Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production
Tiago P. Silva,
Susana M. Paixão* and
Luís Alves*
LNEG – Laboratório Nacional de Energia e Geologia, IP, Unidade de Bioenergia, Estrada do Paço do Lumiar, 22, 1649-038 Lisboa, Portugal. E-mail: susana.alves@lneg.pt; luis.alves@lneg.pt
First published on 10th June 2016
Abstract
Currently, carotenoids are valuable bioactive molecules for several industries, such as chemical, pharmaceutical, food and cosmetics, due to their multiple benefits as natural colorants, antioxidants and vitamin precursors. Hence, the increasing interest on these high added-value products has led to the search of alternatives, more cost-effective and with better yields, towards their industrial production. Indeed, microbial metabolism offers a promising option for carotenoids production. Herein it is shown the potential of the dibenzothiophene desulfurizing bacterium Gordonia alkanivorans strain 1B as a high carotenoid-producer microorganism. The novel carotenoids, produced under different culture conditions, were extracted with DMSO and then further analyzed both through spectrophotometry and HPLC. When grown in glucose-sulfate-light, strain 1B was able of achieving 2015 μg carotenoids per g DCW in shake-flask assays, with about 60% corresponding to lutein, canthaxanthin and astaxanthin. Further optimization studies open a new focus of research aiming to get a hyper pigment-producer strain that may be applied towards different industrial sectors.
I. Introduction
In last two decades, the actinomycete genus Gordonia has attracted much interest. Most species were isolated due to their abilities to degrade xenobiotics; environmental pollutants as polycyclic aromatic hydrocarbons, alkylpyridines, phthalates; or otherwise slowly biodegradable natural polymers, as well as to transform or synthesize organic compounds, such as steroid transformation and carotenoid production. The variety of chemical compounds being transformed, biodegraded, and synthesized by gordoniae makes these bacteria very attractive for environmental and industrial biotechnology.1–4Gordonia alkanivorans strain 1B was isolated by Alves et al.5 from oil contaminated soil samples from Parque das Nações (former Petrogal location, Lisbon, Portugal). It is an aerobic, Gram-positive, catalase-positive, oxidase-negative and pink/orange-pigmented bacterium. Cells were shown to be short branched hyphae, which disintegrated to rods and coccus-like elements when visualized by phase contrast microscopy. They are non-motile cells generally occurring in groups.5
In the last decade, several works have demonstrated the great potential of G. alkanivorans strain 1B towards fossil fuel biodesulfurization (BDS). This bacterium is able of desulfurize dibenzothiophene (DBT) and its alkylated derivatives, as well as mixtures of these compounds (model oils), using the 4S-pathway.5,6 The 4S-pathway is the best pathway for the desulfurization of crude oils since it enables the removal of sulfur without compromising the carbon skeleton of the organo-sulfur compound and therefore without affecting its calorific power, making BDS an industrially interesting process. Enhanced desulfurization by strain 1B was obtained when fructose or fructose-rich materials were used as carbon source.7–10
Clearly, BDS presents advantages as a complementary technique to the commonly oil industry solution (HDS – hydrodesulfurization) towards the production of ultra low sulfur fuels (ULSF) that meets current environmental regulations. However, one of the drawbacks that still hinder the BDS scale up it is the lack of economic viability of this ecofriendly bioprocess.11–14 In this context, measures to make BDS economically competitive include the use of a cost-effective culture medium for biocatalysts production (minimal medium using a cheaper C-source) and the exploitation of high-added value byproducts produced by the desulfurizing microorganisms, such as carotenoids or biosurfactants.8,10,14–16
Several species of the genus Gordonia are known producers of carotenoids, in fact most species present a characteristic color orange/red/pink. Amongst them, the most studied is G. jacobaea, which was the first of this genus to be isolated as a good carotenoid producer, and was later genetically improved to achieve maximum productivity.17,18 G. alkanivorans strain SKF120101, has also been described as producing strong pigmentation, associated with carotenoids, when grown under an intense light.19
Carotenoids in nature are typically C40 tetraterpenoids, which are formed by eight C5 isoprenoid units joined head to tail, with a tail-to-tail linkage at the centre, resulting in a symmetrical structure. They are composed of a central chain, which alternates sequentially between double and single bonds, giving these molecules the antioxidative abilities and the light absorbing qualities responsible for their colours. Carotenoids may be acyclic or have a ring at one or both ends of the molecule,20 and they can be divided into two classes: carotenes and xanthophylls. Carotenes are purely consisted of hydrocarbons, while xanthophylls also have oxygen in their constitution.21 Carotenoids occur generally in photosynthetic systems of higher plants, algae and phototrophic bacteria, but in non-photosynthetic organisms they are important in protecting against photooxidative damage. Indeed, many non-phototrophic bacteria and fungi rely on carotenoids for protection when growing on conditions where light and air are abundant.19,22–24 Their function varies from species-specific coloration, photoprotection, scavenging of reactive oxygen species, light harvesting and membrane stabilization; in more complex organisms they can even be precursors of hormones and vitamins.25,26 In the last decades carotenoids have been the target of much interest due to their antioxidative, anticancer, and anti-inflammatory activities, which have proven influence in human health, as well as their use in the cosmetic industry and food industry as food or feed additives.19,22,24,27,28 Thus, interest in carotenoids has increased considerably due to their multiple benefits towards different industries. The global carotenoid market totalled $1.5 billion in 2014 and is expected to reach nearly $1.8 billion in 2019, with an annual growth rate of 3.9%.29
Most carotenoids in the market are still of synthetic origin, they are produced using petrochemical base materials, which are frowned upon by the general public especially in terms of food supplements and cosmetics.30 The carotenoids production through chemical synthesis or extraction from plants is limited by low yields that results in high production costs. This leads to research of microbial production of carotenoids, as an alternative that has shown better yields.22 In addition, the microbial production of carotenoids could be a better option in terms of production costs, since it may use alternative low-cost substrates. Moreover, carotenoids of microbial origin seem to have some structural differences, which result in higher antioxidant activity.31 This explains the increasing interest in production of microbial carotenoids to substitute the synthetic carotenoids.22,32 In fact, microbial metabolism offers a promising alternative for carotenoids production.
