Synthesis, photophysical and electroluminescent properties of blue-emitting dual core imidazole–anthracene/pyrene derivatives†
Fuli Zhanga,
Weiling Lib,
Donghui Weic,
Xinyu Weid,
Zhongyi Lia,
Shiying Zhanga,
Suzhi Lia,
Bin Wei*b,
Guangxiu Caoa and
Bin Zhai*a
aCollege of Chemistry and Chemical Engineering, Shangqiu Normal University, Shangqiu 476000, P. R. China. E-mail: zhaibin_1978@163.com; Tel: +86-370-2594336
bSchool of Materials Science and Engineering, Shanghai University, Shanghai 200072, P. R. China. E-mail: bwei@shu.edu.cn
cCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450007, P. R. China
dShangqiu Supervision & Testing Center for Quality and Technology, Shangqiu 476000, P. R. China
First published on 17th June 2016
Abstract
Two blue emitting dual core materials consisting of phenanthroimidazole and anthracene or pyrene chromophores were designed and synthesized. 2-(9-Anthracenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (1) and 2-(1-pyrenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (2) were easily synthesized and used as emitters in nondoped OLEDs. Devices based on 1 and 2 show low turn-on voltages (as low as 2.6 and 2.5 V) and stable blue emissions with CIE of (0.16, 0.17) and (0.15, 0.12), respectively. The maximum current efficiencies of 1.15 and 0.90 cd A−1 and maximum external quantum efficiencies of 0.83% and 0.85% with little efficiency roll-off at high current densities were achieved, respectively.
1. Introduction
Organic light emitting diodes (OLEDs) have received significant attention in recent years for their application in flat panel display and solid state lighting as they offer several advantages for self-emitting displays, a wide viewing angle, a thin panel, high contrast, flexible display, bright emission and surface emitting.1–9 Light-emitting materials used in OLEDs can be divided into two general classes, fluorescence and phosphorescence. Phosphorescent systems have been attracting increasing attention due to their harvesting both singlet and triplet excitons for phosphorescence emission to present internal quantum efficiency of 100% in OLED.10–13 Currently, among the three primary RGB colors, highly efficient red and green emitting OLEDs using phosphorescent materials have been realized.14–16 However, the development of highly efficient and long-term stable blue phosphors and corresponding devices is still an ongoing challenge. For blue-emitting phosphorescent materials, the upper lying radiationless deactivation dπdσ* states are much more easily populated from their relatively high lying emitting triplet state. Consequently, the stability and efficiency of blue phosphors are, in many cases, significantly poorer than their green and red counterparts.17 For this reason, devices using blue light-emitting fluorescent OLEDs are still attracting considerable attention.Most existing studies of blue fluorescent emitting chromophores are anthracene,18–21 pyrene,22,23 fluorene,20,21 imidazole,24 benzimidazole,25–27 phenanthroimidazole28,29 and pyrenoimidazole,30 which can be used as core or side moieties in molecules. Nevertheless a single chromophore is incapable of forming stable amorphous thin film due to its tendency to crystallize in the solid state, and its fluorescence can be easily quenched in the solid state, which hamper its application in OLEDs.21,31 Moreover, this kind of molecule can easily form excimer or exciplex through packing because anthracene and pyrene have flat molecular structures, which bring about broad and red-shifted electroluminescent emission.32 Two approaches have been adopted in order to resolve these problems. One is to introduce bulky groups as side moieties to chromophore.18,19 Wang et al. synthesized blue emitters by introducing an additional phenyl ring at the ortho position of a side aromatic ring attached to anthracene. The nondoped devices based on these compounds exhibit low turn-on voltage and stable deep-blue emission with external quantum efficiency of about 3.0%.19 The other strategy is to synthesize dual core chromophore materials by introducing an additional chromophore into a single core chromophore.33–35 Research indicates that the two chromophores in dual core materials are connected orthogonally, which results in the suppression of excimer formation and improvements in the color purity and efficiency of devices.
