Pillar[7]arene-based host–guest complex in water: dual-responsiveness and application in controllable self-assembly†
Li Shao,
Bin Hua,
Jie Yang and
Guocan Yu*
Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China. E-mail: guocanyu@zju.edu.cn; Fax: +86-571-8795-3189; Tel: +86-571-8795-3189
First published on 15th June 2016
Abstract
A dual-responsive supra-amphiphile was successfully constructed between a water-soluble pillar[7]arene and a pyrene-containing guest. The morphologies of the self-assembled materials formed from this supra-amphiphile could be adjusted by changing solution pH or UV irradiation.
Supra-amphiphiles, constructed by non-covalent interactions or dynamic covalent bonds, can self-assemble into well-defined nanostructured soft materials.1 These self-assembled materials formed from supra-amphiphiles possess various interesting stimuli-responsiveness because of the reversible and dynamic nature of non-covalent interactions. Various stimuli have been employed as triggers to tune the amphiphilicity of the supra-amphiphiles, such as pH, redox, enzymes, light and so on.2 Each of these stimuli has its unique advantages. For example, light has many distinctive merits including easy operation, wide availability and few by-products, while enzymes possess excellent biocompatibility and specificity. However, in spite of many reported single-responsive supramolecular systems, it is still a big challenge to fabricate dual- or multi-responsive systems.3
Host–guest systems based on macrocylic compounds have attracted considerable attention in supramolecular chemistry due to their reversible non-convalent interactions between macrocyclic hosts and suitable guests.4 The introduction of host–guest interactions has become an important strategy to construct supra-amphiphiles.5 Pillar[n]arenes, a new kind of macrocyclic hosts next to crown ethers, cyclodextrins, calix[n]arenes and cucurbit[n]urils, have been widely used in host–guest systems because of their facile and high-yield synthesis, symmetrical pillar architecture, and accessible derivatizations.6 Stimuli-responsive supra-amphiphiles based on pillar[5]arenes and pillar[6]arenes have been widely explored.7 However, only a few investigations about pillar[7]arenes-based supra-amphiphiles have been reported although they have larger cavity size and higher binding ability toward some specific guests.8 Previously, Huang and co-workers reported a dual-responsive supra-amphiphilic polypseudorotaxane constructed from a water-soluble pillar[7]arene and an azobenzene-containing random copolymer, realizing the control release by adjusting pH or temperature.8d We reported a supra-amphiphile based on a water-soluble pillar[7]arene and a NDI-containing guest, whose self-assembled morphology can be regulated by changing pH or adding α-cyclodextrin.8c
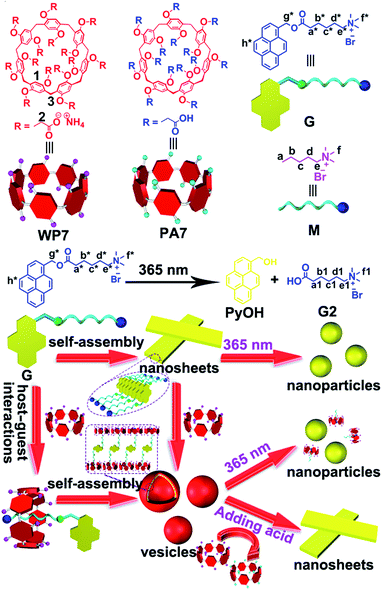
Huang et al. reported a host–guest recognition motif based on a water-soluble pillar[6]arene and a photodegradable guest. The pillar[6]arene was utilized to enhance the drug solubility, while the light-responsive guest was applied for drug release upon irradiation with UV light.9 Inspired by this, we designed a novel supra-amphiphile based on a water-soluble pillar[7]arene (WP7) and a pyrene-containing guest (G). It should be noted that the guest G was responsive to UV irradiation because it contained a photodegradable ester group, which could be used to regulate the self-assembly morphology. Meanwhile WP7 was pH-responsive because its carboxylate groups could be protonated or deprotonated by adding acid or base. Therefore, this supra-amphiphile exhibited pH and light dual-responsiveness. For free guest G, it self-assembled into nanosheets in aqueous solution. Upon the formation of host–guest complex, the self-assembled nanosheets transformed to vesicles. In addition, the vesicular structure of WP7 ⊃ G could be destroyed by adding acid or by UV light irradiation.
