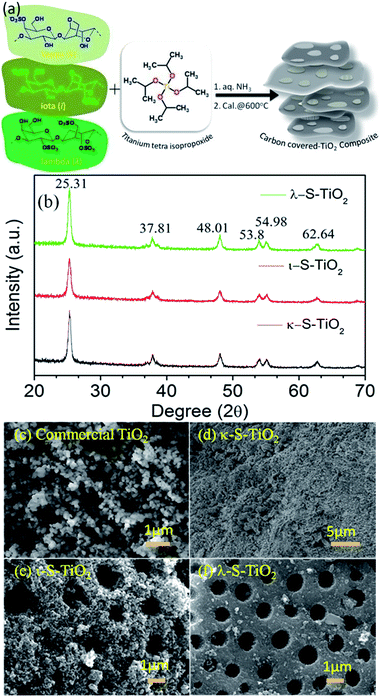
Fabrication of carbon and sulphur-doped nanocomposites with seaweed polymer carrageenan as an efficient catalyst for rapid degradation of dye pollutants using a solar concentrator†
Jai Prakash Chaudharyab,
Ashesh Mahtobc,
Nilesh Vadodariyaab,
Faisal Kholiyaa,
Subarna Maitia,
Sanna Kotrappanavar Nataraj*c and
Ramavatar Meena*ab
aNatural Products & Green Chemistry Division, Central Salt & Marine Chemicals Research Institute, G. B Marg, Bhavnagar-364002, Gujarat, India. E-mail: rmeena@csmcri.org; ramavatar73@yahoo.com; Tel: +91-278-2567760
bAcademy of Scientific and Innovative Research (AcSIR)-Central Salt & Marine Chemicals Research Institute, G. B Marg, Bhavnagar-364002, Gujarat, India
cCentre for Nano and Material Sciences, Jain University, JGI Global Campus, Bangalore-562 112, India. E-mail: sknata@gmail.com; sk.nataraj@jainuniversity.ac.in
First published on 15th June 2016
Abstract
Here we demonstrate direct use of sulphate rich seaweed polysaccharides, carrageenans, namely kappa (κ-), iota (ι-) and lambda (λ-) as source of sulphur and carbon doping in TiO2 photocatalysts. During TiO2 synthesis in the presence of sulphate rich carrageenan leaves, mainly residual carbon and sulphur doping resulted in a highly active photocatalyst. Evaluation of the dye degradation pattern shows rapid degradation of reactive black-5, methylene blue & methyl orange using modified TiO2 nanocomposites in different light sources. Robust dye degradation was achieved between 1 and 4 h under daylight whereas, the use of a solar concentrator reduced the degradation time of MB and RB-5 to <5 min and MO solution was turned colourless within 20 min. The present study elaborates the effect of seaweed carrageenans in inducing heteroatoms like sulphur and residual carbon for the photodegradation of industrially important dyes.
Introduction
At present biomass based materials are receiving great importance from both academic and industrial sectors due to their sustainability and green features. Among several biomaterial sources, marine chemicals and seaweeds possess versatile characteristics due to their inherent saline environmental origin and growth. Further, seaweed cultivation offers both social and economic benefits. Therefore, for commercial aspects seaweeds fulfill the prerequisite of being low cost and easy to use. Carrageenans are one such red seaweed polysaccharide family available in 3 different sulphated forms and have been used in number of applications from food ingredients to water treatment agents. Most importantly, the number of sulphate groups on kappa (κ-, one sulphate), iota (ι-, two sulphates) and lambda (λ-, three sulphates) forms of carrageenans can be utilized effectively for in situ or post-modification of industrially important products.1On the other hand, many researchers have explored the unique properties of several photocatalysts via non-metal doping. There have been several reports in the literature describing the properties and activities of several catalysts being influenced by non-metal dopants like carbon (C-) and sulphur (S-).2,3 It is more evident from the photocatalytic degradation of dyes using TiO2.4 Several combinations of metal and non-metal dopants have been successfully used for doping TiO2 targeting photodegradation applications. In several cases, a textile dye degradation study has been the common objective because textile industries consume large quantities of water and colouring agents in their manufacturing processes.5,6 Modern day textile industries are vibrant and serve as essential part of our day-to-day lives. Despite stringent environmental regulations, industries still lack comprehensive wastewater treatment methods. Annually >500 tons of non-degradable textile colour wastes are being disposed of in natural streams without adequate treatments.7
It is estimated nearly 60 to 70% of hydrolyzed dyes remain in wastewater at the end of the dying process. Therefore, textile wastewater generally characterized by high concentrations of redundant dyes. It is also important to note that modern dye or colour pigments are designed to sustain harsh washing conditions in the consumer products like textile fabrics.8 Among several textile dyes, reactive dyes are being used largely for dyeing cellulosic, wool and nylon fabrics. Hence, reactive dyes make up a large portion of the colouring agents used in the textile industry, some of them are toxic, even suspected to be carcinogenic in nature.9 Once the dye contaminated wastewater is disposed of in large bodies of water, the presence of high concentrations of dyes in wastewater causes a drastic rise in chemical oxygen demand and biological oxygen demand (COD and BOD).8 Ozone oxidation is one of the prominent methods used for breaking double bonded azo-dyes in treatment processes.10,11 Even though, conventionally, adsorption, oxidation, membrane filtration and multi-step biological treatments are being used for the treatment of coloured wastewater, most of these methods are relatively costly. Also, it is well known that methods like membrane treatment, adsorption or coagulation merely concentrate the contaminants instead of completely breaking down the dye molecule chemically to non-toxic constituents. Carrageenan based nanocomposite hydrogels as dye adsorbents have also been reported in literature.12–15 In contrast, catalytic dye degradation is one such method where toxic and hazardous dyes can be degraded to yield non-toxic products.