In this work, we synthesized and systematically examined the characteristics of two blue-emitting compounds 2-(9-anthracenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (1) and 2-(1-pyrenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (2) containing dual core chromophores phenanthroimidazole and anthracene or pyrene. The π-accepting character of the phenanthroimidazole group is expected to increase the electron-transporting mobility of the emitters and to further improve devices performance. OLEDs based on compounds 1 and 2 exhibit high current efficiencies of 1.15 and 0.90 cd A−1 and external quantum efficiencies of 0.83% and 0.85% in blue region, respectively. In addition to low turn-on voltages (as low as 2.6 and 2.5 V), the present devices also show stable emission colors and little efficiency roll-off at high current densities.
2. Experimental
2.1. General experiments
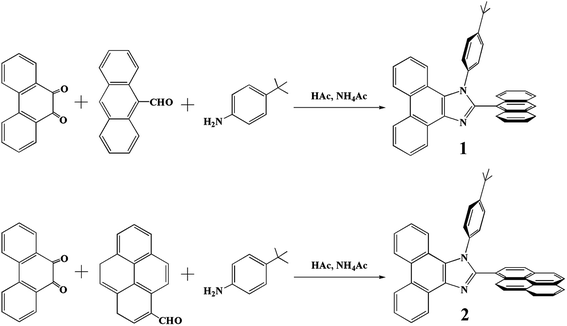
All solvents and materials were used as received from commercial suppliers without further purification. Synthetic routes of the two compounds are outlined in Scheme 1. 2-(9-Anthracenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (1), 2-(1-pyrenyl)-1-(4-tert-butylphenyl)-1H-phenanthro[9,10-d]imidazole (2) were prepared according to the literature.29 All reactions were monitored using precoated thin-layer-chromatography plates (0.20 mm with fluorescent indicator UV254). 1H NMR spectra were recorded on a Bruker DPX 400 spectrometer with tetramethylsilane [Si(CH3)4] as the internal standard. Mass spectrometry (MS) was performed with an Esquire-LC_00136 mass spectrometer. Elemental analysis for carbon, hydrogen, and nitrogen was determined on an Exeter Analytical CE-440 elemental analyzer. TGA thermograms were obtained with STA 449 F3 (NETZSCH). Absorption spectra were recorded with a UV-vis spectrometer (Agilent Cary 60) and photoluminescent (PL) spectra were recorded with a fluorospectrophotometer (Hitachi F-7000). PL quantum yields (at the excitation wavelength of 395 nm) were measured in CH2Cl2 solutions with quinine sulfate (φ = 0.545 in 1 M H2SO4)36 as the standards. Cyclic voltammetry was performed on a CHI 820C electrochemical station (Shanghai Chenhua Co., China) in CH2Cl2 solutions (1 × 10−3 M) at a scan rate of 100 mV s−1 with a platinum plate as the working electrode, a silver wire as the pseudo-reference electrode, and a platinum wire as the counter electrode. The supporting electrolyte was tetra(n-butyl)ammonium hexafluorophosphate (n-Bu4NPF6, 0.1 M) and ferrocene was selected as the internal standard. The solutions were bubbled with argon for 10 min before measurements.2.2. Syntheses
The two compounds 1 and 2 were synthesized from a reported procedure.29 All reactions were performed under argon.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent, yielding a white powder (0.62 g, 0.52 mmol). Yield: 53%. 1H NMR (400 MHz, CDCl3, δ): 8.91–8.77 (m, 3H), 8.46 (s, 1H), 7.96 (d, J = 8.0 Hz, 2H), 7.74–7.67 (m, 4H), 7.55 (dd, J = 8.0 Hz and 4.0 Hz, 1H), 7.41 (dd, J = 8.0 Hz and 4.0 Hz, 4H), 7.30 (d, J = 4.0 Hz, 1H), 7.17 (t, J = 8.0 Hz, 3H), 7.10 (d, J = 8.0 Hz, 2H), 1.14 (s, 9H). MS (MALDI-TOF) [m/z]: 529.0 (M + H)+. Anal. found: C 88.57, H 6.12, N 5.28. Anal. Calcd for C39H32N2: C 88.60, H 6.10, N 5.30.
