Monovalent cation selective crown ether containing poly(arylene ether ketone)/SPEEK blend membranes†
Sinem Tasa,
Bram Zoetebierb,
Mark A. Hempeniusb,
G. Julius Vancsob and
Kitty Nijmeijer*a
aMembrane Science & Technology, Mesa+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands. E-mail: d.c.nijmeijer@tue.nl
bDepartment of Material Science and Technology of Polymers, Mesa+ Institute for Nanotechnology, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands
First published on 27th May 2016
Abstract
Blend membranes of sulfonated poly(ether ether ketone) (SPEEK) and poly(arylene ether ketone) (PAEK) derivatives containing crown ether units in the main chain (CPAEK) were prepared and characterized in terms of water swelling and ion exchange capacity (IEC). The miscibility of the polymers was verified by DSC and HR-SEM. Ion transport characteristics of the membranes were established for the monovalent ions Li+ and K+ and the separation of these ions by the cation exchange membranes was investigated. Diffusion experiments for aqueous KCl, LiCl and their mixtures were carried out with pure SPEEK membranes as well as with the CPAEK/SPEEK membranes. Blending significantly decreased the ion permeability due to cation-crown ether complexation and increased the hydrophobicity of the matrix. The K+ over Li+ selectivity of the SPEEK membrane was enhanced by blending SPEEK with CPAEK by a factor of nearly 4, indicating that the presence of a crown ether polymer changes the relative transport of the ions in the membrane.
Introduction
Cation exchange membranes (CEMs) have been widely used in electrodialysis,1,2 fuel cells3,4 and diffusion dialysis.5,6 CEMs are made from ionic polymers that have negatively charged moieties bound covalently to the polymer backbone. This makes CEMs selectively permeable to positively charged species, yet almost impermeable to anions. Therefore oppositely charged ions can be separated with such membranes. However, the major current challenge in ion exchange membrane development is how to improve the selective transport of a specific ion with a very high degree of specificity.To date, most of the research on cation exchange membranes has focused on monovalent/divalent ion selectivity improvements (e.g. separation of Na+/Mg2+). CEMs with a thin layer of a conducting polymer, such as polypyrrole or polyaniline, were developed for that purpose. The presence of a conducting polymer on the surface of cation exchange membranes decreased the permeation of divalent ions, therefore an improved monovalent over divalent ion selectivity was observed.7,8 In recent studies, layer-by-layer deposition of polyelectrolytes onto CEMs was employed to enhance monovalent over divalent ion selectivity.9–11
Crown ethers, widely used as host molecules, are able to selectively bind specific cationic species due to the ion–dipole interaction of the positively charged metal ion with the negatively polarized oxygen atoms.12 The host–guest interactions between crown ethers and various guests (e.g. cations) generated interest in the development of various well-defined, crown ether containing polymers. For example, Tunca et al. and Alexandratos et al. have reviewed crown ether polymer synthesis and the functionalization of pre-formed polymers with crown ether moieties.13,14 These authors showed that the choice of the polymer matrix for crown ether incorporation greatly influences the complexation ability of crown ethers; for example, it is important to use a hydrophilic polymer matrix to achieve a high degree of ion complexation.14 Van de Water et al. synthesized a polar azathiacrown ether-functionalized poly(glycidyl methacrylate) resin for membrane applications. This hydrophilic resin exhibited selectivity towards Ag+ ions in the presence of Cu2+, Zn2+ and Cd2+ ions.15
Owing to their high ion selectivity, liquid membranes are an interesting alternative to conventional membranes.16 Selective receptor molecules for cationic as well as anionic, organic, or inorganic species are used as carriers in liquid membranes for selective ion transport.17 Carrier-facilitated ionic permeability can be induced by crown ethers as well as by crown ether-containing polymers.18,19 Despite the high selectivity of liquid membranes, their applications are still restricted due to their limited stability.20
Various researchers have prepared self-standing membranes with crown ether polymers.21–24 For instance, to a polymer consisting of alternating co-monomers of ethylene and maleic anhydride, crown ethers were attached as side groups. Simultaneous to the attachment of the crown ethers, carboxylic acid groups were formed which enable active ion transport.24 Another example for self-standing membranes with crown ether polymers consists of a polymer that contains pendant crown ether moieties, synthesized by the reaction of poly(vinyl alcohol) (PVA) with formyl derivatives of crown ethers. This polymer was capable of forming mechanically strong films which had different permeabilities for metal picrates compared with PVA films.22 In addition, Nafion membranes impregnated with crown ethers25–27 were also investigated, but a major drawback of crown ether impregnation for these membranes was that the crown ethers leached out under the applied electric potentials.25
