Nitrogen and sulfur self-doped porous carbon from brussel sprouts as electrode materials for high stable supercapacitors†
Jiangfeng Lia,
Guangtao Zanab and
Qingsheng Wu*a
aDepartment of Chemistry, Tongji University, Shanghai 200092, PR China. E-mail: qswu@tongji.edu.cn; Fax: +86 21 65981097; Tel: +86 21 65982620
bSchool of Materials Science and Engineering, Tongji University, Shanghai 200092, PR China
First published on 19th May 2016
Abstract
Heteroatom self-doped porous carbon materials were synthesized for the first time via freeze drying technique, followed by carbonization of brussel sprouts under a nitrogen atmosphere. The resultant materials were analyzed by scanning electron microscopy (SEM), energy dispersive spectroscopy (EDS) mapping, transmission electron microscopy (TEM), and Brunauer–Emmett–Teller (BET) measurements. Brussel sprouts carbonized at 800 °C exhibit superior capacitive performance, including specific capacitance of 255 F g−1 at a current density of 0.5 A g−1, high rate capability (83% capacitance retention from 0.5 A g−1 to 50 A g−1), and good cycle life (99.5% maintained over 5000 cycles). The highly stable electrochemical performance could be due to the hierarchical porous structure combined with the high specific surface area and heteroatom doping efforts.
Introduction
In order to meet the urgent growing energy demands of current society, meanwhile reducing the environmental pollution and fossil fuel consumption, there has been a requirement for more “greener”, excellent performance and cost-effective energy storage and production systems.1–3 Supercapacitors, known as electrochemical capacitors (ECs), have attracted a lot of attention as an innovative energy storage system due to their higher capacitance, higher power density (103 to 104 W kg−1) and longer cycling life (>106 cycles) than most batteries.4–6 Supercapacitors can be classified into pseudocapacitors and electrical double layer capacitors (EDLCs) due to their charge/discharge mechanism.7,8 The capacitance of pseudocapacitors is generated based on electrical absorption, reversible faradic redox reactions, and intercalation processes.9,10 However, the low electrical conductivity and poor cycle stability of pseudocapacitors limit their practical applications.The energy storage of EDLCs is generally based on the non-faradic surface charge accumulation at electrode and electrolyte interfaces.11 Therefore, high specific surface area and appropriate pore size distribution are two important factors contribute to the improvement of the electrochemical performance of EDLCs. Recently, carbon materials, such as carbon fibers,12,13 porous carbon,9,11 carbon nanotubes,14,15 graphene16,17 and carbide-derived carbons,18,19 are widely used as active electrode materials with excellent EDLCs performance. Amongst them, porous carbon materials are most suitable candidate as electrode materials for supercapacitor.20–22 More often than not, porous carbon materials have been fabricated by various methods such as hard or soft sacrificial template,23,24 chemical activation,2,11 physical activation,25,26 and etc. Owing to the consumption of expensive precursor and high energy, it is a foremost challenge for synthesis of porous carbon materials by a simple and low-cost method. Natural biomass, which is ubiquitous on the planet, has emerged as promising precursor candidate for porous carbon materials owing to its renewable nature and low-cost. So far, several natural biomasses such as pollens,27 leaves,28 eggplant,29 corn husk,30 coconut shell,31 etc. have been successfully utilized to synthesize porous carbon materials with large specific surface area and abundant porosity.
In addition, introducing heteroatom species (e.g., nitrogen,32,33 phosphorus,34,35 boron,36 and sulfur37,38) also can improve the capacitance through additional faradaic reactions by enhancing the surface wettability of porous carbon based electrode materials.39 Heteroatom doping has been widely used in carbon materials by various methods such as thermal annealing method,40 chemical vapor deposition,41 hydrothermal method,42 and etc. Owing to the expensive precursor and complicated technology, it is a meaningful research to reduce the price of the dopants and simplify the doping technology. Recent studies have shown that heteroatoms are successfully doped when some natural biomasses transformed into carbon materials during carbonization process. It provides an effective and “greener” method to reduce the cost of conventional doping technology. According to the literature research, the raw brussel sprouts is composed of water (86%), proteins (3.38%), carbohydrate (8.95%), and other components (1.67%).43 The high water content indicates a flourishing pore structure to store and transport water, while the existence of relative high proteins could provide abundant nitrogen sources in the suit carbonization process. Through an extensive literature survey, there is no report on transformation from the brussel sprouts to porous carbon materials for electrochemical application.
Brussel sprouts, a kind of common vegetable, are cultivated on a large scale in Europe and North America. The composition of brussel sprouts, on average, comprises about 44.83% carbon, 10.19% nitrogen, 43.28% oxygen, 0.38% sulfur, and trace amount of other elements after freeze-drying treatment (Table S1†). In addition, the freeze-dried brussel sprouts has a three dimensionally macroporous structure (Fig. S1†). In this study, we, for the first time, demonstrate the synthesis of porous carbon materials by the pre-treatment of brussel sprouts in KOH solution and subsequent carbonized at high temperature under a N2 atmosphere. The as-synthesized nitrogen and sulfur self-doped brussel sprouts derived porous carbon materials (BSPCs) have larger surface areas and abundant micropores formed by KOH activation. These materials exhibit superior capacitive performance, including high specific capacitance of 255 F g−1 at a current density of 0.5 A g−1, good rate capability (83% capacitance retention from 0.5 A g−1 to 50 A g−1) and cycle life (99.5% capacitance retention after 5000 cycles).