1) as the eluent, yielding a white powder (0.62 g, 0.52 mmol). Yield: 53%. 1H NMR (400 MHz, CDCl3, δ): 8.91–8.77 (m, 3H), 8.46 (s, 1H), 7.96 (d, J = 8.0 Hz, 2H), 7.74–7.67 (m, 4H), 7.55 (dd, J = 8.0 Hz and 4.0 Hz, 1H), 7.41 (dd, J = 8.0 Hz and 4.0 Hz, 4H), 7.30 (d, J = 4.0 Hz, 1H), 7.17 (t, J = 8.0 Hz, 3H), 7.10 (d, J = 8.0 Hz, 2H), 1.14 (s, 9H). MS (MALDI-TOF) [m/z]: 529.0 (M + H)+. Anal. found: C 88.57, H 6.12, N 5.28. Anal. Calcd for C39H32N2: C 88.60, H 6.10, N 5.30.2.3. Quantum chemical calculations
All the calculations were performed using the Gaussian 09 program.37 The B3LYP38,39 density functional was used to optimize the gas-phase geometries of the compounds 1 and 2. The 6-31G(d, p) basis set was applied for C, H, and N atoms. The visualization of molecular orbitals was accomplished using the GaussView program.2.4. Fabrication and characterization of OLEDs
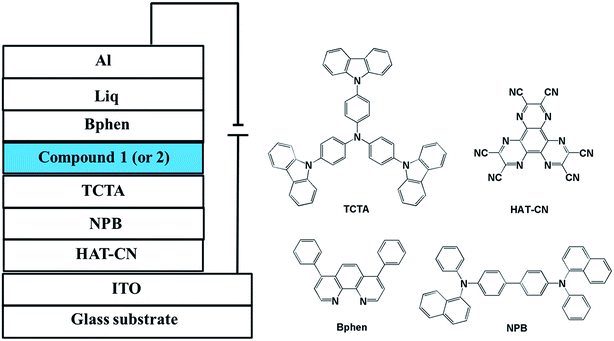
Indium-tin-oxide (ITO) substrates with sheet resistance of 10 Ω sq−1 were sufficiently cleaned using chemical and UV-ozone methods before the deposition of the organic layers. For the electroluminescent (EL) devices, dipyrazino[2,3-f:2′,3′-h]quinoxaline 2,3,6,7,10,11-hexacarbonitrile (HAT-CN) was used for the hole injection layer (HIL), N,N′-bis(naphthalen-1-yl)-N,N′-bis-(phenyl)benzidine (NPB) and 4,4′,4′′-Tris(carbazol-9-yl)-triphenylamine (TCTA) were used for the hole transporting layers (HTL), the synthetic materials 1 and 2 were used in the emitting layer (EML), 4,7-diphenyl-1,10-phenanthroline (Bphen) were used in the electron transporting layer (ETL), 8-hydroxyquinolinolato-lithium (Liq) was used in the electron injection layer (EIL), and ITO was used as the anode and Al as the cathode. All organic layers were deposited under 10−5 Torr, with a rate of deposition of 1 Å s−1 without breaking vacuum between each vacuum-deposition process. The Liq and aluminum layers were continuously deposited under the same vacuum conditions. The luminance–voltage–current density characteristics were measured with a PR655 spectrometer and a Keithley 2400 programmable voltage–current source. The current efficiency and external quantum efficiency characteristics were calculated from electrical parameters, electroluminescent (EL) spectra and luminance. All measurements of the devices were carried out in ambient atmosphere without further encapsulations.3. Results and discussion
3.1. Synthesis and structural characterization
Scheme 1 shows the synthesis of the two compounds. Compounds 1 and 2 were easily synthesized from 9,10-phenanthrenequinone, 9-anthaldehyde, 1-pyrenecarboxaldehyde, 4-tert-butylbenzenamine, and ammonium acetate in glacial acetic acid.29 The details of the syntheses are given in the Experimental section. Both compounds were structurally characterized by 1H NMR, ESI (electron spray ionization) mass spectroscopy and elemental analysis.3.2. Thermal stability
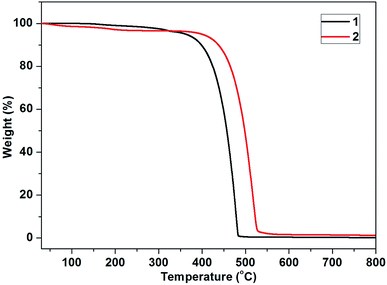
