Bottom-up direct writing approach for controlled fabrication of WS2/MoS2 heterostructure systems†
Rui Donga,
Logan Moorea,
Nozima Aripovab,
Christopher Williamsona,
Robert Schurza,
Yuzi Liuc,
Leonidas E. Ocolac and
Irma Kuljanishvili *a
*a
aDepartment of Physics, Saint Louis University, St. Louis, MO 63103, USA. E-mail: ikuljani@slu.edu
bDepartment of Biology, Saint Louis University, St. Louis, MO 63103, USA
cArgonne National Laboratory, 9700 S. Cass Avenue, Argonne, IL 60439, USA
First published on 7th July 2016
Abstract
The ability to construct heterostructures that consist of layered transition metal dichalcogenides materials (MX2) in a controlled fashion provides an attractive solution for materials design and device applications. For nanotechnology applications it is important to control the shape, geometry and precise position of the grown heterostructure assemblies on a variety of substrates. In this study, we developed a “direct writing” technique to fabricate arrays of WS2/MoS2 and MoS2/WS2/MoS2 heterostructures at predefined locations on a silicon substrate in a controlled fashion. Water based precursor inks were implemented to ensure the formation of quality heterostructure surfaces which are free of residual polymer contaminants. With this technique we demonstrate an attractive and scalable technology with unique capabilities for precise growth of dissimilar MX2 materials, layered either in a vertical or lateral arrangement.
Introduction
The progress of graphene research has stimulated interest in other types of two-dimensional (2D) atomic crystals, such as hexagonal boron nitride, germanene, and layered transition metal dichalcogenides (TMDCs) or MX2 (M: transition metal; X: chalcogen). Although MX2 materials share similar crystalline structures, their physical properties (band gap, light matter interaction, and spin–orbit coupling strength) can vary significantly. Among these 2D materials, the applications of MX2 in logic electronics are promising1–8 because of its sizable bandgaps of around 1–2 eV, comparable to those of conventional groups of semiconductors (groups IV, III–V). In parallel with the study of single layer MX2 materials, van der Waals (vdW) heterostructures that consist of dissimilar MX2 materials, arranged in a vertical direction, have recently been gaining extensive attention. In typical semiconductor heterostructures made of two or more dissimilar materials, highly matched crystalline lattices are required to yield high quality interfaces, hence the designs for multi-compositional conventional semiconductors is complex. In the MX2 heterostructures however, high quality interfaces can be achieved even in the mismatched systems.9 That is due to the nature of the weak vdW forces that are governing the interactions in layered MX2 materials.9–14 Novel properties/phenomena that have been revealed in MX2 heterostructures could trigger a revolution in the design of heterostructure systems for applications such as photovoltaics, optoelectronics, and spontaneous water splitting.10,11Several approaches have been developed to prepare MX2 layers such as micromechanical exfoliation, chemical/electrochemical exfoliation, and chemical vapour deposition (CVD). To date, fabrication of MX2 heterostructures are mainly based on the combination of exfoliation and CVD methods. Huo et al.12,13 micromechanically exfoliated MoS2 and WS2 flakes, and demonstrated the transfer of WS2 layer onto MoS2 flakes using polymethyl methacrylate (PMMA) coating and sodium hydroxide (NaOH) solution etching methods. Yu et al.9 prepared MoS2 and WS2 layer using CVD methods then lifted off and transferred the synthesized MoS2 onto as-grown WS2 layer manually. Chen et al.15 fabricated MoS2 by CVD method, and WS2 layer was obtained by the thermolysis of ammonium tungstate hydrate in an inert gas environment. Zhang et al.16 synthesized MoS2/WS2 heterostructures using core–shell WO3−x/MoO3−x nanowires as precursors. Jung et al.10 deposited and patterned tungsten/molybdenum (W/Mo) and then introduced sulfur (S) vapor for the growth of heterostructure. Gong et al.17 fabricated vertically stacked and in-plane (lateral) WS2/MoS2 heterostructures by controlling the CVD growth temperature and using sulphur, tungsten, and molybdenum oxide as precursors. Although exciting properties have been discovered in these heterostructure systems fabricated with different processing methods, it is challenging to control the shapes, geometry and precise positions of MX2 heterostructure formations at preselected locations. In order to control the position of MoS2 and WS2 layers, additional photo/e-beam lithography is usually required, which inevitably introduces organic/polymer residues and may introduce strain to the interface between the layers and thus could degrade the heterostructure interface quality.
