ||
Then, its behaviors in the presence of F− under different experimental conditions were observed. As shown in Fig. 5, after adding F− from n-Bu4N+ salt to the THF system of 2 (10 μM) for 1 minute, the UV-vis absorption spectra changed from the original broader absorption region to a narrower absorption band with two explicit peaks at 516 nm and 552 nm (Fig. 5a), and the fluorescence spectra changed from the low emission band at 548 nm to a higher characteristic emission band at 595 nm (Fig. 5b). According to the analysis for the spectra of 2 in various solvents (Fig. 3a and 4), the F− induced spectra changes in Fig. 5 could be attributed to that the aggregates of 2 changed into monomers in THF after adding F−.
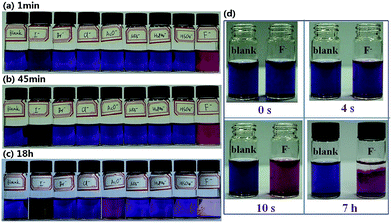
With the successful achievement of F− induced disaggregation of 2 in THF, we then focused our attention to use this system for dual guaranteed detection of F−. First, we investigated the selectivity of 2 toward F−. The UV-vis absorption spectra of 2 (10 μM) in THF treated with the representative anions (2 equiv.) such as AcO−, F−, Cl−, Br−, I−, H2PO4−, HSO4−, and NO3−, all in n-Bu4N+ salts, were also shown in Fig. 5a. Unlike the UV-vis spectra changes after adding F− for 1 minute, no noticeable changes were observed after adding other anions for the same time. Furthermore, as shown in Fig. 5c, when F− was added to the system of 2 (20 μM) in THF, the switch of the color from purple to rose red was clearly observable by the naked eye, but no color changes were observed when the other anions were added. Interestingly, after standing for about 1 day, only the system treated with F− formed an obvious magenta precipitate and gave a colorless transparent solution (Fig. 5d). The distinctive phenomena confirmed the feasibility of using 2 for naked-eye detection of F−.
In order to conveniently study the present F− detection system, 0.05 mM of 2 and 0.1 mM of representative anions (all in n-Bu4N+ salts) in THF were employed to further enhance the visual effects and the results were showed in Fig. 6. Under such conditions, it was clear that two distinct phenomena took place successively. First, as soon as F− was added to the THF system of 2, the color changed from purple to rose red at almost exactly the same time (Fig. 6d), while adding other anions could not induce obvious color change (Fig. 6a). Indeed, within 45 minutes after adding the anions, except for F− which induced a rapid and distinct color change and I− which induced a slow and indistinguishable color change, other anions had an almost negligible impact on the system color, as shown in Fig. 6b. Second, as time went on, after 18 hours, the system containing AcO− also changed to rose red (Fig. 6c), which indicated that the interferences from some strongly basic anions still remained to some extent in the first step for detection of F−; but at the same time a unique magenta precipitate arose from the system containing F−, accompanied with the formation of a colorless transparent solution (Fig. 6c), which provided a second guarantee of extraordinary selectivity for F−. The video for the whole process of naked-eye detection of F− can be found in the ESI.†
In addition, the influence of water on the above F− detection process was also investigated. Unfortunately, even a small amount of water could induce the switch of the color of the system from purple to rose red quickly, which was the same as that induced by F− and had serious interference to the first step of the F− detection process.
To conjecture the mechanism of the second precipitate formation step of the above process, the 1H NMR spectra in CD3OD of n-Bu4N+ salt of 1 (Fig. 7a) which was prepared from the chemical removal of TBDPS groups in 2 by using (n-Bu)4NF, the precipitate from the F− detection process (Fig. 7b) and 2 (Fig. 7c) were given in Fig. 7. Obviously, although having some minor chemical shift changes induced by impurity, to some extent, the 1H NMR spectrum for the precipitate (Fig. 7b) was the combination of the spectra in Fig. 7a and c, indicating that the precipitate from the F− detection process was exactly derived from the F− triggered partially cleavage of Si–O bonds of 2.
