Nanoparticles in sonodynamic therapy: state of the art review
Hongyan Xu,
Xia Zhang,
Rubing Han,
Peimin Yang,
Haifeng Ma*,
Yan Song,
Zhichao Lu,
Weidong Yin,
XiangXia Wu and
Hui Wang
Department of Pharmacy, People′s Hospital of Linzi District, Linzi, Shandong Province 255400, China. E-mail: hfma0533@126.com; Tel: +86-533-7162081
First published on 17th May 2016
Abstract
Sonodynamic therapy (SDT) is a novel therapeutic platform that involves a combination of low-intensity ultrasound and special bioagents called sonosensitizers. The application of nanoparticle technology substantially alters the concepts of traditional SDT. It has great potential to address many shortcomings which frequently trouble the clinical use of SDT, and paves the way for more effective yet safer therapeutic options. In this review, we systematically describe and compare the different individual types of nanoparticle that are being developed for SDT. Recent advances in the use of nanoparticles as sonosensitizers themselves or as carriers for sonosensitizer delivery are highlighted. Furthermore, potential limitations and future perspectives are also presented.
1. Introduction
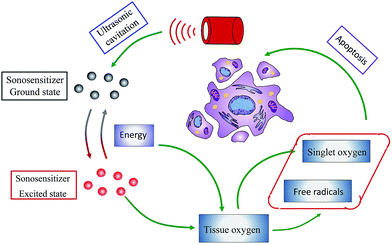
In the past decades, the emergence of drug resistance and treatment-associated toxicities has significantly challenged traditional treatment modalities like chemotherapy.1,2 The research and development of novel treatments have received an increasing interest. Sonodynamic therapy (SDT) is an emerging alternative to conventional therapies. It synergistically uses low intensity ultrasound and special agents termed as sonosensitizers. This treatment mainly applies to the preferential uptake and retention of sonosensitizers in diseased cells and their subsequent activation via ultrasound stimulation.3 As shown in Fig. 1, SDT requires three essential elements: low intensity ultrasound, sonosensitizer and molecular oxygen. Mechanistically, the following three phases are crucial to SDT: excitation of sonosensitizers, generation of reactive oxygen species (ROS) and cell apoptosis. After a predefined time-interval that allows enough sonosensitizers to accumulate in pathological tissue, the diseased site will be exposed to a specific intensity and frequency of ultrasound. In the process, ultrasound vigorously interacts with the surrounding aqueous environment, and results in the presence of a unique phenomenon termed cavitation.4 Sonosensitizer, by the action of ultrasonic cavitation, will be activated from a ground state to an excited state, and then undergoes two kinds of reactions. It may directly react with surrounding oxygen molecules or other substrate molecules, transforming a hydrogen atom to form free radicals. Additionally, on returning to the ground state, such excited state sensitizers can also release energy. The combined action of released energy with surrounding oxygen molecules results in the generation of singlet oxygen, which is thought to be the predominant mediator of sonodynamic activity.5,6 As a highly reactive agent, singlet-state oxygen has the ability to oxidize surrounding substrates, leading to an irreversible destruction in the targeted pathological cells. Once the concentration of singlet molecular oxygen is sufficient, cells will experience a series of biological events, such as the loss of mitochondrial membrane potential, cytoskeletal shrinkage, membrane damage, and DNA fragmentation.7–9 All these physiological responses are ultimately turned into cell death signals. In addition to singlet oxygen, various other free radicals like hydrogen peroxides and superoxide anion radicals can also induce cell damage or apoptosis through chain reaction mechanisms of lipid peroxidation.As an emerging treatment, SDT has been known and investigated less than 30 years. However, the great potential in combating cancer facilitates it as a promising alternative to traditional cancer therapies. SDT is comparatively non-invasive, and the healing process results in little scarring. It is worth noting that sonosensitizers themselves have no inhibitive activity and are very low in toxicity. They are bioactive only after exposure to ultrasonic stimulation. Moreover, the applied ultrasound irradiation can be focused precisely on the tumor site to be treated, with little effect on normal tissues. As a result, the risk of systemic effects that frequently trouble chemotherapy and radiotherapy is significantly reduced. Compared with the other non-invasive modality of photodynamic therapy which uses light instead of ultrasound to activate the sensitizer, SDT is more potent in treating deeply located tumors due to the higher penetrability of ultrasound. In addition, studies have shown that low-intensity ultrasound can affect the cell membrane, resulting in increased permeability to sonosensitizers.10 Recently, SDT is also under investigation for other indications like inflammation and microbial infections. For example, curcumin was proved to be an effective sonosensitizer against various bacteria such as methicillin-resistant Staphylococcus aureus,11 food-borne bacteria Bacillus cereus and Escherichia coli,12 and exhibited potent sonodynamic activity on macrophages,13 the inflammatory cells essential for the formation of atherosclerotic plaques. Following these promising results, SDT with distinct advantages is considered as an excellent platform in future clinical application.
In recent decades, the application of nanotechnology to develop safer and more effective nanomedicines has substantially altered the concepts of conventional disease therapy, and a growing number of innovative nanosized materials are available. As the field of SDT develops, many nano drug delivery systems have been introduced and show great promise. For example, liposome could facilitate the intracellular uptake of loaded artemether, resulting in more efficient sonodynamic therapy.14 Tetra-a-(3-carboxyphenoxyl) zinc(II) phthalocyanine (ZnPcC4) after non-covalent conjugating with bovine serum albumin also exhibited significantly enhanced sonodynamic activity towards tumor cells.15 Among these favorite drug delivery systems, nanoparticles received much attention. They are defined as submicron-sized (<1 μm) colloidal particles. It is known that the size of nanoparticles has great influence on their therapeutic activity. It can significantly affect the mechanism and rate of cellular internalization.16 Compared with large particles, small-sized nanoparticles are uptaken more efficiently by tumor cells, thus allowing an increase of drug concentration at its site of action. Moreover, as the size increases (>300 μm), nanoparticles are more inclined to be recognized and scavenged by phagocytic cells.17 Specifically, the diameter of nanoparticles is preferably below 200 nm, not only for the effective transfer from blood to tumor, but also for a long retention time in tumor tissue.18 For these reasons, the sizes of therapeutic nanoparticles in current investigations are mainly in the range of 1–200 nm. In general, the nanotechnology will deeply change the biological behavior of drugs and offer them many benefits. Potential advantages of therapeutic nanoparticles include: (1) the ability to transport hydrophobic drugs in blood, (2) protection of drugs against degradation, (3) effective improvement in drug biodistribution, (4) enhancement of therapeutic efficacy, (5) large surface area for modification and functionalization.19,20 Therefore, it is undoubted that the introduction of nanoparticles will significantly advance the development of SDT and open a new avenue for future sonosensitizer design. Combining selected examples from literatures, some important classes of nanoparticles that are being developed for SDT will be discussed in this review. Additionally, the mechanism of action, potential limitations as well as promising prospects in SDT will also be presented.