In this study, we have developed a “direct writing” technique to fabricate arrays of WS2/MoS2 heterostructures at predefined locations on silicon substrates using precursor solutions. This technique involves two main steps: (a) the writing of ammonium tetrathiomolybdate ((NH4)2MoS4) precursor inks with cantilever tips (followed by thermal annealing to form MoS2 layer), and (b) the subsequent writing of ammonium tetrathiotungstate ((NH4)2WS4) precursor (followed by thermal annealing to form WS2 layer). The technique is simple, flexible and potentially extendable to variety of substrates and heterostructure systems. Here we demonstrate a sub-micrometer structure formation in a simple two-step table-top process. The reduction in the number of steps in the process of patterning and growth of heterostructure systems carries a significant benefit; it introduces an alternative unconventional approach to the fabrication of sophisticated engineered materials. In contrast to the commonly used conventional lithographic methods, here neither patterned masks or MX2 layer transferring were required in our method, hence contamination between two materials can also be significantly reduced. Our technique demonstrates an attractive technology with unique capabilities for precisely aligning and growing dissimilar MX2 layers in both vertical and lateral directions. This technique can be further extended to a large area fabrication of high quality MX2 heterostructures.
Results and discussion
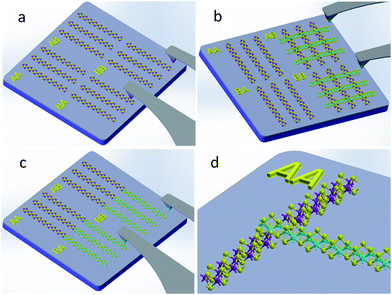
Fig. 1 depicts a schematic overview of the production protocol for creating arrays of WS2/MoS2 heterostructures. Fig. 1a shows (NH4)2MoS4 precursor ink deposited first as an array of ribbons/lines at specific location on SiO2/Si substrate along y-axis. Subsequently, samples with patterned (NH4)2MoS4 precursor structures are processed in the CVD chamber at the required temperature (∼450 °C) to form an array of MoS2 ribbons. Next, a different ink precursor (NH4)2WS4 is patterned atop of MoS2 ribbons (along x-axis) as shown schematically in Fig. 1b. Once again, samples with (NH4)2WS4 patterned precursor structures are subsequently treated in the CVD chamber to complete the formation of the resulting WS2/MoS2 vertical heterostructures. This direct writing technique also provides a unique way to prepare WS2/MoS2 lateral heterostructures where the arrays of (NH4)2WS4 ink precursor can be easily written in the same direction to align with pre-existing MoS2 structures (Fig. 1c). Moreover, writing (NH4)2MoS4 precursor multiple times as shown in Fig. 1d, will produce MoS2/WS2/MoS2 lateral tri-layer heterostructures. These architectures would be challenging to fabricate with conventional lithography and would require multiple additional steps in the fabrication protocol. In contrast, such heterostructures are relatively simple to produce with the use of direct-write scanning probe based nanolithography approach, which effectively offers a nanoscale precision in position and registry with simple mask-free geometry defining capabilities.The inks developed in this study ((NH4)2MoS4 and (NH4)2WS4 precursors) are water based. Since water is a neutral solvent, it is therefore reasonable to conclude that no chemical reactions occur at room temperature under ambient conditions during the entire writing process. The direct writing technique essentially has two main steps for precursor deposition: “inking” and “writing” as shown in Fig. S1.† In the inking step, the tips of an atomic force microscope (AFM) cantilever are dipped into an “ink”, and then, the ink is transferred onto the selected substrate during the writing step. Either single or multi-pen cantilevers can be employed. The non-interacting chemical nature of the developed inks allows us to simplify and understand the kinetic model of the writing steps as follows: (a) the steps of the ink transferring from tip to substrate with the assistance of water meniscus, (b) the ink lateral diffusion on substrate and (c) the stopping of diffusion by surface tension of the substrate. We can characterize our direct writing method as scanning probe based nanolithography, a cousin technique to dip pen nanolithography and other related methods.18–23 In order to obtain large area patterned arrays with high throughput, as required by the semiconductor industry, multi-pen cantilevers had been utilized here for parallel writing. Piezo driven scanners with mounted multi-pen cantilever chip can repeat multiple automated sequences for the production of large area patterns. The use of commercially available custom inkwells with multiple channels enables a convenient solution for improving the patterning efficiency (Fig. S1†).