The thermal stability of emitters is very important for efficient OLEDs. To examine their thermal stabilities, thermogravimetric analysis (TGA, Fig. 1) and differential scanning calorimetry (DSC, Fig. S2, ESI†) were performed under N2 atmosphere. As shown in Fig. 1, the two compounds were very stable under a N2 atmosphere and no significant weight loss was seen below 300 °C. From the TGA curves it can be observed that the decomposition temperature (5% loss of weight) are 363 °C for 1 and 400 °C for 2, respectively. In addition, high glass-transition temperatures (Tgs) are observed in Fig. S2 and S3† for compounds 1 (Tg = 172 °C) and 2 (Tg = 179 °C), which are much higher than those of other blue phenanthroimidazole derivatives.29 This results indicated that these compounds are very stable and suitable for application in OLEDs by vacuum-deposition method.3.3. Photophysical properties
The absorption and PL spectra of the two compounds in CH2Cl2 and solid-state thin films on quartz substrates are shown in Fig. 2, and detailed photophysical characterizations are collected in Table 1. Both compounds exhibit strong absorption from the ultra-violet up to visible range in CH2Cl2. The absorption bands in the region from ∼320 to ∼410 nm are attributed to the delocalized π–π* transition of the phenanthroimidazole.29 The absorption bands before 320 nm might originate from π–π* transitions of the polycyclic aromatic hydrocarbons.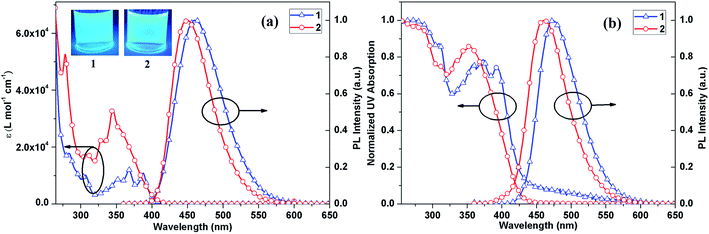 | ||
| Fig. 2 Absorption and emission spectra of compounds 1 and 2 in dilute CH2Cl2 solutions (a) and neat films (b). | ||
| Solutiona | Film on glassb | ϕfd | HOMO (eV) | LUMO (eV) | |||||
|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) (ε (10−4 M−1 cm−1)) | λem, (nm) | FWHMc (nm) | λab, (nm) | λem (nm) | FWHMc (nm) | ||||
| a In CH2Cl2 solutions (1 × 10−5 M).b Neat films were made on quartz substrates with thicknesses of about 100 nm.c Full width at half maximum.d Measured in CH2Cl2 solutions with quinine sulfate as the standard. | |||||||||
| 1 | 306(1.0)/368(1.2) | 459 | 74 | 309/372 | 473 | 66 | 0.40 | −6.20 | −3.11 |
| 2 | 278(5.1)/344(3.3) | 450 | 64 | 310/355 | 461 | 65 | 0.76 | −6.08 | −3.02 |
As depicted in Fig. 2(a), compounds 1 and 2 exhibit blue light emission (peaks at ca. 459 and 450 nm, respectively) with high photoluminescent quantum yields (PLQYs, 0.40 for 1 and 0.76 for 2, respectively) in CH2Cl2 solutions. The full width at half maximums (FWHMs) of compounds 1 and 2 are 74 and 64 nm, which are similar to other blue fluorophores.34,35 The small FWHMs were attributed to near orthogonal structure of t-Bu-benzene ring, phenanthroimidazole, anthracene (in 1) and pyrene (in 2) (see Theoretical calculations section). Such orthogonal structure can improve the performance of the resulting OLED device by assisting the formation of an amorphous film and narrowing the blue emission spectrum. In comparison to the absorption and PL spectra in solution, those in thin film (Fig. 2(b)) are red-shifted, which may be due to closer packing of molecules in the amorphous thin film states. However, both compounds exhibit slight deviation of the emission maximum. This indicates that the highly twisted configuration and nonplanarity of these compounds can effectively suppress the intermolecular π–π stacking in the solid state.