Initially most work was performed on microalgae and yeasts, which were of easier acceptance by the public. However, recently bacteria have been found to have several advantages over other microbes, such as: adaptability to different culture conditions; production of different types of pigments; shorter life spans and easier manipulation in large scale;33 making them very attractive for the industrial production of these bioactive compounds.
In this work, the potential of the DBT desulfurizing bacterium G. alkanivorans strain 1B as a carotenoid-producing microorganism was evaluated. In this context, the influence of different culture conditions (light, carbon source or sulfur source) on pigments production by strain 1B was studied with further characterization of the novel carotenoids.
II. Materials and methods
A. Chemicals
DBT (99%) was obtained from Acros Organics, 2-hydroxybiphenyl (2-HBP) from Sigma and dimethylformamide (DMF) from Riedel-de-Haën. 4-mDBT (96%) from Aldrich Chem. Co. DBT stock solution is prepared by dissolving 150 mM DBT in DMF. Sodium sulfate anhydrous (>99%) was obtained from Merck. Astaxanthin (98%) and β-carotene (≥95%) were from Sigma, canthaxanthin (99%) was from Roche and lutein (10%) was from FloraGLO, Kemin. All other reagents were of the highest grade commercially available.B. Microorganism and culture media
The microorganism used for these studies was the bacterium G. alkanivorans strain 1B, isolated in our laboratory.5 The basal salts medium used for the cultivation/maintenance of this microorganism and further for the desulfurization tests, was a sulfur-free mineral (SFM) culture medium containing 1.22 g L−1 NH4Cl, 2.5 g L−1 KH2PO4, 2.5 g L−1 Na2HPO4·2H2O and 0.17 g L−1 MgCl2·6H2O. This medium was supplemented with 0.5 mL L−1 of a sulfur-free trace elements solution,7 and its final pH was adjusted to 7.5 before autoclaved at 121 °C, 15 psi for 15 min. Filter sterile fructose or glucose solutions (50% w/v) were added to the culture medium to an initial concentration of 10 g L−1, as the only carbon source. Bacterial cultures were performed in 500 mL Erlenmeyer shake-flasks in an orbital incubator (Unitron CH-4103, Infors AG, Bottingen, Switzerland), at 30 °C, pH 7.5 and 150 rpm agitation. The influence of light was assayed by carrying in parallel the bacterial growth assays at ∼3000 lux light (TES-1330 digital light meter, TES Electrical Electronic Corp., Taiwan, R.O.C) and in dark (flasks covered with tinfoil). For the biodesulfurization tests, 400 μM of DBT was added into the sterilized culture medium as sulfur source, while for the remaining growth assays sodium sulfate anhydrous (Na2SO4) was added as the sulfur source (Na2SO4 concentration was adjusted to set the same quantity of sulfur as DBT in BDS assays). All the growth/BDS assays were performed in triplicates.C. Carotenoids extraction
At the end of each growth assay, cells were harvested by centrifugation (8600g at 4 °C, 20 min). The supernatant was discarded and the cells were dried at 55 °C for about 1 h. Then, the pigments were directly pellet-extracted with DMSO, according to the following procedure: first 25 mg cell biomass (cells with about 60% humidity) was transferred to a 1.5 mL Eppendorf tube; 1 mL of DMSO was later added to the biomass, and extraction was performed in an orbital shaker at 50 °C, for 45 min. The tubes were then centrifuged (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300g, 10 min). The supernatant was recovered and the process was repeated until no color was observed in the DMSO from the biomass. The next step was to extract the pigment from the DMSO. Thus, DMSO was mixed with acetone, NaCl solution (20%) and ethyl acetate in the proportion of 4
300g, 10 min). The supernatant was recovered and the process was repeated until no color was observed in the DMSO from the biomass. The next step was to extract the pigment from the DMSO. Thus, DMSO was mixed with acetone, NaCl solution (20%) and ethyl acetate in the proportion of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The mixture was shaken for less than a minute and left to rest for about 1 hour to allow phase separation. After which the ethyl acetate phase (top layer) was recovered. When necessary, more ethyl acetate was added and this process was repeated until no color was left in the lower layers. Finally, samples were left at −20 °C overnight to allow final phase separation and guarantee no water was present in the final extract. Then, the ethyl acetate phase (top layer) was collected and filtered through 0.22 μm filters before carotenoids analysis.
