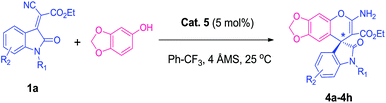Construction of highly enantioselective spiro-oxindole derivatives with fused chromene via organocascade catalysis†
Prathibha Kumariab,
Sekhar Nandiab,
Gaurav Kumarab,
Noorul H. Khan*ab,
Rukhsana I. Kureshyab,
Sayed H. R. Abdiab,
E. Sureshbc and
Hari C. Bajajab
aInorganic Materials and Catalysis Division, Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), G. B. Marg, Bhavnagar, 364002, Gujarat, India. E-mail: Khan251293@yahoo.in
bAcademy of Scientific & Innovative Research (AcSIR), India
cAnalytical Division and Centralized Instrument Facility, Central Salt and Marine Chemicals Research Institute (CSIR-CSMCRI), G. B. Marg, Bhavnagar, 364002, Gujarat, India
First published on 20th May 2016
Abstract
A first highly enantioselective addition of naphthols and sesamol to indolylidene cyanoacetate derived from isatins was carried out in the presence of bi-functional quinine thiourea as organocatalyst. Under optimized reaction conditions the present catalytic system gave spiro-oxindole derivatives with excellent yield and enantioselectivity (up to 99%) at very low catalyst loading (2 mol%). The absolute configuration of 3i was determined by single X-ray crystallographic analysis to be the S isomer of the product. A catalytic cycle is proposed based on NMR, IR and kinetic studies.
Introduction
Chiral spirooxindole derivatives having heterocyclic frameworks are well-known for anomalous biologically active molecules present in numerous natural products as well as pharmaceuticals1–3 and possess excellent binding ability towards many receptors in biological systems.4–10In general, synthesis of the privileged heterocyclic chiral spirooxindole motif remains a significant challenge in organic chemistry. To date numerous asymmetric synthetic methods, various organo-cascade strategies and multistep diastereoselective transformations using chiral auxiliaries have been developed for construction of this class of chiral spiro-oxindole scaffolds fused with heterocyclic rings bearing a four to seven-membered ring system at the C3-position.11–21 In recent years attempts were also made for the synthesis of spiro-oxindoles with fused 4H-chromenes using different basic catalysts.22–24 Despite the enormous developments,25 to the best of our knowledge till date, the enantioselective addition of naphthols and sesamol to oxindole-derived Michael acceptors catalysed by quinine-derived thiourea as organocatalyst has not been explored.26–32 Wang et. al., developed31 rosin thiourea catalysed reaction of coumarins with isatylidene malononitriles and in similar context Zhao et. al., reported30 quinine thiourea catalysed three-component cascade reactions of isatins, malononitrile, and 2-hydroxynaphthalene-1,4-diones. However enantioselective addition of naphthol and sesamol with indolylidene cyanoacetate remain unexplored. With our continuous interest for the development of organocatalytic system in different organic transformations,33 here we are reporting a simple method for the synthesis of spiro-oxindoles with fused chromenes in highly enantioselective manner, through the reaction of indolylidene cyanoacetates with 1-naphthols/sesamol using bi-functional quinine-thiourea as a catalyst.
Results and discussion
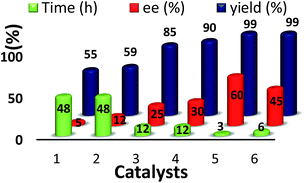
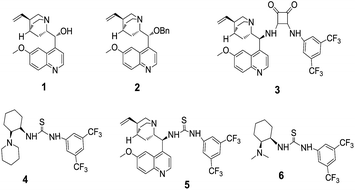
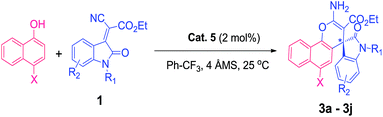
With this backdrop, our initial study begins with commercially available quinine 1 & 2 as organo-catalyst to examine their catalytic activity for enantioselective addition of 1-naphthol with indolylidene cyanoacetates derived from N-benzylisatin 1a, as a model reaction in toluene at RT (25 °C). However, these well-known catalysts were found to be unsuitable for giving the desired product i.e., spiro-oxindoles in terms of yield and enantioselectivity (Fig. 1) even after very long reaction time (48 h). Then, attempts were made to conduct the same reaction with quinine squaramide Cat. 3 which ended up the reaction with good yield but poor enantioselectivity up to 25% (Fig. 1). Thiourea based organocatalysts have shown promising results for many organic transformations, thus we have screened thiourea based bifunctional catalysts 4–6 (Scheme 1) for this reaction. Among all the organo-catalysts screened the Cat. 5 has given better result in terms of yield and enantioselectivity (Fig. 1).Encouraged by the preliminary results with optimized Cat. 5 the optimization of reaction conditions such as choice of solvents and catalyst loading were investigated for enantioselective addition of 1-naphthol with 1a, as these parameters are known to influence the yield and enantioselectivity of the products. Therefore, different solvents viz., dichloromethane, chloroform, diethylether, ethylacetate, toluene, acetonitrile, xylene and Ph–CF3 (α,α,α-trifluoroemethyl benzene) were explored (Table 1, entries 1–7). However, none of these could match the performance (yield 99% and ee 99%) of Ph–CF3 as a solvent of choice (Table 1, entry 7). Next, we varied the catalyst loading from 1 to 10 mol% (Table 1, entries 7–11) and found that only 2 mol% catalyst is sufficient enough to give 99% yield with 99% enantioselectivity of the product 3a (Table 1, entry 10).