Experimental
Materials
The brussel sprouts were purchased from market in Shanghai. Hydrochloric acid, potassium hydroxide, ethanol, graphite powder were purchased from Shanghai Chemical Reagents Co., Ltd. Pure nitrogen was bought from Shanghai BOC Special Gases Sales Service Co., Ltd. Nickel foil was bought from Shanghai Hongxiang Plant. Polytetrafluoroethylene (PTFE) solution was obtained from Shanghai 3F New Materials Co., Ltd. Deionized water was used throughout the experiments.Preparation of BSPCs
The brussel sprouts were cut into pieces, freeze-dried, and pre-carbonized at 450 °C for 2 h in a quartz tube furnace under a nitrogen atmosphere. The pre-carbonized materials were ground to powder, mixed with KOH aqueous solution (weightKOH/weightcarbon = 2), and carbonized in a ceramic crucible at 700, 800 or 900 °C for 2 h under constant nitrogen flow, respectively. The resulting products were immersed into excess HCl solution for 1 h, thoroughly washed with hot deionized water, and then dried in a vacuum. The final porous carbon materials are denoted as BSPC-T, where T is the carbonized temperature.Electrochemical measurements
CHI 660E (ChenHua Corp., Shanghai, China) was used for all electrochemical tests at room temperature. For three-electrode system, the BSPCs based testing electrodes were prepared by mixing the active materials, graphite power and PTFE at a weight ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in deionized water solution. Then, the slurry was pressed onto a nickel foam (1 cm × 1 cm) at 10 MPa and then dried at 80 °C on a vacuum oven over 24 h. A Pt plate and an Hg–HgO were used as the counter electrode and the reference electrode, respectively. 6 M KOH was used as the electrolyte. The specific capacitance was calculated from the galvanostatic charge/discharge values by the following equation:
5 in deionized water solution. Then, the slurry was pressed onto a nickel foam (1 cm × 1 cm) at 10 MPa and then dried at 80 °C on a vacuum oven over 24 h. A Pt plate and an Hg–HgO were used as the counter electrode and the reference electrode, respectively. 6 M KOH was used as the electrolyte. The specific capacitance was calculated from the galvanostatic charge/discharge values by the following equation:where Cs is the specific capacitance (F g−1), I is the discharge current (A), Δt is the discharge time (s), m is the mass of active materials (g), and ΔV is the potential change (V).
A symmetric supercapacitor device was assembled by two BSPCs based electrodes with same weight separated by filter paper. 6 M KOH was used as the electrolyte. The energy density (E) was calculated by: 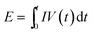 and power density (P) was calculated by: P = E/t. Where I is the discharge current (A), t is the discharge time (s), V is the discharge voltage and m is the total mass of the both electrodes (V).
and power density (P) was calculated by: P = E/t. Where I is the discharge current (A), t is the discharge time (s), V is the discharge voltage and m is the total mass of the both electrodes (V).
Characterization of BSPCs
The porous nature of samples BSPCs were analyzed by nitrogen adsorption–desorption measurement at 77 K using ASAP 2020 Micromeritics volumetric system. The specific surface area and pore size distribution of the samples were obtained by the Brunauer–Emmette–Teller (BET) and Barrette–Joynere–Halenda (BJH) method, respectively. The micropore surface area and micropore volume were calculated by t-plot method. The morphology and elemental composition of the samples were characterized by field emission scanning electron microscope (FESEM, Hitachi S-4800, Japan), X-ray photoelectron spectroscopy (XPS, AXIS Ultra DLD, Japan), scanning electron microscopy (SEM, Phenom Pro-G2), Raman spectroscopy, energy dispersive spectroscope (EDS) and transmission electron microscopy (TEM, JEOL JEM-2100, Japan). Powder X-ray diffraction (XRD) patterns were analyzed from Bruker Focus D 8 diffract-meter with Cu Kα radiation (40 kV, λ = 0.15418 nm) between 10 and 70°.Results and discussion
Structural and morphological characteristics
Scheme 1 illustrates a schematic for the fabrication of brussel sprouts derived porous carbon (BSPC) materials. The clean and freeze-dried brussel sprouts were pre-carbonized at 450 °C for 2 h under a nitrogen atmosphere. The pre-treated carbon was mixed with KOH aqueous solution and then carbonized at 700 °C, 800 °C, and 900 °C under nitrogen atmosphere, respectively.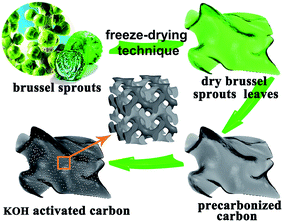 | ||
| Scheme 1 Flow diagram for the fabrication of brussel sprouts derived porous carbon (BSPC) materials. | ||