Titanium dioxide (TiO2) has been one such conventional photocatalyst widely used for photocatalytic degradation of dyes.16–21 TiO2 attracts enormous interest due to its tunable photocatalytic activity22 and it is capable of breaking down the organic species leaving behind colourless solutions.23 Accordingly, TiO2 instigates the degradation process under the influence of ‘light’, generally in the UV-Visible region of the electromagnetic spectrum without undergoing chemical changes during the catalytic process. Interestingly, non-metal dopants like carbon, nitrogen and sulphur have shown remarkable influence on enhancing the photocatalytic activity of TiO2.9,18,19,24–29 Sulfur doping induces strong Brønsted acidic sites on the surface of TiO2 exhibiting a high photocatalytic activity.19,27,28,30 Therefore, it is important to prepare novel nanostructured materials from sustainable resources which can directly induce heteroatom doping to perform efficient photocatalysis in daylight.
The present study utilizes sustainable material derived from seaweeds as a template in combination with a titanium precursor in a one step calcination process resulting in carbon and sulphur-doped TiO2 nanoparticles. Further, we discuss the rapid and efficient daylight photodegradation phenomenon of industrially important dyes like methyl orange, methylene blue and reactive black-5 using the modified photocatalyst.
Experimental section
Chemicals and materials
Titanium isopropoxide was supplied by Sigma Aldrich. Ammonia solution (25%) was purchased from SRL Pvt. Ltd, India. The commercial TiO2 (CASR no-13463-67-7) used in this study was purchased from LABORT, Surat, India. All chemicals were of analytical grade and were used as received. κ-Carrageenan (CAS no.: 11114-20-8), ι-carrageenan (CAS no.: 9062-07-1) and λ-carrageenan (CAS no.: 9064-57-7) were purchased from Tokyo Chemical Industry Co., Ltd, Japan, while reactive black-5, methylene blue & methyl orange (Fig. S1†) were purchased from Sigma Aldrich. These chemicals were used without further purification. Milli Q water was used throughout the process. Fig. S1† shows the molecular structure of the chemicals used in the present study.Properties of carrageenans
The carrageenans, a family of high molecular weight sulphated polysaccharides (average molecular weight ranging from 100 to 1000 kDa) are obtained from certain species of red seaweeds. They consist of galactose and anhydrogalactose units linked through glycosidic bonds. κ-Carrageenan is known to form strong gels in the presence of potassium ions and ι-carrageenan forms soft gels in the presence of calcium ions, while λ-carrageenan is non-gelling in nature.1 The moisture content 10–12%, viscosity 5–10 cPs and ash content 30–40% have been mentioned in the TCI product (carrageenans) catalogue. The carrageenans contain high levels of carbon and sulphur in their matrix along with metal ions including calcium and sodium.31Synthesis of TiO2 nanoparticles
1% w/v of carrageenan aqueous solution (1 g in 100 ml Milli-Q water) was prepared by autoclaving it at 120 °C for 15 min. To the above solution, 14% v/v of titanium isopropoxide (14 mL) was added with continuous stirring and the pH of the reaction mixture was raised to 10 by the drop wise addition of aqueous ammonia (25%) solution at room temperature. The obtained white coloured reaction mixture was placed at 60 °C for 24 h in a hot oven for aging.32 Then the TiO2–carrageenan blend was collected by centrifugation followed by Milli-Q water washing. The washed TiO2–carrageenan blend was dried in an oven at 80 °C overnight to remove moisture and then calcination of each sample was performed at 600 °C in a muffle furnace for 4 h. TiO2 nanocomposites thus prepared by using κ-carrageenan, ι-carrageenan, λ-carrageenan as a green template were designated as κ-S–TiO2, ι-S–TiO2, λ-S–TiO2 respectively. Further, in all photodegradation experiments, commercial TiO2 was used as a reference sample.Instruments and measurements
Fourier transform infra-red (FTIR) spectra of samples were recorded using a Perkin-Elmer Spectrum GX (FT-IR System, USA) instrument. The UV-Vis absorption spectra of standard and degraded dyes were recorded with a Varian CARY 500 UV-VIS-NIR Spectrophotometer. Nitrogen sorption isotherms were obtained using a Beckmann Coulter model SA3100 surface area analyzer at 77 K. Prior to the measurement, the samples were degassed at 393 K for 6 h. Thermogravimetric analysis (TGA) was carried out using a Mettler Toledo Thermal Analyzer, (TGA/SDTA 851e, Switzerland) with a heating rate of 5 °C min−1 under nitrogen atmosphere. The band-gap energy of the nanocrystalline TiO2 was determined using DRS (Shimadzu UV-3101PC) equipped with an integrating sphere; BaSO4 was used as a reference sample. The sulphate content was analysed by (ICP) with a Perkin-Elmer ICP-OES Optima 2000DV machine, and carbon was analysed using a Perkin-Elmer-2400, CHNS/O analyser.The morphology of the TiO2 nanocomposites was determined using a scanning electron microscope (Carl-Zeiss), model LEO 1430 VP, Germany, at an accelerating voltage of 20 kV. A requisite amount of sample was mounted on an aluminum stub and placed in the vacuum chamber. All images were recorded at the same magnification. Transmission electron microscope (TEM) images of the TiO2 nanocomposites were dispersed in acetone and images were recorded with a JEOL HR-TEM (JEOL JEM 2100, Japan) instrument operated under an accelerating voltage of 200 kV. Powder X-ray diffraction (XRD) measurements of the TiO2 samples were recorded using an X’pert MPD powder X-ray diffractometer using Cu-K (λ = 0.15406 nm) radiation operating at 40 kV and 30 mA. Data were collected in the range of 20 to 70° 2θ angle at a scan rate of 0.1° s−1. XPS analysis was carried out using Multi-technique X-ray Photoelectron Spectroscopy (XPS) with a monochromic AlKα-ray source.