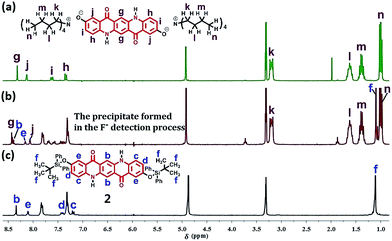 | ||
| Fig. 7 The 1H NMR spectra in CD3OD of tetrabutylammonium salt of compound 1 (a), the precipitate formed in the F− detection process (b) and the compound 2 (c). | ||
Therefore, based on the above analysis of Fig. 5 and 7, a proposed mechanism for the whole process of F− detection was elucidated in Fig. 8. First, when F− was added to the system, the stronger hydrogen bonding interactions between F− and N–H moiety could destroy the intermolecular hydrogen bonds of 2, and the strong F−–π interactions could destroy the π–π stacking interactions in 2. They together made the aggregates (I, Fig. 8) translate to monomers (II, Fig. 8). This first step happened very fast which was the key factor to realize the real-time assay. Second, the F− triggered cleavage of Si–O bonds happened and the corresponding product was very difficult to disperse in THF, generating a precipitate accompanied with a very obvious transparent solution (III, Fig. 8). Although the second step needed several hours, it was very characteristic and could further guarantee the extraordinary selectivity for F−. The mechanism containing two aggregation-state change steps would help us to know how the system of 2 could realize real-time and dual guaranteed detection of F−.
Experimental section
General methods
All reagents were purchased from commercial sources and used without treatment unless otherwise indicated. All reactions were carried out under an argon atmosphere. All reactions were monitored by thin-layer chromatography (TLC) on gel F254 plates using UV light as visualizing agent (if applicable), and a solution of ammonium molybdate tetrahydrate (50 g L−1) in EtOH followed by heating as developing agents.1H NMR and 13C NMR spectra were recorded in DMSO-d6 or CD3OD solution on a Varian 300 MHz instrument (300 MHz for 1H NMR, 75 MHz for 13C NMR) or a Bruker 500 MHz instrument (500 MHz for 1H NMR, 125 MHz for 13C NMR). Chemical shifts were denoted in ppm (δ), and calibrated by using residual undeuterated solvent (DMSO-d5 (2.50 ppm), CHD2OD (3.31 ppm) or tetramethylsilane (0.00 ppm)) as internal reference for 1H NMR and the deuterated solvent (DMSO-d6 (39.51 ppm)) as internal standard for 13C NMR. Coupling constants reported in Hz constitute 3J (H, H) and 4J (H, H) coupling constants. The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, br = broad, m = multiplet. IR spectra were recorded on Nicolet Avatar 360 FT-IR spectrometer. MS data were measured on Agilent 1290-microTOF Q II by means of the ESI technique. Water used in all experiments was doubly distilled and purified by a Milli-Q system (Millipore, USA). Absorption spectra were measured on an AnalitikJena Specord®210 plus UV/VIS spectrophotometer, path length was 1 cm. All fluorescence measurements were carried out on a Shimadzu-RF5301 fluorescence spectrometer.
Synthesis
The compound 1 (Scheme 1) was synthesized according to the steps reported in the literature.19The compound 2 (Scheme 1) was synthesized as follows.
The compound 1 (1.0 g, 2.99 mmol), tert-butylchlorodiphenylsilane (3.30 g, 11.96 mmol, 4.0 equiv.), triethylamine (2.5 mL, 17.94 mmol, 6.0 equiv.) and 4-dimethylaminopyridine (0.73 g, 5.98 mmol, 2.0 equiv.) were added into DMF (50 mL) under argon, and the resulting reaction mixture was stirred at room temperature overnight. After removing DMF under high vacuum while stirring, to the resulting residue was added CH2Cl2 (20 mL) and water (20 mL). Then, the precipitate was filtered, washed with CH2Cl2, water and Et2O, dried in vacuum, affording the compound 2 (1.91 g, 80% yield).