The surface morphologies and porous features of BSPCs were studied by using nitrogen adsorption–desorption analysis shown in Fig. 1A and B. A hysteresis loop extending from P/P0 = 0.4 to 0.9 was obtained for brussel sprouts derived carbon (BSC) by direct carbonized at 800 °C without KOH-pretreated, which indicates its porous nature (Fig. S2A†). The N2 isotherms of BSPCs show typical type-I and type-IV isotherms with a hysteresis loop, which indicates the presence of micropores and mesopores.44 In detail, all the N2 sorption isotherms show an upwards trend at the relative pressure (P/P0 < 0.4), indicating a typical result for microporous materials.9,45 The hysteresis loop was clearly observed at high relative pressure (0.4 < P/P0 < 0.9), indicates the existence of abundant mesopores.46,47 Therefore, these BSPCs all exhibit a hierarchical porous structure of micropores combined with mesopores. The existence of mesopore (2–50 nm) could contribute to the counterions entering the inner pore wall and forming an electric double-cylinder capacitor, while the solvated–desolvated counterions could line up along the inner pore axis and form an electric wire-in-cylinder capacitor in the micropore regime (<2 nm).48 The pore size distribution of BSPCs is shown in Fig. 1B. These BSPCs have a similar pore size distribution ranges from 1.5 to 5 nm, indicating the coexistence of micropore and mesopore structures. During in the electrochemical reaction, mesopores can provide effective paths for the quick transport of electrolyte ions, which could generate a good rate capacity. The pore characteristics of these samples are listed in Table 1. The specific surface areas and pore volumes of KOH-pretreated carbon materials are much larger than the direct carbonized materials without KOH-pretreated, because KOH as active agent can react with carbon at high temperature to generate H2, CO2 and CO gas and then the micropores are formed.49 The specific surface areas of BSPCs carbonized at 700, 800 and 900 °C are determined to be 1715, 2410 and 2267 m2 g−1, while the pore volumes are 1.01, 1.37 and 1.32 cm3 g−1, respectively. The BET specific surface area and microporous volume of BSPC-900 dropped slightly, which may be due to the corrosion of small pores walls during the activation process.9 Higher specific surface area, abundant micropores and heteroatoms (N, S and O) doping of BSPC-800 are advantageous in transport and adsorption of electrolyte ions.50
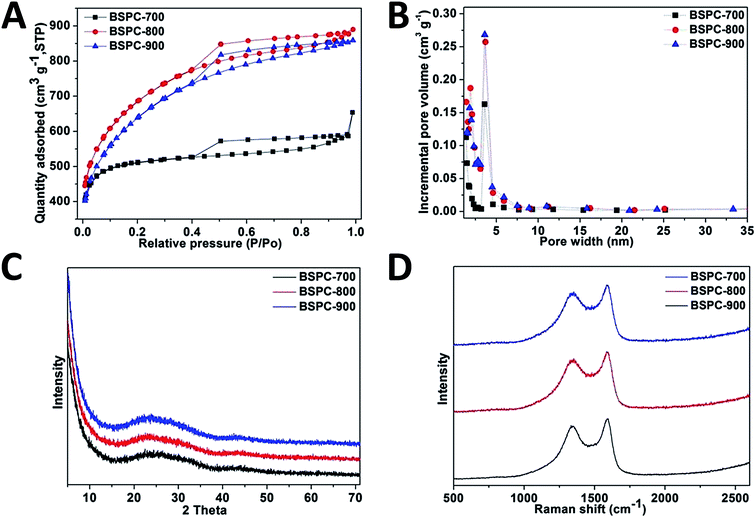 | ||
| Fig. 1 (A) Nitrogen sorption isotherms, (B) pore size distribution profiles, (C) XRD pattern and (D) Raman spectra of BSPCs. | ||
| Samples | SBET [m2 g−1] | Smicro [m2 g−1] | SLangmuir [m2 g−1] | Vpore [cm3 g−1] | Vmicro [cm3 g−1] | Daver [nm] |
|---|---|---|---|---|---|---|
| a SBET: BET surface area; SLangmuir: Langmuir surface area; Smicro: micropore surface area; Vpore: total pore volume; Vmicro: micropore volume (below 2 nm pore size); Daver: average pore size. | ||||||
| BSPC-700 | 1715 | 1639 | 2300 | 1.01 | 0.77 | 2.35 |
| BSPC-800 | 2410 | 2301 | 3353 | 1.37 | 1.17 | 2.28 |
| BSPC-900 | 2267 | 1971 | 3174 | 1.32 | 0.99 | 2.34 |
| BSC | 545 | 460 | 741 | 0.38 | 0.24 | 2.81 |
Fig. 1C shows the powder X-ray patterns of these BSPCs. As depicted in Fig. 1C, two diffraction peaks located at 24.6° (graphitic peak) and 44.3° are clearly observed, which are indexed as graphitic (002) and (100) planes. It indicates that all these samples possess a well-developed graphitic stacking structure, which could improve electrical conductivity.51 The intensity at the low-angle scattering peak indicates the density of micropores.9 Here, BSPC-800 shows a higher density of microporous than the others. The Raman spectroscopy results are depicted in Fig. 1D. There are two clear characteristic peaks located at around 1344 cm−1 and 1582 cm−1, which are ascribed to the D band and G band of carbon materials, respectively. The G band indicates the C–C bond vibrations of sp2 carbon atoms, while the D band is related to the disordered microstructure and defects of carbon material.52 The low D/G intensity ratio indicates the high degree of structural order with respect to a well graphitic structure.9 The D/G intensity ratios of BSPC-700, BSPC-800 and BSPC-900 were deduced to be 1.20, 1.09 and 1.01, respectively. Among all these samples, BSPC-900 shows a lowest D/G intensity ratio, which might be due to the higher carbonized temperature and reduced amount of heteroatom (N, O and S) doping.9
The surface morphology and structure are two key factors influencing the capacitance of carbon based electrochemical materials. Fig. 2A–C shows the FESEM images of BSPC-800 at different angles and magnifications. Compared with BSC, all these BSPCs have a loose and porous structure (Fig. S1C, S1E, S1F† and 2A). As shown in Fig. 2B, the BSPC-800 has a three-dimensional spatial macroporous structure, and the average pore size is less than 1 μm. The existence of three-dimensional spatial macroporous structure could contribute to the electrolyte effectively infiltrating into the internal structure of materials.48 The fracture plane of BSPC-800 was characterized at a higher magnification (Fig. 2C). The loose and porous structure has been clearly observed and the average pore size is less than 20 nm, which could be formed by the activation of KOH at high temperature. The microstructure of BSPC-800 was further characterized by using transmission electron microscope (TEM) (Fig. 2D). As shown in Fig. 2D, abundant mesopores and micropores are randomly distributed on the surface of BSPC-800, which could contribute to the fast absorption and transfer of electrolyte ions. Therefore, these porous carbon materials derived from brussel sprouts could be an ideal candidate as electrode material for supercapacitors due to their high specific area, abundant hierarchical porous structures, and a small amount of heteroatoms doping.