6. The mixture was shaken for less than a minute and left to rest for about 1 hour to allow phase separation. After which the ethyl acetate phase (top layer) was recovered. When necessary, more ethyl acetate was added and this process was repeated until no color was left in the lower layers. Finally, samples were left at −20 °C overnight to allow final phase separation and guarantee no water was present in the final extract. Then, the ethyl acetate phase (top layer) was collected and filtered through 0.22 μm filters before carotenoids analysis.
D. Analytical methods
The cell growth was monitored by measuring the dry cell weight (DCW), the sugars consumption (fructose/glucose) using HPLC9 and the optical density (OD) of the culture broth samples at 600 nm (Thermo Electron Corporation Spectrophotometer, model Genesys 6, Madison, USA). The DBT desulfurization was evaluated by measuring 2-HBP production (the final product of DBT desulfurization). The 2-HBP was extracted from the culture broth, previously acidified with HCl, with ethyl acetate during a 5 min vortex. After phase separation occurred the organic phase was analyzed by gas chromatography (GC) in a gas-chromatograph (Model CP9001, Chrompack, Middelburg, The Netherlands) equipped with a flame ionization detector. An Alltech 10% SE-30 on 80/100 Chromosorb W-HP column was used with nitrogen as the carrier gas. The chromatograph oven start temperature was 190 °C for 2 min and the end temperature 225 °C maintained for 1 min (heating rate 6 °C min−1). The injector and detector temperatures were set for 250 °C and 290 °C, respectively. In all GC measurements, 4-mDBT was used as internal standard to minimize variations.The amount of total carotenoids extracted from each biomass was assessed by spectroscopy, using a Shimadzu UV-Visible spectrophotometer (UV-2401 PC) to carry out the scanning spectra ranging the wavelength from 380 to 700 nm, and the concentration of total carotenoids was calculated based in Lambert–Beer equation accordingly to Nobre et al.,34 but using the value of 2091.4 L/10 g cm−1 for the specific optical extinction coefficient at λ = 477 nm (wavelength of the maximum absorbance of canthaxanthin in ethyl acetate). Specific carotenoid identification and quantification was performed using HPLC (Agilent 1200 Series system, Agilent Technologies, Tokyo) equipped with a μ-Bondapack C18 (250/4.6 mm) column and a detector UV/VIS also from Agilent (λ = 477 nm). In HPLC, the pigment extracts were eluted for 90 min at a flow rate of 0.5 mL min−1, using a mobile phase composed by methanol (with 0.2% water, v/v) and acetonitrile in a 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 ratio. The identification of carotenoids in each sample extract analyzed was performed by comparing the retention times with those obtained for individual standards (astaxanthin, canthaxanthin, lutein and β-carotene), using the LC3D ChemStation software (Rev.A.10.02 [1757], 1990–2003, Agilent Technologies, USA). The pigment results are presented as percentage (%), which correspond to g carotenoid per 100 g DCW.
25 ratio. The identification of carotenoids in each sample extract analyzed was performed by comparing the retention times with those obtained for individual standards (astaxanthin, canthaxanthin, lutein and β-carotene), using the LC3D ChemStation software (Rev.A.10.02 [1757], 1990–2003, Agilent Technologies, USA). The pigment results are presented as percentage (%), which correspond to g carotenoid per 100 g DCW.
III. Results and discussion
A. Growth of G. alkanivorans strain 1B: influence of culture conditions
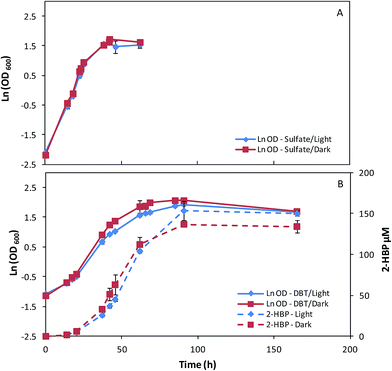
In order to understand the influence of culture conditions on pigments production by G. alkanivorans strain 1B, a set of assays was performed testing different combinations of three key factors: carbon source (fructose/glucose), sulfur source (sulfate/DBT), and light (presence/absence).Since G. alkanivorans strain 1B is a fructophilic microorganism,7 the bacterium was first cultivated with fructose as the preferential carbon source (10 g L−1) in 500 mL shake flasks, with 150 mL of culture medium. Fig. 1 represents the time course profiles of the cell growth of the strain 1B in fructose, with sulfate (Na2SO4, Fig. 1A) or DBT (Fig. 1B) as the sulfur source. For the cultures grown with DBT, the desulfurization profiles were also evaluated (2-HBP production rate). In each of these test combinations, the cultures were grown both under light (∼3000 lux) and dark conditions (absence of light).
In Table 1 is presented the main metabolic parameters associated to these assays. When strain 1B was grown in fructose and sulfate in the presence/absence of light, the bacterium presented similar overall growth profiles (Fig. 1A), attaining maximum OD600 values around 5, but a slightly higher μmax was observed for the cells grown in the dark (0.168 h−1) over those grown in the light (0.154 h−1). When DBT was used as the sulfur source instead sulfate, the bacterial cultures attained higher OD600 values but with lower μmax values. The influence of light (presence/absence) on the growth of these cultures was translated by noticeable differences in terms of overall growth profiles (Fig. 1B). In the dark, strain 1B achieved an OD600 of 7.90 with a μmax of 0.077 h−1, while in presence of light strain 1B achieved an OD600 of 6.82 with a μmax of 0.067 h−1, which corresponds to a 15% lower rate (Table 1). Looking at the 2-HBP production profiles (Fig. 1B), this growth difference did not affect the overall desulfurization ability. Both cultures presented very similar results in terms of 2-HBP production rate and 2-HPP specific production rate (q2-HBP), namely 3.63 μM h−1 corresponding to 5.19 μmol gDCW−1 h−1 for light grown culture, and 3.65 μM h−1 corresponding to 5.31 μmol gDCW−1 h−1 for dark grown culture (Table 1). This indicates that light had little influence on BDS under these culture conditions (10 g L−1 fructose + 400 μM DBT). Additionally, regarding the sugar consumption rates in Table 1, it can be observed that for both tested culture conditions (fructose + sulfate/DBT) strain 1B grown under ∼3000 lux consumed faster the fructose, respectively ∼10% faster in fructose and sulfate, and ∼5% faster in fructose and DBT.
