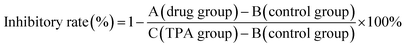Synergistic inhibitory effects of naproxen in combination with magnolol on TPA-induced skin inflammation in mice
Yuan Yue†
a,
Wenfeng Liu†b,
Wei Zhoua,
Min Chena,
Boxin Huanga,
Lanyue Zhanga,
Zhenshi Wanga,
Yan Hea,
Kun Zhangab,
Xi Zhengac and
Zhiyun Du*a
aLaboratory of Natural Medicinal Chemistry & Green Chemistry, Faculty of Light Industry and Chemical Engineering, Guangdong University of Technology, Guangzhou, 510006, China. E-mail: zhiyundu@gdut.edu.cn; Tel: +86-20-3932-2235
bSchool of Chemical & Environmental Engineering, Wuyi University, Jiangmen, 529020, China
cSusan Lehman Cullman Laboratory for Cancer Research, Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers, The State University of New Jersey, Piscataway, NJ 08854, USA
First published on 12th April 2016
Abstract
The combination of naproxen with herbs having a range of pharmacological effects is a potential alternative to improve their bioactivity and limit their adverse effects. Inspired by magnolol, an active constituent found in the bark of Magnolia officinalis, we therefore evaluated the efficacy of naproxen (N) plus magnolol (M) in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin inflammation. During application, treatment with N and M significantly reduced skin inflammation, and the combination of N and M resulted in a synergistic effect. Further mechanistic investigations clearly demonstrated that A3 (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, mol mol−1) markedly suppressed TPA-induced pro-inflammatory cytokines and COX-2 expressions, inhibited NF-κB activity, downregulated IκBα and IκB kinase (IKK) activities, and inhibited PI3K/Akt and PI3K/PKC signaling pathways. These results indicated that a combination of N and M effectively inhibited TPA-induced skin inflammation via blocking PI3K/Akt/IKK and PI3K/PKC/IKK signaling pathways.
3, mol mol−1) markedly suppressed TPA-induced pro-inflammatory cytokines and COX-2 expressions, inhibited NF-κB activity, downregulated IκBα and IκB kinase (IKK) activities, and inhibited PI3K/Akt and PI3K/PKC signaling pathways. These results indicated that a combination of N and M effectively inhibited TPA-induced skin inflammation via blocking PI3K/Akt/IKK and PI3K/PKC/IKK signaling pathways.
1. Introduction
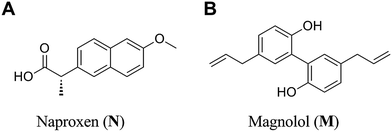
Concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs) and Chinese herbal medicines is frequent. Such interactions may lead to beneficial outcomes.1 In an open-label, randomized controlled trial, patients suffering from acute arthritis with elevated C-reactive protein revealed similar clinical and laboratory improvements when consuming 200 mg diclofenac or 50 mg diclofenac plus Urtica dioica L. 50 g per day. The authors suggested that U. dioica L. enhanced the antirheumatic effectiveness of diclofenac.2 In another trial, co-administration diclofenac and Hu-zhang-tong-feng granule significantly improved the physiological, psychological and social functions.3 More herbs were found to enhance the anti-inflammatory activities of NSAIDs, including Aloe vera L.,4 citral isolated from C. insularimontanum Hayata,5 Ping-wei-san6 and Tridax procumbens L.7Naproxen (Fig. 1A), a potent non-selective cyclooxygenase (COX) inhibitor, has been widely used in the treatment of inflammation associated diseases, such as rheumatoid arthritis,8 joint diseases9 and ankylosing spondylitis.10 It also plays a vital role in the prevention of cancers and possesses analgesic and antipyretic properties.11,12
Magnolol (Fig. 1B), a naturally occurring polyphenol,13 is well known as a dietary supplement or cosmetic additive. Due to its strong antioxidant properties associated with anti-inflammatory activity, magnolol is studied as an anti-tumor and chemo-preventative agent. It was indicated that magnolol had the ability to suppress expression of COX-2 and pro-inflammatory cytokines.14,15 Furthermore, magnolol was reported to attenuate the release of pro-inflammatory cytokines by inhibiting nuclear factor kappa-B (NF-κB) signaling.16 Recent studies suggested that magnolol might block NF-κB activation by attenuation of MAPKs and PI3K/Akt/IKK pathway, which interrupts the degradation of IκBα and further downstream iNOS and COX-2 gene expression to prevent or mitigate chronic inflammation associated condition.17
Although the opportunities for co-treatment of NSAIDs with herbs are considerably high, experimental data on the beneficial interaction between NSAIDs and herbs is currently lacking.1 The primary goals of this study were to compare the anti-inflammatory activity of magnolol, naproxen and the concomitant use of naproxen and magnolol, and to investigate their mechanisms. Firstly, the inhibitory effects regarding the co-administration of naproxen and magnolol were evaluated using a TPA-induced mouse ear oedema model. Secondly, immunohistochemical analysis clearly demonstrated that A3 (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, mol mol−1) was found to have synergistic effects in inhibiting TPA-induced pro-inflammatory cytokines and COX-2 expressions by downregulating NF-κB activation as well as the upstream, PI3K/Akt/IKK and PI3K/PKC/IKK signaling pathways.
