Ultrasensitive sensing performances to sub-ppb level acetone for Pd-functionalized SmFeO3 packed powder sensors
Ling Lia,
Hongwei Qin*a,
Ling Zhangb and
Jifan Hu*a
aSchool of Physics, State Key Laboratory for Crystal Materials, Shandong University, Jinan 250100, China. E-mail: hwqin@sdu.edu.cn; hujf@sdu.edu.cn; Fax: +86-531-88377031; Tel: +86 531 88361560
bSchool of Resource and Environment, University of Jinan, Jinan 250022, China
First published on 26th May 2016
Abstract
Pd-SmFeO3 is functionalized by mixing PdCl2 with nanocrystalline powders and subsequently followed by an annealing at 750 °C. With an increasing amount of Pd2+ in composite powders, the sensing response Rg/Ra for low concentration acetone for Pd-doped SmFeO3 sensors increases at first, reaching a maximum with 3 wt% PdCl2 dopant, and then decreases again. The response for undoped SmFeO3 at 240 °C is 2.26 to 500 ppb acetone, whereas the response for 3 wt% Pd-SmFeO3 at 240 °C is 7.21 to 500 ppb acetone. The 3 wt% Pd-doped SmFeO3 sensor at 240 °C shows a short response time (8 s) and recovery time (15 s) to 500 ppb acetone gas, respectively. Such results show that 3 wt% Pd-doped SmFeO3 sensor is a new promising sensing candidate for detecting low concentrations of acetone. Cross-response to relative humidity has also been considered. With increase of relative humidity (RH), there was a slight reduction in response. When the relative humidity is 80% RH, 3 wt% Pd-SmFeO3 to 500 ppb acetone response is 3.25. The superior response and response to acetone gas offer a potential platform for application in diabetes diagnosis.
1. Introduction
Acetone is regarded as one of the important raw materials, solvents and flammable gases due to its chemical activity, which is extensively used in many fields of industrial production. However, it can cause many health problems when the concentration of inhalation is higher than 173 ppm.1 It has been studied extensively and is generally recognized to have low acute and chronic toxicity if ingested and/or inhaled. The development of acetone gas monitoring sensors is an imperative duty due to the requirements of domestic and industrial safety. When the concentration of acetone gas reaches 1000 ppm in air, uncomfortable symptoms (such as headache, fatigue and even narcosis) may develop in humans. The breath of diabetics is known to be of bad odor with a fruity scent, due to the presence of acetone.2To achieve accurate disease diagnosis using exhaled breath sensors, the minimum detection limit should be at ppb (parts per billion) level, particularly in highly humid atmospheres. In addition, selective detection should be guaranteed to confirm exact recognition of a specific disease. For example, in the case of diabetes diagnosis, the exhaled acetone level of diabetes patients is found to exceed 1800 ppb, which is 2 to 6 fold higher than that (300–900 ppb) of healthy people.3–5 Thus, it is necessary to develop acetone sensing materials and detection devices with high response and selectivity, which is desirable for environmental safety control and daily life, especially for the detection of sub-ppb level acetone concentration.
Perovskite oxides with the general formula ABO3 have attracted much attention for their potential application in many fields, such as giant magneto impedance sensing elements,6,7 catalysis,8 electrodes in solid oxide fuel cells,9 colossal magneto resistance devices,10,11 and chemical sensors.12–27 Acetone sensors based on perovskite-type oxides have been developed. It has been found that the response S = Rgas/Ra to 50 ppm acetone is 7 for La0.68Pb0.32FeO3,28 16 for SmFe0.9Mg0.1O3,29 2.6 for LaFeO3,30 300 for Pd-doped NdFeO3.31 The response S is 204 to 80 ppm acetone for LaFeO3 thick film,32 and 2 to 25 ppm acetone for porous LaFeO3.33 Meanwhile, it has been observed that doping noble metals into inorganic oxides, such as SnO2, ZnO, TiO2, In2O3 and CdSnO3, can dramatically improve the response to reducing gases.34–46 Thus far, several promising research efforts have been conducted for fabrication of highly sensitive sub-ppb level acetone sensors, in particular by combining noble catalysts and common nanostructure sensing materials that make the sensing element have a high surface area and high porosity. These devices include Pd–TiO2 nanofibers,47 PdO–SnO2 nanofibers,48 Pt- and IrO2–WO3 nanofibers,49 Au–TiO2 nanotubes.50 However, accurate cross-response toward exhaled breath containing several gases such as acetone, H2S, toluene, etc., is still a major challenge.
In this work, Pd-doped SmFeO3 packed powder sensors were investigated for the first time using sensing performances to super low concentration acetone test. The response of Pd-SmFeO3 for 500 ppb acetone is high to 7.21, which would be used for diagnosis of diabetes.