2. SDT—current paradigm and shortcomings
As discussed earlier, SDT possesses many distinct advantages and is regarded as a promising alternative to conventional disease therapy. Although various impressive preclinical evidence has emerged over the past decade, the first clinical study of SDT is still awaited. Actually, this therapeutic approach is in its infancy, facing several limitations and shortcomings. In particular, none of the snonsensitizing agents used in SDT are totally satisfactory even though they have been developed with specific characteristics.21,22 Currently, the majority of investigated sonosensitizers are originally applied as photosensitizers, the most common of which are porphyrins and their derivatives such as hematoporphyrin monomethyl ether, protoporphyrin IX and ATX-70.7 These compounds normally have skin sensitivity and cutaneous phototoxicity in PDT studies. This means that such potential issues may also occur with SDT.21 Additionally, some sonosensitizers are derived from clinical drugs like doxorubicin23 and levofloxacin.24 If higher doses are required, they are known to collaterally attack normal cells during killing malignant cells.Meanwhile, most sonosensitizing agents are inherently hydrophobic, and aggregate easily in the physiological environment. The presence of hydrophobic interactions results in strong intermolecular forces between sonosensitizing molecules, which can further reduce the water solubility of sonosensitizers and decrease their ROS production. Another important shortcoming of many sonosensitizers is their poor pharmacokinetic behavior. These agents are likely to be cleared from vasculature, thus significantly decreasing their exposure to pathological sites.25,26 Combined with their indiscriminate biodistribution and weak selectivity for diseased tissues, only a small part of administrated drugs can reach to targeted sites. The resulting intracellular concentration of sonosensitizers may not be enough to produce an effective therapeutic action. Furthermore, the inappropriate tissue half-lives will cause large difficulty to optimize ultrasound schedules, and undesirable normal tissue retention is often challenging for long-term treatment.
However, the development of nanoparticles shows great potential to overcome such shortcomings of classic SDT. They can make the sonosensitizer more water soluble without changing its physiochemical structure. Benefiting from the increased particle size relative to small molecular sonosensitizer, nano-SDT can preferentially accumulate drug in tumor site through the enhanced permeability and retention (EPR) effect,27 which significantly prevents potential damage to normal tissues. If appropriately modified, nanoparticles may not only have the active tumor targeting, but also provide a protective barrier against the activation of sonosensitizers during transport. This effectively avoids the hematologic and cutaneous toxicities. Therefore, combination of nanoparticles and SDT is expected to alter the current paradigm of SDT, ultimately allowing for scientific and clinical advances. While not widely recognized, nanoparticles have been already in use in SDT and may emerge as an active field in the future. Functionally, the use of nanoparticles in SDT can be broadly divided into two classes: “sonosensitizer nanoparticles” and “nanoparticle carriers”. Sonosensitizer nanoparticles refer to the direct involving of nanotechnology with sonosensitizing agents. In this case, the sonosensitizer itself is a nanoparticle. In contrast, the later approach is using nanoparticles as carrier to deliver sonosensitizers.
3. Nanoparticle as sonosensitizer
Sonosensitizer nanoparticles are a type of nanosensitizers that can work as the therapeutics themselves with the assistance of ultrasound stimulation. Their inherent superiorities make them particularly suitable for SDT. These sonosensitizers are nanosized and can disperse in water without additional solubilization, which are conducive to elude the complement system and blood clearance. Besides, the nanoscale can also effectively facilitate the cellular uptake of sonosensitizers, and allows them to penetrate easily into deep tissues. More importantly, the use of nanosensitizers provides an interesting opportunity for nano-SDT where the nanoparticles become an active participant, instead of passive vehicle for sonosensitizer delivery. Among numerous nanosensitizers, titanium dioxide nanoparticles (nano-TiO2) have attracted most interest due to their fascinating properties, such as great biocompatibility, low toxicity and high stability in the physiological condition.28,29 Nano-TiO2 are generally known as photocatalyst to injure bacteria30 and tumor cells.31 Recent studies proved that ultrasound could also activate nano-TiO2 to induce the formation of ROS including hydrogen peroxide (H2O2), hydroxyl radicals (˙OH) and super oxides.32,33 In C32 melanoma-bearing