| Entry | solvent | Catalyst mol% | Time (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction condition: all the reactions were carried out by using 1-naphthol (0.2 mmol) with substrate 1a (0.1 mmol) with Cat. 5 in 2 ml of dry solvent and activated 4 Å MS at 25 °C.b Isolated yields after column chromatography.c ee determined by chiral HPLC using Daicel Chiralpak IB column. | |||||
| 1 | DCM | 10 | 8 | 99 | 60 |
| 2 | CHCl3 | 10 | 8 | 99 | 55 |
| 3 | Et2O | 10 | 10 | 70 | 28 |
| 4 | EtOAc | 10 | 7 | 99 | 94 |
| 5 | CH3CN | 10 | 6 | 95 | 55 |
| 6 | Xylene | 10 | 6 | 95 | 61 |
| 7 | Ph–CF3 | 10 | 0.5 | 99 | 81 |
| 8 | Ph–CF3 | 5 | 1 | 99 | 99 |
| 9 | Ph–CF3 | 2.5 | 1.5 | 99 | 99 |
| 10 | Ph–CF3 | 2 | 1.5 | 99 | 99 |
| 11 | Ph–CF3 | 1 | 3 | 92 | 94 |
With these optimum reaction conditions (Table 1, entry 10), the scope of organo-catalyst Cat. 5 was extended for the enantioselective addition of 1-naphthol to indolylidene cyanoacetate derived from isatins to study the effect of substituents at the different positions of the indolylidene cyanoacetates (1) and data is shown in Table 2.
| a Reaction condition: all the reactions were carried out by using 1-naphthol (0.4 mmol) or 2-naphthol (0.4 mmol) with various substrates (0.2 mmol) using organocatalyst Cat. 5 with 2 mol% or 5 mol%, in 2 ml of dry Ph–CF3 and 30 mg 4 Å MS at 25 °C and ee determined by chiral HPLC using Daicel Chiralpak IA, IB and IC columns. |
|---|
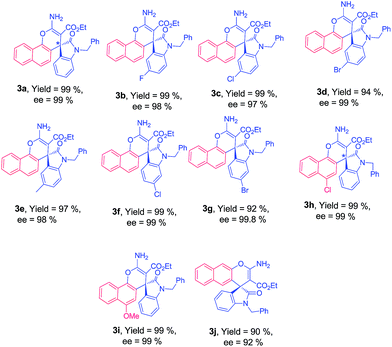 |
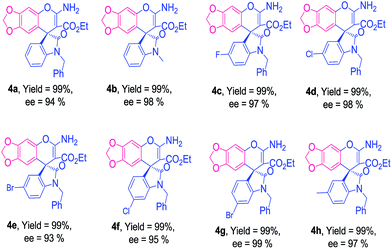
Isatin-derived indolylidene cyanoacetates with benzyl substituents at nitrogen atom (R1 = Bn) was efficiently transformed into the corresponding products with excellent enantioselectivity 99%. Subsequently, we evaluated indolylidene cyanoacetates derived from various substituted N-benzylisatins and obtained the corresponding products 3b–3g in high yields and enantioselectivity (97–99% ee), regardless electronic character of aromatic ring of the isatin and the position of the substituent. The use of different substituted 1-naphthols also afforded the expected products 3h–3i with excellent results 99% ee (Table 2). To further evaluate the generality of our catalytic system we next screened less reactive 2-naphthol as nucleophile with isatin-derived indolylidene cyanoacetate 1a with a catalyst loading of 5 mol%. Good yield with 92% ee was achieved for spiro-oxindole 3j in 12 h. Encouraged by these results, we were delighted to observe that this protocol could be extended for the use of sesamol as the nucleophile. The corresponding products 4a–4h were obtained in good yield with high enantioselectivity (Table 3). The spiro-oxindole sesamol are well known intermediates in many commercially available drugs,25j but till date enantioselective addition of sesamol has been reported only for aldimines and ketimines.25k–m Fortunately, sesamol with indolylidene cyanoacetate derivatives 1a produced spiro-oxindole scaffolds 4a with 98% ee, whereas substituted spiro-oxindole adducts 4b–4h were formed with higher enantioselectivity (95–99% ee).