| Metabolic parameters | Fructose 10 g L−1 | |||
|---|---|---|---|---|
| SO4 | DBT | |||
| Light | Dark | Light | Dark | |
| μmax (h−1) | 0.154 | 0.168 | 0.067 | 0.077 |
| Maximum sugar consumption rate (g L−1 h−1) | 0.193 | 0.176 | 0.154 | 0.148 |
| 2-HBP production rate (μM h−1) | 3.63 | 3.65 | ||
| Maximum 2-HBP production (μM) | 153.24 | 136.84 | ||
| Time for maximum 2-HBP (h) | 90.75 | 90.75 | ||
| q2-HBP (μmol gDCW−1 h−1) | 5.186 | 5.310 | ||
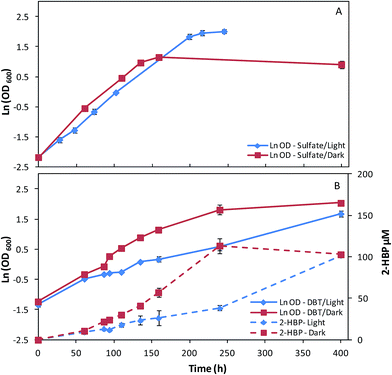
In a second set of assays, fructose was replaced by glucose as carbon-source (10 g L−1) and the remaining key factors were maintained (sulfate/DBT as sulfur source, light/dark growth). The effects of these culture conditions on growth and desulfurization ability of strain 1B can be observed in Fig. 2A and B and in Table 2. For sulfate as the sulfur source, the cultures grown in dark/light conditions presented a different behavior in terms of overall growth curve profiles (Fig. 2A), despite of the μmax obtained be similar for both conditions, about 0.02 h−1 (Table 2). Strain 1B grown in the light achieved a maximum OD600 of 7.46, while the culture grown in the dark abruptly stopped the growth after 160 h, obtaining a maximum OD600 of 3.15, which is less than half (Fig. 2A). In the growth assays with glucose and DBT (Fig. 2B), in dark/light, the strain 1B presented a very distinct behavior. The μmax of 0.017 h−1 obtained by strain 1B in dark conditions was more than 2-fold higher than that obtained under the light (0.007 h−1) (Table 2). In the dark, the growth was almost finished within ∼240 h (OD600 = 6.17), however, when the strain 1B was cultivated with 3000 lux, the growth was much slower (400 h, OD600 = 5.38). These growth differences also affected the DBT desulfurization, which can be observed by the differences within both 2-HBP production profiles (Fig. 2B). Comparing these desulfurization profiles in dark/light conditions, it can be stated that in dark conditions a maximum 2-HBP production of 113.43 μM was achieved at 240 h, corresponding only to a 2-HBP production of 38.20 μM (4-fold lower) by the culture grown in light in the same time period. The light exposed culture took more than 400 h to achieve a maximum 2-HBP concentration of 101.59 μM. These results seem to indicate an inhibitory effect of the light when DBT and glucose are used in the culture medium. In overall, maximum 2-HBP production rates of 0.69 μM h−1 and 0.40 μM h−1 are achieved by strain 1B grown in glucose and DBT at dark and light, respectively. The similarity observed for the corresponding q2-HBP (0.67 vs. 0.61 μmol gDCW−1 h−1) is due to the highest net biomass obtained in the culture grown in dark.
| Metabolic parameters | Glucose 10 g L−1 | |||
|---|---|---|---|---|
| SO4 | DBT | |||
| Light | Dark | Light | Dark | |
| μmax (h−1) | 0.020 | 0.021 | 0.007 | 0.017 |
| Maximum sugar consumption rate (g L−1 h−1) | 0.049 | 0.033 | 0.025 | 0.058 |
| 2-HBP production rate (μM h−1) | 0.40 | 0.69 | ||
| Maximum 2-HBP production (μM) | 101.59 | 113.43 | ||
| Time for maximum 2-HBP (h) | 400 | 240 | ||
| q2-HBP (μmol gDCW−1 h−1) | 0.605 | 0.667 | ||
Moreover, regarding to the sugar consumption rates depicted in Table 2, a different behavior can be stated depending on the sulfur source (sulfate/DBT) used. Similarly to what was observed for the cultures grown in fructose, the culture grown in glucose and sulfate in presence of light presented a carbon source consumption 31% higher than that grown in the dark. Conversely, when DBT was used as sulfur source, this behavior was reversed and the culture grown in the dark presented a sugar consumption rate much higher, more than 2-fold higher than that of cells grown in the light (0.058 versus 0.025 g L−1 h−1, Table 2).