Photocatalytic activities
The photocatalytic activities of seaweed carrageenan modified TiO2 were measured in three different experimental conditions by adding 30 mg of each TiO2 catalyst into 20 mL of each dye solution (25 ppm) in a glass vessel. (i) First, dye degradation studies were performed under a UV lamp with wavelength 254 nm and power 25 W cm−2 at room (30 °C) temperature, (ii) second, experiments were performed under maximum daylight intensity conditions (during 12–2 pm) to evaluate the feasibility of the photocatalysts, and (iii) third, the feasibility of these photocatalysts were further evaluated using a solar concentrator box under maximum daylight intensity. In all experiments 3 commercially important dye samples were exposed with the carrageenan modified photocatalyst and commercial TiO2 was used as the reference photocatalyst.Results and discussion
We used seaweed polysaccharides namely carrageenans as a template due to their high sulphate and high molecular weight with retainable carbon content. In addition, carrageenans contain traces of metal ions including sodium, potassium and calcium.31 To prepare carbon and sulphur rich TiO2, 3-different types of carrageenans namely, kappa, iota & lambda (κ-, ι-, and λ-) were used as initial template precursors as shown in Fig. 1(a). The final mass of the composite TiO2 product resulted under the influence of a hydrothermal calcination procedure in presence of ammonia and was characterized using wide angle X-ray diffraction (XRD). The powder XRD spectra of all the samples analysed are shown in Fig. 1(b), and were in close agreement with the JCPDS 21-1272 standard patterns.17,27 All the prepared TiO2 nanocomposites exhibited major peaks at 2θ angle values of 25.31°, 37.81°, 48.01°, 53.8°, 54.98° and 62.64° corresponding to the (101, d-spacing = 0.3512 nm), (004, d-spacing = 0.2375), (200, d-spacing = 0.1893), (105, d-spacing = 0.1695), (211, d-spacing = 0.1667), (204, d-spacing = 0.1480) of anatase crystal planes. This result revealed the formation of the anatase form of TiO2 in the presence of carrageenans as a template.Further, the morphologies of the TiO2 composites were recorded using a scanning electron microscope (SEM). SEM analysis exhibited a homogeneous mass of the non-metal atom doped TiO2 composite as shown in Fig. 1(c)–(f). However, there is no clear shaped morphology identified even at close magnifications.
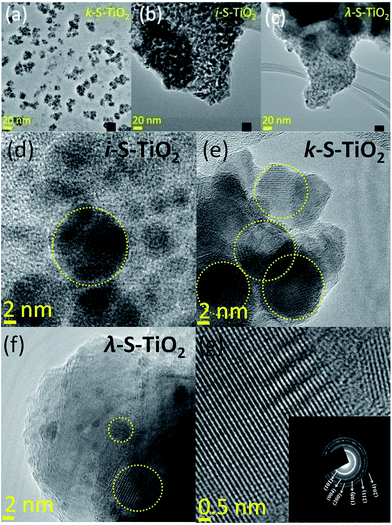
The transmission electron microscope (TEM) images in Fig. 2 give a close view of the composite. Fig. 2(a)–(c) shows no significant difference in the morphology which is almost spherical in shape, obtained with 3 different carrageenan sources. However, there is a notable difference in particle size for all 3 cases, ca. 20.10 nm ± 1.1 nm, ca. 15.43 nm ± 1.1 nm and ca. 5.93 nm ± 1.1 nm for the κ-, ι- and λ-carrageenan precursors, respectively (Fig. 2 & S2†). This finding indicates that different sizes of nanocomposites might be obtained by using carrageenans of different sulphate level. Further, the inset image of the selected area electron diffraction (SAED) pattern in Fig. 2(e) confirms the TiO2 nanoparticles as crystalline anatase phase, which further supports the XRD result.
The thermal stability of all three TiO2 nanocomposites was tested by employing thermogravimetric analysis (TGA) (Fig. S3†). The most notable feature is that almost similar trends in mass losses were obtained up to 800 °C for all the TiO2 nanocomposites prepared from different carrageenan templates. Further, the pattern of mass loss was almost comparable to C–TiO2 used as a reference sample in this study. In all cases the mass loss was obtained in the range of 2 to 4% up to 800 °C and may be attributed to traces of impurities in the form of moisture and some organic species.33 This result indicates that the thermal stability of the resulting TiO2 nanocomposites was comparable to the commercially available TiO2 used in this study as a reference sample. The negligible difference in thermal stability may be due to the availability of varying sulphur groups in the carrageenan templates, which can act as a bridge between the nano-TiO2 particles and the residual graphitic carbon.