3.4. Electrochemical properties and theoretical calculations
The electrochemical behavior of the two compounds was measured in CH2Cl2 solutions containing 0.1 M n-Bu4NPF6 as the supporting electrolyte by cyclic voltammetry. The cyclic voltammograms are shown in Fig. S1 (see ESI†) and the relevant electrochemical data are summarized in Table 1. Both compounds exhibit irreversible oxidation behaviors. On the basis of the onset potential of Eoxonset and the absorption edge data, the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) energy levels can be estimated from the empirical formulae: EHOMO = −e(Eoxonset + 4.74) and ELUMO = EHOMO + Eg (where Eg is the optical energy gap obtained from the absorption threshold).40,41 The HOMO/LUMO levels of the two compounds are −6.20/−3.11 eV for 1 and −6.08/−3.02 eV for 2 (Table 1), which indicates that improving the conjugation of aromatic ring at the 2-position of the phenanthro[9,10-d]imidazole can effectively increase the HOMO/LUMO energy levels but does not impose a noticeable effect on the Eg.Density functional theory (DFT) calculations at the B3LYP/(6-31G(d, p)) level were carried out to gain more insight into the electronic structures of the compounds 1 and 2. Fig. 3 displays the optimized geometry and electron density distribution of the frontier molecular orbitals for compounds 1 and 2. As depicted in Fig. 3, the t-Bu-benzene ring, the anthracene ring in 1 or the pyrene ring in 2 all twisted about the phenanthroimidazole plane, respectively. The twisted configuration and nonplanarity are beneficial for suppressing fluorescence quenching caused by aggregation and maintaining the short wavelength emission in solid state. According to DFT calculations, the HOMO orbitals of both compounds were composed of a mixture of phenanthroimidazole π orbitals and anthracene (or pyrene) π orbitals, which are similar to those of other phenanthroimidazole-based compounds.29 However, their LUMO orbitals were mainly dominated by the anthracene or pyrene units. The calculated HOMO/LUMO levels of compound 1 (−5.25/−1.80 eV) are higher than those of compound 2 (−5.06/−1.64 eV), which is in good agreement with the experimental results (Table 1).
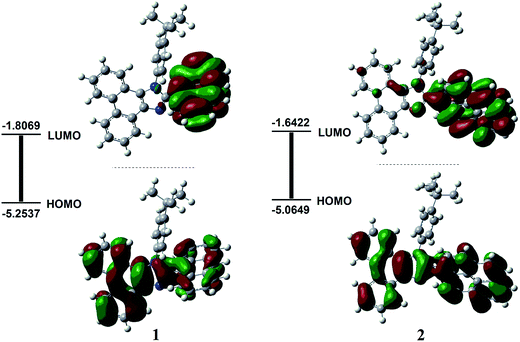 | ||
| Fig. 3 Molecular orbital surfaces of compounds 1 and 2. All the MO surfaces correspond to an isocontour value of |Ψ| = 0.02. | ||
3.5. Electroluminescent properties of OLED devices
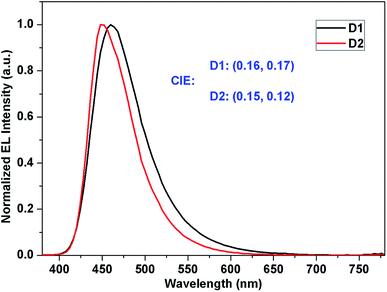
In order to evaluate the electroluminescent properties of both compounds, 1 and 2 were used as emitters to fabricate nondoped OLEDs with the structure of ITO/HAT-CN (30 nm)/NPB (10 nm)/TCTA (10 nm)/compound 1 (for D1) or 2 (for D2) (20 nm)/Bphen (30 nm)/Liq (1 nm)/Al (100 nm). The device architecture, as well as the molecular structure of the materials used are shown in Fig. 4.Fig. 5 depicts the EL spectra of the OLEDs based on compounds 1 and 2. The EL maximum values of the D1 and D2 devices are 460 and 448 nm, respectively. Moreover, D1 and D2 show narrow FWHMs of 66 and 58 nm without excimer or exciplex emission. These values are attributed to the highly twisted structure, which results in steric hindrance toward the phenanthroimidazole unit and the anthracene or pyrene group. According to the CIE data, the color coordinates of compounds 1 and 2 are (0.16, 0.17) and (0.15, 0.12) with the colour rendering index (CRI) of 17 for D1 and 14 for D2, respectively; the y values are satisfactory for blue color applications, according to the National Television System Committee (NTSC) blue color standard.