3, mol mol−1) was found to have synergistic effects in inhibiting TPA-induced pro-inflammatory cytokines and COX-2 expressions by downregulating NF-κB activation as well as the upstream, PI3K/Akt/IKK and PI3K/PKC/IKK signaling pathways.
2. Experimental procedures
2.1 Chemicals and animals
Magnolol (purity >95%) and naproxen were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO). TPA (purity >99.9%) was purchased from Henan Cancer Hospital (Henan, China). All other chemicals used were purchased from commercial sources. Primary antibodies of specific proteins were purchased from various locations as listed below: for IL-1β, IL-6, TNF-α, COX-2, anti-PI3K p85, anti-phospho-PI3K p85, PKC and anti-PKC from Bioss Biotechnology Co. (Beijing, China), for anti-rabbit and anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibodies, anti-Ser473 Akt, anti-IKK, anti-Ser32 IκB-α, anti-Ser536 p65, anti-phospho-Ser473 Akt, anti-phospho-Ser32 IκB-α, and anti-phospho-Ser536 p65 antibodies from Beyotime Biotechnology Co. (Beijing, China).Male BALB/c mice (6 weeks old, 3 per group for each assay) housed (three per cage) and fed standard mouse chow diet and tap water ad libitum. They were supplied from the Experimental Animal Centre of Guang Dong Province (approval documents: SCXK/20130002). For the mouse ear oedema model, both ears of male BALB/c mice were topically treated with 15 μL vehicle (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DCM = 20
DCM = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80), magnolol, naproxen or mixture of magnolol and naproxen (10
80), magnolol, naproxen or mixture of magnolol and naproxen (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–1
1–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) in 15 μL vehicle, 30 min prior to TPA treatment (0.008 nM). The mice were euthanized after 6 h. Ear punches (6 mm in diameter) were taken and weighed. All experiments were performed in compliance with the relevant laws and institutional guidelines, and the Center of Animal Test of SUN Yat-sen University has approved the experiments. The inhibitory rate (%) was calculated by this formula:
10) in 15 μL vehicle, 30 min prior to TPA treatment (0.008 nM). The mice were euthanized after 6 h. Ear punches (6 mm in diameter) were taken and weighed. All experiments were performed in compliance with the relevant laws and institutional guidelines, and the Center of Animal Test of SUN Yat-sen University has approved the experiments. The inhibitory rate (%) was calculated by this formula:
2.2 Determination of IL-1β, IL-6, TNF-α and COX-2
After sacrifice, both ears of BALB/c mice were removed and the deparaffinised skin sections (4 μm) were incubated with 1.2% H2O2 in PBS to quench the endogenous peroxidase activity. The primary antibody of the proliferating cell nuclear antigen was diluted 100 times then applied to each section overnight at 4 °C. After washing with PBS, the sections were incubated with a biotin-conjugated horseradish peroxidase antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) for 1 h at room temperature. Finally, the peroxidase was detected using the 3,3-diaminobenzidine tetrahydrochloride reaction, which produced the brown label in the epidermal tissue. The numbers of positive staining cells were counted in five different fields at both ends as well as in the middle for each section.
200) for 1 h at room temperature. Finally, the peroxidase was detected using the 3,3-diaminobenzidine tetrahydrochloride reaction, which produced the brown label in the epidermal tissue. The numbers of positive staining cells were counted in five different fields at both ends as well as in the middle for each section.