2. Experimental
The SmFeO3 powders were prepared by a sol–gel method. Firstly, a stoichiometric ratio of lanthanum nitrate, ferric nitrate and citrate acid (all analytically pure) were completely mixed in deionized water. After adding some polyethylene glycol (PEG; molecular weight over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), the solution was well stirred for several hours until the sol was formed. The sol was dried into a gel and then the gel was dried into pieces in a baking box. The pieces were ground into a fine powder sample, which was subsequently annealed in an oven at 750 °C for 3 h. Undoped SmFeO3 nanocrystallite powders were obtained. Then, the powders obtained above were mixed with an appropriate amount of palladium chloride (PdCl2) and milled for 3 hours, followed by annealing in an oven at 750 °C for 3 h to obtain the SmFeO3 powders doped with Pd from 1 wt% to 5 wt%. The structure of the resultant powders was characterized by X-ray diffraction with Cu kα radiation. The microstructure of 3 wt% Pd-doped SmFeO3 was observed by field emission scanning electron microscope (FE-SEM). The Pd element distribution on the 3 wt% Pd-doped SmFeO3 sample was analyzed by energy-dispersive X-ray spectroscopy (EDAX). The SmFeO3 powders with different Pd doping levels were mixed with a suitable amount of adhesive, and then the mixture was packed into an Al2O3 ceramic tube onto which two electrodes had been installed at each end. The ceramic tube is about 10 mm in length, 8 mm in outer diameter, and 5 mm in inner diameter. To improve stability and repeatability, the gas sensors were calcined at 400 °C for 3 h. The gas-sensing properties were measured in the temperature range of 100–340 °C. The resistance of a sensor was measured in air and in a test gas. In this work, the response of the sensor to test gas was defined as the sensing response S = Rg/Ra, where Ra is the resistance in air while Rg in a test gas.
000), the solution was well stirred for several hours until the sol was formed. The sol was dried into a gel and then the gel was dried into pieces in a baking box. The pieces were ground into a fine powder sample, which was subsequently annealed in an oven at 750 °C for 3 h. Undoped SmFeO3 nanocrystallite powders were obtained. Then, the powders obtained above were mixed with an appropriate amount of palladium chloride (PdCl2) and milled for 3 hours, followed by annealing in an oven at 750 °C for 3 h to obtain the SmFeO3 powders doped with Pd from 1 wt% to 5 wt%. The structure of the resultant powders was characterized by X-ray diffraction with Cu kα radiation. The microstructure of 3 wt% Pd-doped SmFeO3 was observed by field emission scanning electron microscope (FE-SEM). The Pd element distribution on the 3 wt% Pd-doped SmFeO3 sample was analyzed by energy-dispersive X-ray spectroscopy (EDAX). The SmFeO3 powders with different Pd doping levels were mixed with a suitable amount of adhesive, and then the mixture was packed into an Al2O3 ceramic tube onto which two electrodes had been installed at each end. The ceramic tube is about 10 mm in length, 8 mm in outer diameter, and 5 mm in inner diameter. To improve stability and repeatability, the gas sensors were calcined at 400 °C for 3 h. The gas-sensing properties were measured in the temperature range of 100–340 °C. The resistance of a sensor was measured in air and in a test gas. In this work, the response of the sensor to test gas was defined as the sensing response S = Rg/Ra, where Ra is the resistance in air while Rg in a test gas.