mice, the combination of nano-TiO2 (about 6 nm in diameter) and ultrasound irradiation (1 MHz, 1.0 W cm−2, 2 min duration) achieved obvious inhibition effect on tumor growth.32 Additionally, TiO2 nanoparticles (about 200 nm in diameter) could also exhibited significant sonodynamic activity on human epithelial carcinoma cells in vitro and in vivo. Their cytotoxic efficacy was in a concentration-dependent manner.34Although TiO2 nanoparticles offer a novel promising platform for SDT, their usage in the clinical fields is still confined. The limited dispersibility of nano-TiO2 in water and blood is likely to induce aggregation and precipitation of nanoparticles. As a result, their biodistribution may be suffered, and the side effects on healthy cells are subsequently increased. To prevent such issues, appropriate surface decoration on nano-TiO2 is necessary. It has been demonstrated that chemical modification of nanoparticles with macromolecular polymer materials (e.g. polyethylene glycol, dextran, polyion complex) can successfully improve their water-dispersibility, minimize the immunogenicity and further enhance their biocompatibility.35–39 More importantly, the molecular weight of nanoparticles will significantly increase, which is a pre-requisite for passive targeting. Nano-TiO2 coated with polymers, consequently, are expected to possess prolonged blood circulation time, increased tumor accumulation and enhanced therapeutic activity. However, for potential use of SDT in the clinic, better disease targeting and therapeutic monitoring in vivo are required to minimize adverse effects while maximizing the therapeutic benefits. Besides, owing to the emergence of multidrug resistance (MDR) and complicated molecular basis of many diseases, monotherapy seems powerless for effective and sustained treatment. Therefore, it is desired to develop a highly integrated nanoparticle system with multiple functions such as combination therapy, active tumor-targeting ability and disease diagnosis. Ninomiya et al. constructed targeted TiO2/US treatment, and proved that the combination of TiO2 nanoparticles with targeting moieties is an effective tool in cancer SDT. In such studies, TiO2 nanoparticles were modified with targeting protein, like pre-S1/S2 (model protein with high affinity to hepatocyte)40 or avidin (a ligand of lectins that are over-expressed on the surfaces of many tumor cells).41 These targeted nano-TiO2 could be preferably uptaken byHepG2 cells and MCF-7 cells, and be sonocatalytically activated to perform potent in vitro and in vivo antitumor activities. Using nano-TiO2 as outer shell and magnetic iron oxide (Fe3O4) nanoparticles as inner core, Shen et al. developed a core–shell nanocomposite to carry DOX, and demonstrated its potential superiorities, including efficient ROS generation, long-time tumor retention, extremely high in vivo tumor accumulation and excellent synergistic effect for chemo-sonodynamic therapy. After being integrated with upconversion nanophosphors (NaYF4) and targeting molecule (hyaluronic acid), such nanocomposite exhibited outstanding upconversion luminescence (UCL) imaging effect and active tumor-targeting ability.42 In addition, mesoporous TiO2 nanoparticles after capping with β-cyclodextrin via a ROS sensitive linker were able to entrap docetaxel (DTX) in the pores for combining sonodynamic and chemotherapy (Fig. 2).43 When the system was irradiated by a focused US (1 W cm−2, 40 s), the generated ROS from nano-TiO2 could cleave the ROS-sensitive linker, thereby, DTX was released rapidly to exert therapeutic effects. In S180 tumor-nearing mice, such system showed increased tumor accumulation and prolonged circulating ability with a maximum concentration in tumor at 48 h post injection. Moreover, the tumor volume of mice could be decreased about 60% in the combination treatment of chemotherapy (DTX) and SDT (nano-TiO2).
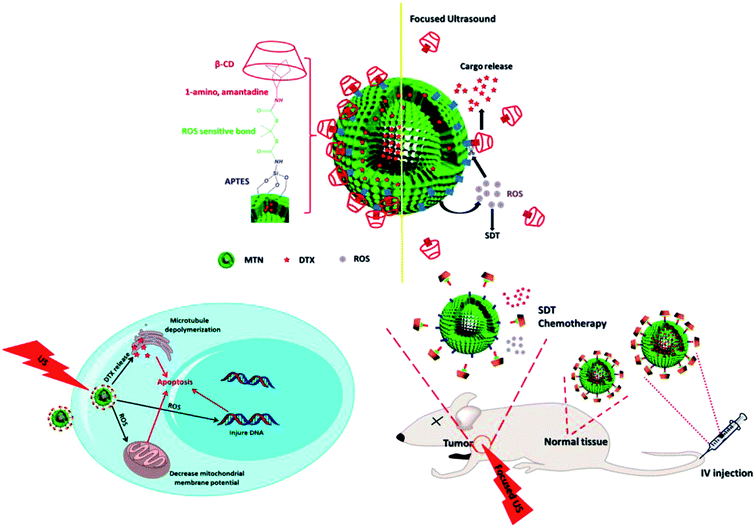 | ||
| Fig. 2 Schematic illustration of DTX-loaded TiO2 nanoparticle and its in vitro and in vivo biomechanism. Reproduced with permission from ref. 43, Copyright (2015) American Chemical Society. | ||
Similar with other treatment modalities like chemotherapy, SDT is facing higher demands and increasing difficulties to achieve efficient and safe therapeutic results. The research on novel sonosensitizing agents provides an alternative approach to improve therapeutic index, and has significantly revitalized the use of emerging nanomaterials in SDT. More recently, a lot of work were devoted to bio-applications of inorganic nanoparticles as potential sonosensitizers. Table 1 summaries some promising examples. Among them, some sonosensitizing nanoparticles, without additional functionalization, can simultaneously integrate targeted drug delivery, bioimaging, and multimodal treatment. Such all-in-one sonosensitizers will become one of the most active research fields in the future.