2.3 Assays of PKC delta, p-PKC delta, PI3k p85, p-PI3k p85, Akt, p-Akt, IKK, IκBα, p-IκBα, p65 and p-p65
NF-κB transcriptional activity, PI3K/Akt and PI3K/PKC signalling pathways were measured using an immunohistochemistry analysis. Mouse ears were removed in toto, fixed in 10% formalin, decalcified in an EDTA buffer, subjected to a series progression of dehydration, and then embedded in paraffin. Samples were serially sectioned at 4 μm and processed routinely for staining. The histological changes were examined under a microscope.2.4 Histological appearance of mouse ears
BALB/c mice were all sacrificed after 6 hours. Both ears were removed in toto, fixed in 10% formalin, decalcified in EDTA buffer, subjected in a series progression of dehydration, and then embedded in paraffin. Samples were serially sectioned at 4 μm and processed routinely for H&E staining. The histological changes were obtained under a microscope.2.5 Scoring the expression of biomarkers
For each male BALB/c mouse, two experienced investigators unaware of the specimens' identity independently scored ≥5 ducts per histologic type of TPA-induced ductal lesion not aware of the (×200). For PKC delta, p-PKC delta, PI3k p85, p-PI3k p85, Akt, p-Akt, IKK, IκBα, p-IκBα, p65 and p-p65, we used integrated optical density (IOD) as the semi-quantitative scoring system.182.6 Statistical analysis
The results are presented as the mean ± SE. Data are presented as mean ± S.D. Comparison of more than two groups was made using a one-way analysis of variance ANOVA followed by a Dunnett t test. P* values less than 0.05 (P* < 0.05), P** values less than 0.01 (P** < 0.01), were considered indicative of significance.3. Results and discussion
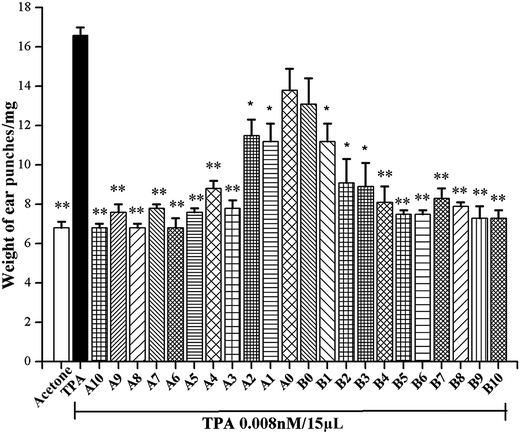
3.1 Effects of naproxen in combination with magnolol on TPA-induced mouse ear oedema
Herb–drug interactions between Chinese herbal medicines and NSAIDs have become an important issue because concomitant use of NSAIDs and herbs are increasing globally.1 It was reported that magnolol had been considered as a no safety concern food additive,19 with strong inflammatory and neuroprotective activities. This study proposes to combine naproxen treatments with magnolol, for the purpose of achieving synergistic anti-inflammatory effects. According to our previous publication, the concentration at which the initial screening anti-inflammatory activities of N and M were conducted was selected as 0.75 μM.20 As shown in Fig. 2, ear oedema was induced by the topical application of TPA alone. Compared with the acetone-treated mouse ears, the average weight of TPA-treated ear punches increased from 6.6 mg to 16.5 mg. Moreover, pre-treatment with N, M and these combinations (concentration ratio of N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M from 10
M from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) showed decreased weight in the TPA-stimulated mouse ear punches. Results indicated that all the combinations of N and M were found to have synergistic effects in suppressing TPA-stimulated mouse ear oedema (Table 1).
10) showed decreased weight in the TPA-stimulated mouse ear punches. Results indicated that all the combinations of N and M were found to have synergistic effects in suppressing TPA-stimulated mouse ear oedema (Table 1).