3. Results and discussion
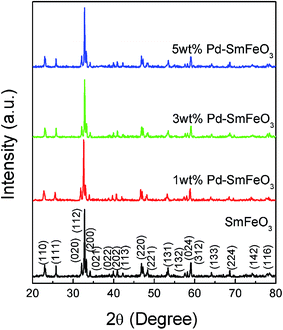
Fig. 1 shows the X-ray diffraction patterns of SmFeO3 with different Pd doping levels annealed at 700 °C. The patterns indicate that the samples have a main phase of orthorhombic perovskite SmFeO3 structure. The diffraction peaks of Pdx+ cannot be distinctly identified for 1–5 wt% Pd-doped SmFeO3 because the doping amount is too low. We deduce that Pd2+ is in the form of PdO phase. The PdO phase was converted from palladium chloride PdCl2 during the calcination at higher temperatures, which would surround on the surface of SmFeO3 nanoparticles formed separately. The oxide PdO2 with the higher valence Pd4+ has much higher Gibbs free energy compared to PdO and is difficult to be formed.Fig. 2 shows the microstructure of SmFeO3 with 1–5 wt% PdCl2 addition sintered at 750 °C for 3 h. There is a distribution of grain size around 45 nm, which is consistent with the average grain size estimated by the Scherrer method. Fig. 3 showed the EDAX mapping result on Pd element distribution for SmFeO3 with 3 wt% PdCl2 addition sintered at 750 °C for 3 h. The red dots represent Pd element. The dispersion of Pd is uniform, which means that the size of PdO grains is very fine, which explains why we could not find a trace of PdO grain in the Fe-SEM photo. In order to confirm the presence of Pd in the doped SmFeO3, XPS analysis was carried out to further investigate the surface compositions and chemical state of Pd-doped SmFeO3. The high-resolution XPS spectra of Sm, Fe, O and Pd are displayed in Fig. 4a–d, respectively. The Sm 3d5/2 at 1083 eV and 1110.5 eV, Fe 2p2/3 at 710.75 eV, Fe 2p1/2 at 725 eV and O 1s peak at 529.27 eV and 531.4 eV, are in good agreement with the values for the lattice oxygen and adsorbed oxygen of SmFeO3, respectively. The low shoulder peak has been proposed for defect sites within the crystal and absorbed oxygen. As the concentration of Pd is low in the sample with 3 wt% Pd-SmFeO3, the Pd 3d signal is deconvoluted into two peaks, as shown in Fig. 4a. The peak at 337 eV is assigned to Pd 3d3/2, while the peak at 335 eV is assigned to elemental palladium Pd0. The Pd 3d peak at 336.7 eV is attributed to Pd2+ of PdO, and the peak at 337.7 eV is attributed to Pd4+ of PdO2. In Fig. 4b, two peaks can be ascribed to Sm 3d. Fig. 4c shows Fe 2p XPS spectra for nanocrystalline Pd-SmFeO3. The peaks located at about 710.5 eV and 724.5 eV could be assigned to 2p3/2 and 2p1/2 states of Fe3+. The O 1s peak in Fig. 4d is asymmetric, and there is a peak at 530.8 eV and a shoulder peak at 532.2 eV. All of these results give insight into the existence of Pd ions and SmFeO3 in the sample.
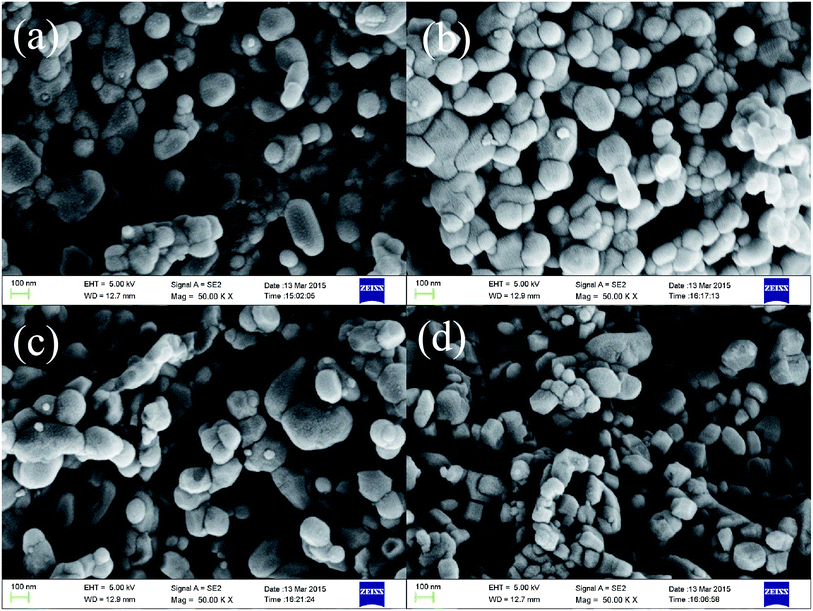 | ||
| Fig. 2 The SEM of SmFeO3 powders with 1–5 wt% PdCl2: (a) SmFeO3; (b) 1 wt% Pd-SmFeO3; (c) 3 wt% Pd-SmFeO3; and (d) 5 wt% Pd-SmFeO3. | ||
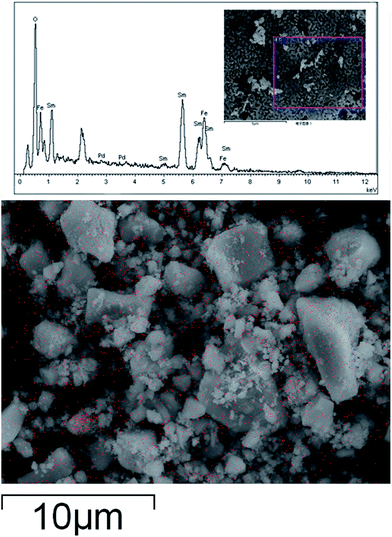 | ||