| Sonosensitizer nanoparticles | Ultrasound | Biological model | Biological effects | Potential functions | Ref. | |
|---|---|---|---|---|---|---|
| W cm−2 | MHz | |||||
| Gold | 2 | 1.1 | CT26 cells | Superior in vivo sonodynamic effects by inhibiting tumor growth and prolonging the cumulative survival time | Photothermal therapy and optical imaging | 44 |
| Fe3O4 | 2 | 1 | MCF-7 cells | Excellent in vitro production of toxic free radicals to induce cell death | Magnetic resonance imaging and hyperthermia | 45 |
| Carbon fluoroxide | 0.4 | 0.04 | 3T3-L1 cells, HuH7 cells | Effective in vitro cell penetration to provoke a complete cell destruction | One- and two-photon excited luminescence cell imaging | 46 |
| Porous silicon | 1–2 | 1–3 | Hep2 cells | Nontoxic up to the concentration of 0.1 mg ml−1 in vitro and doses of 30 mg kg−1 in vivo, strong sonodynamic activity in suppressing cell proliferation | Photoluminescence imaging | 47 |
| Silver | 0.5, 1, 2 | 1 | A2780 cells | Significant decrease of cell viability after US activation | — | 48 |
| Silver copper | 1 | 1 | A2780 cells | Higher sonodynamic activity of phenanthroline-modified nanoparticles than polyvinyl alcohol modification | — | 49 |
4. Nanoparticle as carrier for sonosensitizer delivery
Although sonosensitizer nanoparticles are promising and might represent a new direction for disease nanotechnology, this method is relatively non-universal and cannot be easily applied for every sonosensitizer. Out of all the sonosensitizing agents, the most commonly used are traditional small-molecule compounds. These compounds mainly exist in free single molecules in solution. It is hard, even impossible for them to form nanoparticles on their own. By creating nanoparticles as vehicle to carry sonosensitizers a more flexible approach is proposed. Nanoparticle carriers are well established in the pharmaceutical industry, and applicable to most of drugs. In this mode, the characteristics of nanoparticles will determine the biological and pharmacokinetics of carried molecules. Appropriate nanoparticle design therefore can perform a very far reach where sonosensitizers are concerned. It is noteworthy that sonosensitizers could be carried via physical encapsulation in the inner of nanoparticles as well as covalent conjugation with the nanoparticle substrates, which is originally considered as the basic classification profile in the following discussion.4.1. Encapsulation of sonosensitizer by nanoparticles
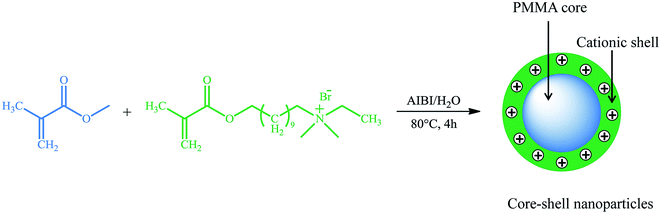
Encapsulation has received tremendous attention as a possible means of delivering sonosensitizers. It has extremely high flexibility, and a large variety of materials and preparation technologies are available. The chemical composition and architecture of nanoparticle matrices can be easily designed to accommodate sonosensitizers with varying degrees of molecular weight, charge and hydrophobicity. By fine-tuning the surface properties, chemical structures and micromorphologies of carrier matrices, nanoparticles can produce specific response according to external changes (e.g. pH, redox potential and enzymes) in physiological environments, thus effectively controlling the release of encapsulated sonosensitizers.50 More importantly, encapsulation protect inner-located sonosensitizers from external harmful environment, and the stability of nanoparticles can be readily tailored for further protecting sonosensitizers against degradation.Investigation into nanoparticles for use in SDT began only recent five years with efforts to improve carrier capacity and enhance therapeutic efficacy. Canaparo et al. engineered poly-methyl methacrylate (PMMA) nanoparticles to carry the sonosensitizers of meso-tetrakis (4-sulfonatophenyl) porphyrin (TPPS).51 These nanoparticles have a core–shell structure (shown in Fig. 3). The cationic external shell plays a significant role in the process that nanoparticles interact with cell membrane, and has been shown to effectively enhance cellular uptake. In vitro results on SH-SY5Y cells revealed an obvious increase in apoptotic (27.45% ± 3.03%) and necrotic (16.91% ± 3.89%) cells after SDT of TPPS nanoparticles with shockwaves (0.43 mJ mm−2 for 500 impulses, 4 impulses per second). Further studies on the mechanism showed that TPPS nanoparticles-mediated sonodynamic activity could significantly increase ROS production, enhance mRNA expression of NRF2 (the main mediator of cellular adaptation to redox stress), down-regulate TIGAR (a checkpoint for glycolysis and apoptosis) and MAP3K5 (a downstream effector in antioxidant responses) genes, and promote the release of cytochrome c. In addition to TPPS, PMMA nanoparticles could also loaded with 64Cu-TPPS for biodistribution evaluation via positron emission tomography (PET) or with Mn(III)-TPPS for tumor accumulation studies through magnetic resonance imaging (MRI).52 Compared with free TPPS solution, such TPPS-loaded nanoparticles performed superior in vivo sonodynamic activity in suppressing tumor growth, and favorable biodistribution with higher and more persistent nanoparticles accumulation at the tumor site. Additionally, polyvinylpyrrolidoned could also be developed as nanocarrier to encapsulate the sonosensitizer of hypocrellin SL052, forming biocompatible nanoparticles.53 Judged by IC50, the nanoparticle system (24 μg ml−1 of SL052) revealed better sonodynamic activity than SL052 solution (60 μg ml−1). For tumor-bearing mice, the survival period was prolonged to about 17 days in nanoparticles-treated group compared to only 7 days in untreated group.
In general, higher MW of nanoparticles is expected to prolong blood circulation and enhance EPR effect. However, regarding of non-biodegradable carriers like PMMA, their MW must be controlled below 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 to ensure total renal elimination. Although tailor-making MW is available to maximize the chance of carrier clear from the body, their inherent non-biodegradability still poses potential safety problems.54,55 These non-biodegradable carriers may accumulate in lysosomes and increase the osmotic pressure, causing a high risk of “lysosomal storage disease” syndrome.56 As a result, naturally occurring carriers with good biocompatibility and biodegradability seem more appealing. For example, chitosan (CS) is a natural polysaccharides, and can be degraded, either hydrolytically or enzymatically, into small molecular saccharides to be excreted from the body. It demonstrates excellent biocompatibility without inducing potential hematotoxicity, genotoxicity or inflammatory response. Because of the unique polyvalent positive-charged property, chitosan could interact with negatively charged sonosensitizers like rose bengal ω-carboxyheptyl ester (RBD), a derivative of rose Bengal (RB).57 These compounds can self-assembly form complex nanoparticles with a particle size of 150–200 nm, and RBS loaded the interior of CS nanoparticles (shown in Fig. 4). These RBD/CS complex nanoparticles exhibited more efficient cellular uptake in HT29 and HepG2 cells than RB, RBD, or CS/RB complex nanoparticles. Higher tumor accumulation and antitumor activity were also observed in CT-26 colon cancer-bearing mice intravenously injected with RBD/CS nanoparticles.