1H NMR (300 MHz, CD3OD): δ = 8.34 (s, 2H), 8.10 (d, J = 2.6 Hz, 2H), 7.85–7.80 (m, 8H), 7.42 (dd, J = 9.0, 2.6 Hz, 2H), 7.34–7.27 (m, 12H), 7.19 (d, J = 9.0 Hz, 2H), 1.11 (s, 18H) ppm; 1H NMR (300 MHz, DMSO-d6, 1.42M): δ = 11.66 (s, 2H), 8.42 (s, 2H), 8.05 (d, J = 2.5 Hz, 2H), 7.82–7.79 (m, 8H), 7.62 (dd, J = 9.0, 2.5 Hz, 2H), 7.39–7.27 (m, 14H), 0.96 (s, 18H) ppm;13C NMR (125 MHz, DMSO-d6): δ = 176.5, 147.7, 137.5, 135.0, 134.7, 134.5, 129.3, 127.5, 127.3, 123.7, 119.0, 117.4, 115.0, 113.0, 26.5, 19.2 ppm; IR: ν = broad band 3680–3110 peaking at 3380, 3075, 3050, 2960, 2930, 2890, 2860, 1615–1550, 1475, 1210, 1090, 880, 700 cm−1; MS (ESI): m/z calcd for C54H52N3O4Si2 [M + H + CH3CN]+: 862.3; found: 862.2 [M + H + CH3CN]+.
For complete experimental procedures and copies of the NMR spectra, see the ESI.†
Conclusions
In conclusion, a novel TBDPS ether substituted quinacridone 2 was synthesized and showed obvious aggregation behavior in several organic solvents. Noteworthily, the aggregates of 2 in THF at proper concentration ranges had high stability. F− was demonstrated to be a good regent to induce two aggregation-state changes (disaggregation and precipitation) of 2 in THF, which was induced by F− triggered intermolecular force (e.g. hydrogen bonding and π–π stacking interactions) changes and the characteristic chemical bond cleavage in chronological order. The above two aggregation-state changes of this system could be applied to an unprecedented dual guaranteed strategy for detecting F−, which could realize real-time naked-eye detection and provide assurance for rapid responsive time and extraordinary selectivity for F−. To the best our knowledge, this promising ingenious process has not been reported before, therefore opening a new door for dual guaranteed strategy for probe design.Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC No. 21302061, 51373068), Jilin Province Science & Technology Development Program (No. 20140520084JH), China Postdoctoral Science Foundation (No. 2013T60318, 2012M510130).Notes and references
- D. Zhai, W. Xu, L. Zhang and Y.-T. Chang, Chem. Soc. Rev., 2014, 43, 2402–2411 RSC.
- C.-C. You, O. R. Miranda, B. Gider, P. S. Ghosh, I.-B. Kim, B. Erdogan, S. A. Krovi, U. H. F. Bunz and V. M. Rotello, Nat. Nanotechnol., 2007, 2, 318–323 CrossRef CAS PubMed.
- M. A. Azagarsamy, P. Sokkalingam and S. Thayumanavan, J. Am. Chem. Soc., 2009, 131, 14184–14185 CrossRef CAS PubMed.
- Y. Takaoka, T. Sakamoto, S. Tsukiji, M. Narazaki, T. Matsuda, H. Tochio, M. Shirakawa and I. Hamachi, Nat. Chem., 2009, 1, 557–561 CrossRef CAS PubMed.
- K. Mizusawa, Y. Ishida, Y. Takaoka, M. Miyagawa, S. Tsukiji and I. Hamachi, J. Am. Chem. Soc., 2010, 132, 7291–7293 CrossRef CAS PubMed.