Thus, comparing the overall results described in Fig. 1 and 2 and Tables 1 and 2, for the different culture conditions combinations studied, it became clear that the culture conditions have a great influence on all bacterial culture metabolic parameters. In fructose as the carbon source, the maximum growth rates were between 4 and 10 times faster, the sugar consumption rates were 2 to 6 times greater and the specific desulfurization rates were approximately 8 times higher than their glucose counterparts, which is in accordance with the fructophilic characteristics attributed to strain 1B.7 It was also noticeable, that the sulfur source had a great influence on the growth rate, especially with fructose. In fact, with this carbon source, cells grown with sulfate presented a growth rate almost 2-fold higher than those grown with DBT. This fact can be supported by the toxic nature of DBT, which can affect the cells even at small concentrations resulting in lower growth rates.35 The sulfur source could also drastically alter the response of the bacterial culture to the light. Cells grown with sulfate and glucose under 3000 lux presented a growth rate about 3-fold higher than those grown with DBT under the same conditions. In contrast, in the corresponding cultures grown in the dark the growth rates only have a difference of about 24% (0.021 versus 0.017 h−1, Table 2).
In overall, the presence of a light source directly influenced both the maximum growth rate, and the maximum sugar consumption rate in every culture condition. When cultivated with 3000 lux, all cultures presented a reduction in μmax, which may be explained by the need to produce the pigmentation in response to the light, resulting in a slower growth rate. However, this might also be the result of photoinhibition from overstimulation by the intense light (3000 lux). With exception of glucose and DBT grown culture, for all the other cultures the sugar consumption rate increased when exposed to light. This is in accordance with what was proposed by Jeon et al.,19 which reported that the bacterium G. alkanivorans SKF120101 produces carotenoids that, under the presence of light, are capable of producing reducing power, increasing glucose consumption for this bacterium. In the absence of light, these carotenoids are not only less produced but also less stimulated, resulting in a loss of reducing power. This fact may possibly explain both the higher sugar consumption rates of cells cultivated under the light and the abrupt stop in growth observed in the culture with glucose and sulfate in dark conditions.
B. Carotenoids production: influence of culture conditions
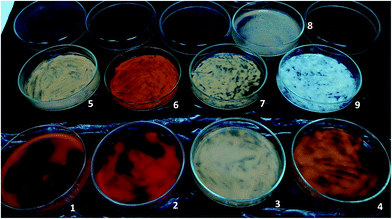
For the different culture conditions above described, besides growth and desulfurization, it was also evaluated the carotenoids production by G. alkanivorans strain 1B. Hence, at the end of each growth an aliquot of biomass was collected and dried in Petri dishes. Fig. 3 illustrates the different color on biomasses obtained in different culture conditions (biomasses 1 to 9). Before pigments extraction from biomasses, an additional test was performed. After growth had ended one flask of the triplicates of the culture grown with fructose and DBT in the dark (biomass 9 in Fig. 3) was placed under the light (3000 lux) for around 72 h, resulting the biomass labeled as 8. A direct analysis of these biomasses revealed very different results in terms of bacterial pigmentation, showing two extremes in terms of color: the completely white biomass obtained with fructose and DBT in the dark (biomass 9), and the very reddish biomass obtained with glucose and sulfate under the light (biomass 2).| Combinations tested | Culture key factors | Carotenoids (%) | |||||
|---|---|---|---|---|---|---|---|
| Fructose | Glucose | DBT | Sulfate | Dark | Light | ||
| #1 | ✗ | ✗ | ✗ | 0.000 | |||
| #2 | ✗ | ✗ | ✗ | 0.019 | |||
| #3 | ✗ | ✗ | ✗ | 0.029 | |||
| #4 | ✗ | ✗ | ✗ | 0.054 | |||
| #5 | ✗ | ✗ | ✗ | 0.043 | |||
| #6 | ✗ | ✗ | ✗ | 0.072 | |||
| #7 | ✗ | ✗ | ✗ | 0.049 | |||
| #8 | ✗ | ✗ | ✗ | 0.200 | |||
Another interesting result was observed for the biomass sample that was grown on fructose and DBT in dark, but put under 3000 lux of light for 72 h after growth has ended (biomass 8, Fig. 3). In contrast with its original growth biomass (biomass 9, Fig. 3), which produced no carotenoids (0%), this biomass achieved a total carotenoids production of 0.027% after light exposed. This value of total carotenoids attained was even higher than that observed from biomass grown in the same conditions under the light (0.019%, see Table 3, #2).
Hence, in overall these results (Table 3) indicate that glucose as carbon source is very important aiming to get the highest pigments production by the strain 1B. For glucose as the best carbon source, light served as a powerful inducer increasing the pigments productivity, and moreover, in the presence of light and glucose, the use of sulfate as sulfur source have still contributed to intensify the carotenoids production to the highest yields achieved by G. alkanivorans strain 1B. The importance of glucose for pigments production had already been observed by Ryu et al.36 with a Cyanobacterium synechocystis sp. PCC 6803. In their work, it was proved that glucose could activate the carotenoid gene expression even under dark conditions, through modifications in intercellular pH. This fact may support the higher results obtained in bacterial biomass grown in glucose, however further study on this issue is needed.