000 g mol−1 to ensure total renal elimination. Although tailor-making MW is available to maximize the chance of carrier clear from the body, their inherent non-biodegradability still poses potential safety problems.54,55 These non-biodegradable carriers may accumulate in lysosomes and increase the osmotic pressure, causing a high risk of “lysosomal storage disease” syndrome.56 As a result, naturally occurring carriers with good biocompatibility and biodegradability seem more appealing. For example, chitosan (CS) is a natural polysaccharides, and can be degraded, either hydrolytically or enzymatically, into small molecular saccharides to be excreted from the body. It demonstrates excellent biocompatibility without inducing potential hematotoxicity, genotoxicity or inflammatory response. Because of the unique polyvalent positive-charged property, chitosan could interact with negatively charged sonosensitizers like rose bengal ω-carboxyheptyl ester (RBD), a derivative of rose Bengal (RB).57 These compounds can self-assembly form complex nanoparticles with a particle size of 150–200 nm, and RBS loaded the interior of CS nanoparticles (shown in Fig. 4). These RBD/CS complex nanoparticles exhibited more efficient cellular uptake in HT29 and HepG2 cells than RB, RBD, or CS/RB complex nanoparticles. Higher tumor accumulation and antitumor activity were also observed in CT-26 colon cancer-bearing mice intravenously injected with RBD/CS nanoparticles.
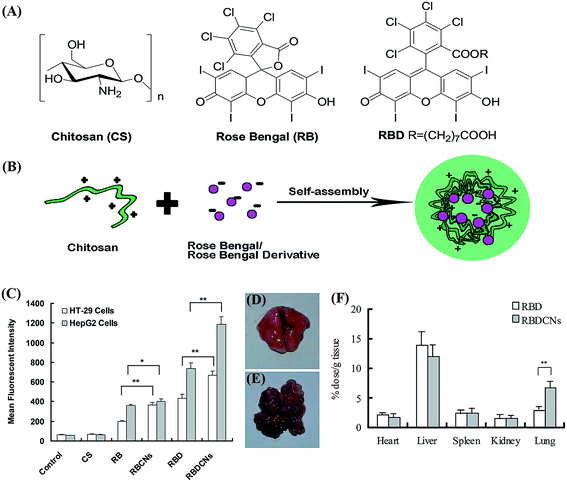 | ||
| Fig. 4 (A) Chemical structures of chitosan, rose Bengal and rose Bengal derivative. (B) Preparation scheme of CS/RB or CS/RBD complex nanoparticles. (C) Mean fluorescence intensity of cells treated with free RB, RBD or their complex nanoparticles. The biodistribution of RBD and RBD complex nanoparticles in the lung of (D) healthy BALB/c mice and (E) CT-26 colon cancer transplanted mouse model. (F) The tissue distribution of complex nanoparticles in CT-26 colon cancer transplanted BALB/c mouse. Each data point represents the mean ± SD (n = 3). *p < 0.05, **p < 0.01. Reproduced with permission from ref. 57. | ||
Following these established examples, encapsulation of sonosensitizer by nanoparticles can be used to enhance therapeutic activity and selectively deliver sonoactive agents to tumor site. In addition to tumor-targeting, the solubilization, functionalization, and assembly of biodegradable nanoparticles are aspects of significant relevance that warrants future research.
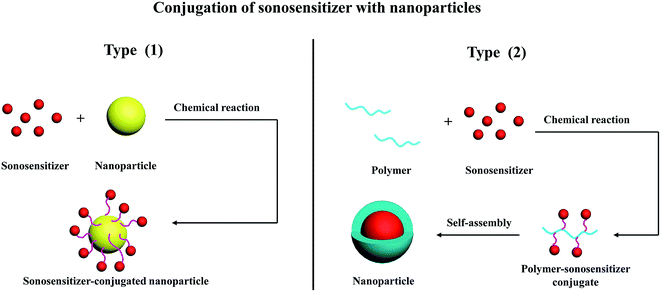
4.2. Conjugation of sonosensitizer with nanoparticles
Conjugation is the other important strategy for combining sonosensitizer with nanoparticle carrier. Different from encapsulation that sonosensitizer is physically entrapped into nanoparticle substrate, sonosensitizer-conjugated nanoparticles are constructed via two types of covalent combination: (1) sonosensitizers are chemically bound on the surface of nanoparticles, (2) sonosensitizers are chemically attached to polymer backbones, the obtained polymer–sonosensitizer conjugates could further self-assemble into nanoparticles (see Fig. 5). Compared with encapsulation, the main advancements of conjugation method are higher drug loading, sustained drug release and better stability without undesirable leaking of sonosensitizer. In such design, the sonosensitizer sometimes remains on the surface of the nanoparticle, but the nanoparticle itself still dominates biodistribution and pharmacokinetics. Theoretically, the ROS would be more available when produced from the surface than from diffusing out of a nanoparticle structure.The activation of sonosensitizer depends on ultrasonic cavitation, and therefore, high intensity ultrasound is necessary for SDT. However, the use of high intensity ultrasound which can also induce side effects on healthy tissues around tumor region is indeed a great challenge in SDT. It has been shown that the addition of particles to a liquid environment provides a nucleation site for ultrasonic cavitation due to their rough surface. This can potentially increase cavitation effect, resulting in enhanced sonochemical reaction.58,59 Therefore, introducing particle as cavitation nuclei is favorable to activate sonosensitizers and improve their sonodynamic activity. As a novel nanomaterial, gold nanoparticles have been proved to serve as nucleation sites for ultrasonic cavitation. They are effective to decrease the cavitation threshold and in turn, increase the cavitation rate.60 Moreover, poor toxicity, good uptake by mammalian cells and antiangiogenetic properties further facilitate gold nanoparticles as attractive carrier for the delivery of sonosensitizers. Compared with free molecular form, protoporphyrin IX (PpIX) after conjugating to the surface of gold nanoparticles can obviously prolong its non-radiative relaxation time, which is important for the efficient generation of singlet oxygen.61 Chemical dosimetric method confirmed that the detected sonoluminescence signal in the gel phantom containing gold nanoparticles-conjugated PpIX was also higher than PpIX alone.62 In colon carcinoma CT26 tumor-bearing mice, such nanoconjugates combined with ultrasound at an intensity of 2 W cm−2 showed significant sonodynamic activity in reducing relative tumor volume (RTV, 3.9 cm3 after 13 days SDT) and increasing average animal survival time (ST, 56.1 days), while 6.3 cm3 RTV and 47.6 days ST were observed for free PpIX plus ultrasound.63,64