Fig. 6 shows the luminance–voltage–current density and current efficiency–current density–external quantum efficiency characteristics of the devices. Detailed electrical characteristics of the devices are summarized in Table 2. The devices have low onset voltage (defined as the voltage required to obtain a luminance of 1 cd m−2) of 2.6 and 2.5 V, respectively, which are much lower than some of pyrenoimidazole-based devices.30 A maximum luminance (Lmax) of 5168 cd m−2, a maximum luminance efficiency (ηc) of 1.15 cd A−1 and a quantum efficiency (ηe) of 0.83% for compound 1, a Lmax of 5118 cd m−2, a ηc of 0.90 cd A−1 and a ηe of 0.85% for compound 2 are achieved. The performance of compounds 1 and 2 based devices are slightly inferior than some of the best blue emitters reported recently.19,29,34 As depicted in Fig. 6(b), the EL efficiencies of devices decrease gradually with a further increase of luminance, which is the so-called EL efficiency roll-off.42,43 However, the EL efficiency roll-off effects in these devices are very mild, which should be attributed to the appropriate energy levels for better charge transporting and carrier mobility.
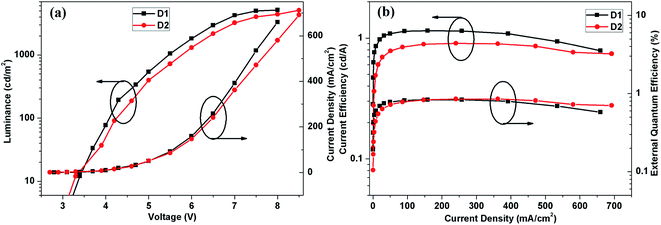 | ||
| Fig. 6 The current density–voltage–luminance (a) and current efficiency–current density–external quantum efficiency characteristics (b) of the devices based on compounds 1 and 2. | ||
4. Conclusion
In conclusion, we have designed and synthesized two blue fluorescent materials (1 and 2) containing two core chromophores phenanthroimidazole and anthracene or pyrene, and investigated their applications in nondoped OLEDs. The fluorophores show high quantum yields and good thermal stability. Devices based on 1 and 2 exhibit low onset voltages of 2.6 and 2.5 V, the maximum current efficiencies of 1.15 and 0.90 cd A−1 and maximum external quantum efficiencies of 0.83% and 0.85% with the CIE coordinates of (0.16, 0.17) and (0.15, 0.12), respectively. Due to their good carrier transporting properties, the devices show little efficiency roll-off at high brightness.Acknowledgements
We would like to thank the National Natural Science Foundation of China (Grant No. 21371114, 21401126, 61274065, 21501117 and 21571123), the Program for Science and Technology Innovation Talents in Universities of Henan Province (2012HASTIT031), the Scientific and Technological Projects of Science and Technology Department of Henan Province (Grant No. 152102210338, 122102210255) and the Key Scientific Research Projects in University of Henan Province (Grant No. 15A150023) for financial support.References
- C. W. Tang and S. A. Vanslyke, Appl. Phys. Lett., 1987, 51, 913–915 CrossRef CAS.