| a Reaction condition: all the reactions were carried out by using sesamol (0.4 mmol) with various substrates (0.1 mmol) with organocatalyst 5 (5 mol%) in 2 ml of dry Ph–CF3 and 30 mg of 4 Å MS at 25 °C. ee determined by chiral HPLC using Daicel Chiralpak IA, IB and IC columns. |
|---|
 |
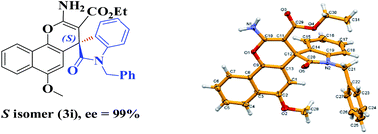
The absolute configuration of product 3i was assigned as (S) on the basis of an X-ray crystal structure analysis34 (Fig. 2). Crystal structure analysis revealed that the product 3i crystallized in orthorhombic system with chiral space group P212121, supporting the good yield and selectivity of the enantiopure product supplemented by other analytical methods.
NMR and IR studies
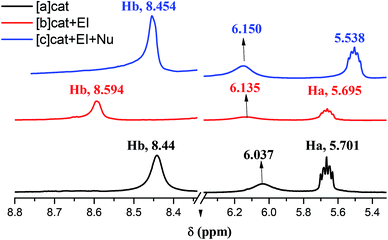
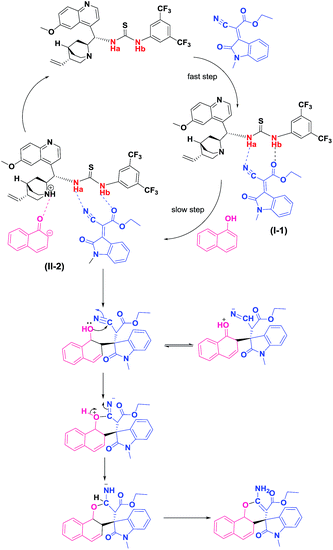
In order to elucidate the reaction mechanism we have conducted NMR spectroscopic experiments using Cat. 5, with N-methylisatin derived indolylidene cyanoacetates (EI) as a representative substrate with 1-naphthol (Nu) as nucleophile. The 1H NMR experiments depicted that the two acidic protons (Ha, Hb) of Cat. 5 appeared at δ 5.70 and δ 8.44, but after interaction with EI the Hb proton of Cat. 5 shifted more down field and lie at δ 8.59 showing the strong interactions of N–H proton of Cat. 5 with EI as shown in Fig. 3. Whereas, a strong up field shift in Ha proton at δ 5.53 of Cat. 5 were observed after mixing the Nu with Cat. 5. These experiments suggest that both EI and Nu get activated through H-bonding with Cat. 5 in stereo-chemical pathway which had a substantial effect on stereoselectivity as shown in Scheme 2.The same trend was also observed in 13C NMR for both substrates and nucleophile after interaction with the catalyst. The chemical shifts for carbon of –CN and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester group present in substrate EI lie at δ 114.11 and δ 164.41 as shown in Fig. 4 and after interaction with Cat. 5 there was a shift in –CN and –C
O of ester group present in substrate EI lie at δ 114.11 and δ 164.41 as shown in Fig. 4 and after interaction with Cat. 5 there was a shift in –CN and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O positions which then lies at δ 114.52 and 164.39 respectively; {[B] is 13C NMR of pure EI, [C] is 13C NMR of substrate EI mixed with Cat. 5 in Fig. 4}. This confirms that the substrate getting interaction with Cat. 5 as shown in the Scheme 2 and possible to form an intermediate-[I-1] in the catalytic cycle as shown in the Scheme 3. In similar manner we have also observed shifting in the phenolic carbon position of Nu which usually comes at δ 151.30 after interaction with Cat. 5 it was shifted to δ 152.61 as shown in Fig. 4. It was observed that after 1 h the substrate 1a was completely consumed (checked by TLC), where we found that complete disappearance of carbon which corresponding to –CN of substrate with the generation of new peak at δ 115.80 which may correspond to carbon atom of the spiro-oxindole product.