Polymer–drug conjugates are a shining platform for drug delivery, and have advanced to the clinical trial stage. Compared to the corresponding parent drugs, these drug conjugates perform demonstrated merits, such as enhanced therapeutic response and reduced adverse effects.65 Generally, polymer–drug conjugates consist of three important components: water-soluble polymer backbone as carrier, hydrophobic drug molecules and biologically responsive linker used to combine polymer and drug. Owing to strong amphiphilic properties, polymer–drug conjugates tend to self-assemble into core–shell nanostructure in aqueous solution.66 Further adding targeting ligand to conjugates is expected to achieve two drug-targeting strategies: EPR effect and active ligand–receptor binding.67 In a study by Tsai et al.,68 doxorubicin (DOX) and galactosamine (an hepatocyte-targeted asialoglycoprotein ligand) were simultaneously conjugated to γ-poly(glutamic acids) (γ-PGA) via carbodiimide chemistry. The attachment of galactosamine (Gal) dramatically increased the uptake of DOX on hepatoma cells but not on fibroblasts. After ultrasound activation, γ-PGA-DOX-Gal conjugate nanoparticles performed enhanced cell-killing efficacy. Such excellent selectivity and improved cytotoxicity facilitate γ-PGA-derived DOX nanoparticles as a promising candidate for clinical SDT use in the future.
5. Conclusion and future perspective
Unequivocally, SDT has emerged as one of the important therapeutic options in the management of cancer and other diseases. It combines low intensity ultrasound and localized sonosensitizers to achieve a synergistic therapeutic effect while influencing as few healthy tissues as possible. The application of nanoparticle technology to develop more effective yet safer nanomedicines has substantially altered the concepts of traditional SDT. Recent researches support that nano-SDT are well on its way to improving biodistribution of sonosensitizers, increasing their targeting ability to pathological sites, enhancing therapeutic efficacy and reducing unwanted side effects.Although the past decades have seen several advancements, the reported nanoparticle-based SDT is yet to gain general clinical acceptance. Several difficulties impede its translation. For example, the accumulation selectivity to pathological targets is not high enough for clinical use. As a result, the actual therapeutic efficiency may be limited. Sometimes, single nano-SDT is also insufficient for effective and sustained therapy because of the molecular complexity of many diseases. Moreover, the reactive oxygen species like singlet oxygen species normally have a lifetime less than 3.5 μs and can diffuse only 0.01 to 0.02 μm during this period.69 Therefore, the rapid degradation rate of nanoparticle carriers and efficient release profile of sonosensitizers are necessary. Beyond the laboratory bench, nanoparticle-based SDT still needs answers like appropriate dosing, exposure times and administration route and frequency that maximize potential therapeutic effectiveness while minimizing undesired side effects.
In the future, many efforts are desirable to develop nanoparticle-based SDT with excellent active targeting ability. Some established homing ligands, such as folic acid, iRGD and monoclonal antibody are encouraged to modify nanoparticles, thereby increasing the accumulation and selectivity of sonosensitizers in tumor cells. Moreover, combination therapy that opens new therapeutic possibilities will be one of an exciting tide. Simultaneous using two or more nano-sensitizers, co-delivering multiple sonosensitizers by single nanoparticle carrier, as well as combining SDT with other treatment modality like chemotherapy are expected to regulate different signaling pathways in diseased cells and improve the therapeutic profiles. Stimuli-responsive drug delivery systems have been proved to trigger the rapid release of drug in specific sites, it is therefore of particular interest to prepare nanoparticles with special chemical structure that can generate specific response to small external changes in physiological environments. In addition, a rational and systematic methodology to design and investigate nanoparticle-based SDT is also important for future research.
References
- Q. Wu, Z. Yang, Y. Nie, Y. Shi and D. Fan, Cancer Lett., 2014, 347, 159–166 CrossRef CAS PubMed.
- M. Di Maio, E. Basch, J. Bryce and F. Perrone, Nat. Rev. Clin. Oncol., 2016, 13, 319–325 CrossRef CAS PubMed.
- M. Kuroki, K. Hachimine, H. Abe, H. Shibaguchi, M. Kuroki, S.-i. Maekawa, J. Yanagisawa, T. Kinugasa, T. Tanaka and Y. Yamashita, Anticancer Res., 2007, 27, 3673–3677 CAS.
- K. Tachibana, L. B. Feril and Y. Ikeda-Dantsuji, Ultrasonics, 2008, 48, 253–259 CrossRef CAS PubMed.
- H. Shibaguchi, H. Tsuru, M. Kuroki and M. Kuroki, Anticancer Res., 2011, 31, 2425–2429 Search PubMed.
- M. Trendowski, Cancer Metastasis Rev., 2014, 33, 143–160 CrossRef CAS PubMed.
- X. Pang, C. Xu, Y. Jiang, Q. Xiao and A. W. Leung, Pharmacol. Ther., 2015 DOI:10.1016/j.pharmthera.2015.12.004.
- J. Xu, X. Xia, A. W. Leung, J. Xiang, Y. Jiang, H. Yu, D. Bai, X. Li and C. Xu, Ultrasonics, 2011, 51, 480–484 CrossRef CAS PubMed.
- H. Jin, X. Zhong, Z. Wang, X. Huang, H. Ye, S. Ma, Y. Chen and J. Cai, J. Cell. Biochem., 2011, 112, 169–178 CrossRef CAS PubMed.