This mask free approach can potentially reduce the amount of residue between the layers of MoS2 and WS2 since no polymer resists were required in the process of writing. An additional advantage of this approach is that there is no need for MX2 materials transfer from growth surfaces to the desirable substrates which has been commonly used and reported in the MX2 heterostructure preparations.12–14 The solvent chosen for the precursor inks was water, which is normally removed at low annealing temperatures (∼200 °C), prior to the formation of MX2 materials (see the discussion of MoS2 and WS2 formation below). Heat-treatment of patterned (NH4)2MoS4 and (NH4)2WS4 structures with the presence of hydrogen gas (H2) in the CVD furnace has shown to lower the required temperature24,25 at which MoS2 and WS2 crystalline structures are formed (∼800 °C to ∼450 °C) as described in the eqn (1) and (2) in the Experimental section.
It is possible to generate more complex structures with the combination of two basic patterns, i.e. dots and ribbons/lines using the software protocol sequencing. Arrays of dots were produced by holding the inked cantilever in contact with the substrate so that inks diffuse out in a radial direction to form a circular dot pattern. Then the tip was moved to the next position and the process was repeated as shown in Fig. S2.† By controlling the humidity in the working chamber (environmental cell), the tip moving speed and the dwell time, various shapes of MoS2 and WS2 structures (dot arrays and L-shaped ribbons) were fabricated on SiO2/Si substrates (Fig. S2†). Other substrates (not shown in this report) have also been successfully tested.
The fabrication of MoS2 and WS2 ribbons/lines is more challenging as compared to the dot patterns where the AFM tip must continuously move along the sample as shown schematically on the Fig. 1a. Therefore, a dynamic balance needs to be maintained between the ink transfer (from cantilever to substrate), the ink lateral diffusion and stopping of the ink diffusion process in order to modulate the formation of ribbons/lines on a substrate. Although the “writing” of desired ribbons/lines might also be adjusted by modulating the environmental humidity and temperature, all studies of the patterns in this work were performed at a fixed humidity (50%) and temperature (23 °C) for the consistency of the analyses. The writing of MoS2 and WS2 ribbons was studied on SiO2/Si substrates. Surface of SiO2/Si substrates is easy to modify and ensures that the key parameters of the ribbons, i.e. width and thickness, could also be precisely controlled and optimized. Once parameters were optimized for individual ribbons, more complex systems such as WS2/MoS2 bilayers and MoS2/WS2/MoS2 tri-layer heterostructures were created.
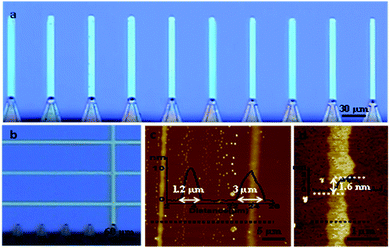
It was established that single and multiple MX2 ribbons with controlled parameters can be fabricated in this direct writing fashion. Multiple parallel ribbons with selected widths and thicknesses were prepared by employing multi-pen cantilever as shown in the optical image (Fig. 2a). In the case where one specific cantilever tip was inked, individual ribbons with fixed width and thickness were written directly at pre-selected locations as shown in Fig. 2b. Due to the fact that the processes of ink transfer (from cantilever to substrate) and diffusing (lateral diffusion on substrate) occur simultaneously when tip is in contact with the substrate, it was found that control of the tip movement speed was the most optimal way to determine the width of the resulting MoS2 and WS2 ribbons. With relatively faster tip speed (in the range of 1–5 μm s−1), the process of lateral diffusion of ink on the substrate can be controlled hence narrow ribbons can be obtained. For example, the width of WS2 ribbons were decreased from 3 μm to 1.2 μm when tip moving speed increased from 2 μm s−1 to 5 μm s−1, as shown in the Fig. 2c. However, it should be noted that at significantly slower tip moving speeds such as 0.1 μm s−1 (the slowest speed achievable with this tool), the ink meniscus will have more time to diffuse laterally and also produce thicker vertical deposits on the substrate. Table S1† in the ESI shows the relationship between the tip speed and the width of the MoS2 ribbons.