| Fig. 3 The EDAX mapping result on Pd element distribution for SmFeO3 with 3 wt% PdCl2 addition sintered at 750 °C for 3 h. The red dots represent Pd element. | ||
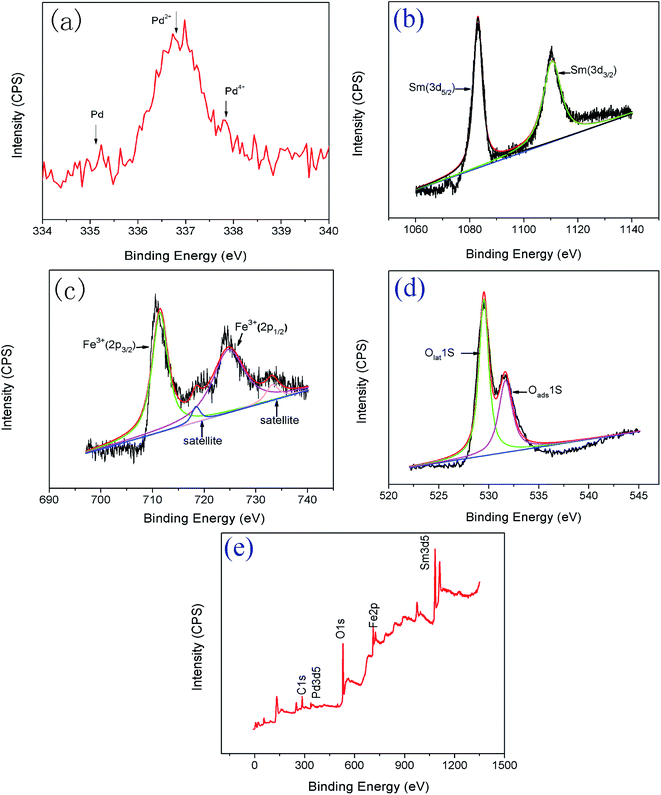 | ||
| Fig. 4 XPS spectra of 3 at% Pd-doped SmFeO3, (a) Pd 3d, (b) Sm 3d, (c) Fe(2p) and (d) O 1s. (e) Survey spectrum and high resolution spectra. | ||
The temperature dependence of the response to 500 ppb acetone gas for different Pd-doped amounts of SmFeO3 are shown in Fig. 5. It can be seen that the maximum response to acetone gas is about 7.21 when the PdCl2 content in SmFeO3 powders is 3 wt%. Obviously, the appropriate amount of PdO doping is beneficial to increase the acetone gas response of sensors. However, over-doping reduces the sensing response. It seems that Pd-doped SmFeO3 could serve as a sensitive sensor for low concentrations of acetone. The optimum operating temperature is around 240 °C. The maximum response S to 500 ppb acetone is 2.45, 5.62, 7.21 and 5.04 for SmFeO3 with doped PdCl2 concentration of 0, 1, 3 and 5 wt%, respectively. The enhancement of similar sensing response has also been found in Pd–SnO2 to reducing gases such as CO, H2, CH4, and other hydrocarbon gases.39–41,43–46
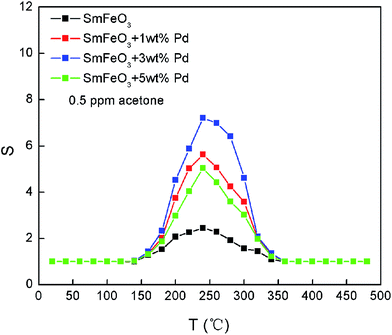 | ||
| Fig. 5 The temperature dependence of the response to 500 ppb acetone gas for samples SmFeO3 with different Pd doping levels. | ||
The temperature dependence of response to different concentrations of acetone gas for 3 wt% Pd-SmFeO3 sensor is shown in Fig. 6. The optimum operating temperature is around 240 °C. The maximum sensitivities to 500 ppb, 750 ppb, 1.0 ppm and 5 ppm acetone gas for 3 wt% Pd-doped SmFeO3 sensor are about 7.21, 10.12, 12.34 and 19.56 respectively. The response increases with increasing acetone concentration. The response is largely enhanced by the PdCl2 modification on the surface of SmFeO3 grains.
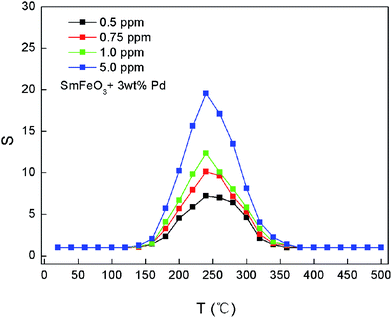 | ||
| Fig. 6 The temperature dependence of response to different concentrations of acetone gas for 3 wt% Pd-doped SmFeO3 sensor. | ||
Water vapor is present in ambient atmosphere, especially in breathed air. Many types of semiconducting oxide sensors have been used for measuring humidity.51–59 such as ZnO,51,52 BaTiO3,53,54 TiO2,55 SnO2,.56–58 The gas sensing properties of the semiconductor sensors vary with the humidity of the atmosphere. For some medical science and chemical industries, it is very important to consider a sensor capable of detecting low concentrations of acetone in the presence of water vapor at high temperatures. The temperature dependence of the response to 500 ppb acetone for sample SmFeO3 with 3 wt% Pd doping levels in different relative humidity (20% RH, 40% RH, 80% RH) were shown in Fig. 7. It can be seen that with the relative humidity increased, the response decreased. When the relative humidity is 20% RH, 40% RH, 80% RH, S was 7.21, 4.84, 3.25, respectively. In fact, the adsorption of water vapor-related species on SmFeO3 powder surfaces, it will restrain the gas sensitive reaction. The acetone sensing process is that O−ads (adsorbed O species) reacts with acetone gas, and the reaction products are CO2 and H2O. Therefore, with an increase of humidity, the response S decreased.