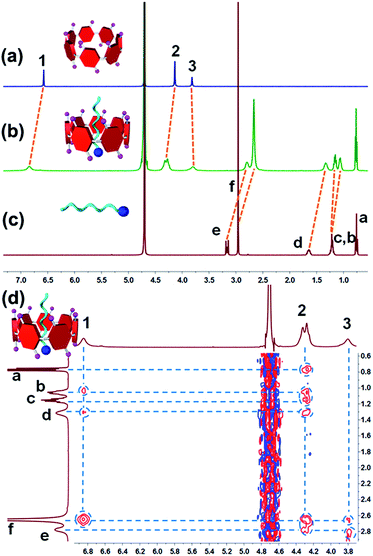
In order to study the host–guest interactions between WP7 and G, a model compound M (Scheme 1) was employed because of the poor water-solubility of G. The host–guest complexation between WP7 and M was firstly studied by 1H NMR spectroscopy. As shown in Fig. 1b, after the addition of equimolar WP7 to a solution of M, chemical shift changes of the signals of some protons on WP7 and M appeared. Among them, the chemical shift changes related to the protons on M were calculated: Δδ = −0.37, −0.30, −0.32, −0.14 and −0.07 ppm for He, Hf, Hd, Hb and Hc, respectively, while negligible shift changes were monitored for the signal of proton Ha. Moreover, the peaks corresponding to the protons on WP7 also exhibited slight chemical shift changes owing to the interactions between WP7 and M (Fig. 1b). The peaks related to H1 and H2 shifted downfield (Δδ = 0.28 and 0.17 ppm, respectively), and the peaks of H3 shifted upfield slightly (Δδ = −0.02 ppm). These phenomena suggested that linear guest M threaded into the cavity of cyclic host WP7 to form a [2]pseudorotaxane with its positive trimethyl ammonium head close to the carboxylate groups of WP7, the middle four methylene groups located in the cavity, and the rest of the tail out of the cavity.10 2D NOESY NMR experiment was conducted to study the relative positions of the components in the host–guest inclusion complex (Fig. 1d). NOE correlation signals were observed between protons H1–3 on WP7 and Ha–f on M, which indicated that M threaded deeply into the cavity of WP7, resulting in the formation of a [2]pseudorotaxane-type inclusion complex.
 | ||
| Scheme 1 Chemical structures of the building blocks and schematic representation of the dual-responsive self-assembly in water. | ||
To estimate the association constant (Ka) for the complex between WP7 and M, isothermal titration calorimetry (ITC) experiment was conducted. ITC is a useful tool to explore the inclusion complexation, which not only provides the association constant (Ka) but also yields its thermodynamic parameters (enthalpy ΔH° and entropy changes ΔS°).11 From Fig. S4,† the Ka value of WP7 ⊃ M was determined to be (6.58 ± 0.58) × 105 M−1 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation, which was higher than those of WP6 ⊃ M (Fig. S10, ESI†) and WP5 ⊃ M.12 Furthermore, the enthalpy and entropy changes were obtained (ΔH° < 0; TΔS° > 0), indicating that this complexation was driven by both enthalpy change and entropy change.
1 complexation, which was higher than those of WP6 ⊃ M (Fig. S10, ESI†) and WP5 ⊃ M.12 Furthermore, the enthalpy and entropy changes were obtained (ΔH° < 0; TΔS° > 0), indicating that this complexation was driven by both enthalpy change and entropy change.