O positions which then lies at δ 114.52 and 164.39 respectively; {[B] is 13C NMR of pure EI, [C] is 13C NMR of substrate EI mixed with Cat. 5 in Fig. 4}. This confirms that the substrate getting interaction with Cat. 5 as shown in the Scheme 2 and possible to form an intermediate-[I-1] in the catalytic cycle as shown in the Scheme 3. In similar manner we have also observed shifting in the phenolic carbon position of Nu which usually comes at δ 151.30 after interaction with Cat. 5 it was shifted to δ 152.61 as shown in Fig. 4. It was observed that after 1 h the substrate 1a was completely consumed (checked by TLC), where we found that complete disappearance of carbon which corresponding to –CN of substrate with the generation of new peak at δ 115.80 which may correspond to carbon atom of the spiro-oxindole product.
To further support the interactions of the catalyst, substrate and nucleophile observed in NMR experiments, we have also conducted the IR experiments in order to establish an appropriate mechanistic path of the reaction. A band which was coming around 2210 cm−1 of –CN group of EI and after interaction with Cat. 5 it was shifted towards lower wave number and lie at 2146 cm−1 which confirm the interaction of –CN group with Cat. 5 {[B] is IR of pure substrate EI, [E] is IR of both the substrates after interaction with Cat. 5 in Fig. 5}. As the reaction proceeded the peak correspond to –CN started diminishing {[F] IR of reaction after 5 min in Fig. 5} and after completion of the reaction there was complete disappearance of –CN peak {[G] reaction after 10 min} which confirm the formation of the spirooxindole product.
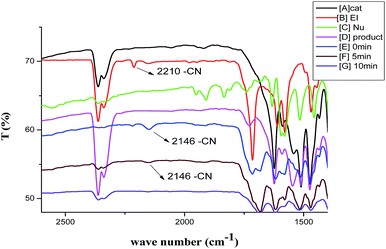 | ||
| Fig. 5 Reaction was carried out with stoichiometric amount of 1-naphthol (Nu), substrate (EI) and catalyst (Cat. 5) in equal ratios in DCM. | ||
Kinetics study
The kinetics study of enantioselective addition of 1-naphthol to indolylidene cyanoacetate was carried out in detail by conducting the kinetic experiments with N-benzylisatin derived indolylidene cyanoacetates 1a as a model substrate and as a function of concentration of Cat. 5 and 1-naphthol. In the initial experiments, the plot of formation of the product with time was found to be linear (Fig. 6).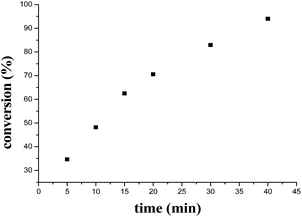 | ||
| Fig. 6 Plot of time versus concentration of product at 0 °C, [Cat. 5] = 0.0003 M, [substrate] = 0.005 M, [1-naphthol 0.01 M]. | ||
The kinetics of reaction was evaluated by performing the experiments at optimized reaction condition with different concentrations of Cat. 5 and substrates concentrations, from those corresponding kinetics data as shown in Table 4, the graphs were plotted with respect to the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kobs obtained and logarithm of concentration of catalyst as well as substrates concentrations as indicated in Fig. 7–9. From the Fig. 7 and 9, a linear plot of log
kobs obtained and logarithm of concentration of catalyst as well as substrates concentrations as indicated in Fig. 7–9. From the Fig. 7 and 9, a linear plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kobs of the product formation versus log[catalyst] and log[1-naphthol] with unit slopes was obtained indicates that reaction is of first order with respect to the concentration of the Cat. 5 and 1-naphthol and Fig. 8 indicate the zero order dependence of substrate concentration.
kobs of the product formation versus log[catalyst] and log[1-naphthol] with unit slopes was obtained indicates that reaction is of first order with respect to the concentration of the Cat. 5 and 1-naphthol and Fig. 8 indicate the zero order dependence of substrate concentration.