- S. Lamansky, P. Djurovich, D. Murphy, F. Abdel-Razzaq, H. E. Lee, C. Adachi, P. E. Burrows, S. R. Forrest and M. E. Thompson, J. Am. Chem. Soc., 2001, 123, 4304 CrossRef CAS PubMed.
- S. Lamansky, P. I. Djurovich, F. Abdel-Razzaq, S. Garon, D. L. Murphy and M. E. Thompson, J. Appl. Phys., 2002, 92, 1570–1575 CrossRef CAS.
- N. Rehmann, C. Ulbricht, A. Köhnen, P. Zacharias, M. C. Gather, D. Hertel, E. Holder, K. Meerholz and U. S. Schubert, Adv. Mater., 2008, 20, 129–133 CrossRef CAS.
- H. B. Wu, G. J. Zhou, J. H. Zou, C. L. Ho, W. Y. Wong, W. Yang, J. B. Peng and Y. Cao, Adv. Mater., 2009, 21, 4181–4184 CrossRef CAS.
- J. H. Zou, H. Wu, C. S. Lam, C. D. Wang, J. Zhu, C. M. Zhong, S. J. Hu, C. L. Ho, G. J. Zhou, H. B. Wu, W. C. H. Choy, J. B. Peng, Y. Cao and W. Y. Wong, Adv. Mater., 2011, 23, 2976–2980 CrossRef CAS PubMed.
- G. J. Zhou, W. Y. Wong and S. Suo, J. Photochem. Photobiol., C, 2010, 11, 133–156 CrossRef CAS.
- M. A. Baldo, M. E. Thompson and S. R. Forrest, Nature, 2000, 403, 750–753 CrossRef CAS PubMed.
- B. W. D'Andrade, R. J. Holmes and S. R. Forrest, Adv. Mater., 2004, 16, 624–628 CrossRef.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4 CrossRef CAS.
- W. Y. Wong and C. L. Ho, Coord. Chem. Rev., 2009, 253, 1709–1758 CrossRef CAS.
- W. Y. Wong and C. L. Ho, J. Mater. Chem., 2009, 19, 4457–4482 RSC.
- G. J. Zhou, W. Y. Wong and X. L. Yang, Chem.–Asian J., 2011, 6, 1706–1727 CrossRef CAS PubMed.
- H. Fu, Y.-M. Cheng, P.-T. Chou and Y. Chi, Mater. Today, 2011, 14, 472–479 CrossRef CAS.
- P. T. Chou, Y. Chi, M. W. Chung and C. C. Lin, Coord. Chem. Rev., 2011, 255, 2653–2665 CrossRef CAS.
- L. X. Xiao, Z. J. Chen, B. Qu, J. X. Luo, S. Kong, Q. H. Gong and J. J. Kido, Adv. Mater., 2011, 23, 926–952 CrossRef CAS PubMed.
- X.-L. Chen, R. Yu, Q.-K. Zhang, L.-J. Zhou, X.-Y. Wu, Q. Zhang and C.-Z. Lu, Chem. Mater., 2013, 25, 3910–3920 CrossRef CAS.
- H. Park, J. Lee, I. Kang, H. Y. Chu, J.-I. Lee, S.-K. Kwon and Y.-H. Kim, J. Mater. Chem., 2012, 22, 2695–2700 RSC.
- Z.-Q. Wang, C. Xu, W.-Z. Wang, L.-M. Duan, Z. Li, B.-T. Zhao and B.-M. Ji, New J. Chem., 2012, 36, 662–667 RSC.
- M.-S. Gong, H.-S. Lee and Y.-M. Jeon, J. Mater. Chem., 2010, 20, 10735–10746 RSC.
- C.-H. Wu, C.-H. Chien, F.-M. Hsu, P.-I. Shih and C.-F. Shu, J. Mater. Chem., 2009, 19, 1464–1470 RSC.
- S.-L. Lai, Q.-X. Tong, M.-Y. Chan, T.-W. Ng, M.-F. Lo, S.-T. Lee and C.-S. Lee, J. Mater. Chem., 2011, 21, 1206–1211 RSC.