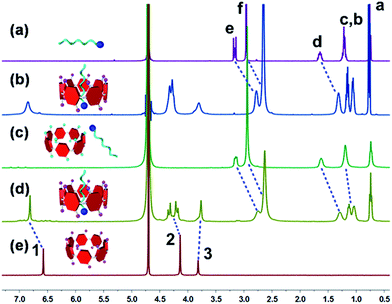
The pH responsive host–guest complexation was further demonstrated by 1H NMR spectra. As shown in Fig. 2, after the addition of an aqueous DCl solution into the WP7 ⊃ M solution, the signals of the protons on WP7 disappeared, and the resonance peaks related to the protons on M returned to their original positions just as free guest M (Fig. 2a). The reason was that the anionic carboxylate groups on WP7 were protonated into neutral carboxylic acid groups and precipitated from the solution, resulting in the disassociation of the inclusion complex.2c On the other hand, it was obvious that the insoluble carboxylic acid groups would be deprotonated when the solution was changed to basic and the macrocyclic host would become soluble in water again after the addition of NaOD. Meanwhile the peaks corresponding to protons Hb–e on M shifted upfield and became broad as shown in the 1H NMR spectrum (Fig. 2d), indicating the reformation of the threading structure between WP7 and M. These studies confirmed that the complex WP7 ⊃ M exhibited pH responsiveness controlled by adding acid or base.
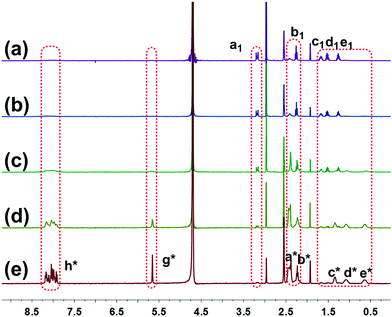
As mentioned before, guest G possessed unqiue photodegradation property. It can be photo-cleaved into PyOH and G2 upon irradiation with UV light (Scheme 1).2f,9 1H NMR spectra were conducted to monitor the gradual degradation process of G. As shown in Fig. 3, the peaks related to pyrene protons Hh* and the methylene protons Hg* next to pyrene diminished gradually upon UV irradiation. Moreover, the peaks (Ha*–e*) related to the alkyl chain part of G disappeared and the new peaks (Ha1–e1) corresponding to the alkyl chain part of G2 became more and more explicit with the irradiation of UV light. The reason was that with the photodegradation process of G into PyOH and G2, PyOH precipitated from the solution, while G2 dissolved in the aqueous solution. Moreover, the color of the solution containing G changed gradually upon UV irradiation (Fig. S5, ESI†), confirming the occurrence of the photodegradation behavior. The 1H NMR spectra indicated that G was cleaved into PyOH and G2 completely after irradiation with UV light (8 W medium-pressure Hg lamp using a UV filter) for 10 min.