| [Cat] × 10−3 M | [Electrophile] × 10−2 M | [Naphthol] × 10−2 M | kobs × 10−3 mM min−1 |
|---|---|---|---|
| Catalyst variation | |||
| 1.5 | 5 | 10 | 7.29 ± 03 |
| 2.5 | 5 | 10 | 14.48 ± 02 |
| 3.5 | 5 | 10 | 16.96 ± 03 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Electrophile variation | |||
| 2.5 | 3 | 10 | 13.73 ± 03 |
| 2.5 | 5 | 10 | 14.48 ± 02 |
| 2.5 | 7 | 10 | 12.60 ± 03 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 1-Naphthol | |||
| 2.5 | 5 | 5 | 6.00 ± 0.02 |
| 2.5 | 5 | 10 | 14.48 ± 0.02 |
| 2.5 | 5 | 15 | 17.66 ± 0.04 |
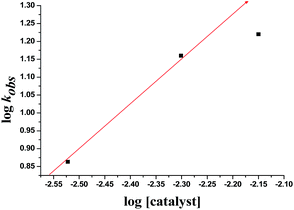 | ||
Fig. 7 Plot of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kobs versus log[catalyst] at 0 °C, log[substrate] = 5 × 10−2 M, [1-naphthol] = 10 × 10−2 M. kobs versus log[catalyst] at 0 °C, log[substrate] = 5 × 10−2 M, [1-naphthol] = 10 × 10−2 M. | ||
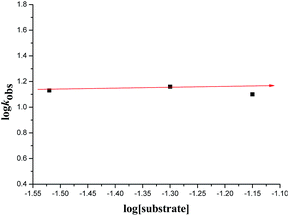 | ||
Fig. 8 Plot of substrate 1a concentration versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kobs at 0 °C, [1-naphthol] = 10 × 10−2 M, [catalyst] = 2.5 × 10−3 M. kobs at 0 °C, [1-naphthol] = 10 × 10−2 M, [catalyst] = 2.5 × 10−3 M. | ||
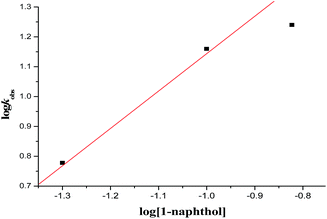 | ||
Fig. 9 Plot of 1-naphthol concentration versus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kobs at 0 °C, log[substrate] = 5 × 10−2 M = 10 × 10−2 M, [catalyst] = 2.5 × 10−3 M. kobs at 0 °C, log[substrate] = 5 × 10−2 M = 10 × 10−2 M, [catalyst] = 2.5 × 10−3 M. | ||
Proposed catalytic cycle
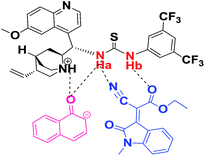
Based on the NMR, IR experiment and kinetics study, a general hypothesis of mechanism for the synthesis of enantioselective spiro-oxindole derivative with bi-functional quinine-thiourea catalyst was proposed as shown in Scheme 2. The kinetics study revealed that reaction performs zero order with respect to substrate EI and the interaction of EI with Cat. 5 assumed to formed in fast step of the reaction as an intermediate [I-1] of Scheme 3, whereas 1-naphthol is following first order and possible to form an intermediate [II-2] (Scheme 3) in slow step of the reaction constituting C–C bond formation product which intern undergoes intra-molecular cyclization to produce spiro-oxindole product with the regeneration of the active catalyst and this catalytic cycle proceeds till the complete consumption of the starting materials.Conclusions
In the present study, we have demonstrated the first effective bi-functional quinine thiourea catalytic system for the reaction of naphthols and sesamol with indonylidene cyanoacetate towards the synthesis of chiral spiro-oxindole products in good yield (up to 99%) with excellent enantioselectivity (<99%). The absolute configuration of compound 3i was ascertained as S-isomer on the basis of X-ray crystallographic analysis. In this context we have also studied the kinetics of the reaction and NMR experiments were also conducted in order to know the origin of catalytic mechanism for this reaction.Acknowledgements
CSIR-CSMCRI registration no is 186/2015. Authors are thankful to UGC and Indus Magic Project CSC0123 for financial support. Prathibha Kumari, Sekhar Nandi and Gaurav kumar is thankful to AcSIR for Ph. D. enrolment. Analytical Discipline and Centralized Instrumental Facility is gratefully acknowledged for providing instrumental facilities.Notes and references
- V. Glover, J. M. Halket, P. J. Watkins, A. Clow, B. L. Goodwin and M. J. Sandler, J. Neurochem., 1998, 51, 656 CrossRef.