Higher ink concentration in this study implies the amount of reactant ammonium tetrathiomolybdate ((NH4)2MoS4) or ammonium tetrathiotungstate ((NH4)2WS4), used for “writing” on the substrate was increased. In order to prepare thinner/thicker structures the amount of reactant was correspondingly decreased/increased by adjusting as-prepared ink concentration (see more details in Experimental section and ESI†). Therefore, to optimize the process it was concluded that the most convenient approach to determine the thicknesses of the resulting MoS2 and WS2 ribbons is to adjust precursor ink composition or concentration and perform the writing at tip speeds in the range between 2 μm s−1 to 5 μm s−1. The influence of ink concentration is clearly observed in the AFM images (Fig. 2d), where double layers WS2 ribbon (∼1.6 nm thick and ∼0.7 μm wide) was produced by controlling the ink concentration. Table S2† in the ESI shows the relationship between the ink concentrations and the thicknesses of the MoS2 ribbons.
This direct writing approach provides additional flexibility for precise patterning of MoS2 and WS2 ribbons aligned to prefabricated structures on the substrate. Fig. 3a shows AFM images of the WS2 ribbon structures formed between prefabricated Al/Ti electrodes on SiO2/Si substrate. The connection between Al/Ti electrodes and WS2 ribbons is continuous and robust and can be observed in the enlarged AFM image (Fig. 3b). An AFM image of the surface of WS2 ribbon is shown in Fig. 3c and the height profile measurement indicates an approximately 1 nm average surface roughness (inset of Fig. 3c). The three dimensional AFM representation of the same area is shown in Fig. 3d. The robust connection between WS2 and Al/Ti electrode has been further confirmed with the transport measurement at room temperature in vacuum at approximate pressure of 10−4 Torr. Linear current–voltage behaviour was recorded, as shown in Fig. 3e. The resistance obtained here is comparable to the devices reported elsewhere, where electrodes are usually deposited on top of the grown WS2 structures.16,26 These results demonstrate that with the proposed bottom-up fabrication method robust connection between MX2 ribbons and prefabricated electrodes is achievable. Moreover, the surface quality of MX2 ribbons is uniform and the surface roughness of ∼1 nm is better than typical roughness for the materials derived from the solution based methods. The high-resolution transmission electron microscopy (HRTEM) image in Fig. 3f clearly reveals the periodic atom arrangement of the MoS2 film at a selected location. HRTEM characterization also demonstrates that heat-treatment of patterned (NH4)2MoS4 structures with the presence of argon and hydrogen gas atmosphere would indeed lower the required MoS2 crystallization temperature (see ESI† for more details on TEM and X-ray diffraction (XRD) data).