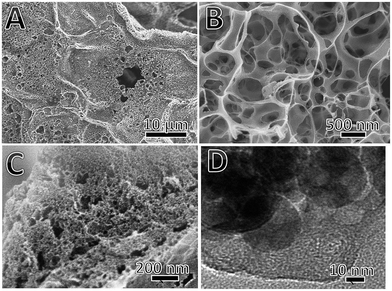 | ||
| Fig. 2 (A–C) FESEM images of BSPC-800 at different angles and magnification; (D) TEM image of BSPC-800. | ||
Table 2 summarizes the chemical compositions for these BSPCs materials characterized by EDS and XPS (see Fig. S3†). The chemical compositions of BSPCs were found to consist of C, N, O and S. From the results of the EDS and XPS spectra, the contents of these heteroatoms (O, N and S) are gradually decreased with the increasing activation temperature. Fig. S3† shows the high resolution C1s, N1s, O1s, and S2p spectra. In the C1s spectra of BSPCs, a narrow trend is clearly observed with the increased activation temperature, which indicates an improved degree of graphitic order.50,53 The binding energy centered at 284.4 eV in the C1s spectra belongs to sp2-hybridised carbon atoms bound to carbon atoms or hydrogen, which could improve the obtained carbon materials' conductivity.54 Meanwhile, two smaller peaks centered at 285.4 eV and 288.1 eV are assigned to carbon atoms bound to nitrogen or sulfur, and carbonyl groups, which could attribute to the pesudocapacitance.55 In the N1s spectra, two binding energies located at around 398.5 eV and 400.4 eV belong to pyridinic and pyrrolic nitrogen species, which could impose nitrogen related faradaic reactions and improve the samples' electrochemical performance.9 In addition, the peak centered at 401 eV belongs to quaternary nitrogen species, which could improve the carbon materials' conductivity. In the O1s spectra, three binding energies centered at 531.5 eV, 533.2 eV and 536 eV indicate the presence of oxygen atoms in carbonyl groups and various other oxygen groups, which could induce faradaic reactions and improve the wettability of carbon materials.56 The remnant sulfur appears at around 163.5 eV (C–S–S–C–), 164.9 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) and 168.3 eV (–C–SOX–C–) can also contribute to the pesudocapacitance.57 The EDS mapping of as-obtained samples are measured and shown in Fig. 3. The EDS elemental mapping indicates that S and O are homogeneously distributed on the surface of BSPC-800, while N is distributed dispersedly.
S) and 168.3 eV (–C–SOX–C–) can also contribute to the pesudocapacitance.57 The EDS mapping of as-obtained samples are measured and shown in Fig. 3. The EDS elemental mapping indicates that S and O are homogeneously distributed on the surface of BSPC-800, while N is distributed dispersedly.
| Samples | EDS (wt%) | XPS (atom%) | ||||||
|---|---|---|---|---|---|---|---|---|
| C | O | N | S | C | O | N | S | |
| BSPC-700 | 84.09 | 9.49 | 5.74 | 0.67 | 83.45 | 14.88 | 1.47 | 0.2 |
| BSPC-800 | 90.96 | 5.46 | 3.35 | 0.23 | 87.58 | 11.26 | 1.13 | 0.03 |
| BSPC-900 | 92.45 | 4.19 | 3.11 | 0.25 | 91.86 | 7.4 | 0.57 | 0.17 |
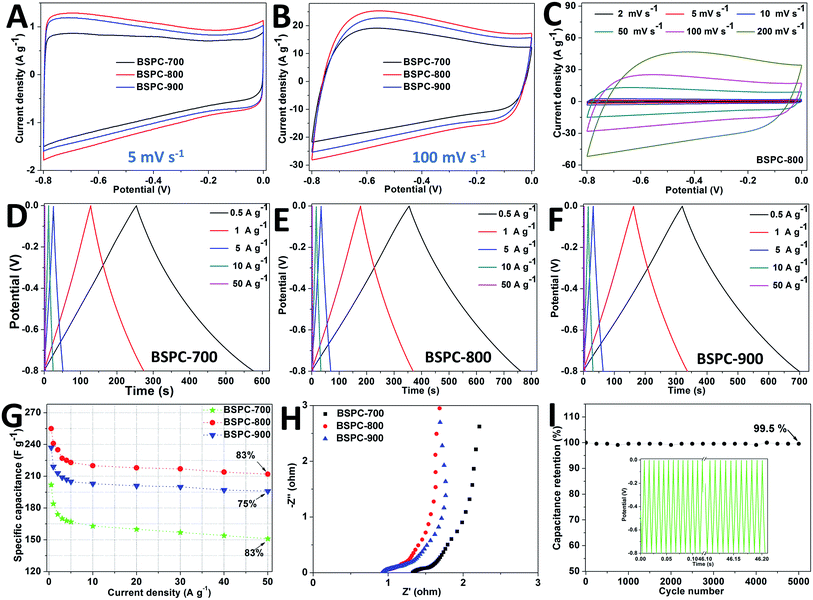
The performance of these BSPC materials as electrodes was characterized by cyclic voltammetry (CV) measurement in 6 M KOH electrolyte (Fig. 4A–C). All the CV curves of these BSPCs exhibit a rectangular shape (typical capacitive behavior) from −0.8 to 0 V at low scan rate (5 mV s−1), indicating a typical double-layer capacitive behavior. Even at a high scan rate of 100 mV s−1, these CV curves can also hold a symmetric and rectangular shape, which indicates the corresponding electrodes have low resistance and quick charge propagation.58 Compared with BSPC-700 and BSPC-900, BSPC-800 exhibits a more symmetric and rectangular shape at a high current density, due to its higher specific surface area and more abundant porosity (Fig. 4A and B). It shows that BSPC-800 is a promising electrode material for supercapacitors. As depicted in Fig. 4C, BSPC-800 still exhibits a rectangular shaped CV curve even at a high current density of 200 mV s−1.