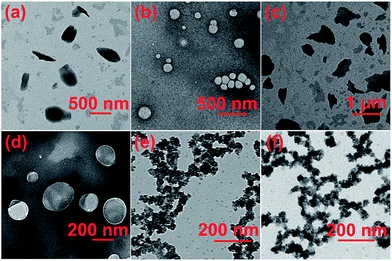
With the molecular recognition motif between WP7 and M in hand, the dual-responsive self-assembly behaviour of the supra-amphiphile WP7 ⊃ G was investigated. By using the concentration-dependent conductivity, the critical aggregation concentration (CAC) of G was determined to be 1.48 × 10−6 M (Fig. S6, ESI†). The morphology of the self-assembly structure of G could be visualized by transmission electron microscopy (TEM). As shown in Fig. 4a, the amphiphilic guest G self-assembled into nanosheets. After the addition of WP7, the CAC value increased to be 4.65 × 10−6 M (Fig. S7, ESI†), which was ascribed to the host–guest complexation.13 Moreover, the self-assembly morphology transformed from nanosheets to vesicles with an average diameter about 200 nm (Fig. 4b). Dynamic light scattering (DLS) was further employed to confirm the size of the aggregates formed by WP7 ⊃ G. As shown in Fig. S8,† the main diameter distribution of the aggregates was around 172 nm, which was in accordance with the corresponding TEM images. After the addition of HCl solution, WP7 was protonated and precipitated from the solution. As a consequence, G dethreaded from the cavity of WP7 and formed nanosheets again (Fig. 4c). Then after the addition of NaOH solutions, the vesicles reappeared again, exhibiting reversibility of the pH responsiveness (Fig. 4d). On the other hand, after UV irradiation of WP7 and G, the photodegradation process of G occurred, accompanied with the transformation of the self-assembly morphology from vesicles to nanoparticles (Fig. 4e). For comparison, the TEM image of G after the UV irradiation for 10 min was obtained (Fig. 4f), which was in accordance with the microstructure formed by WP7 ⊃ G after UV irradiation.
A probable mechanism was proposed to explain the morphology changes of this dual-responsive supra-amphiphile. The micro-assembled structure of the aggregates formed by amphiphiles is determined by the curvature of the membrane.14 G self-assembled into nanosheets because of the hydrophobic effect and the strong π–π stacking interactions between the pyrene aromatic rings. After the addition of WP7, the trimethylamine group threaded into the cavity of WP7 upon forming a [2]pseudorotaxane. The π–π stacking interactions decreased, while the steric hindrance and the electrostatic repulsion increased, resulting in the formation of vesicles with higher curvature. The morphology could be adjusted after the addition of HCl or NaOH solution because of the decomplexation or complexation process between WP7 and G. Moreover, upon irradiating the WP7 ⊃ G solution with UV light for about 10 min, the self-assembly morphology changed to nanoparticles. The TEM image of PyOH (Fig. S9, ESI†) was similar to that of WP7 ⊃ G after UV irradiation, which was confirmed that WP7 ⊃ G was cleaved into PyOH and WP7 ⊃ G2 upon UV irradiation.
In conclusion, a dual-responsive supra-amphiphile based on a water-soluble pillar[7]arene host and a pyrene derivative guest was constructed successfully and its stimuli-responsive self-assembly in water was studied. The free guest self-assembled in water to form nanosheets. After the addition of WP7, the nanosheets transformed to vesicles arising from the formation of supra-amphiphile. Furthermore, this supra-amphiphile exhibited pH- and light-responsiveness: upon addition of HCl/NaOH, the reversible transformation from nanosheets to vesicles could be observed; the vesicles can be also destroyed after the irradiation of the supra-amphiphile with UV light for about 10 min. In the future, we will focus on the development of smart nanomaterials based on this novel host–guest recognition motif for the applications in controlled release.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities.Notes and references
- (a) C. Wang, Z. Wang and X. Zhang, Acc. Chem. Res., 2012, 45, 608–618 CrossRef CAS PubMed; (b) G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed.
- (a) K. Patel, S. Angelos, W. R. Dichtel, A. Coskun, Y.-W. Yang, J. I. Zink and J. F. Stoddart, J. Am. Chem. Soc., 2008, 130, 2382–2383 CrossRef CAS PubMed; (b) J. Hu, G. Zhang and S. Liu, Chem. Soc. Rev., 2012, 41, 5933–5949 RSC; (c) G. Yu, X. Zhou, Z. Zhang, C. Han, Z. Mao, C. Gao and F. Huang, J. Am. Chem. Soc., 2012, 134, 19489–19497 CrossRef CAS PubMed; (d) Q. Duan, Y. Cao, Y. Li, X. Hu, T. Xiao, C. Lin, Y. Pan and L. Wang, J. Am. Chem. Soc., 2013, 135, 10542–10549 CrossRef CAS PubMed; (e) W. Xia, X.-Y. Hu, Y. Chen, C. Lin and L. Wang, Chem. Commun., 2013, 49, 5085–5087 RSC; (f) J. Yang, G. Yu, D. Xia and F. Huang, Chem. Commun., 2014, 50, 3993–3995 RSC; (g) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577–8589 CrossRef CAS PubMed; (h) X.-F. Hou, Y. Chen and Y. Liu, Soft Matter, 2015, 11, 2488–2493 RSC; (i) P. Xing, H. Chen, M. Ma, X. Xu, A. Hao and Y. Zhao, Nanoscale, 2016, 8, 1892–1896 RSC.