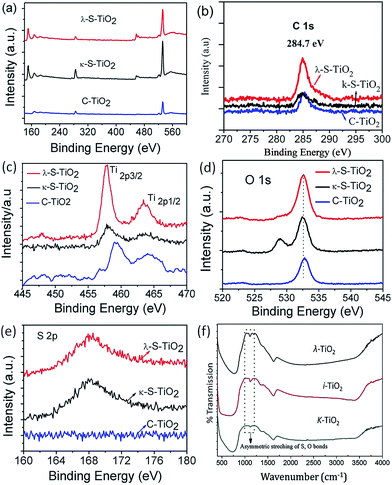
The X-ray photospectroscopic (XPS) analysis was performed for the characterization of the carbon and sulphur doped TiO2 nanocomposite. Fig. 3(a) gives the XPS spectra for κ-TiO2, λ-S–TiO2 and the control commercial (C–TiO2) sample. With a survey of these spectra which show intensity as a function of binding energy (BE), we can identify the presence of C1s, O1s, S2p and Ti2p3/2, Ti2p1/2 spin-orbital splitting photoelectrons by peaks corresponding to their binding energies of 286.0, 532.6, 168, 457.31 & 463.5 eV respectively. It can be seen that all the samples contain Ti, O, S and C except C–TiO2 which is devoid of sulphur. The appearance of a peak at 284.7 eV in Fig. 3(b) corresponds to the binding energy of C1s. The Ti2p spectra (Fig. 3(c)) exhibit two peaks corresponding to binding energies of Ti3+ 2p3/2 at 457.31 eV and Ti4+ 2p1/2 at 463.5 eV. For the S-doped TiO2 nanocomposite, the slight shift of the Ti2p3/2 peak with respect to that of Ti4+ in pure anatase TiO2 (458.5–459.7 eV) indicates the presence of Ti3+. The peak observed at 532.6 eV in Fig. 3(d) clearly indicates presence of O1s. Fig. 3(e) clearly shows the S2p level centered at 168 eV which corresponds to sulfur bonded to oxygen, confirming the presence of sulphate groups. It is also important to note that due to the absence of peaks in the region 160–163 eV, it can be inferred that no new Ti–S bond is forming at the expense of Ti–O bonds.34 Also, sulphur is present in the form of metal sulphate which is confirmed by the presence of a peak at 168 eV.
In Fig. 3(e) the S2p spectrum indicates that sulphur is present in different oxidation states as two isolated peaks at binding energies of 168 and 164.4 eV correspond to the chemical states of S4+ and S0. Higher calcination temperatures (600 °C) lead to the disappearance of the S0 peak (164.4 eV) and an intense peak at ∼168 eV is observed. This is an indication of the conversion of S0 to a higher oxidation state at elevated temperatures. These sulphate ions can also form O–S–O bonds on the TiO2 surface, creating an unbalanced charge on the Ti and vacancies/defects on the surface of the TiO2 nanoparticles. The C1s peak is centered at 287.4 eV and is graphitic in nature.35
The FTIR spectra of the nanocomposites (Fig. 3(f)) and TEM-EDX (Fig. S4†) further confirm the presence of the sulphur group in the carrageenan templated TiO2 composites. The sulphate presence is evident from the peak at 1125 cm−1 which is due to the asymmetric stretching frequency of S–O bonds in the SO42− groups while the presence of peaks below 1000 cm−1 is due to TiO2 crystal lattice vibrations.28 It must be noted that in the case of λ-S–TiO2, a peak at 1100 cm−1 can be observed which is due to the symmetric stretching of the S–O bonds. For ι-S–TiO2 and κ-S–TiO2 intensity of this peak is so low that it merges with the peak due to asymmetric stretching (1125 cm−1). This is probably due to the presence of more SO42− in λ-S–TiO2 as compared to the other 2 samples. Also, the intensity of the S–O bond asymmetric stretching band increases from κ-S–TiO2 to λ-S–TiO2 which may be due to the same reason. The presence of the broad adsorption bands that appear in the range 500–700 cm−1 are characteristic of a Ti–O–Ti symmetric stretching vibration mode.36 Therefore, the presence of a less electronegative S having high energy level molecular orbitals compared to O effectively reduces the band gap in the composite TiO2 compared to commercial TiO2. Further, ICP analysis confirms that λ-TiO2 contains highest sulphate content (0.649% in the form SO42−). Surface area and pore size measurements using N2 adsorption–desorption isotherms showed an increase in the trend from κ-TiO2 to λ-TiO2 as seen in Table 1.
| Sample | BET-SA (m2 g−1) | Langmuir SA (m2 g−1) | BJH-SA (cm3 g−1) | BJHD-SA (cm3 g−1) | Pore volume (cm3 g−1) | Pore size (nm) | % carbon by elemental analysis | % SO42− by ICP |
|---|---|---|---|---|---|---|---|---|
| κ-S–TiO2 | 25.72 | 39.07 | 24.83 | 31.07 | 0.141 | 21.93 | 0.10 | 0.144 |
| ι-S–TiO2 | 36.49 | 56.50 | 33.11 | 43.72 | 0.167 | 18.36 | 0.17 | 0.248 |
| λ-S–TiO2 | 40.35 | 50.63 | 44.74 | 46.41 | 0.184 | 28.55 | 0.25 | 0.649 |
| C–TiO2 | 5.26 | 7.21 | 6.16 | 7.48 | 0.02 | 15.61 | NA | NA |
Evaluation of dye degradation
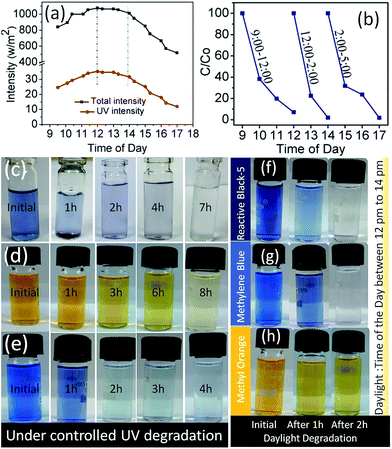
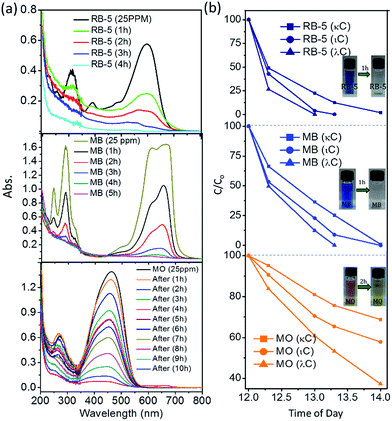
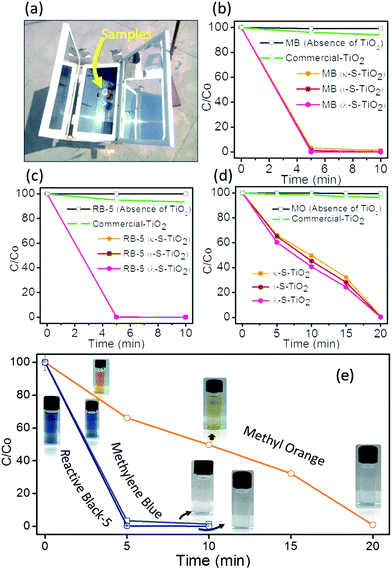