Fig. 4H shows the Nyquist plots of these BSPC carbon based electrode materials in the frequency range from 10−1 Hz to 106 Hz. The Nyquist plot shows the plot of imaginary component (Z′) against the real component (Z′′) of impedance, which indicates the frequency response of the electrode/electrolyte system.59 The theoretical Nyquist plot can be separated into three sections: (a) a semicircle in the high frequency region, (b) a straight line in the middle frequency region, and (c) a vertical line in the low frequency region. As depicted in Fig. 4H, an almost vertical line is clearly observed at a low frequency region that indicates a typical double-layer charge storage behavior.60 The Nyquist plot is relatively 45° in the middle frequent region, which is a typical character of porous carbon-based electrodes.61 The wettability for the diffusion of electrolyte ions into the inner through the three-dimensional macroporous structure of the electrode acts on this phase.9,62 The semicircle in the high frequency region reflects the charge transfer resistance (Rct), while the first intercept of the plots with the real axis reflects the solution resistance of the electrochemical system (Rs). The values of Rs and Rct both can be obtained from the Nyquist plot (Fig. 4H). The BSPC-700, BSPC-800 and BSPC-900 show solution resistances of 1.32 Ω, 0.93 Ω and 0.91 Ω with charge transfer resistances of 0.26 Ω, 0.22 Ω and 0.16 Ω, respectively. The reduced resistances could be due to the improved degree of carbon materials and reduced heteroatoms doping with increasing carbonization temperature. All those samples show low series equivalent resistances (Rct + Rs) on account of high specific surface area, abundant porosity and heteroatom doping, which could considerably improve the electrolyte ion transport rate and obtain perfect rate capability.
The galvanostatic charge–discharge (GCD) technique has been used to investigate the performance of these BSPC-T electrode materials at various current densities in a three-electrode system. Fig. 4D–G shows the GCD curves and specific capacitances of BSPCs at different current densities. All these samples exhibit a charge–discharge curve in isosceles triangular shape with a little IR drop at low current densities due to their high graphitization and good conductivity.63 At a current density of 0.5 A g−1, BSPC-800 shows a specific capacitance of 255 F g−1, whereas BSPC-700 and BSPC-900 have lower specific capacitance values of 202 and 237 F g−1, respectively (Fig. 4G). The specific capacitance of these BSPCs slightly decreased with increasing current density due to an inadequate time for electrolyte diffusion and electron transfer. However, even at a current density of 50 A g−1, BSPC-800 still maintains specific capacitance of 212 F g−1 (83% capacitance retention from 0.5 A g−1 to 50 A g−1). This excellent electrochemical stability might be due to the high surface area and high pore volume of the carbon materials which provides a quick ion transfer pathway to electrolyte access into the microporous area. At the same time, the doping of heteroatoms (N, O and S) can improve the surface wettability of the electrode materials and then induce pseudocapacitive behavior.
Furthermore, the long cycle stability of the BSPC-800 electrode was tested by using GCD technology at a current density of 10 A g−1. Fig. 4I shows the retention of specific capacitance with cycle number. The specific discharge capacitance has a slight fluctuation in long time cyclic process due to the effects of heteroatoms doping or in situ activation of the electrode.64 BSPC-800 exhibits a good rate capability of 99.5% capacitance retention at a current density of 10 A g−1 over 5000 cycles, indicates an amazing cycle performance.
Table 3 compared the specific capacitance, specific capacitance retention and cyclic stability of various porous carbon materials derived from different plant. The porous carbon material synthesized from brussel sprouts shows an excellent performance in comparison to many reported porous carbon derived from watermelon, bagasse, water hyacinth, water bamboo, etc. The superior electrochemical performance of BSPC-800, such as high specific capacitance retention and excellent cyclic stability, can be attributed to high specific area, proper pore size distribution, and a small amount of heteroatoms doping.