| No. | N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M (mol mol−1) M (mol mol−1) |
Inhibition (%) | No. | N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M (mol mol−1) M (mol mol−1) |
Inhibition (%) |
|---|---|---|---|---|---|
| a Each bar represents the mean ± SE from 3 mice. Statistical significance relative to TPA group was indicated, *P < 0.05, **P < 0.01. | |||||
| A0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
28.6 ± 7.8 | B0 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
35.7 ± 6.6 |
| A1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55.1 ± 5.4* | B1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
55.1 ± 5.4* |
| A2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
55.7 ± 4.8* | B2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
76.5 ± 7.2* |
| A3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
93.8 ± 2.4** | B3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
78.6 ± 7.2* |
| A4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
83.5 ± 2.4** | B4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
86.7 ± 4.8** |
| A5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
95.9 ± 1.2** | B5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92.8 ± 1.2** |
| A6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
100 ± 3.0** | B6 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
92.8 ± 1.2** |
| A7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
86.6 ± 1.2** | B7 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
84.7 ± 3.0** |
| A8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
100 ± 1.2** | B8 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
88.8 ± 1.2** |
| A9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
88.7 ± 2.4** | B9 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94.9 ± 3.6** |
| A10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
100 ± 1.2** | B10 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
94.9 ± 2.4** |
In addition, Table 1 also shows that among these combinations, 19 combinations were found to be more potent than N and M in decreasing in the average weight of TPA-induced ear punches (55.1–100% decrease), and 9 combinations exhibited up to a 90% decrease in the average weight of TPA-induced ear punches. Therefore, A3 (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), significantly decreased the weight of ear punches by 93.3%, and was selected for further assessment of the anti-inflammatory mechanism.
3), significantly decreased the weight of ear punches by 93.3%, and was selected for further assessment of the anti-inflammatory mechanism.
3.2 Histology
Histological sections of the ears submitted to a single TPA application revealed a significant increase in the dermis thickness and infiltrating inflammatory cells (Fig. 3B). As described in Fig. 3C–E, when compared to the acetone-treated ears (Fig. 3A, vehicle), treatment with N and M, either alone or in combination, decreased the level of inflammatory cell infiltration. Furthermore, A3 showed more significant morphological alterations than in N and M alone.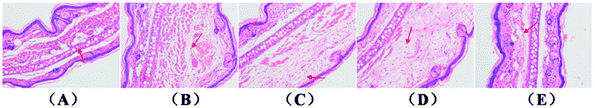 | ||
| Fig. 3 H&E staining for histological changes of TPA-induced mouse ears. (A) Acetone, (B) TPA, (C) magnolol, (D) naproxen, (E) A3. Magnification 200×. | ||
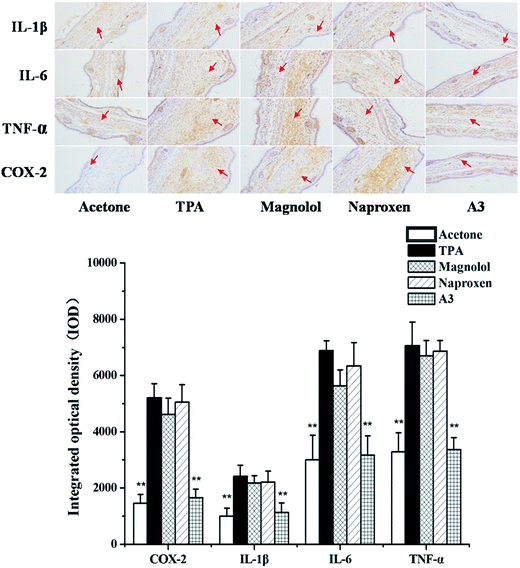
3.3 Inhibition of A3 on TPA-induced pro-inflammatory cytokines and COX-2 expressions
It was reported that pro-inflammatory cytokines and COX-2 play a key role in the pathogenesis of inflammation.21–23 Therefore, to assess the inhibitory effects of the single compound, N, M, and the combination of N with M on the IL-1β, IL-6, TNF-α and COX-2 expressions, the levels of IL-1β, IL-6, TNF-α and COX-2 were evaluated using an immunohistochemical analysis. As shown in Fig. 4, the expression of pro-inflammatory cytokines and COX-2 increased after TPA exposure. However, pre-treatments with N and M alone, or their combinations (A3), effectively inhibited TPA-induced pro-inflammatory cytokines and COX-2 expression. In all cases, the pre-treatment with A3 (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) led to a significant decrease in the expression of pro-inflammatory cytokines and COX-2.
3) led to a significant decrease in the expression of pro-inflammatory cytokines and COX-2.