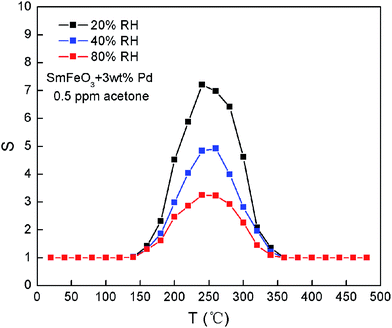 | ||
| Fig. 7 The temperature dependence of the response to 500 ppb acetone for sample SmFeO3 with 3% Pd doping with different relative humidity. | ||
Fig. 8 shows the resistance of sensors based on 3 wt% Pd-doped SmFeO3 to 500 ppb acetone with increasing relative humidity. With increasing relative humidity, the resistance of the packed powder sensor gradually decreased.
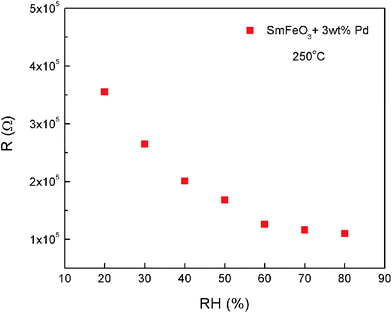 | ||
| Fig. 8 The resistance of sensors based on 3 wt% Pd-doped SmFeO3 to 500 ppb acetone with increasing relative humidity. | ||
The dynamic sensing characteristics of undoped and 3 wt% Pd-doped SmFeO3 sensor exposed to 500 ppb acetone gas in ambient air are shown in Fig. 9. The operating temperature was 240 °C. The resistance of 3 wt% Pd-doped SmFeO3 sensor increases obviously when acetone gas was introduced, and when acetone gas was removed, the resistance of the 3 wt% Pd-doped SmFeO3 sensor drops. The response time has been defined as the time taken to attain 90% of the final value, and the recovery time as the time taken to regain 10% of the base value. The response time and recovery time for 3 wt% Pd-doped SmFeO3 sensor to 0.5 ppm acetone gas are about 4 and 2 seconds, respectively.
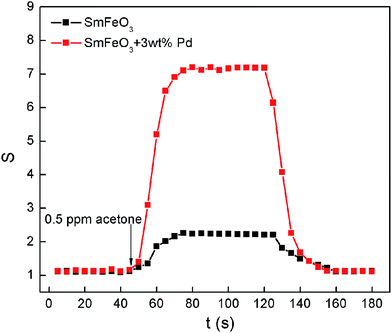 | ||
| Fig. 9 The dynamic sensing characteristic of undoped and 3 wt% Pd-doped SmFeO3 sensor exposed to 500 ppb acetone gas in ambient air. | ||
Fig. 10 shows the response of the 3 wt% Pd-doped SmFeO3 sensor to different gases (acetone, ethanol, gasoline, HCHO and CO). The SmFeO3 sensor exhibits high selectivity to ethanol gas. However, the 3 wt% Pd-doped SmFeO3 sensor exhibits increased selectivity to acetone gas.
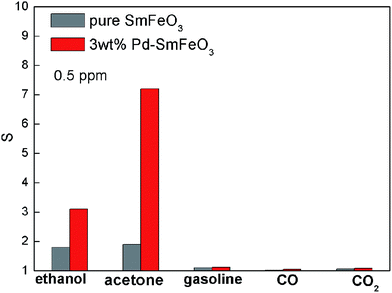 | ||
| Fig. 10 The response of sensors based on undoped and 3 wt% Pd-doped SmFeO3 to 500 ppb acetone, ethanol, gasoline, CO and CO2. | ||
In air, the surfaces of SmFeO3 are usually adsorbed by oxygen species. Before introduction of reducing gas, oxygen molecules capture electrons from the surface of SmFeO3, forming the oxygen species and decreasing the resistance of the SmFeO3 sensor. With increasing the temperature in air, the state of oxygen adsorbed on the surface of semiconductor undergoes the following changes:
| O2(gas) → O2−(ads) |
| O2−(ads) → O−(ads) |
| O−(ads) → O2−(ads) |
After the reducing gas is introduced, it would interact with the oxygen species adsorbed on SmFeO3, releasing electrons back to the surface of SmFeO3 and increase the resistance of the SmFeO3 sensor. When ethanol or acetone gas is introduced, the following reaction may occur, respectively.42,60
| CH3COCH + 8O−(ads) → 3CO2 + 2H2O + 8e− |
| CH3COCH3 + 8O−(ads) → 3CO2 + 3H2O + 8e− |
The releasing electron would return back to the surface of SmFeO3, and are annihilated with the holes
| h* + e− → null. |
It decreases the number of holes, thus increasing the resistance of the p-type SmFeO3 sensor. There are two different mechanisms for gas-sensing enhancement by doping noble metals, which are the electronic and chemical sensitization.43–46 There have been many papers discussing the role of nobel metal in the improvement of the sensing response to reducing gas. In the present work, when PdCl2 was doped with nanoparticle SmFeO3, the nanoparticle PdO located on the surface of SmFeO3 would be adsorbed by lots of oxygen species, accompanying the dissociation of oxygen molecule. This process enhances the rate of dissociation and diffusion of oxygen species on the surface of SmFeO3, which is the “spill-over” effect.43