| Materials | Electrolyte | Maximum capacitance (F g−1) | Rate capability (%) | Cycling stability (%) |
|---|---|---|---|---|
| Brussel sprouts | 6 M KOH | 255 (0.5 A g−1) | 83 (up to 50 A g−1) | 99.5 (5000 cycles) |
| Melaleuca barks65 | 1 M H2SO4 | 233 (2 mV s−1) | ∼80 (up to 500 mV s−1) | 98 (3000 cycles) |
| Broad beans66 | 1 M H2SO4 | 202 (0.5 A g−1) | 64 (up to 10 A g−1) | 90 (3000 cycles) |
| Watermelon67 | 6 M KOH | 337 (0.5 A g−1) | 66 (up to 6 A g−1) | 96 (1000 cycles) |
| Bagasse68 | 6 M KOH | 268 (2 mV s−1) | 75 (up to 200 mV s−1) | — |
| Shiitake69 | 6 M KOH | 300 (1 A g−1) | 77 (up to 30 A g−1) | — |
| Water hyacinth70 | 6 M KOH | 273 (1 A g−1) | 77 (up to 50 A g−1) | 99 (5000 cycles) |
| Loofah sponge71 | 6 M KOH | 304 (1 A g−1) | 60.2 (up to 50 A g−1) | 98 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles) 000 cycles) |
| Lignin72 | 1 M H2SO4 | 165 (0.05 A g−1) | 74 (up to 10 A g−1) | 97 (5000 cycles) |
| Biochar73 | 6 M KOH | 260 (0.6 A g−1) | 88 (up to 1 A g−1) | 99 (2000 cycles) |
| Liquefied wood74 | 1 M H2SO4 | 280 (0.5 A g−1) | 81.8 (up to 10 A g−1) | 99.3 (2000 cycles) |
| Water bamboo75 | 6 M KOH | 268 (0.5 A g−1) | 83 (up to 10 A g−1) | 97 (5000 cycles) |
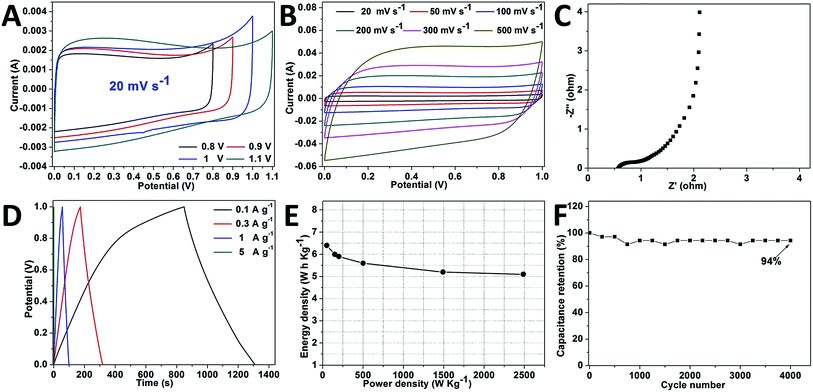
A symmetric two-electrode cell was assembled to further investigate the electrochemical performance of BSPC-800 in 6 M KOH. When the potential widow increases from 0.8 V up to 1.0 V, the CV curves exhibit a rectangular shape of a capacitive behavior (Fig. 5A). As shown in Fig. 5B, the CV curves of BSPC-800 exhibit a relatively symmetrical rectangular shape even at a scan rate as high as 500 mV s−1, indicating a super stable capacitor behavior of the mentioned electrode. Fig. 5C provides the Nyquist plot of BSPC-800 in the frequency range from 10−1 Hz to 106 Hz. As shown in Fig. 5C, the Nyquist plot consists of a semicircle at the mid frequency region and accompanied with a straight line, which indicates a low solution and charge transfer resistance of BSPC-800 based electrode. The GCD curves (Fig. 5D) maintain a symmetric triangle shape at different current densities with a small IR drop, revealing good capacitive characteristics. The Ragone plot of the BSPC-800//BSPC-800 symmetric supercapacitor shows that the energy density can reach up 6.4 W h kg−1 at a power density of 50 W kg−1 and 5 W h kg−1 at a power density of 2486 W kg−1 (Fig. 5E). The long-term cyclic stability of the symmetrical supercapacitor was evaluated from the galvanostatic charge/discharge at a current density of 10 A g−1. As shown in Fig. 5F, the symmetrical supercapacitor retains 94% of its initial capacitance after 4000 cycles, indicates an exhibits excellent stability of BSPC-800 based electrode.
Conclusion
In summary, nitrogen and sulfur self-doped porous carbon were synthesized via pre-carbonization and activation of brussel sprouts. The resulting carbon materials activated with KOH have a hierarchical porous (macro-, meso-, and micro-) structure and possess a high specific surface area of 2410 m2 g−1. In addition, these materials activated at 800 °C exhibit superior capacitive performance, including specific capacitance of 255 F g−1 at a current density of 0.5 A g−1, good rate capability (83% capacitance retention from 0.5 A g−1 to 50 A g−1) and negligible capacitance loss over 5000 cycles. This high stable supercapacitor performance can be attributed to micro/mesoporosity combined with high effective surface area and a small amount of heteroatoms (N, O and S) doping.Acknowledgements
We appreciate the financial support of the National Natural Science Foundation of China (No. 21471114), the State Major Research Plan (973) of China (No. 2011CB932404).References
- J. Li, G. Zan and Q. Wu, J. Mater. Chem. A, 2016, 4, 9097–9105 CAS.
- P. Hao, Z. Zhao, J. Tian, H. Li, Y. Sang, G. Yu, H. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120–12129 RSC.
- J. Xue, G. Zan and Q. Wu, Inorg. Chem. Front., 2016, 3, 354–364 RSC.
- Z. S. Wu, K. Parvez, X. Feng and K. Müllen, Nat. Commun., 2013, 4, 2487 Search PubMed.
- J. R. Miller and P. Simon, Sci. Mag., 2008, 321, 651–652 CAS.
- A. S. Arico, P. Bruce, B. Scrosati, J. Tarascon and W. Van Schalkwijk, Nat. Mater., 2005, 4, 366–377 CrossRef CAS PubMed.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- W. Qian, F. Sun, Y. Xu, L. Qiu, C. Liu, S. Wang and F. Yan, Energy Environ. Sci., 2014, 7, 379–386 CAS.