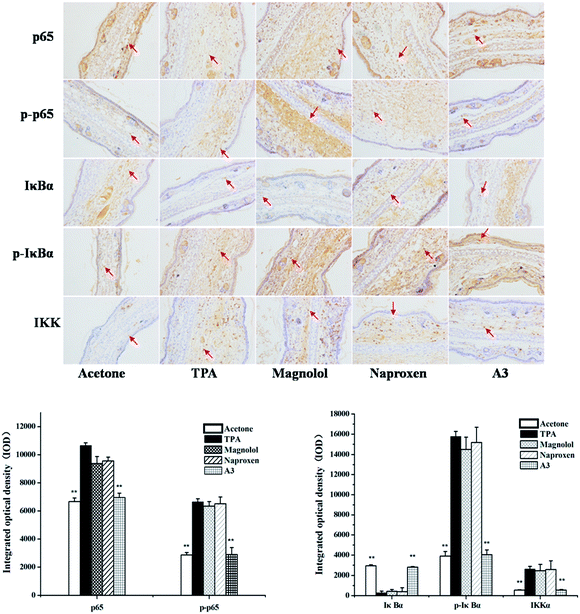
3.4 Effects of A3 on TPA-induced NF-κB activation by targeting IKK
NF-κB was reported to be critical for regulating gene expression in TPA-induced inflammatory responses.24 p65, one of NF-κB subunits, was distributed mostly in the cytoplasm in unstimulated conditions. After stimuli by TPA, the level of p65 was significantly increased, which was markedly reduced by treatment with A3. In addition, combining the naproxen treatment with magnolol (A3) also resulted in a synergistic effect on the level of phosphorylated p65. It was suggested that the A3 treatment significantly inhibited TPA-stimulated NF-κB promoter activity.It was demonstrated that IKK, stimulated by TPA, activated and phosphorylates IκBα, which form complex with NF-κB in normal conditions.25 As described in Fig. 5, TPA treatment resulted in activated IKK and IκBα phosphorylation compared to the acetone-treated control group, and A3 treatment suppressed the activities of IKK and IκBα and IκBα phosphorylation.
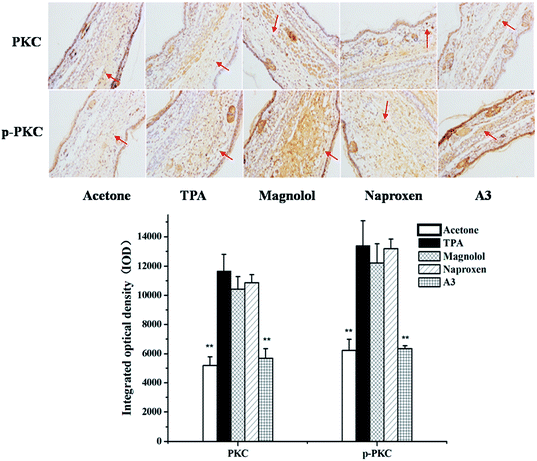
3.5 Effects of A3 on the TPA-induced activation and phosphorylation of PKC
It was reported that PKC is well connected with TPA.26 Therefore, in order to investigate whether the combination of N with M has any influence on TPA-stimulated activation and phosphorylation of PKC an immunohistochemistry analysis for the expression of PKC was performed. As shown in Fig. 6, TPA treatment induced PKC expression, while N, M and the combination of N with M (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) inhibited the expression and phosphorylation of PKC, and the synergistic effect was significant with the combination treatment (A3).
3) inhibited the expression and phosphorylation of PKC, and the synergistic effect was significant with the combination treatment (A3).