When a reducing gas is introduced, the reducing molecule would interact with oxygen species and PdO, where PdO is transform to Pd through the reduction process,45 releasing electrons to the surface of SmFeO3 and resulting in the increase of resistance of the SmFeO3 sensor. The enhancement of sensing resistivity to reducing gas of SmFeO3 by doping of PdO is mainly associated with the “spill-over” effect of oxygen species and reduction process of PdO to Pd. The enhancement of sensing response signals due to nobel metal doping have often been attributed to either a “spillover effect” or a “Fermi energy control”. If the spillover effects would dominate, metallic clusters must activate the gaseous species. The metal phase (Pd nanoparticles) could catalyze the dissociation of oxygen molecules into ions, forming oxygen species adsorbed on the surface of semiconductor oxide, which results in an increase of the active sites for the reaction with the gas molecule. For the sensing mechanism controlled by “Fermi energy control” model, in comparison to semiconductor oxide, PdO has a higher value for “Fermi energy control”. The Fermi energy level of semiconductor oxide is lower than that of PdO, leading to the transfer of some free electrons from the semiconductor oxide nanoparticles to the PdO particles, since the two systems attain a thermodynamic equilibrium and a new Fermi energy level is created. The decrease of electrons of p-type semiconductor oxide leads to the evident enhancement of resistance.61–65 For our work, metal Pd0 and PdO coexist in the sample. In this way, both mechanisms of “spillover effect” and “Fermi energy control” are present simultaneously. Since the amount of PdO is much more than that of Pd in present samples, the “Fermi energy control” model is dominant for the mechanism of enhancement of sensing response.
4. Conclusion
PdCl2 is mixed with nanocrystalline powders SmFeO3 and subsequently followed by annealing at 750 °C. A PdO phase is formed and distributes almost uniformly on the surface of SmFeO3 nanoparticles. EDAX mapping shows that Pd element almost distributes uniformly on the whole sample. It seems that superfine PdO nanoparticles locate around the surface of SmFeO3 nanoparticles. With an increase of PdO amounts in composite powders, there is an enhancement of sensing response Rg/Ra to low concentration of acetone or ethanol for Pd-doped SmFeO3 sensors, which is mainly associated with the “spill-over” effect of oxygen species and reduction process of PdO to Pd. The 3 wt% Pd-doped SmFeO3 sensor exhibits highest sensing response to acetone. The response for SmFeO3 at 240 °C is 2.26 to 500 ppb acetone, whereas the response for 3 wt% Pd-doped SmFeO3 at 240 °C is 7.21 to 500 ppb acetone. It is clear that appropriate Pd doping in SmFeO3 improves the behavior of low concentration gas sensing. The effect of the water vapor was also investigated in this work, with the RH increased, the response S gradually decreased. The 3 wt% Pd-doped SmFeO3 sensor at 240 °C shows very short response time (8 s) and recovery time (15 s) to 500 ppb acetone gas, respectively.Acknowledgements
This work was supported by Shandong Natural Science Foundation (No. ZR2013MM016), National Natural Science Foundation of China (No: 51472145, 51472150, 51272133 and J1103212)References
- Q. Jia, H. Ji, Y. Zhang, Y. Chen, X. Sun and Z. Jin, J. Hazard. Mater., 2014, 276, 262–270 CrossRef CAS PubMed.
- C. Turner, C. Walton, S. Hoashi and M. Evans, J. Breath Res., 2009, 30, 46004 CrossRef PubMed.
- N. Makisimovich, V. Vorotyntsev, N. Nikitina, O. Kaskevich, P. Karabun and F. Martynenko, Sens. Actuators, B, 1996, 36, 419–421 CrossRef CAS.
- A. M. Diskin, P. Spanel and D. Smith, Physiol. Meas., 2003, 24, 107–119 CrossRef PubMed.
- C. H. Deng, J. Zhang, X. F. Yu, W. Zhang and X. M. Zhang, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2004, 810, 269–275 CrossRef CAS.
- J. Hu and H. Qin, J. Magn. Magn. Mater., 2001, 234, 419–422 CrossRef CAS.