Before using direct sunlight or daylight for direct dye degradation, the UV intensity percentage in sunlight was measured. The intensity of sunlight and the UV percentage varies with place and the time of the day. To reduce the degradation time and the influence of intensity, maximum intensity timings were measured for both UV and total daylight as shown in Fig. 4(a). The intensity of the average daylight in the month of July was recorded in two fragments (i) total intensity and (ii) UV intensity fraction. It was measured that in a maximum (>1000 W m−2) total daylight intensity UV light intensity was ∼30 W m−2 (3% of total daylight). Firstly, the random dye degradation pattern for RB-5 was analysed by exposing the vial containing dye solution and photocatalyst (λ-S–TiO2) to direct daylight. At three different time periods of the day from 0900 hours to 1700 hours, the colour change of the solution in the vial was monitored at different intervals as shown in Fig. 4(b). Remarkably, >99% RB-5 degradation was achieved in <2 h between 1200 hours to 1400 hours. Further, controlled dye degradation experiments were carried out to infer the effect of UV radiation on RB-5, MB and MO. The 3 dyes were subjected to a degradation process using λ-TiO2 under controlled UV light exposure using a standard UV lamp of wavelength 254 nm and power 25 W cm−2 at room temperature. The photographs in Fig. 4(c)–(e) give the degradation pattern which lasted between 4 and 8 h under UV radiation. On the other hand, similar experiments were performed under direct daylight exposure with sample vials containing the above 3 dye solutions and the photocatalyst which resulted in rapid colour change. Photographs Fig. 4(f)–(h) showed TiO2 (κ-S–TiO2) activity at its peak between 1200 hours and 1400 hours and a drastic colour change was noticed for RB-5 and MB within ∼1 h. However, MO colour remains intense even after 2 h under daylight exposure. Even though MO retained some colour during the UV lamp experiment, substantial decolouring was noticed in vial. It is also important to note that among the three composites, κ-S–TiO2, ι-S–TiO2 & λ-S–TiO2 derived from different carrageenans (kappa, iota and lamda), λ-S–TiO2 exhibited the fastest dye degradation activity. A detailed study as shown in Fig. 5(a) and (b) reveal that λ-S–TiO2 activity was rapid and instant in comparison to κ-S–TiO2 and ι-S–TiO2 in degrading MB and RB-5 which took <1 h to get clear water.The rapid degradation achieved through carbon and sulphur doped TiO2 follows the trend; λ > ι > κ precursors. These trends may be due to number of sulphate groups inducing an increasing order of sulphur content in the final TiO2 composite from the parent κ-carrageenan to λ-carrageenan. To best of our knowledge, solar concentrators have been utilized for the first time to induce high intensity daylight, thereby accelerating the dye degradation process. A portable solar concentrator box with a flexible lenses panel was used to focus the sunrays to a sample chamber as shown in Fig. 6(a). In the set-up, the sample solution temperature variation due to sunray concentration was measured to be 55–60 °C for a period of 20 min, whereas the temperature outside the concentrator was recorded to be 42 ± 2 °C for the same time period. Therefore, the concentrator box attains an elevated ∼15 °C due to concentrated intensity of daylight. Once the solar concentrator box was optimized, dye samples with 3 different S–TiO2, commercial TiO2 and a control dye sample without TiO2 were placed inside the chamber.
Fig. 6(b)–(d) shows the dye degradation pattern for the first 20 min in the chamber. It is noted that in all cases, the control dye sample without any TiO2 and with commercial TiO2 showed no significant colour change. Whereas, MB and RB-5 become completely colourless in <5 min and MO took ∼20 min to undergo complete degradation. Reported literature on the synthesis of metal and non-metal doped TiO2 & their photocatalytic performance has been summarised in Table 2.
| Doped element | Dopant source | Light source | Dye used | Degradation time (min) | Reference |
|---|---|---|---|---|---|
| C | Activated carbon | UV | MO | 140 min | 2 |
| B, C, N, P, S, Fe, Zn, Zr, Sb, Ce | Boric acid, CTAB, NH3, H3PO4, ethylene glycol, citric acid, non metal salts | Sunlight | MB | >60 min | 18 |
| S | Sodium thiosulphate, sodium and dodecylbenzene sulphonate | Sunlight | MB | 330 min | 19 |
| Ce, N, S | Ammonium cerium sulphate, urea | Fluorescent lamp | MO | 90 min | 26 |
| C, S | Sulfur hydrosol, tetrabutyl orthotitanate, | Visible light | MB | 20 min | 28 |
| C, S | κ-, ι-, & λ-carrageenans | Solar concentrator | RB-5 | <5 min | Present study |
| MB | <5 min | ||||
| MO | <20 min |
Fig. 6(e) shows comparative analysis by using only λ-S–TiO2 as an efficient photocatalyst for the degradation of dyes in a solar concentrator. In the presence of commercial TiO2, MB, RB-5 and MO have shown a colour change (C/Co) < 5% in the solar concentrator box whereas all three dyes under the influence of κ-S–TiO2, ι-S–TiO2 and λ-S–TiO2 have undergone rapid and robust degradation to yield colourless solutions.