Sulfonated poly(ether ether ketone) (SPEEK) has been used as cation exchange membrane material owing to its excellent chemical and mechanical stability.28–30 Unfortunately, SPEEK membranes lack specific ion selectivity and the need for specificity has driven efforts aimed at their modification.31,32 Polymer blending is a versatile and cost-effective method for creating new functional materials,33 but obtaining homogeneous blends is limited by the degree of miscibility of the polymers.32,34–36 Although the use of SPEEK membranes for monovalent–divalent ion separation has been reported,31,37 there are only few reports in the literature on the use of ion exchange membranes for the separation of two monovalent ionic species.25,26 We synthesized, for the first time, novel hydrolytically stable main-chain crown ether containing poly(arylene ether ketone) (CPAEK) high performance polymers. Since cation exchange membranes require the presence of negatively charged groups, several options exist for introducing such species in the polymer matrix. Sulfonate groups can be introduced by copolymerizing sulfonate-bearing monomers with crown ether-functional monomers.23 Blending CPAEK with SPEEK, a hydrophilic sulfonated polymer, constitutes an alternative and thus far unexplored method for creating ion selective, crown ether bearing membranes. This approach allows one to readily tune the degree of sulfonation of the overall blend. In the present study, we demonstrate the successful blend formation of CPAEK polymers with SPEEK to prepare hydrophilic membranes with tailorable monovalent ion transport properties. To this end, membranes with different blending ratios of PAEK/SPEEK and CPAEK/SPEEK were prepared. The miscibility of these polymers was investigated by DSC and HR-SEM. Moreover, single and mixed ion transport properties of the SPEEK and CPAEK/SPEEK membranes for K+ and Li+ ions were assessed by diffusion dialysis.
Experimental
Materials
For poly(arylene ether ketone) (PAEK) and crown ether poly(arylene ether ketone) (CPAEK) synthesis, 4,4′-difluorobenzophenone (DFBP, 99%), bisphenol A (BPA, ≥99%), 1-methyl-2-pyrrolidinone (NMP, anhydrous, 99.5%), and K2CO3 (99.9%) were obtained from Sigma-Aldrich and used as received. Sulfonated poly(ether ether ketone) (SPEEK) with a sulfonation degree of 40% (SD 40) was obtained from FumaTech GmbH. For the diffusion experiments, LiCl (99%) and KCl (99%) were obtained from Sigma-Aldrich and used without further purification. 4,4′(5′)-Di(hydroxybenzo)-18-crown-6 was prepared as described in previous work.23Synthesis of PAEK and CPAEK
PAEK, and CPAEK containing 25 mol% of dibenzo-18-crown-6 units in the main chain were synthesized according to literature23 and details of gel permeation chromatography measurements and molar mass characteristics of the polymers are summarized in the ESI (Table S1†).Blend membrane preparation
The blend membranes were defined as CPAEK/SPEEK (x/y) and PAEK/SPEEK (x/y), where x and y represent the weight percentages of the different polymers. A 20 wt% polymer solution was prepared with the desired ratio of CPAEK and SPEEK (SD 40) or PAEK and SPEEK (SD 40) in NMP. The viscous solution was cast onto a glass plate with a 0.5 mm casting knife. After casting, the solvent was evaporated under a nitrogen atmosphere for 5 days at room temperature, followed by 5 days at 60 °C under a nitrogen atmosphere and 2 days at 110 °C under vacuum (9 mbar).The membranes were peeled off the glass plate after immersion into water. Prior to diffusion experiments they were converted into their sulfonic acid (H+) form with 1.0 M HCl; the excess HCl was removed by washing in Milli-Q water.
The SPEEK, CPAEK/SPEEK (40/60), CPAEK/SPEEK (60/40) and PAEK/SPEEK (40/60) membranes have thicknesses of 50 ± 5 μm, 45 ± 3 μm, 38 ± 5 μm and 40 ± 5 μm, respectively.
Blend membrane characterization
The membranes obtained were characterized by DSC, TGA and HR-SEM. The miscibility of the polymers in the blend was verified by DSC and HR-SEM. The membranes obtained were also characterized in terms of water swelling and ion exchange capacity (IEC). Thermogravimetric analysis (TGA) measurements were carried out with a Perkin Elmer TGA 4000 instrument. Samples of 10 mg were heated under a nitrogen atmosphere at 20 °C min−1 over a 50–900 °C temperature range. Differential scanning calorimetry (DSC) measurements were performed on a Perkin Elmer DSC 8000 (Perkin-Elmer, Waltham, MA, USA). Specimens with a mass of 10 mg were cooled and heated at a rate of 20 °C min−1 from 30 °C to 220 °C under a nitrogen atmosphere. The Tg of the membranes was determined from the second heating cycle at 20 °C min−1.The microstructure of the membranes was analyzed with high-resolution scanning electron microscopy (HR-SEM) (Zeiss Merlin, GeminiSEM, Oberkochen, Germany). Sample cross sections were prepared by freezing the films in liquid nitrogen and subsequently breaking them. After drying under vacuum, the samples were coated with gold.
Swelling measurements were performed by immersing the prepared membranes (2 cm × 2 cm) in Milli-Q water for 24 hours to measure the wet weight of the membranes. The wet membranes were subsequently dried at 60 °C for 12 hours. Membrane swelling was calculated using the following equation. Four samples were measured for each membrane and the average results were reported.
 | (1) |
A titration method was used to determine the membrane ion exchange capacity (IEC).38 First, the membranes were converted into the sulfonic acid (H+) form by stirring the membrane in a 1.0 M HCl solution for at least 15 hours. Subsequently the membranes were converted back into the sodium form by immersing them in a 2.0 M NaCl solution for 3 hours. The released amount of H+ in the solution was then determined by titration with 0.1 M NaOH. The IEC (mmol gdry membrane−1) values were calculated with the following equation:
 | (2) |
Diffusion dialysis
Diffusion experiments were performed with a glass diffusion cell (70 mL) in which the two compartments were separated by the membrane under investigation, with an effective area of 12 cm2. For the single-ion diffusion experiments (Fig. 1a), the receiving chamber contained an aqueous 10 mM HCl solution, while the feed chamber contained aqueous solutions of 10 mM KCl or LiCl. For the mixed-ion diffusion experiments (Fig. 1b), an aqueous solution of 10 mM KCl and 10 mM LiCl was added to the feed chamber, while the receiving chamber contained an aqueous solution of 20 mM HCl. | ||
| Fig. 1 Schematic illustration of the glass diffusion cell for (a) single-ion and (b) mixed-ion diffusion experiments. | ||
The two compartments were stirred vigorously throughout the experiments. Every 15 min, a 0.1 mL sample was taken and analyzed. The potassium and lithium ion concentrations were measured with a BWB-XP flame photometer (BWB Technologies, Newbury, UK).
The specific ion flux through the membrane was calculated from the concentration change of the specific ion in the receiving compartment, as follows:
 | (3) |
 | (4) |
Results and discussion
Polymer blend and membrane characterization
PAEK and CPAEK were synthesized according to a literature procedure.23 The molecular structures of the polymers are shown in Fig. 2. Polymer blend membranes of PAEK/SPEEK and CPAEK/SPEEK were prepared by solution-casting. | ||
| Fig. 2 Molecular structure of (a) PAEK, (b) CPAEK, and (c) SPEEK polymers used for the preparation of the CPAEK/SPEEK and PAEK/SPEEK blend membranes. | ||
The thermal characteristics of the membranes were determined with DSC and TGA under a nitrogen atmosphere. A detailed understanding of the thermal properties is needed to be able to correlate these properties with the miscibility of the polymers. Thermodynamically, miscible polymer blends can be achieved when the free energy of mixing has a negative value, a condition induced by specific interactions such as hydrogen bonding, dipole–dipole interactions or acid–base interactions between the polymers in the mixture.39–41 DSC is often employed to study the miscibility of the polymers of interest by analyzing the glass transition temperature (Tg) of the blend.40 A single Tg, intermediate to the Tg values of the individual components, is often taken as an evidence of polymer/polymer miscibility.41 However, the glass transition temperature is not an ideal indicator for the thermodynamic miscibility of the polymers. Tg is rather a measure of the state of dispersion.42
Moreover, a single Tg indicates that the domain size of the homogeneous polymer/polymer dispersion is at least smaller than the proposed resolution limit of DSC for domains.39,42,43 Generally, a minimum detectable domain size for DSC is in the range of 10–50 nm.40,43,44 Partially miscible blends exhibit two Tg values, however in such cases, Tg values differ slightly from those of the pure polymers.43,45 In case of completely immiscible blends, two Tg values can be observed and they are similar to those of the individual components.43
Table 1 lists the Tg values of the starting materials and the blend membranes prepared in this study (Fig. S1, ESI†). SPEEK exhibits a glass transition temperature around 205 °C, which is in good agreement with literature values.46 The pure CPAEK and PAEK polymers show a glass transition temperature of 144 °C and 162 °C, respectively. The CPAEK/SPEEK (40/60) blend exhibits two Tg values. The lower Tg is similar to that of CPAEK. The higher Tg is decreased by 26 °C compared with the Tg of pure SPEEK. For CPAEK/SPEEK (60/40), also two Tg values are observed, although the depression of the higher Tg in this case is about 20 °C. The shifts in glass transition temperature provide indication for the partial miscibility of CPAEK and SPEEK. In the CPAEK/SPEEK blend systems, the first Tg indicates the presence of a CPAEK phase. The shifts of the second, higher glass transitions towards lower temperatures indicate partial miscibility of CPAEK in the SPEEK rich phase.47 The PAEK/SPEEK blend exhibits immiscible characteristics. In this case, the blend clearly demonstrates the two Tg values that correspond to the respective pure components.
| Tg,1 (°C) | Tg,2 (°C) | |
|---|---|---|
| PAEK | 162 | — |
| CPAEK | 144 | — |
| SPEEK | — | 205 |
| CPAEK/SPEEK (40/60) | 145 | 179 |
| CPAEK/SPEEK (60/40) | 144 | 185 |
| PAEK/SPEEK (40/60) | 159 | 202 |
Fig. 3a shows thermogravimetric curves for SPEEK, CPAEK and PAEK. The first thermal decomposition of SPEEK around 290 °C is associated with the degradation of sulfonic acid groups and the second decomposition stage at 527 °C is related to thermal degradation of aromatic moieties.48,49 PAEK exhibits a one-stage degradation with a maximum weight loss around 550 °C indicating the degradation of aromatics.49,50 CPAEK is more sensitive to thermal degradation as compared with PAEK and shows two main degradation steps. The first weight loss step around 400–450 °C is due to the loss of crown ether units from the polymer.23,51 This is followed by decomposition of thermally stable aromatic moieties.49,50
Fig. 3b shows the thermal decomposition behavior of the blend membranes. A three-stage thermal degradation profile was observed for the CPAEK/SPEEK (40/60) membrane. In contrast to the SPEEK membranes, desulfonation started at a slightly higher temperature (310 °C) and occurred over a more narrow temperature range. This was probably caused by an interaction between the crown ether units of the CPAEK and the sulfonic acid groups of SPEEK. When the CPAEK weight ratio in the blend was increased, the CPAEK/SPEEK (60/40) membrane showed no weight loss around 310 °C. Instead, the thermal degradation started at 454 °C, which is possibly associated with the loss of sulfonic acid and crown ether groups. The PAEK/SPEEK (40/60) membrane exhibited a two stage thermal degradation profile. The first weight loss around 300 °C is ascribed to the loss of sulfonic acid groups and continues with decomposition of the thermally stable aromatic groups.
The microstructure of the blend membranes was investigated using HR-SEM. Photographs of sample cross sections are displayed in Fig. 4. In Fig. 4a, images of a PAEK/SPEEK (40/60) blend are shown at different magnifications, revealing the presence of micrometer-size, droplet-shaped PAEK domains (dotted line), surrounded by SPEEK. For comparison, the morphology of pure SPEEK is displayed in Fig. 4b. Clearly, the PAEK/SPEEK (40/60) membrane exhibited macroscopic phase separation. PAEK and SPEEK apparently lack the specific interactions necessary to form miscible blends of these polymers.33,52
In contrast, CPAEK/SPEEK (40/60) and (60/40) membranes did not exhibit any signs of macroscopic phase separation. HR-SEM images of these membranes showed smooth morphologies where micrometer-size, droplet-like features were completely absent (Fig. 4c and d).
These results are in agreement with the DSC observations and indicate that favorable intermolecular interactions exist between CPAEK and SPEEK. The SPEEK polymer in the CPAEK/SPEEK membranes was in the sulfonic acid (H+) form. In an aqueous environment, protonation of water results in formation of hydronium ions (H3O+) which can form proton bridges with the six oxygen atoms present in the 18-crown-6 cavity, creating a stable complex.53,54 In our case, the ionic groups of SPEEK and the dibenzo-18-crown-6 moieties of CPAEK can interact with H3O+ simultaneously, which may favor miscibility of the two polymers (Fig. 5).
In CPAEK/SPEEK systems, the crown ether and the sulfonic acid groups together can accommodate H3O+ ions or metal cations. Such ion–dipole interactions are known to play a role in forming compatible blends.55 Ion–dipole interactions have also been reported for poly(ethylene oxide)/polystyrene sulfonate (PEO/PSS) blends in the presence of a lithium salt.36 PEO is able to bind alkali metals just as crown ethers do; the lithium ion is situated in the electron-rich environment of the ethers' oxygen atoms of the PEO chain and also interacts with the ionic groups of PSS.36 Another study showed that disulfonated poly(arylene ether sulfone) and poly(ethylene glycol) (PEG) form compatible blends due to strong ion–dipole interactions between potassium ions located between the sulfonate groups of poly(arylene ether sulfone) and PEG.56,57
Water swelling, ion exchange capacity and ion transport properties of the blend membranes
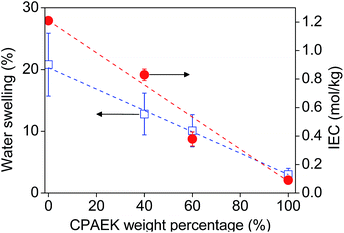
Fig. 6 shows the water swelling, indicated by blue squares, and the ion exchange capacity (IEC), represented by red dots, of the SPEEK and CPAEK/SPEEK membranes as a function of the CPAEK mass ratio. In agreement with the literature, the SPEEK membranes exhibit 20 wt% swelling in water.3,49 CPAEK is a hydrophobic polymer and the CPAEK film shows only 3 wt% swelling in water. Despite the large measurement error, the SPEEK membranes exhibit higher water uptake than the blend membranes. Blending SPEEK with CPAEK increases the hydrophobicity of the membranes and the water content of the membranes linearly decreases with increasing CPAEK mass percentage.The IEC of the blend membranes decreases with increasing CPAEK content. As the ionic groups (sulfonate groups) become increasingly surrounded by hydrophobic domains, relatively less sulfonate groups are available for ion exchange.32
The Li+ and K+ transport properties of the SPEEK and CPAEK/SPEEK blend membranes were evaluated with a Donnan dialysis setup, with the membrane under investigation positioned between the feed compartment and the receiving compartment. The ion concentration difference between the feed compartment and the receiving compartment causes a transport of potassium (or lithium) ions from the feed side to the receiving side. Table 2 shows the Li+ and K+ fluxes at the initial stage of the operation for the single-ion and mixed-ion diffusion experiments.
| Membrane | JLi+,single 15 min (mol (cm−2 s−1)) 1010 | JK+,single 15 min (mol (cm−2 s−1)) 1010 | JLi+,mixed 15 min (mol (cm−2 s−1)) 1010 | JK+,mixed 15 min (mol (cm−2 s−1)) 1010 |
|---|---|---|---|---|
| SPEEK | 47 ± 5 | 113 ± 8 | 24 ± 4 | 65.8 ± 1.0 |
| CPAEK/SPEEK (40/60) | 7.8 ± 1 | 31 ± 13 | 2.7 ± 0.5 | 37.6 ± 0.1 |
| CPAEK/SPEEK (60/40) | 5.1 ± 0.4 | 7.1 ± 1 | ∼0 | 3.9 ± 0.1 |
The Li+ ion has a larger hydrated radius (0.34 nm) than the K+ ion (0.23 nm).58,59 The mobility of the Li+ ions in Nafion membranes is greater than that of the K+ ions.60,61 However, for single ion diffusion, SPEEK and blend membranes exhibit lower ion fluxes for Li+ ions than for K+ ions.
As a general trend, it is observed that blending CPAEK with SPEEK reduces the ion flux of both ions, compared with pure SPEEK membranes. This is partially caused by the dependence of the ion flux on the membrane water content, since ion mobility is closely related to the extent of water swelling of the matrix and diffusion rate of water molecules in the membrane.62 The water molecules facilitate the transport of cationic species (K+ and Li+) from one sulfonate group to the next.63 Increasing the CPAEK content in the membrane significantly reduces the water uptake of the membranes; therefore ion fluxes decrease.
Another reason for the reduced ion flux is the cation complexation by the crown ether moieties in the CPAEK polymer backbone. In previous research, we reported that the K+ flux for sulfonated poly(arylene ether ketone) (SPAEK) membranes is almost 4 times higher than for SPAEK membranes with crown ether moieties incorporated in the main chain.23 Kimura et al. obtained similar results for PVA-based poly(crown ether)s, which were ascribed to the strong interactions between crown ethers and cations.22 Bhattacharyya et al. investigated the mobility of alkali metal ions in dibenzo-18-crown-6-loaded Nafion-117 membranes. In these membranes, the mobility of Li+ and Cs+ ions was found to be extremely low relative to their mobility in pure Nafion-117 membranes.26 These results indicate that the cation complexing ability of crown ethers changes the mobility of these ions in the membranes.
Dibenzo-18-crown-6 can form complexes with K+, Li+ and Na+ ions, however the K+ ion complex with dibenzo-18-crown-6 is stronger than that with Li+ and Na+ ions.64,65 Therefore, increasing the CPAEK content influences the K+ ion flux more significantly than the Li+ ion flux. For the CPAEK/SPEEK (60/40) membrane, the K+ ion flux decreased 16 times compared to that of the pure SPEEK membranes, against only 9 times for Li+ ions. Therefore, single ion diffusion experiments confirm the potassium selective nature of the CPAEK polymer.
Ion fluxes in mixed-ion experiments with Li+ and K+ ions are lower than in the single-ion transport experiments. This may be related to a competition between Li+ and K+ ions for the available ion exchange sites (SO3− groups) in the membrane.60,66 Since ion fluxes are related to the gradient of fractional occupancy of the SO3− sites by a specific ion, one can speculate that in the competition between Li+ and K+ ions for the available ion exchange sites, the increased hydrophobicity of the membrane and the crown ether-ion complexation66 lead to lower ion fluxes.
Fig. 7 shows the K+/Li+ selectivity change in time for the single-ion and mixed-ion diffusion experiments. For single-ion diffusion, SPEEK and CPAEK/SPEEK membranes show almost similar selectivities. The SPEEK membrane exhibits the same K+/Li+ selectivity in the mixed-ion experiment as in the single-ion experiment.
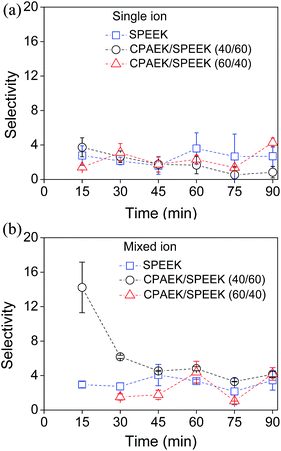 | ||
| Fig. 7 Membrane potassium over lithium ion selectivity change in time during (a) single-ion diffusion and (b) mixed-ion diffusion. | ||
In the mixed-ion experiment, the K+/Li+ selectivity of the CPAEK/SPEEK (40/60) membrane is 3.8 times greater than for SPEEK membranes in the initial stage of the diffusion. The CPAEK/SPEEK (60/40) membrane is only permeable to K+ ions during the first 15 minutes of the operation. As mentioned before, Li+ and K+ ions compete for the available SO3− sites in the SPEEK membrane.67 This applies to CPAEK/SPEEK membranes as well, but in this case, transport is also affected by complex formation between Li+ and K+ ions and dibenzo-18-crown-6. These interactions combined determine the selectivity of the membranes.
The blend membranes only show the higher selectivity in the initial stage of operation. This can be most probably explained by the decreasing availability of crown ether sites for complex formation with the ions during Donnan dialysis, diminishing the complexation rate due to the lack of available crown ether binding sites at longer operation times. The CPAEK/SPEEK membrane's selectivity decreases to values comparable to those of the pure SPEEK membrane from 45 minutes on. The results of the mixed-ion experiments suggest that not only the presence of crown ether moieties in the membrane matrix, but also the increased hydrophobicity of the membrane, influences the relative mobility of K+ and Li+ ions in the initial stage of the operation. Overall, the CPAEK/SPEEK blend membranes have demonstrated their utility in the separation of monovalent ions. Regeneration of these mechanically robust membranes by acid treatment allows one to restore their selectivity, which implies that their arrangement in series could lead to an effective, continuous ion separation process.
Conclusions
This study explored the selective permeability of crown-ether-containing PAEK blend membranes for monovalent ions (K+/Li+). CPAEK/SPEEK and PAEK/SPEEK membranes were prepared by solution blending the two polymers of interest in different weight ratios, and their morphology and thermal characteristics were investigated. Membranes made from PAEK and SPEEK exhibited macroscopic phase separation, as was established by DSC and HR-SEM. CPAEK and SPEEK, however, were found to be partially miscible. The IEC and water content of the membranes decreased with increasing CPAEK content in the membrane. Single and mixed ion transport characteristics of SPEEK and CPAEK/SPEEK membranes for K+ and Li+ ions were assessed by Donnan dialysis. The presence of the hydrophobic crown ether polymer in the matrix resulted in a lower ion transport for both single and mixed ion diffusion cases. However, in mixed-ion diffusion experiments, CPAEK/SPEEK membranes exhibited selectivity towards K+ ions over Li+ ions.Acknowledgements
Sinem Tas acknowledges the Industrial Partnership Program (IPP) “Spectroscopic analysis of particles in water” of the Stichting voor Fundamenteel Onderzoek der Materie (FOM), which is financially supported by the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO). This IPP is co-financed by Wetsus, Centre of Excellence for Sustainable Water Technology, as part of Wetsus' TTI program. Wetsus is co-funded by the Dutch ministry of economics, agriculture and innovation. Bram Zoetebier acknowledges NanoNextNL, a micro and nanotechnology consortium of the government of the Netherlands and 130 partners. We especially thank Mark Smithers for HR-SEM analysis.References
- P. Dlugolecki, B. Anet, S. J. Metz, K. Nijmeijer and M. Wessling, J. Membr. Sci., 2010, 346, 163–171 CrossRef CAS.
- J. J. Krol, M. Wessling and H. Strathmann, J. Membr. Sci., 1999, 162, 145–154 CrossRef CAS.
- M. Gil, X. L. Ji, X. F. Li, H. Na, J. E. Hampsey and Y. F. Lu, J. Membr. Sci., 2004, 234, 75–81 CrossRef CAS.
- C. Wang, D. W. Shin, S. Y. Lee, N. R. Kang, G. P. Robertson, Y. M. Lee and M. D. Guiver, J. Mater. Chem., 2012, 22, 25093–25101 RSC.
- H. Miyoshi, Chem. Eng. Sci., 1997, 52, 1087–1096 CrossRef CAS.
- C. Agarwal, A. Mhatre and A. Goswami, Ind. Eng. Chem. Res., 2015, 54, 3445–3450 CrossRef CAS.
- T. Sata, T. Funakoshi and K. Akai, Macromolecules, 1996, 29, 4029–4035 CrossRef CAS.
- T. Sata, Y. Ishii, K. Kawamura and K. Matsusaki, J. Electrochem. Soc., 1999, 146, 585–591 CrossRef CAS.
- N. White, M. Misovich, A. Yaroshchuk and M. L. Bruening, ACS Appl. Mater. Interfaces, 2015, 7, 6620–6628 CAS.
- S. Abdu, M. C. Marti-Caatayud, J. E. Wong, M. Garcia-Gabaldon and M. Wessling, ACS Appl. Mater. Interfaces, 2014, 6, 1843–1854 CAS.
- H. N. Deng, Z. X. Wang, W. Zhang, B. S. Hu and S. F. Zhang, J. Appl. Polym. Sci., 2015, 132, 41488–41495 Search PubMed.
- T. J. Marrone, D. S. Hartsough and K. M. Merz, J. Phys. Chem., 1994, 98, 1341–1343 CrossRef CAS.
- U. Tunca and Y. Yagci, Prog. Polym. Sci., 1994, 19, 233–286 CrossRef CAS.
- S. D. Alexandratos and C. L. Stine, React. Funct. Polym., 2004, 60, 3–16 CrossRef CAS.
- L. G. A. van de Water, F. ten Hoonte, W. L. Driessen, J. Reedijk and D. C. Sherrington, Inorg. Chim. Acta, 2000, 303, 77–85 CrossRef CAS.
- M. F. S. Roman, E. Bringas, R. Ibanez and I. Ortiz, J. Chem. Technol. Biotechnol., 2010, 85, 2–10 CrossRef.
- J. P. Behr, M. Kirch and J. M. Lehn, J. Am. Chem. Soc., 1985, 107, 241–246 CrossRef CAS.
- K. H. Wong, K. Yagi and J. Smid, J. Membr. Biol., 1974, 18, 379–397 CrossRef CAS PubMed.
- T. B. Stolwijk, E. J. R. Sudhölter and D. N. Reinhoudt, J. Am. Chem. Soc., 1987, 109, 7042–7047 CrossRef CAS.
- A. H. P. Skelland and X. Q. Meng, AIChE J., 1996, 42, 547–561 CrossRef CAS.
- K. L. Thunhorst, R. D. Noble and C. N. Bowman, J. Membr. Sci., 1999, 156, 293–302 CrossRef CAS.
- K. Kimura, M. Yoshinaga, S. Kitazawa and T. Shono, J. Polym. Sci., Part A: Polym. Chem., 1983, 21, 2777–2785 CrossRef CAS.
- B. Zoetebier, S. Tas, G. J. Vancso, K. Nijmeijer and M. A. Hempenius, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2786–2793 CrossRef CAS.
- K. Kimura, H. Sakamoto, M. Yoshinaga and T. Shono, J. Chem. Soc., Chem. Commun., 1983, 24, 978–979 RSC.
- S. Chaudhury, A. Bhattacharyya and A. Goswami, Ind. Eng. Chem. Res., 2014, 53, 8804–8809 CrossRef CAS.
- A. Bhattacharyya and A. Goswami, J. Phys. Chem. B, 2009, 113, 12958–12963 CrossRef CAS PubMed.
- T. Hayashita, J. C. Lee and R. A. Bartsch, J. Membr. Sci., 1996, 116, 243–251 CrossRef CAS.
- A. Reyna-Valencia, S. Kaliaguine and M. Bousmina, J. Appl. Polym. Sci., 2005, 98, 2380–2393 CrossRef CAS.
- H. S. Dang and D. Kim, J. Power Sources, 2013, 222, 103–111 CrossRef CAS.
- Y. Zhang, G. Zhang, Y. Wan, C. J. Zhao, K. Shao, H. T. Li, M. M. Han, J. Zhu, S. A. Xu, Z. G. Liu and H. Na, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5824–5832 CrossRef CAS.
- J. Balster, O. Krupenko, I. Punt, D. F. Stamatialis and M. Wessling, J. Membr. Sci., 2005, 263, 137–145 CrossRef CAS.
- F. G. Wilhelm, I. G. M. Punt, N. F. A. van der Vegt, H. Strathmann and M. Wessling, J. Membr. Sci., 2002, 199, 167–176 CrossRef CAS.
- J. Kerres, Blend concepts for fuel cell membranes, Springer, US, 2009, pp. 185–221 Search PubMed.
- F. Sabzi and A. Boushehri, J. Appl. Polym. Sci., 2006, 101, 492–498 CrossRef CAS.
- B. Nandan, L. D. Kandpal and G. N. Mathur, Polymer, 2003, 44, 1267–1279 CrossRef CAS.
- A. Eisenberg and M. Hara, Polym. Eng. Sci., 1984, 24, 1306–1311 CAS.
- G. S. Gohil, R. K. Nagarale, V. V. Binsu and V. K. Shahi, J. Colloid Interface Sci., 2006, 298, 845–853 CrossRef CAS PubMed.
- S. Heiner, Preparation and characterization of ion exchange membranes, Elsevier, 2004 Search PubMed.