- J. Hu and H. Qin, Solid State Commun., 2000, 116, 159–162 CrossRef CAS.
- S. J. Skinner, Fuel Cell. Bull., 2001, 4, 6–12 CrossRef.
- J. G. McCarty and H. Wise, Catal. Today, 1990, 8, 231–248 CrossRef CAS.
- S. Jin, T. H. Tiefel, M. McCormack, R. A. Fastnacht, R. Ramesh and L. H. Chen, Science, 1994, 264, 413–415 CrossRef CAS PubMed.
- R. von Helmolt, J. Wecker, B. Holzapfel, L. Schultz and K. Samwer, Phys. Rev. Lett., 1993, 71, 2331–2333 CrossRef CAS PubMed.
- J. Qin, Z. Cui, X. Yang, S. Zhu, Z. Li and Y. Liang, Sens. Actuators, B, 2015, 209, 706–713 CrossRef CAS.
- Y. M. Zhang, J. Zhang, J. L. Chen, Z. Q. Zhu and Q. J. Liu, Sens. Actuators, B, 2014, 195, 509–514 CrossRef CAS.
- H. Fan, T. Zhang, X. Xu and N. Lv, Sens. Actuators, B, 2011, 153, 83–88 CrossRef CAS.
- L. Zhang, J. Hu, P. Song, H. Qin and M. Jiang, Sens. Actuators, B, 2006, 114, 836–840 CrossRef CAS.
- S. Huang, H. Qin, P. Song, X. Liu, L. Li, R. Zhang, J. Hu, H. Yan and M. Jiang, J. Mater. Sci., 2007, 42, 9973–9977 CrossRef CAS.
- M. L. Post, B. W. Sanders and P. Kennepohl, Sens. Actuators, B, 1993, 13, 272–275 CrossRef CAS.
- E. Traversa, S. Matsushima, G. Okada, Y. Sadaoka, Y. Sakai and K. Watanabe, Sens. Actuators, B, 1995, 25, 661–664 CrossRef CAS.
- H. Hao and R. Liu, J. Rare Earths, 2014, 32, 23 CrossRef CAS.
- M. C. Carotta, M. A. Butturi, G. Martinelli, Y. Sadaoka, P. Nunziante and E. Traversa, Sens. Actuators, B, 1997, 44, 590–594 CrossRef CAS.
- Z. Mu, L. Zhang, X. Li and J. Hu, J. Rare Earths, 2011, 29, 374 CrossRef CAS.
- H. Aono, E. Traversa, M. Sakamoto and Y. Sadaoka, Sens. Actuators, B, 2003, 94, 132–139 CrossRef CAS.
- K. Fan, H. Qin, L. Wang, L. Ju and J. Hu, Sens. Actuators, B, 2013, 177, 265–269 CrossRef CAS.
- P. Song, Q. Wang, Z. Zhang and Z. Yang, Sens. Actuators, B, 2010, 147, 248–254 CrossRef CAS.
- M. Siemons, A. Leifert and U. Simon, Adv. Funct. Mater., 2007, 17, 2189–2197 CrossRef CAS.
- Y. Wang, J. Chen and X. Wu, Mater. Lett., 2001, 49, 361–364 CrossRef CAS.
- J. W. Fergus, Sens. Actuators, B, 2007, 123, 1169–1179 CrossRef CAS.
- L. Zhang, H. Qin, P. Song, J. Hu and M. Jiang, Mater. Chem. Phys., 2006, 98, 358 CrossRef CAS.
- X. Liu, J. Hu, B. Cheng, H. Qin and M. Jiang, Sens. Actuators, B, 2008, 134, 483 CrossRef CAS.
- T. Chen, Z. Zhou and Y. Wang, Sens. Actuators, B, 2009, 143, 124–131 CrossRef.
- Z.-L. Wu, R. Zhang, M. Zhao, S.-M. Fang, Z.-X. Han, J.-F. Hu and K.-Y. Wang, Int. J. Miner., Metall. Mater., 2012, 19, 141 CrossRef CAS.
- X. Liu, H. Ji, Y. Gu and M. Xu, Mater. Sci. Eng., B, 2006, 133, 98–101 CrossRef CAS.
- P. Song, H. Zhang, D. Han, J. Li, Z. Yang and Q. Wang, Sens. Actuators, B, 2014, 196, 140–146 CrossRef CAS.
- L. Liu, T. Zhang, S. C. Li, L. Y. Wang and Y. X. Tian, Mater. Lett., 2009, 63, 1975 CrossRef CAS.
- T. Zhang, L. Liu, Q. Qi, S. Li and G. Lu, Sens. Actuators, B, 2009, 139, 287–291 CrossRef CAS.
- M. Zhang, Z. Yuan, J. Song and C. Zheng, Sens. Actuators, B, 2010, 148, 87–92 CrossRef CAS.
- M. Zhang, T. Ning, S. Zhang, Z. Li, Q. Cao and Z. Yuan, Mater. Sci., 2014, 20, 375–380 Search PubMed.