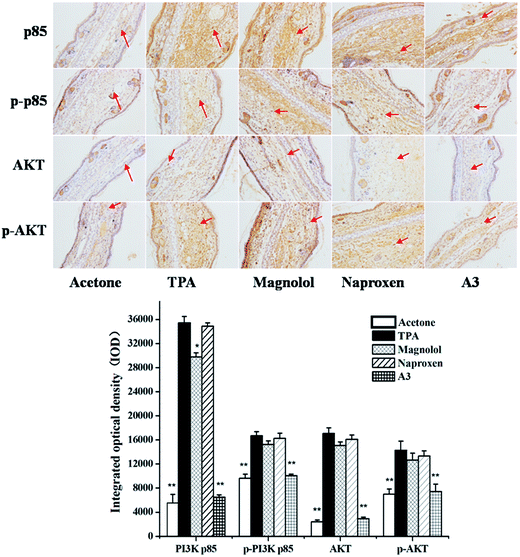
3.6 Effects of A3 on TPA-induced PI3K/Akt signalling pathway
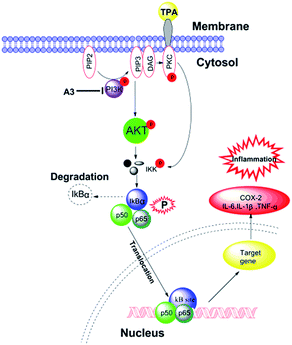
It was demonstrated that an active PI3K/Akt signalling pathway resulted in NF-κB activation.27,28 Herein, to determine whether such synergism is specific to the PI3K/Akt signalling pathway TPA-induced mouse ear oedema model, we also performed the A3 treatment in the TPA-induced mouse ear oedema model. The results clearly revealed that p85, p-p85, Akt, and p-Akt expressions were markedly up-regulated by TPA-induction. As described in Fig. 7, A3 showed significant synergistic suppression of p85 and Akt expressions. Additionally, A3 had synergistic inhibitory effects on the phosphorylations of p85 and Akt. Furthermore, on-going experiments are being conducted to further study these synergistic anti-inflammatory effects. Taken together, these results revealed that proposed mechanism of naproxen in combination with magnolol (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M = 1
M = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) is presented in Fig. 8.
3) is presented in Fig. 8.
4. Conclusions
In summary, combining naproxen treatment with magnolol, which is a naturally occurring polyphenol with significantly anti-inflammatory activity, is urgent need to use for transdermal application to enhance their anti-inflammatory activity. The results indicated that naproxen in combination with magnolol have synergistic inhibitory effects on TPA-induced inflammation by blocking PI3K/PKC/IKK and PI3K/Akt/IKK pathways. Bioactive constituents, found in traditional Chinese medicines, possess various pharmacological activities. Herein, combining them with naproxen may be a beneficial therapy for the treatment of inflammation-associated diseases. Moreover, it is well-recognized that combining naproxen treatment with active constituents that occur naturally in herbs, plants and citrus fruits lead to limited side effects.5 Hence, inspired by magnolol, which is an active constituent identified as a neurologically active agent with anxiolytic, sedative, neuroprotective and anti-convulsant results in animal models,29–31 a combination of naproxen and magnolol may be an attractive strategy to retain naproxen's anti-inflammatory properties while avoiding side effects.Acknowledgements
Financial support was provided by the National Natural Science Foundation of PR China (Grant No. 21272043 and 81272452) and the Project of Guangdong Science & Technology Collaboration (2012b091000170). We thank LetPub for its linguistic assistance during the preparation of this manuscript.Notes and references
- S. Y. Fong, T. H. Efferth and Z. Zuo, Expert Opin. Drug Metab. Toxicol., 2014, 10, 1711–1739 CrossRef CAS PubMed.
- S. Chrubasik, W. Enderlein, R. Bauer and W. Grabner, Phytomedicine, 1997, 4, 105–108 CrossRef CAS PubMed.
- X. Y. Xiao, Y. F. Wang and R. Xu, Zhongguo Zhongxiyi Jiehe Zazhi, 2012, 32, 620–623 Search PubMed.
- V. Velam, P. R. Yalavarthi, C. Sundaresan, K. Vandana, T. B. Dudala, H. Kodavatikanti and H. C. Vadlamudi, Int. J. Pharm. Invest., 2013, 3, 212–216 CrossRef PubMed.
- M. I. Ortiz, M. P. González-García, H. A. Ponce-Monter, G. Castañeda-Hernández and P. Aguilar-Robles, Phytomedicine, 2010, 18, 74–79 CrossRef CAS PubMed.
- C. Tiong, H. Y. Liu and W. L. Liang, J. Food Drug Anal., 2011, 19, 509–516 CAS.
- S. Das, M. K. Das and S. P. Basu, J. Pharm. Sci. Res., 2009, 1, 123–126 CAS.
- C. S. Crowson, K. P. Liao, J. M. Davis, D. H. Solomon, E. L. Matteson, K. L. Knutson, M. A. Hlatky and S. E. Gabrie, Am. Heart J., 2013, 166, 622–628 CrossRef CAS PubMed.
- E. F. Ekman, J. S. Gimbel, A. E. Bello, M. D. Smith, D. S. Keller, K. M. Annis, M. T. Brown, C. R. West and K. M. Verburg, J. Rheumatol., 2014, 41, 2249–2259 CrossRef CAS PubMed.