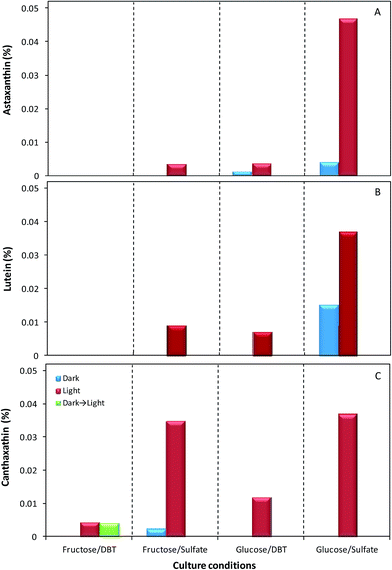
Fig. 4A–C presents the relative abundance of the three identified carotenoids within the total carotenoids produced by strain 1B on different culture condition. These results show that, in overall, astaxanthin was the carotenoid with the lowest concentrations, except both in biomass cultivated with glucose and sulfate under the light, the best condition, where it was the highest of the three carotenoids achieving 0.047%, and in biomass grown with glucose and DBT in the dark, where it was the only identifiable pigment (Fig. 4A). The maximum value of astaxanthin obtained in the optimal conditions was much higher than the values of astaxanthin obtained in the other combinations of key culture factors studied (carbon source; sulfur source and presence/absence of light), ranging from 0.0000% to 0.0038%. Furthermore, in fructose as carbon source, this carotenoid was only detected when the culture was grown with sulfate as sulfur source and under the light. The production of lutein by strain 1B occurred in the same culture conditions as for astaxanthin, with the exception of glucose and DBT in the dark, where no production of lutein was detected (Fig. 4B). Lutein was produced in a higher concentration than astaxanthin, whenever the two pigments were observed, except for maximum concentration, with glucose and sulfate under the light, where lutein only achieved 0.034%, i.e. about 28% lower than the astaxanthin produced. Relatively to canthaxanthin, in Fig. 4C can be observed that the highest production of this carotenoid was achieved when the strain 1B was grown with sulfate and light, independently of the carbon source. For these two key factors, the cells produced 0.037% and 0.035% of canthaxanthin, with glucose and fructose, respectively. Moreover, this carotenoid was the only identifiable pigment produced in cells grown with: (a) fructose and DBT under the light (0.0042% canthaxanthin); (b) fructose and DBT in the dark and afterward exposed to light (0.0041% canthaxanthin); and (c) fructose with sulfate in the dark (0.0021% canthaxanthin). In fact, the posteriori exposure to light of biomass that had no pigments induced the production of canthaxanthin by the cells to the level of that produced by cells grown in the same culture conditions but under light, demonstrating the great importance of light factor for canthaxanthin production.
Once again, these results highlight the great influence of the key culture factors towards the production of carotenoids by strain 1B, both in quantity and quality, since different concentrations and types of carotenoids may be obtained depending on the culture conditions used.
For each culture condition studied, the comparison between the two concentration values obtained for carotenoids production by strain 1B, both spectrophotometrically and by HPLC (Table 3 and Fig. 4), shows that, for most of the cases, the sum concentration of the three carotenoids identified is less than half of the total carotenoids production. In fact, these two concentrations calculated were only very close in the extracts from cells grown with fructose and sulfate under the light (0.054% – Table 3 versus 0.047% – Fig. 4). Even in extracts from biomass grown in the best conditions for the production of these three carotenoids, their overall net concentration (0.1209%) only corresponds to 60% of the total carotenoids concentration (0.2015%) produced by strain 1B. In addition both concentration values determined may be under evaluated since further optimization studies are required towards maximal pigments extraction from bacterial biomass. Indeed, much of the work performed on carotenoids is focused in the extraction methods. Carotenoids are lipophilic and usually present low solubility in water, so the most common method of extraction is through the use of organic solvents. Due to the characteristics of the different carotenoids, it is important to choose the most appropriate solvent for extraction.20 Hexane is the most common option; however acetone, ethyl acetate, petroleum ether, methanol and ethanol are also widely used. In this study, preliminary results indicated that DMSO extraction, followed by a transfer to ethyl acetate, resulted in a maximum extraction. However, even after successive extractions the bacterial biomass still maintained a remaining color, possibly indicating the presence of carotenoids with very different polarities.37 Thus, towards maximal yield on pigment extraction from strain 1B is essential to further explore alternative extraction methods, either using new solvents, or different technologies, such as extraction with ionic liquids, or in super critical conditions.37,38
In overall, these results obtained for carotenoids production by G. alkanivorans strain 1B are very promising, even when compared with those obtained for another species of the same genera, Gordonia jacobaea, known for its high pigments production. The wildtype of this strain was able to produce 227 μg gDCW−1, which is about 9-fold less carotenoids than what strain 1B achieved without any genetic enhancements (0.2015% carotenoids = 2015 μg gDCW−1). Furthermore, even the hyper pigmented G. jacobaea mutants, described by de Miguel et al.18 are only slightly better than the strain 1B in terms of total carotenoids productivity, achieving 2500 μg gDCW−1. Recently, Kim et al.39 also report the carotenoids production by another species of Gordonia, the G. ajoucoccus A2T, in a bioreactor under optimized conditions with hexadecane in which achieved 2900 μg gDCW−1. This is about 44% more pigments than those obtained by strain 1B, however the carotenoids production results for strain 1B were obtained only from shake-flask assays without any optimization. Furthermore, strain 1B is also known for its ability to grow on alternative carbon sources, based on agro-industrial materials.8,40–42 The use of low-cost feedstocks is commonly recognized as a promising strategy aiming to get a reduction of costs associated with the production of microbial carotenoids and a fundamental step to ensure a sustainable process.33,43
Therefore, this study opens a new focus of research on the further optimization of carotenoids production by strain 1B, either with optimization of the culture conditions in bioreactor, or with improvements in the pigments extraction from microbial biomass and in pigments identification, aiming to get a hyper pigment-producer strain that may be applied at an industrial level. Additionally, the ability of the desulfurizing strain 1B to produce high added-value products may be also a commercial benefit that may contribute for a more cost-effective downstream BDS scale up into a petroleum refinery using this bacterium to get ULSF.14,44
In this context, analyzing both metabolic parameters (Tables 1 and 2) and carotenoids production (Fig. 4) it is possible to choose the best conditions for further optimization of the envisaged bioprocess (BDS and/or carotenoids industry). If the bacterial biomass is intended as biocatalysts for fossil fuels desulfurization, the cells should be cultivated in fructose with DBT in the dark, and only after be exposed to light. This would make the process more efficient, since, at an industrial level, it is easier to cultivate cells in the dark, and the subsequently exposition to light would then allow the production of the carotenoids. The market for carotenoids continues to increase, and the exploitation of these added-value bioactive products could be crucial to make BDS process economically sustainable, as above referred. So, further studies must be performed in this field to determine the best conditions towards both the highest BDS and carotenoids production.