- (a) F. Rouatbi, M. Askri, F. Nana and G. Kirsch, Tetrahedron Lett., 2015, 57, 163–167 CrossRef; (b) G. Kumari, Nutan, M. Modi, S. K. Gupta and R. K. Singh, Eur. J. Med. Chem., 2011, 46, 1181–1188 CrossRef CAS PubMed; (c) R. Karmakar, U. Kayal, B. Bhattacharya and G. Maiti, Tetrahedron Lett., 2014, 55, 1370–1372 CrossRef CAS; (d) X. Jiang, Y. Cao, Y. Wang, L. Liu, F. Shen and R. Wang, J. Am. Chem. Soc., 2010, 132, 15328–15333 CrossRef CAS PubMed; (e) J. Liu, Y. Sun, X. Zhang, X. Liang, Y. Wu, Y. Wang and X. Jiang, Inflammation and Cell Signaling, 2014, 1, 372, DOI:10.14800/ics.372; (f) R. Murugan, S. Anbazhagan and S. S. Narayanan, Eur. J. Med. Chem., 2009, 44, 3272–3279 CrossRef CAS PubMed; (g) R. Kumar, R. C. Bansal and A. Mahmood, Biog. Amines, 1993, 9, 281 CAS.
- See several reviews: (a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748–8758 CrossRef CAS PubMed; (b) L. Honga and R. Wang, Adv. Synth. Catal., 2013, 355, 1023–1052 CrossRef; (c) M. M. M. Santos, Tetrahedron, 2014, 70, 9735–9757 CrossRef CAS; (d) S. Gao, C. H. Tsai, C. Tseng and C. F. Yao, Tetrahedron, 2008, 64, 9143 CrossRef CAS; (e) L. L. Wang, J. F. Bai, L. Peng, L. W. Qi, L. N. Jia, Y. L. Guo, X. Y. Luo, X. Y. Xu and L. X. Wang, Chem. Commun., 2012, 48, 5175–5177 RSC; (f) Y. Cao, X. Jiang, L. Liu, F. Shen, F. Zhang and R. Wang, Angew. Chem., Int. Ed., 2011, 50, 9124–9127 CrossRef CAS PubMed; (g) Y. L. Liu, X. Wang, Y. L. Zhao, F. Zhu, X. P. Zeng, L. Chen, C. H. Wang, X. L. Zhao and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 13735–13739 (Angew. Chem., 2013, 125, 13980) CrossRef CAS PubMed; (h) S. Kato, T. Yoshino, M. Shibasaki, M. Kanai and S. Matsunaga, Angew. Chem., Int. Ed., 2012, 51, 7007–7010 (Angew. Chem., 2012, 124, 7113) CrossRef CAS PubMed; (i) W. Guo, X. Wang, B. Zhang, S. Shen, X. Zhou, P. Wang, Y. Liu and C. Li, Chem.–Eur. J., 2014, 20, 8545–8550 CrossRef CAS PubMed; (j) M. X. Zhao, H. Zhou, W. H. Tang, W. S. Qu and M. Shi, Adv. Synth. Catal., 2013, 355, 1277–1283 CrossRef CAS.
- A. E. Medvedev, A. Clow, M. Sandler and V. Glover, Biochem. Pharmacol., 1996, 52, 385 CrossRef CAS PubMed.
- D. J. F. M. Silva, S. J. Garden and A. C. Pinto, J. Braz. Chem. Soc., 2001, 12, 273 CrossRef.
- A. H. Abdel-Rahman, E. M. Keshk, M. A. Hanna and M. E. B. Sh, Bioorg. Med. Chem., 2004, 12, 2483 CrossRef CAS PubMed.
- K. Hiramoto, A. Nasuhara, K. Michiloshi, T. Kato and K. Kikugawa, Mutat. Res., 1997, 47, 395 Search PubMed.
- K. C. Joshi, R. Jain and S. Arora, J. Indian Chem. Soc., 1988, 65, 277 CAS.
- (a) K. C. Joshi, R. Jain and K. Sharma, J. Indian Chem. Soc., 1988, 65, 202 CAS; (b) A. G. A. Elagamay and F. M. A. A. E. -Taweel, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 1990, 29, 885 Search PubMed.