- H. Smi and C. Chen, J. Mater. Chem., 2012, 22, 13204 RSC.
- D.-J. Yang, I. Kamienchick, D. Y. Young, A. Rothschild and I.-D. Kim, Adv. Funct. Mater., 2010, 20, 4258–4264 CrossRef CAS.
- A. Cabot, J. Arbiol, J. R. Morante, U. Weimer, N. Barsan and W. Gopel, Sens. Actuators, B, 2000, 70, 87 CrossRef CAS.
- M. Batzill and U. Diebold, Prog. Surf. Sci., 2005, 79, 47 CrossRef CAS.
- A. Rajgure, J. Patil, R. Pawar, C. Lee and S. Suryavanshi, Ceram. Int., 2013, 39, 87–92 CrossRef CAS.
- A. Kolmakov, D. Klenov, Y. LiSmch, S. Stemmer and M. Moskovits, Nano Lett., 2002, 5, 667 CrossRef PubMed.
- Y. Lee, H. Huang, O. K. Tan and M. S. Tse, Sens. Actuators, B, 2008, 132, 239 CrossRef CAS.
- M. V. Vaishampayan, R. G. Deshmukh and I. S. MulSm, Sens. Actuators, B, 2008, 131, 665 CrossRef CAS.
- P. Menini, F. Parret, M. Gueerero, K. SouSmntica, L. Erades, A. Maisonnat and B. Chaudret, Sens. Actuators, B, 2004, 103, 111 CrossRef CAS.
- J. Moon, J. A. Park, S.-J. Lee, T. Zyung and I.-D. Kim, Sens. Actuators, B, 2010, 149, 301–305 CrossRef CAS.
- D. J. Yang, I. Kamienchick, D. Y. Youn, A. Rothschild and I. D. Kim, Adv. Funct. Mater., 2010, 20, 4258–4264 CrossRef CAS.
- J. Shin, S. J. Choi, D. Y. Youn and I. D. Kim, J. Electroceram., 2012, 29, 106–116 CrossRef CAS.
- S. Yuasa, M. Kida, T. Kanmura, Y. Huh, J. Yamazoe and N. K. Shimanoe, J. Ceram. Soc. Jpn., 2011, 119, 884–889 CrossRef.
- B. C. Yadav, R. Srivastava, C. D. Dwivedi and P. Pramanik, Sens. Actuators, B, 2008, 131, 216–222 CrossRef CAS.
- S. Pokhrel, B. Jeyaraj and K. S. Nagaraja, Mater. Lett., 2003, 57, 3543–3548 CrossRef CAS.
- J. Wang, Q. H. Lin, R. Q. Zhou and B. K. Xu, Sens. Actuators, B, 2002, 81, 248–253 CrossRef CAS.
- A. C. Caballero, M. Villegas, J. F. Fernandez, M. Viviani, M. T. Buscaglia and M. Leoni, J. Mater. Sci. Lett., 1999, 18, 1297–1299 CrossRef CAS.
- K. P. Biju and M. K. Jain, Thin Solid Films, 2008, 516, 2175–2180 CrossRef CAS.
- Q. Kuang, C. Lao, Z. Wang, Z. Xie and L. Zheng, J. Am. Chem. Soc., 2007, 129, 6070–6071 CrossRef CAS PubMed.
- F. Pourfayaz and Y. Mortazavi, Sens. Actuators, B, 2008, 130, 625–629 CrossRef CAS.
- W. P. Tai and H. Oh, Sens. Actuators, B, 2002, 85, 154–157 CrossRef CAS.
- L. Li, H. Qin, C. Shi, L. Zhang, Y. Chen and J. Hu, RSC Adv., 2015, 5, 103073–103081 RSC.
- X. Liu, B. Cheng, J. Hu, H. Qin and M. Jiang, Sens. Actuators, B, 2008, 129, 53–58 CrossRef CAS.
- A. Daryakenari, A. Apostoluk and J. Delaunay, Phys. Status Solidi C, 2013, 10, 1297–1300 CAS.
- W. Lu, S. Gao and J. Wang, J. Phys. Chem. C, 2008, 112, 16792 CrossRef CAS.
- J. Zhang, X. Liu, L. Wang, T. Yang, X. Guo, S. Wu, S. Wang and S. Zhang, Nanotechnology, 2011, 22, 185501 CrossRef PubMed.
- L. L. Xing, C. H. Ma, Z. H. Chen, Y. J. Chen and X. Y. Xuel, Nanotechnology, 2011, 22, 215501 CrossRef PubMed.
- J. Kappler, N. Bârsan, U. Weimar, A. Dièguez, J. L. Alay, A. Romano-Rodriguez, J. R. Morante and W. Göpel Fresenius, J. Anal. Chem., 1998, 361, 110–114 CAS.
| This journal is © The Royal Society of Chemistry 2016 |