Recovery and reuse of photocatalysts
The stability, recovery and sustainability of any catalyst is very important to make the product & process economically feasible. The reusability of the TiO2 samples was tested by recovering samples from each dye solution after achieving complete degradation of the dye and colourless water (Fig. 7(a)). For recovery of the catalysts, samples were centrifuged and catalyst was separated as residue. Subsequently the recovered residue was washed with milli-Q water and acetone 3–4 times followed by oven drying to obtain the pure catalyst. The recovered catalyst was characterized for structure and integrity using FTIR and XRD prior to dye degradation experiments (Fig. 7(b)–(e)). The catalytic efficiency was tested up to 6 cycles and it was found that the degradation efficiency was maintained at >97% (Fig. 7(a)). This result makes the nanocomposite more cost-effective as well as eco-friendly for industrial applications.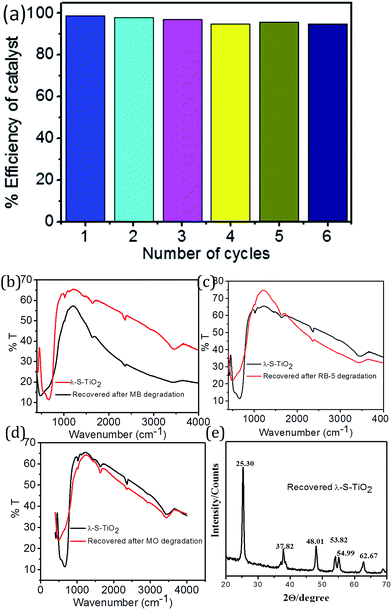 | ||
| Fig. 7 (a) Recyclability of photocatalyst for RB-5 dye using λ-S–TiO2, (b–e) catalyst recovery and characterization after each dye degradation. | ||
Degradation mechanism, effect of residual carbon and doped sulphur on catalytic activity
The degradation mechanisms of photocatalysis exhibited by pristine TiO2 (rutile or anatase) as well as transition metal or main group element doped TiO2 are well known and documented.23,37–39 Anatase TiO2 absorbs electromagnetic radiation in the near-UV region, which is about 3% of the total daylight (Fig. 4(a)). Due to this intrinsic constrain on TiO2 nanoparticles, direct utilization of solar energy is inefficient for photocatalysis. To broaden the photoresponsive region of TiO2 nanoparticles, non-metals are being used as dopants.Here, we have doped or induced graphitic carbon in TiO2 which was confirmed by XPS and SEM images. The TiO2 nanoparticles are embedded in these graphitic layers as can be seen in the HRTEM images (Fig. 2) which increases the available surface area for photocatalysis. This residual carbon can be thought of as a few layers of graphite which acts as a support and an interconnecting media for the TiO2 nanoparticles.40 There is a synergistic effect between the nanoparticles and carbon which results in the exfoliation of the graphitic sheets due to intercalation of the TiO2 nanoparticles and formation of a graphitic matrix which binds the TiO2 nanoparticles together.
The role sulphur plays here is less straightforward. It must be emphasised that the role of sulphur and carbon or for that matter any other non-metal dopant depends completely on whether the visible radiation photoresponse of doped titania outweighs a modest drop in the efficiency of the undoped catalyst using UV radiation. In 2001, Asahi and co-workers proposed delocalised mixing of the O 2p and N 2p orbitals resulting in the rise of the valence band level as compared to undoped TiO2.41 Current research supports an alternative explanation in which dopant atom orbitals generate a mid-gap level above the valence band.37,42–45 As the bulk valence band energy level rises, the oxidising power of the photochemically generated holes decreases which in turn limits the performance of the doped TiO2. In other words, there will be certain reactions in which only the pristine TiO2 photocatalyst could perform.
We propose a mechanism for dye degradation in typical anatase TiO2, in which the O2p and Ti3d orbitals contribute to a valence band (VB) and a conduction band (CB), respectively. In pristine TiO2 daylight absorption has a negligible effect on the photocatalytic activity (Fig. 6(b)–(d)). On the other hand, sulphur doped TiO2 results in indirect moderation of the band gap by forming S-centers from which electrons may fall. This will lower the oxidizing power of the holes; relative to undoped TiO2 assuming charge migration is faster compared to reaction with the dye molecule. However, the consequences of this theory are still a matter of discussion.40 From the DRS study it was found that the band gap energy is lowest for λ-S–TiO2 and highest for κ-S–TiO2 (Fig. S5†). The presence of graphitic carbon induces a larger surface area which enhances the adsorption and light absorption capacity of the S–TiO2 nanocomposites. Continued exposure to daylight effectively separates holes with the strong oxidation power in VB and electrons in CB. Therefore, MB and RB-5 get completely reduced to colourless solutions in ∼5 min though, MO underwent slow degradation reaching a completely colourless state at around ∼20 min.
Conclusion
In summary, seaweed heteroatom doped TiO2 nanoparticles were successfully designed to perform photodegradation on frequently used dyes. The residual carbon and S-doping from the seaweed carrageenan precursor in the TiO2 composite meant that the composite exhibited a larger surface area, which helped in robust dye degradation in <20 min in daylight. The λ-S–TiO2 has a precursor of lambda carrageenan with 3-suphlate groups and from the parent compound showed relatively higher surface area and faster photodegradation activity in daylight and with a solar concentrator. The enhanced daylight activity of λ-S–TiO2 could be explained on the basis of the large surface area induced by the graphite like carbon residue, higher sulphur content and good anatase crystallinity. Therefore, the present study illustrates the effective use of low-cost, sustainable marine resources as doping agents in addressing environmental issues related to coloured wastewaters.Acknowledgements
SKN and AM gratefully acknowledge DST, Government of India for the DST-INSPIRE Fellowship and Research Grant (IFA12-CH-84). RM and JPC are thankful to DST, New Delhi, Government of India (SB/EMEQ-052/2013) and CSIR, New Delhi, India (CSC0130) for financial support.Notes and references
- Li. Li, R. Ni, Y. Shao and S. Mao, Carrageenan and its applications in drug delivery, Carbohydr. Polym., 2014, 103, 1–11 CrossRef CAS PubMed.