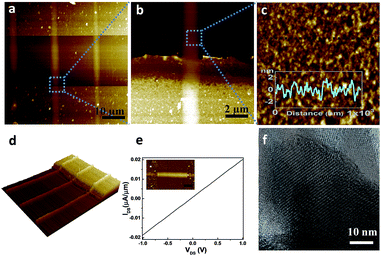 | ||
| Fig. 3 Direct write of WS2 ribbon at predefined locations of the selected substrate. (a) AFM images of the WS2 ribbon structures formed between the prefabricated Al/Ti electrodes on SiO2/Si substrate; (b) enlargement of AFM images of connection between Al/Ti electrodes and WS2 ribbon; (c) enlargement of AFM images and the height profile measurement (inset); (d) AFM 3D image of the area shown in (a); (e) transport measurement of WS2 ribbon fabricated by direct write technique atop of metal electrodes (inset: AFM images of the measured device; a horizontal ribbon of WS2 between two electrode is shown, the scale bar is 5 μm). (f) High-resolution TEM image for the MoS2 polycrystalline film (see ESI† for more details). | ||
The success in controlled production of MX2 ribbons with specific thickness and width further enables the fabrication of more complex MX2 structures directly on existing devices. The arrays of WS2/MoS2 heterostructures (in vertical and lateral geometries) have been written at predefined locations between pre-deposited electrodes. As shown in the Fig. 4a and b, nine vertical heterostructure regions of WS2/MoS2 were prepared by cross-patterning of the arrays of MoS2 ribbons (x-axis direction) and the array of WS2 ribbons (y-axis direction) in the 3 × 3 arrangement. Representative AFM image (3D view) of the cross-pattered WS2/MoS2 vertical heterostructures is shown in Fig. 4c, where spacing between heterostructures regions is approximately ∼20 μm (see more details in ESI in Fig. S3†). In addition, the lateral heterostructures of WS2/MoS2 were also prepared by connecting patterns of MoS2 ribbons (x-axis direction) with WS2 ribbons (y-axis direction), in 3 × 3 L-shaped junction arrangement, as shown in the Fig. 4d and e. Furthermore, we have shown that our approach has proven to be a versatile and enables the fabrication of more complicated MX2 tri-layer heterostructures, i.e., MoS2/WS2/MoS2 alternating structures directly patterned on prefabricated devices. See ESI† for representative examples of vertical tri-layer structure of MoS2/WS2/MoS2 shown in Fig. S4a and b,† and the lateral tri-layer structure of MoS2/WS2/MoS2 shown in Fig. S4c and d.†
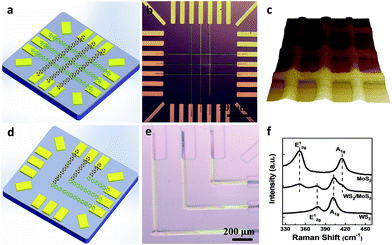 | ||
| Fig. 4 Direct write of various WS2/MoS2 heterostructures on pre-fabricated devices. (a) The production scheme for the arrays of WS2/MoS2 bilayer vertical heterostructures; (b) optical image of WS2/MoS2 bilayer vertical heterostructures; (c) representative AFM 3D image of patterned cross-bar WS2/MoS2 vertical heterostructures (see more details in ESI in Fig. S3†); (d) the production scheme for the arrays of WS2/MoS2 bilayer lateral heterostructures; (e) optical image of WS2/MoS2 bilayer lateral heterostructures; (f) representative resonant Raman spectroscopy of the fabricated ribbons of MoS2, WS2 and WS2/MoS2 vertical heterostructures. | ||
Resonant Raman spectroscopy was also utilized to characterize the fabricated ribbon arrays of MoS2, WS2 and WS2/MoS2 vertical heterostructures (Fig. 4f). Raman spectra acquired from the pure MoS2 region which exhibited two peaks located at 382 cm−1 and 407 cm−1, corresponding to E12g and A1g modes. Raman spectra from the pure WS2 region show two peaks at 352 cm−1 and 419 cm−1, also typical signals of E12g and A1g modes. The spectra collected at these regions show the characteristic peaks A1g and E12g with a wave-number difference of Δ ∼ 25 cm−1 and Δ ∼ 67 cm−1 which corresponds to the multilayer values for MoS2 and WS2 as reported previously.11 In contrast, the Raman spectra collected at the vertical heterostructure region show four discrete peaks, which match well to the above E12g and A1g modes of multilayer MoS2 and WS2. The relative Raman intensities of E12g and A1g modes of MoS2 are maintained without considerable changes before and after the formation of WS2, demonstrating the feasibility for the application of direct write lithography for the production of complex MX2 heterostructures. It was suggested that Raman spectral signatures from vertical heterostructures should not be different from the Raman plots of the lateral ones, although if a strain/stress is present at the lateral heterostructure interface, it may potentially alter Raman spectral characteristics.15 One can further improve on the quality (crystallinity) of such polycrystalline materials by increasing the base temperature of the thermal treatment. This is a common approach that could be applied to most polycrystalline systems. The relatively low temperatures were chosen in this study to allow for testing the concept of direct fabrication of precursor inks on metallic devices and even flexible substrates (not shown in this report). We note that high temperature thermal treatment of MX2 thin films may induce the sulfur deficiently, which might subsequently degrade their quality. Liu et al.27 suggested that introducing sulfur vapor during the thermal treatment (∼1000 °C) would significantly improve MX2 material quality.