- (a) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042–6065 RSC; (b) K. Wang, C.-Y. Wang, Y. Wang, H. Li, C.-Y. Bao, J.-Y. Liu, S. X.-A. Zhang and Y.-W. Yang, Chem. Commun., 2013, 49, 10528–10530 RSC; (c) N. Song and Y.-W. Yang, Chem. Soc. Rev., 2015, 44, 3474–3504 RSC.
- (a) F. Huang and H. W. Gibson, J. Am. Chem. Soc., 2004, 126, 14738–14739 CrossRef CAS PubMed; (b) F. Wang, J. Zhang, X. Ding, S. Dong, M. Liu, B. Zheng, S. Li, L. Wu, Y. Yu, H. W. Gibson and F. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090–1094 CrossRef CAS PubMed; (c) Y. Kou, H. Tao, D. Cao, Z. Fu, D. Schollmeyer and H. Meier, Eur. J. Org. Chem., 2010, 6464–6470 CrossRef CAS; (d) G. Chen and M. Jiang, Chem. Soc. Rev., 2011, 40, 2254–2266 RSC; (e) D.-S. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907–5921 RSC; (f) K.-D. Zhang, J. Tian, D. Hanifi, Y. Zhang, A. C.-H. Sue, T.-Y. Zhou, L. Zhang, X. Zhao, Y. Liu and Z.-T. Li, J. Am. Chem. Soc., 2013, 135, 17913–17918 CrossRef CAS PubMed; (g) J. Zhou, M. Chen and G. Diao, ACS Appl. Mater. Interfaces, 2014, 6, 18538–18542 CrossRef CAS PubMed; (h) Y. Wu, Y. Lan, J. Liu and O. A. Scherman, Nanoscale, 2015, 7, 13416–13419 RSC; (i) Y. Chen and Y. Liu, Adv. Mater., 2015, 27, 5403–5409 CrossRef CAS PubMed; (j) G. Wang, B. Tang, Y. Liu, Q. Gao, Z. Wang and X. Zhang, Chem. Sci., 2016, 7, 1151–1155 RSC; (k) P. Wang, Z. Gao, M. Yuan, J. Zhu and F. Wang, Polym. Chem., 2016, 7, 3664–3668 RSC.
- (a) Z. Ge and S. Liu, Chem. Soc. Rev., 2013, 42, 7289–7325 RSC; (b) Z. Qin, D.-S. Guo, X.-N. Gao and Y. Liu, Soft Matter, 2014, 10, 2253–2263 RSC; (c) Y. Kang, Z. Cai, X. Tang, K. Liu, G. Wang and X. Zhang, ACS Appl. Mater. Interfaces, 2016, 8, 4927–4933 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T.-a. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) X.-B. Hu, Z. Chen, G. Tang, J.-L. Hou and Z.-T. Li, J. Am. Chem. Soc., 2012, 134, 8384–8387 CrossRef CAS PubMed; (c) W. Chen, Y. Zhang, J. Li, X. Lou, Y. Yu, X. Jia and C. Li, Chem. Commun., 2013, 49, 7956–7958 RSC; (d) L. Wu, Y. Fang, Y. Jia, Y. Yang, J. Liao, N. Liu, X. Yang, W. Feng, J. Ming and L. Yuan, Dalton Trans., 2014, 43, 3835–3838 RSC; (e) W.-B. Hu, H.-M. Yang, W.-J. Hu, M.-L. Ma, X.-L. Zhao, X.-Q. Mi, Y. A. Liu, J.-S. Li, B. Jiang and K. Wen, Chem. Commun., 2014, 50, 10460–10463 RSC; (f) C. Li, Chem. Commun., 2014, 50, 12420–12433 RSC; (g) W. Si, Z.-T. Li and J.-L. Hou, Angew. Chem., Int. Ed., 2014, 53, 4578–4581 CrossRef CAS PubMed.