- Y. Li, X. Li, J. Li and J. Yin, Photocatalytic degradation of methyl orange by TiO2-coated activated carbon and kinetic study, Water Res., 2006, 40, 1119–1126 CrossRef CAS PubMed.
- Z. Yang, Z. Yao, G. Li, G. Fang, H. Nie, Z. Liu, X. Zhou, X. Chen and S. Huang, Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction, ACS Nano, 2012, 6, 205–211 CrossRef CAS PubMed.
- K. Vinodgopal, D. E. Wynkoop and P. V. Kamat, Environmental Photochemistry on Semiconductor Surfaces:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Photosensitized Degradation of a Textile Azo Dye, Acid Orange 7, on TiO2 Particles Using Visible Light, Environ. Sci. Technol., 1996, 30, 1660–1666 CrossRef CAS.
Photosensitized Degradation of a Textile Azo Dye, Acid Orange 7, on TiO2 Particles Using Visible Light, Environ. Sci. Technol., 1996, 30, 1660–1666 CrossRef CAS. - B. Neppolian, C. Heechul, S. Sakthivel, B. Arabindoo and V. Murugesan, Solar/UV-induced photocatalytic degradation of three commercial textile dyes, J. Hazard. Mater., 2002, 89, 303–317 CrossRef CAS PubMed.
- I. K. Konstantinou and T. A. Albanis, TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations: A review, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS.
- A. Ajmal, I. Majeed, R. N. Malik, H. Idriss and M. A. Nadeem, Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview, RSC Adv., 2014, 4, 37003–37026 RSC.
- N. Barka, A. Assabbane, A. Nounah and Y. Ait-Ichou, Photocatalytic degradation of indigo carmine in aqueous solution by TiO2-coated non-woven fibres, J. Hazard. Mater., 2008, 152, 1054–1059 CrossRef CAS PubMed.
- Y. D. Shi, Q. Guo and Y. S. Xie, The preparation of C, N, S tri-doped TiO2 and visible-light photo-degradation of methylene blue and dyes, Adv. Mater. Res., 2011, 287–290, 1735–1743 CrossRef CAS.
- H.-Y. Shu and C.-R. Huang, Degradation of commercial azo dyes in water using ozonation and UV enhanced ozonation process, Chemosphere, 1995, 31, 3813–3825 CrossRef CAS.
- A. Gutowska, J. Kałużna-Czaplińska and W. K. Jóźwiak, Degradation mechanism of Reactive Orange 113 dye by H2O2/Fe2+ and ozone in aqueous solution, Dyes Pigm., 2007, 74, 41–46 CrossRef CAS.
- G. R. Mahdavinia and A. Asgari, Synthesis of kappa-carrageenan-g-poly(acrylamide)/sepiolite nanocomposite hydrogels and adsorption of cationic dye, Polym. Bull., 2013, 70, 2451–2470 CrossRef CAS.
- G. R. Mahdavinia, S. Iravani, S. Zoroufi and H. Hosseinzadeh, Magnetic and K+-cross-linked kappa-carrageenan nanocomposite beads and adsorption of crystal violet, Iran. Polym. J., 2014, 23, 335–344 CrossRef CAS.
- G. R. Mahdavinia, F. Bazmizeynabad and B. Seyyedi, kappa-Carrageenan beads as new adsorbent to remove crystal violet dye from water: adsorption kinetics and isotherm, Desalin. Water Treat., 2015, 53, 2529–2539 CrossRef CAS.
- G. R. Mahdavinia, A. Massoudi, A. Baghban and E. Shokri, Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels, J. Environ. Chem. Eng., 2014, 2(3), 1578–1587 CrossRef CAS.
- Y. Liu, J. Goebl and Y. Yin, Templated synthesis of nanostructured materials, Chem. Soc. Rev., 2013, 42, 2610–2653 RSC.
- K. Pathakoti, S. Morrow, C. Han, M. Pelaez, X. He, D. D. Dionysiou and H.-M. Hwang, Photoinactivation of Escherichia coli by Sulfur-Doped and Nitrogen–Fluorine-Codoped TiO2 Nanoparticles under Solar Simulated Light and Visible Light Irradiation, Environ. Sci. Technol., 2013, 47, 9988–9996 CrossRef CAS PubMed.
- R. R. Bhosale, R. S. Pujari, G. G. Muley, S. S. Patil, M. F. Shaikh and A. B. Gambhire, Solar photocatalytic degradation of methylene blue using doped TiO2 nanoparticles, Sol. Energy, 2014, 103, 473–479 CrossRef CAS.
- R. Ghosh Chaudhuri and S. Paria, Visible light induced photocatalytic activity of sulfur doped hollow TiO2 nanoparticles, synthesized via a novel route, Dalton Trans., 2014, 43, 5526–5534 RSC.
- J. Yan and F. Zhou, TiO2 nanotubes: Structure optimization for solar cells, J. Mater. Chem., 2011, 21, 9406–9418 RSC.
- S. Dutta, A. K. Patra, S. De, A. Bhaumik and B. Saha, Self-Assembled TiO2 Nanospheres By Using a Biopolymer as a Template and Its Optoelectronic Application, ACS Appl. Mater. Interfaces, 2012, 4, 1560–1564 Search PubMed.