On the other hand, if the biomass is intended for canthaxanthin production, fructose with sulfate under the light should be used. Under this culture condition, the growth is much faster than with glucose, and the canthaxanthin produced is roughly at the same concentration. Moreover, in these growth conditions, canthaxanthin is the primary carotenoid produced by strain 1B, which will require less purification costs. But, if the biomass is used for overall carotenoids production, the bacterium should be cultivated with glucose and sulfate under the light. In this combination of key culture factors, the growth will be slower and cells will not desulfurize, but the biomass will present the highest pigments productivity.
IV. Conclusions
Prior works have already reported the influence of light in the pigments production by actinomycetes, however, this is the first study that correlates the carotenoids production with three key culture factors: light, carbon source and sulfur source. This work highlighted the influence of these factors on several metabolic parameters (growth, DBT desulfurization, carotenoids). G. alkanivorans strain 1B was able of achieving 2015 μg gDCW−1 of total carotenoids growing in glucose-sulfate-light, with about 60% corresponding to lutein, canthaxanthin and astaxanthin. Further optimization studies open a new focus of research towards the exploitation of these microbial high added value bioactive compounds.Acknowledgements
This work was financed by FEDER funds through POFC-COMPETE and by national funds through FCT (Fundação para a Ciência e a Tecnologia) in the scope of the project Carbon4Desulf – FCOMP-01-0124-FEDER-013932. T. P. Silva also acknowledges to FCT for his Ph.D. financial support (SFRH/BD/104977/2014).Notes and references
- M. Arenskötter, D. Bröker and A. Steinbüchel, Appl. Environ. Microbiol., 2004, 70, 3195–3204 CrossRef PubMed.
- D. Bröker, M. Arenskötter, A. Legatzki, D. H. Nies and A. Steinbüchel, J. Bacteriol., 2004, 186, 212–225 CrossRef.
- N. K. Harner, T. L. Richardson, K. A. Thompson, R. J. Best, A. S. Best and J. T. Trevors, J. Ind. Microbiol. Biotechnol., 2011, 38, 1761–1775 CrossRef CAS PubMed.
- P. W. G. Liu, T. C. Chang, L. M. Whang, C. H. Kao, P. T. Pan and S. S. Cheng, Int. Biodeterior. Biodegrad., 2011, 65, 1119–1127 CrossRef.
- L. Alves, R. Salgueiro, C. Rodrigues, E. Mesquita, J. Matos and F. M. Gírio, Appl. Biochem. Biotechnol., 2005, 120, 199–208 CrossRef CAS PubMed.
- L. Alves, M. Melo, D. Mendonça, F. Simões, J. Matos, R. Tenreiro and F. M. Gírio, Enzyme Microb. Technol., 2007, 40, 1598–1603 CrossRef CAS.
- L. Alves and S. M. Paixão, New Biotechnol., 2014, 31, 73–79 CrossRef CAS PubMed.
- L. Alves and S. M. Paixão, Appl. Biochem. Biotechnol., 2014, 172, 3297–3305 CrossRef CAS PubMed.
- S. M. Paixão, P. D. Teixeira, T. P. Silva, A. V. Teixeira and L. Alves, New Biotechnol., 2013, 30, 598–606 CrossRef PubMed.
- T. P. Silva, S. M. Paixão, J. C. Roseiro and L. Alves, J. Appl. Microbiol., 2015, 118, 609–618 CrossRef CAS PubMed.
- J. J. Kilbane, Curr. Opin. Biotechnol., 2006, 17, 305–314 CrossRef CAS PubMed.
- G. Mohebali and A. S. Ball, Microbiology, 2008, 154, 2169–2183 CrossRef CAS PubMed.
- V. C. Srivastava, RSC Adv., 2012, 2, 759–783 RSC.