- G. Harrison and E. Balcer-Kubiczek, Ultrasound Med. Biol., 1991, 17, 627–632 CrossRef CAS PubMed.
- X. Wang, M. Ip, A. W. Leung and C. Xu, Ultrasonics, 2014, 54, 2109–2114 CrossRef CAS PubMed.
- X. Wang, M. Ip, A. W. Leung, Z. Yang, P. Wang, B. Zhang, S. Ip and C. Xu, Ultrasonics, 2015, 62, 75–79 CrossRef CAS PubMed.
- F. Wang, Q. Gao, S. Guo, J. Cheng, X. Sun, Q. Li, T. Wang, Z. Zhang, W. Cao and Y. Tian, BioMed Res. Int., 2012, 2013, 737264 Search PubMed.
- H.-J. Chen, X.-R. Huang, X.-B. Zhou, B.-Y. Zheng and J.-D. Huang, Chem. Commun., 2015, 51, 4681–4684 RSC.
- H.-N. Xu, H.-J. Chen, B.-Y. Zheng, Y.-Q. Zheng, M.-R. Ke and J.-D. Huang, Ultrason. Sonochem., 2015, 22, 125–131 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS PubMed.
- C. He, Y. Hu, L. Yin, C. Tang and C. Yin, Biomaterials, 2010, 31, 3657–3666 CrossRef CAS PubMed.
- D. K. Chatterjee, L. S. Fong and Y. Zhang, Adv. Drug Delivery Rev., 2008, 60, 1627–1637 CrossRef CAS PubMed.
- T. Sun, Y. S. Zhang, B. Pang, D. C. Hyun, M. Yang and Y. Xia, Angew. Chem., Int. Ed., 2014, 53, 12320–12364 CAS.
- N. Bertrand, J. Wu, X. Xu, N. Kamaly and O. C. Farokhzad, Adv. Drug Delivery Rev., 2014, 66, 2–25 CrossRef CAS PubMed.
- H. Chen, X. Zhou, Y. Gao, B. Zheng, F. Tang and J. Huang, Drug Discovery Today, 2014, 19, 502–509 CrossRef CAS PubMed.
- D. Costley, C. Mc Ewan, C. Fowley, A. P. McHale, J. Atchison, N. Nomikou and J. F. Callan, Int. J. Hyperthermia, 2015, 31, 107–117 CrossRef CAS PubMed.
- T. Yoshida, T. Kondo, R. Ogawa, L. B. Feril Jr, Q.-L. Zhao, A. Watanabe and K. Tsukada, Cancer Chemother. Pharmacol., 2008, 61, 559–567 CrossRef CAS PubMed.
- B. Liu, J. Wang, X. Wang, B.-M. Liu, Y.-M. Kong, D. Wang and S.-K. Xu, J. Fluoresc., 2010, 20, 985–992 CrossRef CAS PubMed.
- A. Vargas, B. Pegaz, E. Debefve, Y. Konan-Kouakou, N. Lange, J.-P. Ballini, H. van den Bergh, R. Gurny and F. Delie, Int. J. Pharm., 2004, 286, 131–145 CrossRef CAS PubMed.
- Y. N. Konan, R. Gurny and E. Allémann, J. Photochem. Photobiol., B, 2002, 66, 89–106 CrossRef CAS.
- H. Maeda, J. Wu, T. Sawa, Y. Matsumura and K. Hori, J. Controlled Release, 2000, 65, 271–284 CrossRef CAS PubMed.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- H. Shi, R. Magaye, V. Castranova and J. Zhao, Part. Fibre Toxicol., 2013, 10, 1–33 CrossRef CAS PubMed.
- Y. H. Tsuang, J. S. Sun, Y. C. Huang, C. H. Lu, W. H. S. Chang and C. C. Wang, Artif. Organs, 2008, 32, 167–174 CrossRef CAS PubMed.
- Y. Yin, W.-W. Zhu, L.-P. Guo, R. Yang, X.-S. Li and Y. Jiang, J. Phys. Chem. B, 2012, 117, 125–131 CrossRef PubMed.
- Y. Harada, K. Ogawa, Y. Irie, H. Endo, L. B. Feril, T. Uemura and K. Tachibana, J. Controlled Release, 2011, 149, 190–195 CrossRef CAS PubMed.
- K. Ninomiya, K. Noda, C. Ogino, S.-i. Kuroda and N. Shimizu, Ultrason. Sonochem., 2014, 21, 289–294 CrossRef CAS PubMed.
- O. Itano, T. Onishi, S. Matsuda, T. Fujimura, H. Jinno, M. Kitagou, M. Shinoda, S. Kawachi, M. Tanabe and C. Ogino, Cancer Res., 2012, 72, 4341 Search PubMed.
- S. Naghibi, H. R. M. Hosseini and M. A. F. Sani, Ceram. Int., 2013, 39, 8377–8384 CrossRef CAS.
- S. Yamaguchi, H. Kobayashi, T. Narita, K. Kanehira, S. Sonezaki, N. Kudo, Y. Kubota, S. Terasaka and K. Houkin, Ultrason. Sonochem., 2011, 18, 1197–1204 CrossRef CAS PubMed.
- A. Harada, M. Ono, E. Yuba and K. Kono, Biomater. Sci., 2013, 1, 65–73 RSC.
- S. S. Mano, K. Kanehira, S. Sonezaki and A. Taniguchi, Int. J. Mol. Sci., 2012, 13, 3703–3717 CrossRef CAS PubMed.
- S. Yamamoto, E. Yuba, A. Harada and K. Kono, Langmuir, 2015, 31, 8583–8588 CrossRef CAS PubMed.
- K. Ninomiya, C. Ogino, S. Oshima, S. Sonoke, S.-i. Kuroda and N. Shimizu, Ultrason. Sonochem., 2012, 19, 607–614 CrossRef CAS PubMed.
- K. Ninomiya, A. Fukuda, C. Ogino and N. Shimizu, Ultrason. Sonochem., 2014, 21, 1624–1628 CrossRef CAS PubMed.