- M. Lu, F. Beguin and E. Frackowiak, Supercapacitors: materials, systems and applications, John Wiley & Sons, 2013 Search PubMed.
- D. Bhattacharjya and J. Yu, J. Power Sources, 2014, 262, 224–231 CrossRef CAS.
- C. Fu, A. Mahadevegowda and P. S. Grant, J. Mater. Chem. A, 2015, 3, 14245–14253 CAS.
- S. Pan, J. Deng, G. Guan, Y. Zhang, P. Chen, J. Ren and H. Peng, J. Mater. Chem. A, 2015, 3, 6286–6290 CAS.
- X. Zhou, A. Wang, Y. Pan, C. Yu, Y. Zou, Y. Zhou, Q. Chen and S. Wu, J. Mater. Chem. A, 2015, 3, 13011–13015 CAS.
- W. J. Lee, U. N. Maiti, J. M. Lee, J. Lim, T. H. Han and S. O. Kim, Chem. Commun., 2014, 50, 6818–6830 RSC.
- X. Zang, X. Li, M. Zhu, X. Li, Z. Zhen, Y. He, K. Wang, J. Wei, F. Kang and H. Zhu, Nanoscale, 2015, 7, 7318–7322 RSC.
- Y. Wang, J. Chen, J. Cao, Y. Liu, Y. Zhou, J. Ouyang and D. Jia, J. Power Sources, 2014, 271, 269–277 CrossRef CAS.
- T. Kim, G. Jung, S. Yoo, K. S. Suh and R. S. Ruoff, ACS Nano, 2013, 7, 6899–6905 CrossRef CAS PubMed.
- J. Chmiola, C. Largeot, P. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480–483 CrossRef CAS PubMed.
- K. Song, W. Song and L. Fan, J. Mater. Chem. A, 2015, 3, 16104–16111 CAS.
- Z. Li, W. Lv, C. Zhang, B. Li, F. Kang and Q. Yang, Carbon, 2015, 92, 11–14 CrossRef CAS.
- M. Biswal, A. Banerjee, M. Deo and S. Ogale, Energy Environ. Sci., 2013, 6, 1249–1259 CAS.
- J. Zhu, L. Yu and J. Zhao, J. Power Sources, 2014, 270, 411–417 CrossRef CAS.
- Q. Wang, J. Yan, Y. Xiao, T. Wei, Z. Fan, M. Zhang and X. Jing, Electrochim. Acta, 2013, 114, 165–172 CrossRef CAS.
- P. Nowicki, J. Kazmierczak, K. Sawicka and R. Pietrzak, Int. J. Environ. Sci. Technol., 2015, 12, 2233–2244 CrossRef CAS.
- M. Ulaganathan, A. Jain, V. Aravindan, S. Jayaraman, W. C. Ling, T. M. Lim, M. P. Srinivasan, Q. Yan and S. Madhavi, J. Power Sources, 2015, 274, 846–850 CrossRef CAS.
- L. Zhang, F. Zhang, X. Yang, K. Leng, Y. Huang and Y. Chen, Small, 2013, 9, 1342–1347 CrossRef CAS PubMed.
- M. Biswal, A. Banerjee, M. Deo and S. Ogale, Energy Environ. Sci., 2013, 6, 1249–1259 CAS.
- G. Zan and Q. Wu, Adv. Mater., 2016, 28, 2099–2147 CrossRef CAS PubMed.
- S. Song, F. Ma, G. Wu, D. Ma, W. Geng and J. Wan, J. Mater. Chem. A, 2015, 3, 18154–18162 CAS.
- X. Geng, L. Li, M. Zhang, B. An and X. Zhu, J. Environ. Sci., 2013, 25, S110–S117 CrossRef.
- M. Zhou, F. Pu, Z. Wang and S. Guan, Carbon, 2014, 68, 185–194 CrossRef CAS.
- X. Y. Chen, C. Chen, Z. J. Zhang, D. H. Xie and X. Deng, Ind. Eng. Chem. Res., 2013, 52, 10181–10188 CrossRef CAS.
- C. Wang, Y. Zhou, L. Sun, P. Wan, X. Zhang and J. Qiu, J. Power Sources, 2013, 239, 81–88 CrossRef CAS.
- D. Hulicova-Jurcakova, A. M. Puziy, O. I. Poddubnaya, F. Suárez-García, J. M. Tascón and G. Q. Lu, J. Am. Chem. Soc., 2009, 131, 5026–5027 CrossRef CAS PubMed.
- H. Guo and Q. Gao, J. Power Sources, 2009, 186, 551–556 CrossRef CAS.
- D. Zhang, L. Zheng, Y. Ma, L. Lei, Q. Li, Y. Li, H. Luo, H. Feng and Y. Hao, ACS Appl. Mater. Interfaces, 2014, 6, 2657–2665 CAS.
- M. Seredych and T. J. Bandosz, J. Mater. Chem. A, 2013, 1, 11717–11727 CAS.
- S. Yang, L. Zhi, K. Tang, X. Feng, J. Maier and K. Müllen, Adv. Funct. Mater., 2012, 22, 3634–3640 CrossRef CAS.
- G. Lota, K. Lota and E. Frackowiak, Electrochem. Commun., 2007, 9, 1828–1832 CrossRef CAS.
- B. Cao, B. Zhang, X. Jiang, Y. Zhang and C. Pan, J. Power Sources, 2011, 196, 7868–7873 CrossRef CAS.