- P. M. Peloso, A. Gammaitoni, S. S. Smugar, H. Wang and A. R. Moore, BMC Musculoskeletal Disord., 2011, 12, 165 CrossRef CAS PubMed.
- G. J. Leung, K. D. Rainsford and W. F. J. Kean, J. Pharm. Pharmacol., 2014, 66, 347–357 CrossRef CAS PubMed.
- R. N. Brogden, R. C. Heel, T. M. Speight and G. S. Avery, Drugs, 1979, 18, 241–277 CrossRef CAS PubMed.
- M. Fujita, H. Itokawa and Y. Sashida, Chem. Pharm. Bull., 1972, 20, 212–213 CrossRef CAS.
- C. S. Lai, Y. S. Lai, D. H. Kuo, C. H. Wu, C. T. Ho and M. H. Pan, J. Funct. Foods, 2011, 3, 198–206 CAS.
- S. Y. Lee, D. Y. Yuk, H. S. Song, D. Y. Yoon, J. K. Jung, D. C. Moona, B. S. Leed and J. T. Honga, Eur. J. Pharmacol., 2008, 582, 17–25 CrossRef CAS PubMed.
- J. Luo, Y. Xu, M. Zhang, L. Gao, C. Fang and C. Zhou, Inflammation, 2013, 36, 997–1003 CrossRef CAS PubMed.
- D. H. Kuo, Y. S. Lai, C. Y. Lo, A. C. Cheng, H. Wu and M. H. Pan, J. Agric. Food Chem., 2010, 58, 5777–5783 CrossRef CAS PubMed.
- N. Ouyang, J. L. Williams, G. J. Tsioulias, J. J. Gao, M. J. Iatropoulos, L. Kopelovich, K. Kashfi and B. Rigas, Cancer Res., 2006, 66, 4503–4511 CrossRef CAS PubMed.
- M. Greenberg, P. Urnezis and M. Tian, J. Agric. Food Chem., 2007, 55, 9465–9469 CrossRef CAS PubMed.
- W. F. Liu, Y. Yue, Y. L. Li, X. Zheng, K. Zhang and Z. Y. Du, MedChemComm, 2015, 6, 2129–2139 RSC.
- A. R. J. Brecher, J. Drugs Dermatol., 2002, 1, 44–47 Search PubMed.
- J. E. Rundhaug and S. M. Fischer, Photochem. Photobiol., 2008, 84, 322–329 CrossRef CAS PubMed.
- Y. Li, Q. Wu, Y. Deng, H. Lv, J. Qiu, G. Chi and H. Feng, Int. Immunopharmacol., 2015, 26, 286–294 CrossRef CAS PubMed.
- J. B. Méric, S. Rottey, K. Olaussen, J. C. Soria, D. Khayat, O. Rixea and J. P. Spanoa, Crit. Rev. Oncol. Hematol., 2006, 59, 51–64 CrossRef PubMed.
- S. Maeda and M. Omata, Cancer Sci., 2008, 99, 836–842 CrossRef CAS PubMed.
- G. F. Passos, R. Medeiros, R. Marcon, A. F. Nascimento and J. B. Calixto, Eur. J. Pharmacol., 2013, 698, 413–420 CrossRef CAS PubMed.
- L. V. Madrid, M. W. Mayo, J. Y. Reuther and A. S. Baldwin, J. Biol. Chem., 2001, 276, 18934–18940 CrossRef CAS PubMed.
- J. Á. F. Vara, E. Casado, J. de Castro, P. Cejas, C. Belda-Iniesta and M. González-Barón, Cancer Treat. Rev., 2004, 30, 193–204 CrossRef CAS PubMed.
- Y. R. Lin, H. H. Chen, C. H. Ko and M. H. Chan, Neuropharmacology, 2005, 49, 542–550 CrossRef CAS PubMed.
- H. Ma, C. S. Kim, Y. Ma, S. Y. Nam, D. S. Kim, S. S. Woo, J. T. Hong and K. W. Oh, Phytother. Res., 2009, 23, 1340–1344 CrossRef CAS PubMed.
- C. R. Chen, R. Tan, W. M. Qu, Z. Wu, Y. Wang, Y. Urade and Z. L. Huang, Br. J. Pharmacol., 2011, 164, 1534–1546 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |