Discovery of lipoic acid-4-phenyl-1H-pyrazole hybrids as novel bifunctional ROCK inhibitors with antioxidant activity†
Ya-lin Tuabc,
Qiu-he Chenabc,
Sheng-nan Wangabc,
Asko Urid,
Xiao-hong Yangabc,
Jia-qi Chuabc,
Jing-kao Chenabc,
Bing-ling Luobe,
Xiao-hong Chenf,
Shi-jun Wen*be and
Rong-biao Pi*abcg
aSchool of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, China. E-mail: pirb@mail.sysu.edu.cn
bInternational Joint Laboratory (SYSU-PolyU HK) of Novel Anti-Dementia Drugs of Guangdong, Guangzhou 510006, China
cNational and Local United Engineering Lab of Druggability and New Drugs Evaluation, Sun Yat-sen University, Guangzhou 510006, China
dInstitute of Chemistry, University of Tartu, Ravila St., Tartu 50411, Estonia
eSun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng East Road, Guangzhou 510060, China
fDepartment of Neurology, The Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou, China
gGuangdong Province Key Laboratory of Brain Function and Disease, Zhongshan School of Medicine, Sun Yat-sen University, Guangzhou, 510080, China
First published on 13th June 2016
Abstract
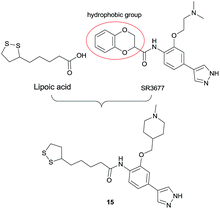
A series of lipoic acid (LA) and 4-phenyl-1H-pyrazole hybrids as bifunctional Rho-associated kinase (ROCK) inhibitors were designed, synthesized and evaluated. Compound 15 is identified to be a novel potent bifunctional ROCK inhibitor with antioxidant activity and neuroprotection.
Central nervous system (CNS) progressive neurodegenerative diseases, such as Alzheimer's disease (AD), Parkinson's disease (PD) and amyotrophic lateral sclerosis (ALS), are identified as multifactorial diseases, bringing not only physical and mental suffering to the patients, but also heavy financial burden to society and families.1 However, due to the particularity of the brain, e.g., the blood-brain-barrier (BBB), and especially the complex network of interconnected pathological processes, the discovery of therapeutic drugs to effectively fight against these diseases remains a longstanding challenge.
The pathological mechanisms of CNS diseases are complex, involving known and unknown signaling cascades, oxidative stress, protein misfolding and so on.2 One of the culprits, reactive oxygen species (ROS) is considered as a major determinant of the pathogenesis and progression of CNS diseases.3,4 Consequently, compounds with antioxidative activity have been proposed either for the prevention or the treatment of CNS diseases. Alpha lipoic acid (LA, Fig. 1) possesses vigoroso capability to scavenge free radicals and neuroprotection, which is beneficial for patients with CNS diseases.5–7 However, “one-drug one-target” strategy is not good to hit the multiple targets implicated in CNS complex diseases, combining LA with other agents or using LA as a pharmacophore in designing new multi-target ligands (MTLs) may be more effective and safety although more difficult to fulfill.8,9
Rho-associated kinases (ROCK1 and ROCK2), downstream effectors of the small GTPase Rho A, play remarkable roles in numerous cellular processes, such as cell motility, contraction, survival, differentiation, invasion and migration.10 Mounting evidences show that there is an abnormal activation of the Rho/ROCK pathway in several CNS diseases, and inhibiting the Rho/ROCK pathway is considered as a promising approach for the treatment of these diseases.11,12 For example, it is believed that ROCK inhibitors show significant beneficial effects on AD by reducing the production of amyloid-beta protein (Aβ), decreasing inflammation response, and regulating synaptic function and plasticity, etc.13,14
Given of the failures in fighting against CNS diseases in the past decades, now researchers realize that single-target drugs are limited in the treatment of complex diseases such as CNS diseases, which have multiple pathogenic mechanisms and are found to overlap and influence one another.15 Therefore, it is of high interest to seek molecules that are able to influence on two or more complementary targets involving complex diseases. To date, the MTLs strategy have brought us lights of success on the treatment of CNS diseases.15,16
SR3677 (Fig. 1) is one of potently selective ROCK2 inhibitors, and has been shown to dramatically reduce Aβ production as well as the levels in vitro and in vivo.17,18 SR3677 and its derivatives contain a hydrophobic moiety that interacts with the hydrophobic pocket under the P-loop of ROCK, contributing to their high potency.17 However, according to the reported results, we found the hydrophobic moiety permits more flexibility.19–23 Basing MTLs strategy, in this work, we combined LA as an antioxidative hydrophobic moiety with ROCK inhibition fragments in the hope of discovering novel bifunctional ROCK inhibitors, which exhibit a balanced bifunctional profile covering antioxidant and ROCK inhibition. Herein, the synthesis and biological evaluation of a series of designed novel hybrids for CNS diseases were described.
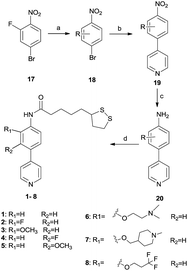
Compounds 1–8 were prepared as the Scheme 1. The side chain groups (R) was introduced by nucleophilic substitution following the reported procedure to provide the nitrobenzene derivatives 18 (ref. 17) (or obtained from commercial sources). Coupling of 18 with pyridine-4-boronic acid pinacol ester using Pd[P(Ph)3]4 as the catalyst in the presence of K2CO3 were applied to provide 19. The intermediates were then reduced with RANEY®-nickel and hydrazine to arylamine 20. 1–8 were obtained after an amide formation on the newly formed amino group with LA, in which HATU was used as the coupling reagent with the presence of DIEA in DMF.
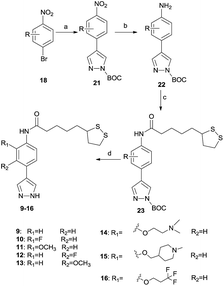
Similar to the synthetic process of 1–8, 9–16 were prepared as the Scheme 2. The intermediates contained side chain (18) prepared in the Scheme 1 were used directly or synthesized as mentioned. N-Boc-protected pyrazole-4-boronic acid pinacol ester was used as another material, and a Suzuki coupling reaction using PdCl2(dppf) as the catalyst in the presence of Cs2CO3 was likewise applied to provide 21. The intermediates were then reduced with Pd/H2 to arylamine 22. Amide reaction were taken place as mentioned above to give 23, and 9–16 were obtained after the N-Boc group of 23 were removed using TFA in DCM.
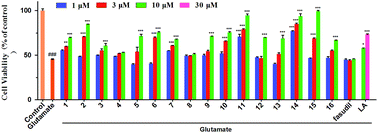
Glutamate (Glu) induced neurotoxicity in HT22 cells, is considered to be one of excellent models for studying the consequences of oxidative stress.24 And neuroprotective drugs are able to protect cells against Glu-induced damage.25 Therefore, firstly, we examined neuroprotective effects of compounds against Glu-induced cell death by MTT assay (Fig. 2).
Pretreatment with test compounds, except 4 and 8, showed better protection against Glu-induced HT22 cell death than that with LA, while fasudil, a clinical used ROCK inhibitor, did not exert protective effect under similar concentrations. Moreover, several compounds (2, 6, 7, 10, 11, 14 and 15) showed satisfactory effects even at low concentration, at which LA had no effects. Compounds 11, 14 and 15 could completely reverse Glu-induced cell death at 10 μM.
Obviously, as mentioned at the beginning, our goal is to discover bifunctional compounds that not only exhibit potent selective ROCK inhibition but also possess moderate antioxidative effects. So that, we detected the kinases inhibition of some of our compounds that have good to moderate antioxidative effect in vitro. As shown in Table 1, pyrazoles (1, 2, 3, 6 and 7) were more potent inhibitory against ROCK than pyridines (9, 10, 11, 14 and 15) and compounds that possess an alkaline side chain have better inhibitory against ROCK2. These results were consistent with the previous report.22 The IC50 of the most potent compound 15 for ROCK2 was 0.8 μM, which was close to the value of fasudil (0.46 μM in this assay), and had a better selectivity against PKA (∼453-fold) and PKG (∼30-fold).
| Compound | ROCK2 IC50 (μM) | PKA IC50 (μM) | PKG IC50 (μM) |
|---|---|---|---|
| 1 | 156 | >5000 | 76 |
| 2 | 70 | >5000 | 60 |
| 3 | 92 | >5000 | 105 |
| 6 | 49 | >5000 | 14 |
| 7 | 5 | 252 | 6 |
| 9 | 64 | >5000 | 81 |
| 10 | 46 | >5000 | 168 |
| 11 | 37 | >5000 | 147 |
| 14 | 10 | 256 | 33 |
| 15 | 0.8 | 363 | 24 |
| Fasudil | 0.46 | 5.3 | 2.5 |
There are sufficient evidences indicating that the beneficial effects of ROCK inhibitors for CNS diseases, especially AD, are mainly attributed to the ROCK2 inhibition while ROCK1 inhibition may be more closely associated with their side effects.18,26 Therefore, we determined the subtype selectivity of 15. The result (Fig. S1†) indicated that 15 displayed modulate ROCK2 selectivity against ROCK1 (IC50 = 2.110 μM to ROCK1, IC50 = 0.437 μM to ROCK2 in this assay, there were some subtle differences between two methods†).
In order to understand the interactions between 15 and ROCK2 in the active site in detail, a Molecular Operating Environment (MOE) program was used for molecular docking. Through the best scoring pose (Fig. 3 and S2†), we found that the interaction model between 15 and ROCK2 was very similar to the reported SR3677.17 The two H-bonds from pyrazole ring and two amino acid residues of ROCK2 are proposed necessary for the potency of 15. And the H-bond between the protonated tertiary amine of the 1-methyl-4-(oxymethyl)piperidine group of 15 and the Asp-176 carboxylate side chain is crucial to the selectivity of 15 against other kinases. Docking score of 7, the pyridine analogue of 15, is lower than 15, and there is only one hydrogen bond between 7 and ROCK2 on the bottom of the pocket (data not shown). This may explain the higher potency of pyrazoles than pyridines. The longish alkyl chain and the weaker hydrophobicity of LA-group, compared to aromatic ring or heterocyclic aromatics, may explain the reason for the milder activity of 15. However, dramatically, it accomplished the balance between the antioxidative effect and ROCK inhibition.
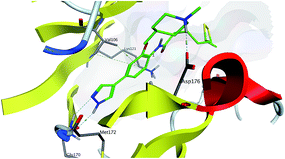 | ||
| Fig. 3 Docking pose of compound 15 (green) in the catalytic domain of ROCK2 (PDB ID: 2F2U). Interactions are displayed by light blue dotted lines. Key residues that could form interactions with 15 are shown. Direct hydrogen bond interactions are formed between the pyrazole ring of 15 and Glu 170, Met 172 on the bottom of the pocket as well as the protonated tertiary amine of the 1-methyl-4-(oxymethyl)piperidine group of 15 and the Asp-176 carboxylate side chain. | ||
Better BBB penetration capacity plays a crucial role in the effect of CNS drugs. To explore whether our compounds could penetrate into the brain, we used a parallel artificial membrane permeation assay for the BBB (PAMPA-BBB), similar to the procedure described by Di et al.27 The results (Tables S1 and S2†) showed that the selected compounds except 1 and 9 were able to cross the porcine brain lipid, which indicated these compounds could penetrate the BBB.
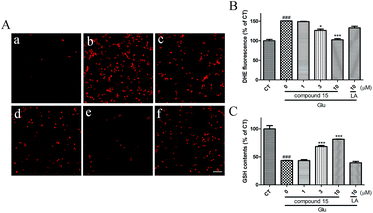
From the neuroprotective experiment, we were pleased to find that most of targeted compounds displayed a better neuroprotective capacity compared to LA. Furthermore, those compounds were predicted to be able to penetrate the BBB. Considering the inhibitory activity against ROCK, we selected compound 15 for further study. LA exhibits its antioxidant activity not only by directly scavenging free radicals, but also by regenerating other endogenic antioxidants, including glutathione (GSH).28 Thus, to examine the effects of 15 on ROS, we measured the free radical and the intracellular GSH levels by radical fluorescent probe dihydroethidium (DHE) and a GSH assay kit respectively. The results indicated that Glu caused an significant increase of ROS, and 15 potently scavenged free radical compared with LA (Fig. 4A and B). Furthermore, Glu dramatically decreased the intracellular level of GSH, while 15 remarkably reversed the process at 3 and 10 μM but LA did not show any effect even at 10 μM (Fig. 4C) and merely slight increase the level of GSH at 30 μM in our previous report.29
The significant blood pressure (BP) reduction associated with ROCK inhibitions is one of the main challenges in the development of ROCK inhibitors for systemic applications and it is speculated that the side effect of BP reduction is mainly associated with ROCK1 inhibition.26 However, on the flip side, increasing research results indicate that appropriate vasodilatation of brain is beneficial for some CNS diseases, e.g. AD.30,31 The IC50 value of 15 for ROCK1 was 2.11 μM, while for ROCK2 was 0.437 μM as mentioned above. It indicates that 15 may possess a moderate vasodilatation activity. To confirm the ROCK inhibit activity and the effects of compound 15 on blood vessels, we evaluated the vasorelaxant effect of 15 by a rat aorta assay. Similar to our previous report,29 pro-contracting of aorta was induced by KCl (60 mM) and fasudil was used as a positive control. As shown in Fig. 5, compared with the vehicle control group, compound 15 did not exert a significant relaxation at 10 μM, and only had a mild relaxation (23%) even at 30 μM compared to that of fasudil (53%).
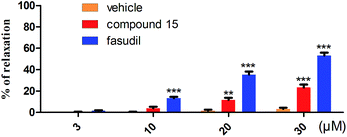 | ||
| Fig. 5 Effects of compound 15 on aortic rings contracted by KCl. ***P < 0.001, **P < 0.01 vs. vehicle control group (n = 6). | ||
In summary, we synthesized a series of LA based bifunctional compounds and evaluated their biological activity from antioxidant to ROCK inhibition. Compounds 7, 14 and 15 exhibited a balanced bifunctional profile covering antioxidant and ROCK inhibition with good BBB penetration property. Notably, compound 15 displayed a remarkable neuroprotective activity by prominently scavenging free radicals and increasing the level of intracellular GSH. Furthermore, 15 was identified as a potent ROCK2 inhibitor with good selectivity against ROCK1, so that exhibited milder vasorelaxant effect compared to fasudil. The satisfactory inchoate biological evaluation results strongly encourage further pharmacological studies and optimization of compound 15 in the development of bifunctional ROCK inhibitors for specified CNS disease. Such studies have been undertaken, and the progress will be reported in short future.
Acknowledgements
This work was supported by grants (to R. Pi) from Guangdong Provincial International Cooperation Project of Science & Technology (No. 2013B051000038), National Natural Scientific Foundation Committee (No. 31371070) and the Fundamental Research Funds for the Central Universities (No. 15ykjc08b).Notes and references
- X. Ning, Y. Guo, X. Wang, X. Ma, C. Tian, X. Shi, R. Zhu, C. Cheng, Y. Du, Z. Ma, Z. Zhang and J. Liu, J. Med. Chem., 2014, 57, 4302–4312 CrossRef PubMed.
- M. P. Mattson and T. Magnus, Nat. Rev. Neurosci., 2006, 7, 278–294 CrossRef CAS PubMed.
- P. J. Vernon and D. Tang, Antioxid. Redox Signaling, 2013, 18, 677–691 CrossRef CAS PubMed.
- M. Valko, D. Leibfritz, J. Moncol, M. T. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS PubMed.
- K. Hager, A. Marahrens, M. Kenklies, P. Riederer and G. Munch, Arch. Gerontol. Geriatr., 2001, 32, 275–282 CrossRef CAS PubMed.
- F. Di Domenico, E. Barone, M. Perluigi and D. A. Butterfield, Expert Rev. Neurother., 2015, 15, 19–40 CrossRef CAS PubMed.
- D. P. De Araujo, F. Lobato Rde, J. R. Cavalcanti, L. R. Sampaio, P. V. Araujo, M. C. Silva, K. R. Neves, M. M. Fonteles, F. C. Sousa and S. M. Vasconcelos, Int. J. Neurosci., 2011, 121, 51–57 CrossRef PubMed.
- O. Prezzavento, E. Arena, C. Parenti, L. Pasquinucci, G. Arico, G. M. Scoto, S. Grancara, A. Toninello and S. Ronsisvalle, J. Med. Chem., 2013, 56, 2447–2455 CrossRef PubMed.
- A. Di Stefano, P. Sozio, A. Cocco, A. Iannitelli, E. Santucci, M. Costa, L. Pecci, C. Nasuti, F. Cantalamessa and F. Pinnen, J. Med. Chem., 2006, 49, 1486–1493 CrossRef CAS PubMed.
- F. Amigoni, E. Legnaghi and P. Pevarello, Pharm. Pat. Anal., 2012, 1, 177–192 CrossRef CAS PubMed.
- B. K. Mueller, H. Mack and N. Teusch, Nat. Rev. Drug Discovery, 2005, 4, 387–398 CrossRef CAS PubMed.
- M. Chen, A. Liu, Y. Ouyang, Y. Huang, X. Chao and R. Pi, Expert Opin. Invest. Drugs, 2013, 22, 537–550 CrossRef CAS PubMed.
- N. Hensel, S. Rademacher and P. Claus, Front. Neurosci., 2015, 9, 198 Search PubMed.
- R. Lefort, Neurotherapeutics, 2015, 12, 19–28 CrossRef CAS PubMed.
- H. Zheng, M. Fridkin and M. Youdim, Pharmaceuticals, 2014, 7, 113–135 CrossRef PubMed.
- C. Berk, G. Paul and M. Sabbagh, Expert Opin. Invest. Drugs, 2014, 23, 837–846 CrossRef CAS PubMed.
- Y. Feng, Y. Yin, A. Weiser, E. Griffin, M. D. Cameron, L. Lin, C. Ruiz, S. C. Schurer, T. Inoue, P. V. Rao, T. Schroter and P. Lograsso, J. Med. Chem., 2008, 51, 6642–6645 CrossRef CAS PubMed.
- J. H. Herskowitz, Y. Feng, A. L. Mattheyses, C. M. Hales, L. A. Higginbotham, D. M. Duong, T. J. Montine, J. C. Troncoso, M. Thambisetty, N. T. Seyfried, A. I. Levey and J. J. Lah, J. Neurosci., 2013, 33, 19086–19098 CrossRef CAS PubMed.
- X. Fang, Y. Yin, Y. T. Chen, L. Yao, B. Wang, M. D. Cameron, L. Lin, S. Khan, C. Ruiz, T. Schroter, W. Grant, A. Weiser, J. Pocas, A. Pachori, S. Schurer, P. Lograsso and Y. Feng, J. Med. Chem., 2010, 53, 5727–5737 CrossRef CAS PubMed.
- R. Li, M. P. Martin, Y. Liu, B. Wang, R. A. Patel, J. Y. Zhu, N. Sun, R. Pireddu, N. J. Lawrence, J. Li, E. B. Haura, S. S. Sung, W. C. Guida, E. Schonbrunn and S. M. Sebti, J. Med. Chem., 2012, 55, 2474–2478 CrossRef CAS PubMed.
- Y. T. Chen, T. D. Bannister, A. Weiser, E. Griffin, L. Lin, C. Ruiz, M. D. Cameron, S. Schurer, D. Duckett, T. Schroter, P. LoGrasso and Y. Feng, Bioorg. Med. Chem. Lett., 2008, 18, 6406–6409 CrossRef CAS PubMed.
- Y. Yin, M. D. Cameron, L. Lin, S. Khan, T. Schroter, W. Grant, J. Pocas, Y. T. Chen, S. Schurer, A. Pachori, P. LoGrasso and Y. Feng, ACS Med. Chem. Lett., 2010, 1, 175–179 CrossRef CAS PubMed.
- Y. Feng, P. V. LoGrasso, O. Defert and R. Li, J. Med. Chem., 2016, 59, 2269–2300 CrossRef CAS PubMed.
- A. Pfeiffer, M. Jaeckel, J. Lewerenz, R. Noack, A. Pouya, T. Schacht, C. Hoffmann, J. Winter, S. Schweiger, M. K. Schafer and A. Methner, Br. J. Pharmacol., 2014, 171, 2147–2158 CrossRef CAS PubMed.
- M. Koufaki, E. Theodorou, D. Galaris, L. Nousis, E. S. Katsanou and M. N. Alexis, J. Med. Chem., 2006, 49, 300–306 CrossRef CAS PubMed.
- Y. Feng and P. V. LoGrasso, Expert Opin. Ther. Pat., 2014, 24, 295–307 CrossRef CAS PubMed.
- L. Di, E. H. Kerns, K. Fan, O. J. McConnell and G. T. Carter, Eur. J. Med. Chem., 2003, 38, 223–232 CrossRef CAS PubMed.
- M. Jalali-Nadoushan and M. Roghani, Brain Res., 2013, 1505, 68–74 CrossRef CAS PubMed.
- M. Chen, Q. Liu, A. Liu, M. Tan, Z. Xie, A. Uri, Z. Chen, G. Huang, Y. Sun, H. Ge, P. Liu, M. Li, X. Li, S. Wen and R. Pi, RSC Adv., 2014, 4, 37266–37269 RSC.
- Y. Zhao, J. H. Gu, C. L. Dai, Q. Liu, K. Iqbal, F. Liu and C. X. Gong, Front. Aging Neurosci., 2014, 6, 10 Search PubMed.
- A. ElAli, P. Theriault, P. Prefontaine and S. Rivest, Acta Neuropathol. Commun., 2013, 1, 75 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12081d |
| This journal is © The Royal Society of Chemistry 2016 |