- L. Chen, Z. Huang, H. Liang, W. Yao, Z. Yu and S. Yu, Energy Environ. Sci., 2013, 6, 3331–3338 CAS.
- USDA, National nutrient database for standard reference, release 28, US Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory, 2016, p. 9040 Search PubMed.
- Y. Su and A. Manthiram, Nat. Commun., 2012, 3, 1166 CrossRef PubMed.
- M. Liu, J. Qian, Y. Zhao, D. Zhu, L. Gan and L. Chen, J. Mater. Chem. A, 2015, 3(21), 11517–11526 CAS.
- X. Ma, L. Gan, M. Liu, P. K. Tripathi, Y. Zhao, Z. Xu, D. Zhu and L. Chen, J. Mater. Chem. A, 2014, 2, 8407–8415 CAS.
- P. K. Tripathi, M. Liu, Y. Zhao, X. Ma, L. Gan, O. Noonan and C. Yu, J. Mater. Chem. A, 2014, 2, 8534–8544 CAS.
- J. Huang, B. G. Sumpter and V. Meunier, Chem.–Eur. J., 2008, 14, 6614–6626 CrossRef CAS PubMed.
- J. Li, G. Zan and Q. Wu, New J. Chem., 2015, 39, 8165–8171 RSC.
- Y. Zhao, M. Liu, X. Deng, L. Miao, P. K. Tripathi, X. Ma, D. Zhu, Z. Xu, Z. Hao and L. Gan, Electrochim. Acta, 2015, 153, 448–455 CrossRef CAS.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87–92 CrossRef CAS PubMed.
- D. Wang, F. Li, J. Zhao, W. Ren, Z. Chen, J. Tan, Z. Wu, I. Gentle, G. Q. Lu and H. Cheng, ACS Nano, 2009, 3, 1745–1752 CrossRef CAS PubMed.
- H. Zhu, X. Wang, X. Liu and X. Yang, Adv. Mater., 2012, 24, 6524–6529 CrossRef CAS PubMed.
- J. P. Paraknowitsch, B. Wienert, Y. Zhang and A. Thomas, Chem.–Eur. J., 2012, 18, 15416–15423 CrossRef CAS PubMed.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- L. Hao, X. Li and L. Zhi, Adv. Mater., 2013, 25, 3899–3904 CrossRef CAS PubMed.
- J. P. Paraknowitsch, B. Wienert, Y. Zhang and A. Thomas, Chem.–Eur. J., 2012, 18, 15416–15423 CrossRef CAS PubMed.
- G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446–1450 CrossRef CAS PubMed.
- B. Sethuraman, K. K. Purushothaman and G. Muralidharan, RSC Adv., 2014, 4, 4631–4637 RSC.
- P. Guo, Y. Gu, Z. Lei, Y. Cui and X. S. Zhao, Microporous Mesoporous Mater., 2012, 156, 176–180 CrossRef CAS.
- K. Wang, Q. Meng, Y. Zhang, Z. Wei and M. Miao, Adv. Mater., 2013, 25, 1494–1498 CrossRef CAS PubMed.
- Y. Qu, G. Zan, J. Wang and Q. Wu, J. Mater. Chem. A, 2016, 4, 4296–4304 CAS.
- H. Zhu, X. Wang, X. Liu and X. Yang, Adv. Mater., 2012, 24, 6524–6529 CrossRef CAS PubMed.
- H. Sun, L. Cao and L. Lu, Energy Environ. Sci., 2012, 5, 6206–6213 CAS.
- Q. Luo, L. Huang, X. Gao, Y. Cheng, B. Yao, Z. Hu, J. Wan, X. Xiao and J. Zhou, Nanotechnology, 2015, 26, 304004 CrossRef PubMed.
- G. Xu, J. Han, B. Ding, P. Nie, J. Pan, H. Dou, H. Li and X. Zhang, Green Chem., 2015, 17, 1668–1674 RSC.
- X. Wu, T. Wen, H. Guo, S. Yang, X. Wang and A. Xu, ACS Nano, 2013, 7, 3589–3597 CrossRef CAS PubMed.
- P. Hao, Z. Zhao, J. Tian, H. Li, Y. Sang, G. Yu, H. Cai, H. Liu, C. P. Wong and A. Umar, Nanoscale, 2014, 6, 12120–12129 RSC.
- P. Cheng, S. Gao, P. Zang, X. Yang, Y. Bai, H. Xu, Z. Liu and Z. Lei, Carbon, 2015, 93, 315–324 CrossRef CAS.
- K. Wu, B. Gao, J. Su, X. Peng, X. Zhang, J. Fu, S. Peng and P. K. Chu, RSC Adv., 2016, 6, 29996–30003 RSC.
- Y. Luan, L. Wang, S. Guo, B. Jiang, D. Zhao, H. Yan, C. Tian and H. Fu, RSC Adv., 2015, 5, 42430–42437 RSC.
- W. Zhang, H. Lin, Z. Lin, J. Yin, H. Lu, D. Liu and M. Zhao, ChemSusChem, 2015, 8, 2114–2122 CrossRef CAS PubMed.
- H. Jin, X. Wang, Z. Gu and J. Polin, J. Power Sources, 2013, 236, 285–292 CrossRef CAS.
- Z. Jin, X. Yan, Y. Yu and G. Zhao, J. Mater. Chem. A, 2014, 2, 11706–11715 CAS.
- J. Li and Q. Wu, New J. Chem., 2015, 39, 3859–3864 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra08428a |
| This journal is © The Royal Society of Chemistry 2016 |