In conclusion, we report on a unique approach which allows for an easy formation of high quality WS2/MoS2 heterostructures with controllable lengths, widths and thicknesses. An important aspect of this study was the demonstration of the selective growth of heterostructures and the formation of various architectures on the substrate, which is presently not easily achievable by other fabrication methods that are commonly used. In this report we have demonstrated several heterostructure geometries and hybrid device architectures. Our approach can potentially be extrapolated to produce an entire new range of heterostructured systems. This versatile and scalable mask free technology combines the simplicity of solution based processing and the precision of scanning probe based nanolithography which makes it a promising tool for nanoscale materials engineering future applications.
Experimental
Preparation of (NH4)2MoS4 precursor
Ammonium thiomolybdates ((NH4)2MoS4) powder (Alfa Aesar, purity of 99.95%; 0.28 g) was added into 60 mL of deionized (D.I.) water. The obtained precursors were sonicated for 30 min then filtered with 0.2 μm PTFE membranes to get highly dispersed clear solutions. The obtained (NH4)2MoS4 solution was used as ink precursor for the formation of MoS2 structures.Preparation of (NH4)2WS4 precursor
Ammonium tetrathiotungstate ((NH4)2WS4) powder (Sigma-Aldrich, purity of 99.9%; 0.026 g) was added into 60 mL of D.I. water. The obtained precursors were sonicated for 30 min then filtered with 0.2 μm PTFE membranes to get highly dispersed clear solutions. The obtained (NH4)2WS4 solution was used as ink for the formation of WS2 structures. The diluted inks with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 (as-prepared ink
9 (as-prepared ink![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) deionized water) was used to produce double layer WS2 ribbon.
deionized water) was used to produce double layer WS2 ribbon.
Direct write patterning process
The patterning of (NH4)2MoS4 and ((NH4)2WS4) precursor inks were performed with a custom made patterning platform with motorized piezo-stages with a resolution of approximately 20 nm for all three xyz axes. The sample holding stage is also equipped with tilt correction capabilities. The writing tool is essentially a cantilever array of twelve tips with approximately 60 μm inter-tip spacing mounted on the tip holder. The tips and the inkwells with matching pitch were purchased from Advanced Creative Solutions Technology LLC. Si substrates (with 300 nm SiO2 layer) were cleaned by 10 min sonication of acetone, IPA and D.I. water respectively. Cantilevers and substrates were additionally cleaned in ozone cleaner to render them hydrophilic. Alphabetical markers on Si/SiO2 substrates were deposited via standard e-beam lithography and lift-off process (Pt/Ti metal with thickness of 70 nm/5 nm respectively was used for metallization). Samples with the metalized alphabetical markers were additionally cleaned in the furnace with the mixture of Ar/H2 at 450 °C to remove any possible polymer residuals.Preparation of MX2 heterostructures
Ribbons of (NH4)2MoS4 precursor ink were patterned on cleaned SiO2/Si substrate and then transferred into CVD system for crystallization of MoS2. The formation of MoS2 was performed in ambient condition, annealed in a mixture of Ar/H2 with the respective flow rates of 400 sccm/100 sccm. The annealing at lower temperatures (∼200 °C) can efficiently remove the residual D.I. water. The subsequently higher temperature annealing (∼450 °C) orders crystallinity of MoS2. Ribbons of (NH4)2WS4 precursor ink were patterned atop of MoS2 ribbons (to form vertical bilayer heterostructures) and adjacent to MoS2 ribbons (to form lateral bilayer heterostructures). The formation of WS2/MoS2 heterostructures was also performed in ambient condition and annealed in a mixture of Ar/H2 with the respective flow rates of 400 sccm/100 sccm. A slightly lower temperature of 400 °C was used for crystallization of WS2 layer. Same protocol was used for the multi-layered patterned structures. Eqn (1) and (2) show the resections in presence of H2 gas.| (NH4)2MoS4 + H2 → 2NH3 + 2H2S + MoS2 | (1) |
| (NH4)2WS4 + H2 → 2NH3 + 2H2S + WS2 | (2) |
Device characterization
AFM topographic images were acquired in non-contact mode with a Park NX10 system. Raman spectroscopy was obtained with a Renishaw InVia Raman Spectrometer with the laser excitation wavelength of 532 nm. The Si peak at 520 cm−1 was used as reference for wavenumber calibration in all Raman spectral data. A JEOL-2100F system working at 200 kV was employed for the HRTEM microstructure characterization.Acknowledgements
Use of the Center for Nanoscale Materials was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02 06CH11357. I. K. acknowledges support of NSF MRI program (Award No. 1338021), and the Saint Louis University seed funds.Notes and references
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- S. Das, R. Gulotty, A. V. Sumant and A. Roelofs, Nano Lett., 2014, 14, 2861–2866 CrossRef CAS PubMed.