- R. Ballini, G. Bosica, M. L. Conforti, R. Maggi, A. Mazzacanni, P. Righi and G. Sartori, Tetrahedron, 2001, 57, 1395 CrossRef CAS.
- (a) A. P. Antonchick, C. Gerding-Reimers, M. Catarinella, M. Schurmann, H. Preut, S. Ziegler, D. Rauh and H. Waldmann, Nat. Chem., 2010, 2, 735–740 CrossRef CAS PubMed; (b) Y.-M. Wang, H.-H. Zhang, C. Li, T. Fana and F. Shi, Chem. Commun., 2016, 52, 1804–1807 RSC; (c) J. J. Zhao, S. B. Sun, S. H. He, Q. Wu and F. Shi, Angew. Chem., Int. Ed., 2015, 54, 5460–5464 CrossRef CAS PubMed; (d) J.-J. Zhao, Y.-C. Zhang, M.-M. Xu, M. Tang and F. Shi, J. Org. Chem., 2015, 80, 10016–10024 CrossRef CAS PubMed.
- G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104–6155, DOI:10.1021/cr300135y.
- D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743–762 CrossRef CAS.
- N. R. Ball-Jones, J. J. Badillo and A. K. Franz, Org. Biomol. Chem., 2012, 10, 5165–5181 CAS.
- J.-Q. Zhao, Z.-J. Wu, M.-Q. Zhou, X.-Y. Xu, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2015, 17, 5020–5023 CrossRef CAS PubMed.
- Y.-J. Xie, J. Sun and C.-G. Yan, ACS Comb. Sci., 2014, 16, 271–280 CrossRef CAS PubMed.
- D. B. Ramachary, C. Venkaiah and R. Madhavachary, Org. Lett., 2013, 15, 12 Search PubMed.
- L.-W. Qi, Y. Yang, Y.-Y. Gui, Y. Zhang, F. Chen, F. Tian, L. Peng and L.-X. Wang, Org. Lett., 2014, 16, 6436–6439 CrossRef CAS PubMed.
- W. Dai, X.-L. Jiang, Q. Wu, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 5737–5744 CrossRef CAS PubMed.
- D. Jiang, S. Dong, W. Tang, T. Lu and D. Du, J. Org. Chem., 2015, 80, 11593–11597 CrossRef CAS PubMed.
- Y. Wang, F. Shi, X.-X. Yao, M. Sun, L. Dong and S.-J. Tu, Chem.–Eur. J., 2014, 20, 15047–15052 CrossRef CAS PubMed.
- (a) S. Abbaraju, N. Ramireddy, N. K. Rana, H. Arman and J. C.-G. Zhaoa, Adv. Synth. Catal., 2015, 357, 2633–2638 CrossRef CAS; (b) Z.-C. Geng, S.-Y. Zhang, N.-K. Li, N. Li, J. Chen, H.-Y. Li and X.-W. Wang, J. Org. Chem., 2014, 79, 10772–10785 CrossRef CAS PubMed; (c) F. Tan, L.-Q. Lu, Q.-Q. Yang, W. Guo, Q. Bian, J.-R. Chen and W.-J. Xiao, Chem.–Eur. J., 2014, 20, 3415–3420 CrossRef CAS PubMed; (d) G. Shanthi, G. Subbulakshmi and P. T. Perumal, Tetrahedron, 2007, 63, 2057–2063 CrossRef CAS; (e) S. L. Zhu, S. J. Ji and Y. Zhanga, Tetrahedron, 2007, 63, 9365 CrossRef CAS.
- L.-M. Wang, N. Jiao, J. Qiu, J.-J. Yu, J.-Q. Liu, F.-L. Guo and Y. Liu, Tetrahedron, 2010, 66, 339 CrossRef CAS.
- A. R. Karimi and F. Sedaghatpour, Synthesis, 2010, 10, 1731 CrossRef.