- W. U. Gedde, Polymer Physics, Springer-Science+Business Media, B.V, 1999, pp. 70–73 Search PubMed.
- A. K. Kulshreshtha, Handbook of polymer blends and composites, Smither Rapra Technology, 2002, vol. 3, pp. 1–13 Search PubMed.
- X. Lu and R. A. Weiss, Macromolecules, 1992, 25, 3242–3246 CrossRef CAS.
- L. A. Utracki, Adv. Polym. Technol., 1985, 5, 33–39 CrossRef.
- A. Newman, D. Engers, S. Bates, I. Ivanisevic, R. C. Kelly and G. Zografi, J. Pharm. Sci., 2008, 97, 4840–4856 CrossRef CAS PubMed.
- B. Van Eerdenbrugh, M. Lo, K. Kjoller, C. Marcott and L. S. Taylor, Mol. Pharmaceutics, 2012, 9, 1459–1469 CAS.
- D. Bikiaris, J. Prinos, M. Botev, C. Betchev and C. Panayiotou, J. Appl. Polym. Sci., 2004, 93, 726–735 CrossRef CAS.
- S. M. J. Zaidi, S. D. Mikhailenko, G. P. Robertson, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2000, 173, 17–34 CrossRef CAS.
- N. Marin and B. D. Favis, Polymer, 2002, 43, 4723–4731 CrossRef CAS.
- W. L. Harrison, F. Wang, J. B. Mecham, V. A. Bhanu, M. Hill, Y. S. Kim and J. E. McGrath, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2264–2276 CrossRef CAS.
- F. Wang, M. Hickner, Y. S. Kim, T. A. Zawodzinski and J. E. McGrath, J. Membr. Sci., 2002, 197, 231–242 CrossRef CAS.
- G. Montaudo, C. Puglisi and F. Samperi, Macromol. Chem. Phys., 1994, 195, 1241–1256 CrossRef CAS.
- E. M. Maya, A. E. Lozano, J. G. de la Campa and J. de Abajo, Macromol. Rapid Commun., 2004, 25, 592–597 CrossRef CAS.
- W. Zhang, C. M. Tang and J. Kerres, Sep. Purif. Technol., 2001, 22–3, 209–221 CrossRef.
- M. Buhl, R. Ludwig, R. Schurhammer and G. Wipff, J. Phys. Chem. A, 2004, 108, 11463–11468 CrossRef.
- P. Hurtado, F. Gamez, S. Hamad, B. Martinez-Haya, J. D. Steill and J. Oomens, J. Phys. Chem. A, 2011, 115, 7275–7282 CrossRef CAS PubMed.
- T. Okada, J. Phys. Chem. B, 1998, 102, 3053–3059 CrossRef CAS.
- C. H. Lee, D. VanHouten, O. Lane, J. E. McGrath, J. B. Hou, L. A. Madsen, J. Spano, S. Wi, J. Cook, W. Xie, H. J. Oh, G. M. Geise and B. D. Freeman, Chem. Mater., 2011, 23, 1039–1049 CrossRef CAS.
- C. H. Lee, J. Spano, J. E. McGrath, J. Cook, B. D. Freeman and S. Wi, J. Phys. Chem. B, 2011, 115, 6876–6884 CrossRef CAS PubMed.
- Y. Marcus, Biophys. Chem., 1994, 51, 111–127 CrossRef CAS.
- M. A. Izquierdo-Gil, V. M. Barragan, J. P. G. Villaluenga and M. P. Godino, Chem. Eng. Sci., 2012, 72, 1–9 CrossRef CAS.
- A. Stenina, P. Sistat, A. I. Rebrov, G. Pourcelly and A. B. Yaroslavtsev, Desalination, 2004, 170, 49–57 CrossRef.
- T. Okada, H. Satou, M. Okuno and M. Yuasa, J. Phys. Chem. B, 2002, 106, 1267–1273 CrossRef CAS.
- H. L. Yeager and A. Steck, J. Electrochem. Soc., 1981, 128, 1880–1884 CrossRef CAS.
- M. Saito, N. Arimura, K. Hayamizu and T. Okada, J. Phys. Chem. B, 2004, 108, 16064–16070 CrossRef CAS.
- Y. Takeda, R. Kohno, Y. Kudo and N. Fukuda, Bull. Chem. Soc. Jpn., 1989, 62, 999–1003 CrossRef CAS.
- E. Shchori, N. Nae and J. Jagurgrodzinski, J. Chem. Soc., Dalton Trans., 1975, 56, 2381–2386 RSC.
- S. Sodaye, C. Agarwal and A. Goswami, J. Membr. Sci., 2008, 314, 221–225 CrossRef CAS.
- Y. F. Li, S. F. Wang, G. W. He, H. Wu, F. S. Pan and Z. Y. Jiang, Chem. Soc. Rev., 2015, 44, 103–118 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra11566g |
| This journal is © The Royal Society of Chemistry 2016 |