- G. Fiori, F. Bonaccorso, G. Iannaccone, T. Palacios, D. Neumaier, A. Seabaugh, S. K. Banerjee and L. Colombo, Nat. Nanotechnol., 2014, 9, 768–779 CrossRef CAS PubMed.
- F. Schwierz, J. Pezoldt and R. Granzner, Nanoscale, 2015, 7, 8261–8283 RSC.
- F. H. L. Koppens, T. Mueller, P. Avouris, A. C. Ferrari, M. S. Vitiello and M. Polini, Nat. Nanotechnol., 2014, 9, 780–793 CrossRef CAS PubMed.
- R. Dong, Z. Guo, J. Palmer, Y. Hu, M. Ruan, J. Hankinson, J. Kunc, S. K. Bhattacharya, C. Berger and W. A. d. Heer, J. Phys. D: Appl. Phys., 2014, 47, 094001 CrossRef.
- A. C. Ferrari, F. Bonaccorso, V. Fal'ko, K. S. Novoselov, S. Roche, P. Boggild, S. Borini, F. H. L. Koppens, V. Palermo, N. Pugno, J. A. Garrido, R. Sordan, A. Bianco, L. Ballerini, M. Prato, E. Lidorikis, J. Kivioja, C. Marinelli, T. Ryhanen, A. Morpurgo, J. N. Coleman, V. Nicolosi, L. Colombo, A. Fert, M. Garcia-Hernandez, A. Bachtold, G. F. Schneider, F. Guinea, C. Dekker, M. Barbone, Z. Sun, C. Galiotis, A. N. Grigorenko, G. Konstantatos, A. Kis, M. Katsnelson, L. Vandersypen, A. Loiseau, V. Morandi, D. Neumaier, E. Treossi, V. Pellegrini, M. Polini, A. Tredicucci, G. M. Williams, B. Hee Hong, J.-H. Ahn, J. Min Kim, H. Zirath, B. J. van Wees, H. van der Zant, L. Occhipinti, A. Di Matteo, I. A. Kinloch, T. Seyller, E. Quesnel, X. Feng, K. Teo, N. Rupesinghe, P. Hakonen, S. R. T. Neil, Q. Tannock, T. Lofwander and J. Kinaret, Nanoscale, 2015, 7, 4598–4810 RSC.
- B. Radisavljevic, A. Radenovic, J. Brivio, V. Giacometti and A. Kis, Nat. Nanotechnol., 2011, 6, 147–150 CrossRef CAS PubMed.
- Y. Yu, S. Hu, L. Su, L. Huang, Y. Liu, Z. Jin, A. A. Purezky, D. B. Geohegan, K. W. Kim, Y. Zhang and L. Cao, Nano Lett., 2015, 15, 486–491 CrossRef CAS PubMed.
- Y. Jung, J. Shen, Y. Sun and J. J. Cha, ACS Nano, 2014, 8, 9550–9557 CrossRef CAS PubMed.