- U. G. Akpan and B. H. Hameed, Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review, J. Hazard. Mater., 2009, 170, 520–529 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates, Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- F. Li, X. L. Yin, M. M. Yao and J. Li, Investigation on F–B–S tri-doped nano-TiO2 films for the photocatalytic degradation of organic dyes, J. Nanopart. Res., 2011, 13, 4839–4846 CrossRef CAS.
- P. Wang, P.-S. Yap and T.-T. Lim, C–N–S tridoped TiO2 for photocatalytic degradation of tetracycline under visible-light irradiation, Appl. Catal., A, 2011, 399, 252–261 CrossRef CAS.
- A. Charanpahari, S. S. Umare and R. Sasikala, Effect of Ce, N and S multi-doping on the photocatalytic activity of TiO2, Appl. Surf. Sci., 2013, 282, 408–414 CrossRef CAS.
- M. A. Abdullah, M. S. Al-Amoudic, A. B. Saleh, A. A. Abdulmajid, A. A. Khalid and A. Sambandam, Enhanced visible light photodegradation of water pollutants over N-, S-doped titanium dioxide and n-titanium dioxide in the presence of inorganic anions, J. Saudi Chem. Soc., 2014, 18, 155–163 CrossRef.
- P. Xu, T. Xu, J. Lu, S. Gao, N. S. Hosmane, B. Huang, Y. Dai and Y. Wang, Visible-light-driven photocatalytic S- and C-codoped meso/nanoporous TiO2, Energy Environ. Sci., 2010, 3, 1128–1134 Search PubMed.
- X. E. Peng, M.-M. Yao, F. Li and X.-H. Sun, Microstructures and Photocatalytic Properties of S Doped Nanocrystalline TiO2 Films, Part. Sci. Technol., 2012, 30, 81–91 CrossRef CAS.
- Z. H. Dong, Y. Li Wei, W. Shi and G. A. Zhang, Characterisation of doped polypyrrole/manganese oxide nanocomposite for supercapacitor electrodes, Mater. Chem. Phys., 2011, 131, 529–534 CrossRef CAS.
- J. Eskil Trudso, Kappa carrageenan, WO 2009002727 A1, 2008.
- A. K. Patra, S. K. Das and A. Bhaumik, Self-assembled mesoporous TiO2 spherical nanoparticles by a new templating pathway and its enhanced photoconductivity in the presence of an organic dye, J. Mater. Chem., 2011, 21, 3925–3930 RSC.
- M. Hema, A. Y. Arasi, P. Tamilselvi and R. Anbarasan, Titania Nanoparticles Synthesized by Sol–Gel Technique, Chem. Sci. Trans., 2013, 2, 239–245 CrossRef.
- S. U. Khan, M. Al-Shahry and W. B. Ingler Jr, Efficient photochemical water splitting by a chemically modified n-TiO2, Science, 2002, 297, 2243 CrossRef CAS PubMed.
- Y. Xie and M. A. S. Peter, Ultrahigh Purity Graphite Electrode by Core Level and Valence Band XPS, Surf. Sci. Spectra, 1992, 1, 367–372 CrossRef CAS.
- J. Rubio, J. L. Oteo, M. Villegas and P. Duran, Characterization and Sintering Behaviour of Submicrometre Titanium Dioxide Spherical Particles Obtained by GasPhase Hydrolysis of Titanium Tetrabutoxide, J. Chin. Chem. Soc., 1997, 32, 643–652 CAS.
- E. M. Rockafellow, L. K. Stewart and W. S. Jenks, Is sulfur-doped TiO2 an effective visible light photocatalyst for remediation?, Appl. Catal., B, 2009, 91, 554–562 CrossRef CAS.
- C. Chen, X. Li, W. Ma and J. Jhao, Effect of Transition Metal Ions on the TiO2-Assisted Photodegradation of Dyes under Visible Irradiation:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A Probe for the Interfacial Electron Transfer Process and Reaction Mechanism, J. Phys. Chem. B, 2002, 106, 318–324 CrossRef CAS.
A Probe for the Interfacial Electron Transfer Process and Reaction Mechanism, J. Phys. Chem. B, 2002, 106, 318–324 CrossRef CAS. - X. Chen and S. S. Mao, Titanium Dioxide Nanomaterials:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Synthesis, Properties, Modifications, and Applications, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed.
Synthesis, Properties, Modifications, and Applications, Chem. Rev., 2007, 107, 2891 CrossRef CAS PubMed. - Q. Xiang, J. Yu and M. Jaroniec, Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles, J. Am. Chem. Soc., 2012, 134, 6575–6578 CrossRef CAS PubMed.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides, Science, 2001, 293, 269–271 CrossRef CAS PubMed.
- H. Liu, A. Imanishi and Y. Nakato, Mechanisms for Photooxidation Reactions of Water and Organic Compounds on Carbon-Doped Titanium Dioxide, as Studied by Photocurrent Measurements, J. Phys. Chem. C, 2007, 111, 8603–8610 CrossRef CAS.
- H. Irie, Y. Watanabe and K. Hashimoto, Nitrogen-Concentration Dependence on Photocatalytic Activity of TiO2–xNx Powders, J. Phys. Chem. B, 2003, 107, 5483–5486 CrossRef CAS.
- S. Sakthivel, M. Janczarek and H. Kisch, Visible Light Activity and Photoelectrochemical Properties of Nitrogen-Doped TiO2, J. Phys. Chem. B, 2004, 108, 19384–19387 CrossRef CAS.
- D. V. Cristiana, P. Gianfranco and S. Annabella, Theory of Carbon Doping of Titanium Dioxide, Chem. Mater., 2005, 17, 6656–6665 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10317k |
| This journal is © The Royal Society of Chemistry 2016 |