- (a) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed; (b) Z. Zhang, Y. Luo, J. Chen, S. Dong, Y. Yu, Z. Ma and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1397–1401 CrossRef CAS PubMed; (c) J. Fan, H. Deng, J. Li, X. Jia and C. Li, Chem. Commun., 2013, 49, 6343–6345 RSC; (d) H. Zhang and Y. Zhao, Chem.–Eur. J., 2013, 19, 16862–16879 CrossRef CAS PubMed; (e) M. Ni, X. Hu, J. Jiang and L. Wang, Chem. Commun., 2014, 50, 1317–1319 RSC; (f) Y. Wang, J.-F. Xu, Y.-Z. Chen, L.-Y. Niu, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Commun., 2014, 50, 7001–7003 RSC; (g) W.-B. Hu, H.-M. Yang, W.-J. Hu, M.-L. Ma, X.-L. Zhao, X.-Q. Mi, Y. A. Liu, J.-S. Li, B. Jiang and K. Wen, Chem. Commun., 2014, 50, 10460–10463 RSC; (h) J. Bi, X. Zeng, D. Tian and H. Li, Org. Lett., 2016, 18, 1092–1095 CrossRef CAS PubMed; (i) X. Mao, T. Liu, J. Bi, L. Luo, D. Tian and H. Li, Chem. Commun., 2016, 52, 4385–4388 RSC.
- (a) C. Han, Z. Zhang, X. Chi, M. Zhang, G. Yu and F. Huang, Acta Chim. Sin., 2012, 70, 1775–1778 CrossRef CAS; (b) J. Fan, Y. Chen, D. Cao, Y.-W. Yang, X. Jia and C. Li, RSC Adv., 2014, 4, 4330–4333 RSC; (c) L. Shao, J. Zhou, B. Hua and G. Yu, Chem. Commun., 2015, 51, 7215–7218 RSC; (d) X. Chi, X. Ji, D. Xia and F. Huang, J. Am. Chem. Soc., 2015, 137, 1440–1443 CrossRef CAS PubMed.
- G. Yu, W. Yu, Z. Mao, C. Gao and F. Huang, Small, 2015, 11, 919–925 CrossRef CAS PubMed.
- Z. Zhang, Y. Luo, B. Xia, C. Han, Y. Yu, X. Chen and F. Huang, Chem. Commun., 2011, 47, 2417–2419 RSC.
- G. Yu, B. Hua and C. Han, Org. Lett., 2014, 16, 2486–2489 CrossRef CAS PubMed.
- B. Hua, L. Shao, J. Zhou and G. Yu, RSC Adv., 2016, 6, 47281–47284 RSC.
- Q. Zhou, H. Jiang, R. Chen, F. Qiu, G. Dai and D. Han, Chem. Commun., 2014, 50, 10658–10660 RSC.
- (a) R. A. Salkar, D. Mukesh, S. D. Samant and C. Manohar, Langmuir, 1998, 14, 3778–3782 CrossRef CAS; (b) C. Wang, S. Yin, S. Chen, H. Xu, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2008, 47, 9049–9052 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, ESI mass spectra and ITC. See DOI: 10.1039/c6ra12183g |
| This journal is © The Royal Society of Chemistry 2016 |