- (a) For a review, see: F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; For recent selected examples, see: (b) P. Chauhan and S. S. Chimni, Chem.–Eur. J., 2010, 16, 7709 CrossRef CAS PubMed; (c) X.-N. Wang, Y.-Y. Zhang and S. Ye, Adv. Synth. Catal., 2010, 352, 1892 CrossRef CAS; (d) Q. Guo, M. Bhanushali and C.-G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 9460 CrossRef CAS PubMed; (e) T. Bui, N. R. Candeias and C. F. Barbas III, J. Am. Chem. Soc., 2010, 132, 5574 CrossRef CAS PubMed; (f) X. Li, S. Luo and J.-P. Cheng, Chem.–Eur. J., 2011, 16, 14290 CrossRef PubMed; (g) K. Zheng, C. Yin, X. Liu, L. Lin and X. Feng, Angew. Chem., Int. Ed., 2011, 50, 2573 CrossRef CAS PubMed; (h) Z. Zhang, W. Zheng and J. C. Antilla, Angew. Chem., Int. Ed., 2011, 50, 1135 CrossRef CAS PubMed; (i) F. Zhong, X. Han, Y. Wang and Y.-X. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed; (j) B. Tan, N. R. Candeias and C. F. Barbas III, Nat. Chem., 2011, 3, 473 CAS; (k) Z. Liu, P. Gu, M. Shi, P. McDowell and G. Li, Org. Lett., 2011, 13, 2314 CrossRef CAS PubMed; (l) A.-B. Xia, C. Wu, T. Wang, Y.-P. Zhang, X.-H. Du, A.-G. Zhong, D.-Q. Xu and Z.-Y. Xua, Adv. Synth. Catal., 2014, 356, 1753–1760 CrossRef CAS; (m) M. M.-C. Magraner, R. Vila, G. Cantýn, I. Blay, M. Fernndez, C. MuÇoz and J. R. Pedro, Angew. Chem., Int. Ed., 2015, 54, 6320–6324 CrossRef PubMed; (n) P. Chauhan and S. S. Chimni, Tetrahedron Lett., 2013, 54, 4613–4616 CrossRef CAS; (o) S. Bai, Y. Liao, L. Lin, W. Luo, X. Liu and X. Feng, J. Org. Chem., 2014, 79, 10662–10668 CrossRef CAS PubMed.
- Z.-M. Liu, N.-K. Li, X.-F. Huang, B. Wu, N. Li, C.-Y. Kwok, Y. Wang and X.-W. Wang, Tetrahedron, 2014, 70, 2406–2415 CrossRef CAS.
- A. Quintavalla, F. Lanza, E. Montroni, M. Lombardo and C. Trombini, J. Org. Chem., 2013, 78, 12049–12064 CrossRef CAS PubMed.
- H. R. Safaei Shekouhy, M. S. Rahmanpur and A. Shirinfeshan, Green Chem., 2012, 14, 1696 RSC.
- R. Ghahremanzadeh, G. Hosseini, R. Akbarzadeh and A. Bazgira, J. Heterocycl. Chem., 2013, 50, 272 CrossRef CAS.
- H.-W. Zhao, B. Li, T. Tian, W. Meng, Z. Yang, X.-Q. Song, X.-Q. Chen and H.-L. Pang, Eur. J. Org. Chem., 2015, 3320–3326 CrossRef CAS.
- X. Jiang, Y. Sun, J. Yao, Y. Cao, M. Kai, N. He, X. Zhang, Y. Wang and R. Wanga, Adv. Synth. Catal., 2012, 354, 917–925 CrossRef CAS.
- X.-M. Shi, W. P. Dong, L.-P. Zhu, X.-X. Jiang and R. Wang, Adv. Synth. Catal., 2013, 355, 3119–3123 CrossRef CAS.
- (a) S. Saravanan, A. Sadhukhan, N. H. Khan, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, J. Org. Chem., 2012, 77, 4375 CrossRef CAS PubMed; (b) S. Saravanan, N. H. Khan, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, ACS Catal., 2013, 3, 2873 CrossRef CAS; (c) A. Sadhukhan, S. Saravanan, N. H. Khan, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, J. Org. Chem., 2012, 77, 7076 CrossRef CAS PubMed; (d) D. Ghosh, S. H. R. Abdi, N. H. Khan and H. C. Bajaj, Eur. J. Org. Chem., 2015, 2801–2806 CrossRef CAS; (e) P. Kumari, S. Barik, N. H. Khan, B. Ganguly, R. I. Kureshy, S. H. R. Abdi and H. C. Bajaj, RSC Adv., 2015, 5, 69493–69501 RSC.
- CCDC number 1434825 (3j) contains the ESI† crystallographic data for this paper.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, HPLC profiles and X-ray crystallographic CIF file are given. CCDC 1434825. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra06812j |
| This journal is © The Royal Society of Chemistry 2016 |