- S. Shen, X. Guo, L. Wu, M. Wang, X. Wang, F. Kong, H. Shen, M. Xie, Y. Ge and Y. Jin, J. Mater. Chem. B, 2014, 2, 5775–5784 RSC.
- J. Shi, Z. Chen, B. Wang, W. Lei, T. Lu and Z. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 28554–28565 Search PubMed.
- A. Sazgarnia, A. Shanei, A. R. Taheri, N. T. Meibodi, H. Eshghi, N. Attaran and M. M. Shanei, J. Ultrasound. Med., 2013, 32, 475–483 Search PubMed.
- A. E. Fard, A. Zarepour, A. Zarrabi, A. Shanei and H. Salehi, J. Magn. Magn. Mater., 2015, 394, 44–49 CrossRef.
- A. Kharin, O. Syshchyk, A. Geloen, S. Alekseev, A. Rogov, V. Lysenko and V. Timoshenko, Sci. Technol. Adv. Mater., 2015, 16, 44601–44606 CrossRef.
- L. Osminkina, A. Nikolaev, A. Sviridov, N. Andronova, K. Tamarov, M. Gongalsky, A. Kudryavtsev, H. Treshalina and V. Y. Timoshenko, Microporous Mesoporous Mater., 2015, 210, 169–175 CrossRef CAS.
- V. Bernard, V. Mornstein, J. Jaroš, M. Sedláčková and J. Škorpíková, J. Appl. Biomed., 2014, 12, 137–145 CrossRef.
- V. Bernard, O. Zobač, J. Sopoušek and V. Mornstein, J. Cancer Res., 2014, 2014, 971769 Search PubMed.
- J. Du, L. A. Lane and S. Nie, J. Controlled Release, 2015, 219, 205–214 CrossRef CAS PubMed.
- R. Canaparo, G. Varchi, M. Ballestri, F. Foglietta, G. Sotgiu, A. Guerrini, A. Francovich, P. Civera, R. Frairia and L. Serpe, Int. J. Nanomed., 2013, 8, 4247 Search PubMed.
- G. Varchi, F. Foglietta, R. Canaparo, M. Ballestri, F. Arena, G. Sotgiu, A. Guerrini, C. Nanni, G. Cicoria and G. Cravotto, Nanomedicine, 2015, 10, 3483–3494 CrossRef CAS PubMed.
- Y. Meng, C. Zou, R. Madiyalakan, T. Woo, M. Huang, X. Yang, E. Swanson, J. Chen and J. Z. Xing, Nanomedicine, 2010, 5, 1559–1569 CrossRef CAS PubMed.
- L. Aragao-Santiago, H. Hillaireau, N. Grabowski, S. Mura, T. L. Nascimento, S. Dufort, J.-L. Coll, N. Tsapis and E. Fattal, Nanotoxicology, 2016, 10, 292–302 CrossRef CAS PubMed.
- R. Duncan and M. J. Vicent, Adv. Drug Delivery Rev., 2013, 65, 60–70 CrossRef CAS PubMed.
- R. Duncan and S. C. Richardson, Mol. Pharm., 2012, 9, 2380–2402 CrossRef CAS PubMed.
- Y. Gao, Z. Li, C. Wang, J. You, B. Jin, F. Mo, J. Chen, Y. Zheng and H. Chen, RSC Adv., 2015, 5, 17915–17923 RSC.
- T. Tuziuti, K. Yasui, M. Sivakumar, Y. Iida and N. Miyoshi, J. Phys. Chem. A, 2005, 109, 4869–4872 CrossRef CAS PubMed.
- C. H. Farny, T. Wu, R. G. Holt, T. W. Murray and R. A. Roy, Acoust. Res. Lett. Online, 2005, 6, 138–143 CrossRef.
- A. Shanei, A. Sazgarnia, M. Hassanzadeh-Kayyat, H. Eshghi, S. Soudmand and N. Attaran Kakhki, Iranian Journal of Medical Physics, 2012, 9, 41–50 Search PubMed.
- J. J. Pérez, A. Cruz-Orea, E. Ramón-Gallegos, R. G. Fuentes and J. S. Ramirez, Eur. Phys. J.: Spec. Top., 2008, 153, 353–356 CrossRef.
- A. Sazgarnia, A. Shanei, H. Eshghi, M. Hassanzadeh-Khayyat, H. Esmaily and M. M. Shanei, Ultrasonics, 2013, 53, 29–35 CrossRef CAS PubMed.
- A. Sazgarnia, A. Shanei, N. T. Meibodi, H. Eshghi and H. Nassirli, J. Ultrasound. Med., 2011, 30, 1321–1329 Search PubMed.
- A. Shanei, A. Sazgarnia, N. T. Meibodi, H. Eshghi, M. Hassanzadeh-Khayyat, H. Esmaily and N. A. Kakhki, Iran. J. Basic Med. Sci., 2012, 15, 759–767 CAS.
- X. Pang, H.-L. Du, H.-Q. Zhang, Y.-J. Zhai and G.-X. Zhai, Drug Discovery Today, 2013, 18, 1316–1322 CrossRef CAS PubMed.
- X. Pang, Y. Jiang, Q. Xiao, A. W. Leung, H. Hua and C. Xu, J. Controlled Release, 2016, 222, 116–129 CrossRef CAS PubMed.
- H. Xu, H. Ma, P. Yang, X. Zhang, X. Wu, W. Yin, H. Wang and D. Xu, Colloids Surf., B, 2015, 136, 729–734 CrossRef CAS PubMed.
- W.-B. Tsai, H.-Y. Lai, J.-L. Lee, C.-W. Lo and W.-S. Chen, Langmuir, 2014, 30, 5510–5517 CrossRef CAS PubMed.
- S. Hatz, J. D. Lambert and P. R. Ogilby, Photochem. Photobiol. Sci., 2007, 6, 1106–1116 CAS.
| This journal is © The Royal Society of Chemistry 2016 |