- S. M. Paixão, T. P. Silva, B. F. Arez and L. Alves, in Applying Nanotechnology to the Desulfurization Process in Petroleum Engineering, ed. T. Saleh, IGI Global, 2016, ch. 13, pp. 390–425, DOI:10.4018/978-1-4666-9545-0.ch013.
- S. Bandyopadhyay, R. Chowdhury, C. Bhattacharje and S. Pan, Fuel, 2013, 113, 107–112 CrossRef CAS.
- T. P. Silva, S. M. Paixão, A. V. Teixeira, J. C. Roseiro and L. Alves, J. Chem. Technol. Biotechnol., 2013, 88, 919–923 CrossRef CAS.
- T. de Miguel, C. Sieiro, M. Poza and T. G. Villa, Int. Microbiol., 2000, 3, 107–111 CAS.
- T. de Miguel, C. Sieiro, M. Poza and T. G. Villa, J. Agric. Food Chem., 2001, 49, 1200–1202 CrossRef CAS PubMed.
- B. Y. Jeon, B. Y. Kim, I. L. Jung and D. H. Park, J. Microbiol. Biotechnol., 2012, 22, 1471–1477 CrossRef PubMed.
- D. B. Rodriguez-Amaya, Food Carotenoids: Chemistry, Biology and Technology, Wiley Blackwell, 2016 Search PubMed.
- S. Kiokias, C. Proestos and T. Varzakas, Curr. Res. Nutr. Food Sci., 2016, 4, 25–37 CrossRef.
- L. C. Mata-Gómez, J. C. Montañez, A. Méndez-Zavala and C. N. Aguilar, Microb. Cell Fact., 2014, 13, 12 CrossRef PubMed.
- B. Tian, Z. Xu, Z. Sun, J. Lin and Y. Hua, Biochim. Biophys. Acta, 2007, 1770, 902–911 CrossRef CAS PubMed.
- W. Stahl and H. Sies, Biochim. Biophys. Acta, 2005, 1740, 101–107 CrossRef CAS PubMed.
- P. C. Lee and C. Schmidt-Dannert, Appl. Microbiol. Biotechnol., 2003, 60, 1–11 CrossRef PubMed.
- T. Tanaka, M. Shnimizu and H. Moriwaki, Molecules, 2012, 17, 3202–3242 CrossRef CAS PubMed.
- A. V. Rao and L. G. Rao, Pharmacol. Res., 2007, 55, 207–216 CrossRef CAS PubMed.
- R. Esteban, J. F. Moran, J. M. Becerril and J. I. García-Plazaola, Environ. Exp. Bot., 2015, 119, 63–75 CrossRef CAS.
- BCC Research, The Global Market for Carotenoids, July 2015, Report ID: FOD025E.
- H. Ernst, Pure Appl. Chem., 2002, 74, 1369–1382 CrossRef CAS.
- B. Capelli, D. Bagchi and G. R. Cysewski, Nutrafoods, 2013, 12, 145–152 CrossRef CAS.
- E. A. Johnson and W. A. Schroeder, in Adv Biochl Eng Biotech, ed. A. Fiecher, Springer-Verlage, Heidelberg, 1995, vol. 53, pp. 119–178 Search PubMed.
- C. K. Venil, C. A. Aruldass, L. Dufossé, Z. A. Zakaria and W. A. Ahmad, RSC Adv., 2014, 4, 39523 RSC.
- B. Nobre, F. Marcelo, R. Passos, L. Beirão, A. Palavra, L. Gouveia and R. Mendes, Eur. Food Res. Technol., 2006, 223, 787–790 CrossRef CAS.
- L. Alves and S. M. Paixão, Bioresour. Technol., 2011, 102, 9162–9166 CrossRef CAS PubMed.
- J. Y. Ryu, J. Y. Song, J. M. Lee, S. W. Jeong, W. S. Chow, S. B. Choi, B. J. Pogson and Y. I. Park, J. Biol. Chem., 2004, 279, 25320–25325 CrossRef CAS PubMed.
- E. Yara-Varón, A. S. Fabiano-Tixier, M. Balcells, R. Canela-Garayoa, A. Bily and F. Chemat, RSC Adv., 2016, 6, 27750–27759 RSC.
- A. Singh, S. Ahmadb and A. Ahmad, RSC Adv., 2015, 5, 62358–62393 RSC.
- J. H. Kim, S. H. Kim, J. H. Yoon and P. C. Lee, Appl. Microbiol. Biotechnol., 2014, 98, 3759–3768 CrossRef CAS PubMed.
- L. Alves, S. Marques, J. Matos, R. Tenreiro and F. M. Gírio, Chemosphere, 2008, 70, 967–973 CrossRef CAS PubMed.
- T. P. Silva, S. M. Paixão, A. V. Teixeira, J. C. Roseiro and L. Alves, J. Chem. Technol. Biotechnol., 2013, 88, 919–923 CrossRef CAS.
- T. P. Silva, S. M. Paixão, J. C. Roseiro and L. Alves, J. Appl. Microbiol., 2015, 118, 609–618 CrossRef CAS PubMed.
- S. M. T. Gharibzahedi, S. H. Razavi and M. Mousavi, RSC Adv., 2013, 3, 23495–23502 RSC.
- L. Alves, S. M. Paixão, R. Pacheco, A. F. Ferreira and C. M. Silva, RSC Adv., 2015, 5, 34047–34057 RSC.
| This journal is © The Royal Society of Chemistry 2016 |