- S. Tongay, W. Fan, J. Kang, J. Park, U. Koldemir, J. Suh, D. S. Narang, K. Liu, J. Ji, J. Li, R. Sinclair and J. Wu, Nano Lett., 2014, 14, 3185–3190 CrossRef CAS PubMed.
- N. Huo, J. Kang, Z. Wei, S.-S. Li, J. Li and S.-H. Wei, Adv. Funct. Mater., 2014, 24, 7025–7031 CrossRef CAS.
- N. Huo, Z. Wei, X. Meng, J. Kang, F. Wu, S.-S. Li, S.-H. Wei and J. Li, J. Mater. Chem. C, 2015, 3, 5467–5473 RSC.
- G. R. Bhimanapati, Z. Lin, V. Meunier, Y. Jung, J. Cha, S. Das, D. Xiao, Y. Son, M. S. Strano, V. R. Cooper, L. Liang, S. G. Louie, E. Ringe, W. Zhou, S. S. Kim, R. R. Naik, B. G. Sumpter, H. Terrones, F. Xia, Y. Wang, J. Zhu, D. Akinwande, N. Alem, J. A. Schuller, R. E. Schaak, M. Terrones and J. A. Robinson, ACS Nano, 2015, 9, 11509–11539 CrossRef CAS PubMed.
- K. Chen, X. Wan, J. Wen, W. Xie, Z. Kang, X. Zeng, H. Chen and J.-B. Xu, ACS Nano, 2015, 9, 9868–9876 CrossRef CAS PubMed.
- Q. Zhang, X. Xiao, R. Zhao, D. Lv, G. Xu, Z. Lu, L. Sun, S. Lin, X. Gao, J. Zhou, C. Jin, F. Ding and L. Jiao, Angew. Chem., Int. Ed., 2015, 54, 8957–8960 CrossRef CAS PubMed.
- Y. Gong, J. Lin, X. Wang, G. Shi, S. Lei, Z. Lin, X. Zou, G. Ye, R. Vajtai, B. I. Yakobson, H. Terrones, M. Terrones, K. T. Beng, J. Lou, S. T. Pantelides, Z. Liu, W. Zhou and P. M. Ajayan, Nat. Mater., 2014, 13, 1135–1142 CrossRef CAS PubMed.
- I. Kuljanishvili, D. A. Dikin, S. Rozhok, S. Mayle and V. Chandrasekhar, Small, 2009, 5, 2523–2527 CrossRef CAS PubMed.
- R. D. Piner, J. Zhu, F. Xu, S. Hong and C. A. Mirkin, Science, 1999, 283, 661–663 CrossRef CAS PubMed.
- B. Wu, A. Ho, N. Moldovan and H. D. Espinosa, Langmuir, 2007, 23, 9120–9123 CrossRef CAS PubMed.
- S. Rozhok, R. Piner and C. A. Mirkin, J. Phys. Chem. B, 2003, 107, 751–757 CrossRef CAS.
- J. Li, C. Lu, B. Maynor, S. Huang and J. Liu, Chem. Mater., 2004, 16, 1633–1636 CrossRef CAS.
- B. W. Maynor, Y. Li and J. Liu, Langmuir, 2001, 17, 2575–2578 CrossRef CAS.
- G. Alonso, M. Del Valle, J. Cruz, A. Licea-Claverie, V. Petranovskii and S. Fuentes, Catal. Lett., 1998, 52, 55–61 CrossRef CAS.
- J. L. Brito, M. Ilija and P. Hernández, Thermochim. Acta, 1995, 256, 325–338 CrossRef CAS.
- N. Huo, S. Yang, Z. Wei, S.-S. Li, J.-B. Xia and J. Li, Sci. Rep., 2014, 4, 5209 CAS.
- K.-K. Liu, W. Zhang, Y.-H. Lee, Y.-C. Lin, M.-T. Chang, C.-Y. Su, C.-S. Chang, H. Li, Y. Shi, H. Zhang, C.-S. Lai and L.-J. Li, Nano Lett., 2012, 12, 1538–1544 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra12576j |
| This journal is © The Royal Society of Chemistry 2016 |