- K. Mizusawa, Y. Takaoka and I. Hamachi, J. Am. Chem. Soc., 2012, 134, 13386–13395 CrossRef CAS PubMed.
- D. Amado Torres, M. Garzoni, A. V. Subrahmanyam, G. M. Pavan and S. Thayumanavan, J. Am. Chem. Soc., 2014, 136, 5385–5399 CrossRef CAS PubMed.
- P. Anees, S. Sreejith and A. Ajayaghosh, J. Am. Chem. Soc., 2014, 136, 13233–13239 CrossRef CAS PubMed.
- J. Guo, J. Zhuang, F. Wang, K. R. Raghupathi and S. Thayumanavan, J. Am. Chem. Soc., 2014, 136, 2220–2223 CrossRef CAS PubMed.
- Y. B. Ruan, A. F. Li, J. S. Zhao, J. S. Shen and Y. B. Jiang, Chem. Commun., 2010, 46, 4938–4940 RSC.
- C. Chen, H. Dong, Y. Chen, L. Guo, Z. Wang, J. J. Sun and N. Fu, Org. Biomol. Chem., 2011, 9, 8195–8201 CAS.
- C. Chen, R. Wang, L. Guo, N. Fu, H. Dong and Y. Yuan, Org. Lett., 2011, 13, 1162–1165 CrossRef CAS PubMed.
- H. Zhu, J. Fan, H. Chen, Z. Tang, G. Wang and N. Fu, Dyes Pigm., 2015, 113, 181–188 CrossRef CAS.
- J. Liu, X. Yang, K. Wang, R. Yang, H. Ji, L. Yang and C. Wu, Chem. Commun., 2011, 47, 935–937 RSC.
- G. Lincke, Dyes Pigm., 2000, 44, 101–122 CrossRef CAS.
- G. Lincke, Dyes Pigm., 2002, 52, 169–181 CrossRef CAS.
- S. Q. Lomax, Stud. Conserv., 2005, 50, 19–29 CrossRef.
- Y. Zhou, J. F. Zhang and J. Yoon, Chem. Rev., 2014, 114, 5511–5571 CrossRef CAS PubMed.
- P. H. Liu, T. He and C. P. Chang, J. Photochem. Photobiol., A, 2000, 137, 99–104 CrossRef CAS.
- J. Wu, A. Fechtenkotter, J. Gauss, M. D. Watson, M. Kastler, C. Fechtenkotter, M. Wagner and K. Mullen, J. Am. Chem. Soc., 2004, 126, 11311–11321 CrossRef CAS PubMed.
- K. Ye, J. Wang, H. Sun, Y. Liu, Z. Mu, F. Li, S. Jiang, J. Zhang, H. Zhang, Y. Wang and C. M. Che, J. Phys. Chem. B, 2005, 109, 8008–8016 CrossRef CAS PubMed.
- H. Sun, K. Ye, C. Wang, H. Qi, F. Li and Y. Wang, J. Phys. Chem. A, 2006, 110, 10750–10756 CrossRef CAS PubMed.
- J. Wang, Y. Zhao, C. Dou, H. Sun, P. Xu, K. Ye, J. Zhang, S. Jiang, F. Li and Y. Wang, J. Phys. Chem. B, 2007, 111, 5082–5089 CrossRef CAS PubMed.
- C. Wang, K. Wang, Q. Fu, J. Zhang, D. Ma and Y. Wang, J. Mater. Chem. C, 2013, 1, 410 RSC.
- J. Mizuguchi and T. Senju, J. Phys. Chem. B, 2006, 110, 19154–19161 CrossRef CAS PubMed.
- S. De Feyter, A. Gesquière, F. C. De Schryver, U. Keller and K. Müllen, Chem. Mater., 2002, 14, 989–997 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization date and available video. See DOI: 10.1039/c6ra01487a |
| This journal is © The Royal Society of Chemistry 2016 |