- T. M. Figueira-Duarte, P. G. Del Rosso, R. Trattnig, S. Sax, E. J. W. List and K. Müllen, Adv. Mater., 2010, 22, 990–993 CrossRef CAS PubMed.
- H.-H. Chou, Y.-H. Chen, H.-P. Hsu, W.-H. Chang, Y.-H. Chen and C.-H. Cheng, Adv. Mater., 2012, 24, 5867–5871 CrossRef CAS PubMed.
- S. Gong, Y. Zhao, M. Wang, C. Yang, C. Zhong, J. Qin and D. Ma, Chem.–Asian J., 2010, 5, 2093–2099 CrossRef CAS PubMed.
- W.-Y. Hung, L.-C. Chi, W.-J. Chen, Y.-M. Chen, S.-H. Chou and K.-T. Wong, J. Mater. Chem., 2010, 20, 10113–10119 RSC.
- M.-Y. Lai, C.-H. Chen, W.-S. Huang, J. T. Lin, T.-H. Ke, L.-Y. Chen, M.-H. Tsai and C.-C. Wu, Angew. Chem., Int. Ed., 2008, 47, 581–585 CrossRef CAS PubMed.
- W.-C. Chen, Y. Yuan, G.-F. Wu, H.-X. Wei, L. Tang, Q.-X. Tong, F.-L. Wong and C.-S. Lee, Adv. Opt. Mater., 2014, 2, 626–631 CrossRef CAS.
- Y. Zhang, S.-L. Lai, Q.-X. Tong, M.-F. Lo, T.-W. Ng, M.-Y. Chan, Z.-C. Wen, J. He, K.-S. Jeff, X.-L. Tang, W.-M. Liu, C.-C. Ko, P.-F. Wang and C.-S. Lee, Chem. Mater., 2012, 24, 61–70 CrossRef.
- D. Kumar, K. R. J. Thomas, C.-C. Lin and J.-H. Jou, Chem.–Asian J., 2013, 8, 2111–2124 CrossRef CAS PubMed.
- M. Zhu, Q. Wang, Y. Gu, X. Cao, C. Zhong, D. Ma, J. Qin and C. Yang, J. Mater. Chem., 2011, 21, 6409–6415 RSC.
- S. H. Kim, I. Cho, M. K. Sim, S. Park and S. Y. Park, J. Mater. Chem., 2011, 21, 9139–9148 RSC.
- Y. Park, S. Kim, J.-H. Lee, D. H. Jung, C.-C. Wu and J. Park, Org. Electron., 2010, 11, 864–871 CrossRef CAS.
- B. Kim, Y. Park, J. Lee, D. Yokoyama, J.-H. Lee, J. Kido and J. Park, J. Mater. Chem. C, 2013, 1, 432–440 RSC.
- H. Lee, B. Kim, S. Kim, J. Kim, J. Lee, H. Shin, J.-H. Lee and J. Park, J. Mater. Chem. C, 2014, 2, 4737–4747 RSC.
- W. H. Melhuish, J. Phys. Chem., 1961, 65, 229–235 CrossRef CAS.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- P. E. Burrows, Z. Shen, V. Bulovic, D. M. McCarty, S. R. Forrest, J. A. Cronin and M. E. Thompson, J. Appl. Phys., 1996, 79, 7991–8006 CrossRef CAS.
- B. Liang, L. Wang, Y. H. Xu, H. H. Shi and Y. Cao, Adv. Funct. Mater., 2007, 17, 3580–3589 CrossRef CAS.
- Y.-C. Zhu, L. Zhou, H.-Y. Li, Q.-L. Xu, M.-Y. Teng, Y.-X. Zheng, J.-L. Zuo, H.-J. Zhang and X.-Z. You, Adv. Mater., 2011, 23, 4041–4046 CrossRef CAS PubMed.
- Q.-L. Xu, X. Liang, S. Zhang, Y.-M. Jing, X. Liu, G.-Z. Lu, Y.-X. Zheng and J.-L. Zuo, J. Mater. Chem. C, 2015, 3, 3694–3701 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra04958c |
| This journal is © The Royal Society of Chemistry 2016 |