Facile one-pot synthesis of a NiMoO4/reduced graphene oxide composite as a pseudocapacitor with superior performance
Yongfeng Li*ab,
Jianming Jiana,
Yun Fana,
Hui Wanga,
Lin Yua,
Gao Chenga,
Junli Zhouab and
Ming Suna
aKey Laboratory of Clean Chemistry Technology of Guangdong Higher Education Institutions, School of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou, 510006, P. R. China. E-mail: gdliyf@163.com
bSchool of Chemical & Biomolecular Engineering and RBI at Georgia Tech, Georgia Institute of Technology, 500 10th Street NW, Atlanta, GA 30332-0620, USA
First published on 18th July 2016
Abstract
A hybrid NiMoO4/rGO composite was successfully synthesized by a facile one-pot hydrothermal method. A series of characterization techniques: SEM, TEM, XRD, Raman, XPS and N2 adsorption/desorption isotherms were used to verify the special nanostructure and unique composition of the as-prepared products, in which the NiMoO4 nanowires are homogenously distributed in the interconnected rGO network, and at the same time the NiMoO4 nanowires are encapsulated within the rGO sheets, further preventing the rGO nanosheets from agglomerating and restacking. As an electrode material for pseudocapacitors, the NiMoO4/rGO composite exhibited a maximum specific capacitance of 1202 F g−1 at 1 A g−1, and still remained as high as 775 F g−1 at 10 A g−1 and 592 F g−1 at 20 A g−1, which are superior to those of pure NiMoO4 nanowires. The enhanced capacitive performance of the as-prepared NiMoO4/rGO composite is closely related to the form of an internal mesoporous structure by utilizing the high electrical conductivity and large surface area of rGO nanosheets, which could provide more electroactive sites, a larger electrode–electrolyte contact area and accelerate effective ion and electron transport in the whole electrode. The results suggest that such a hybrid electrode has great potential applications in high performance energy-storage systems.
1 Introduction
Supercapacitors are considered as one of the most promising candidates for next generation power devices owing to their ultralong cycling stability, high power density and fast charge/discharge rate.1–3 Generally, on the basis of the energy storage mechanism, supercapacitors can be classified into the electrical double layer capacitors (EDLCs) and pseudocapacitors. EDLCs only involve physical adsorption of ions without any chemical reactions. Carbon materials with high surface areas, such as activated carbon, carbon nanotube and graphene, generally display double-layered capacitive behaviour, and are usually highly conductive and maintain particularly stable during the long running process.4–6 But for pseudocapacitors, the energy storage capability is determined by the fast and reversible faradic reaction near the surface.7 Transition metal compounds, including oxides,8–11 hydroxides,12–14 sulfides,15–17 phosphides,18,19 and nitrides20–22 are important electrode materials for pseudocapacitors, which usually exhibit larger specific capacitance than EDLCs and have attracted much attention in recent years. Among these materials, the transition metal molybdates, such as NiMoO4,23–25 CoMoO4 (ref. 26 and 27) and MnMoO4,28,29 have received considerable research attention due to their low cost, abundant resources, high redox activity, well-defined redox behaviour and environmental compatibility. However, some challenges are still obstructing the application of the reported electrode materials based on transition metal oxide, and one of the major drawbacks is their low electrical conductivity.30 Therefore, considering the high conductivity of carbonaceous materials, the pseudocapacitors can be commercialized in hope of a perfect combination of these transition metal materials with the high-surface-area carbon materials is expected.31–33Recently, graphene has been considered as an ideal electrode material for supercapacitor because of its outstanding electronic properties, good chemical stability and high surface area.34–36 Previous studies have exhibited that graphene (or reduced graphene oxide (rGO)) can be combined with different kinds of transition metal compounds and used as high performance supercapacitors.37–44 Herein, we prepared nanocomposites consisting of NiMoO4 nanowires and conductive rGO sheets using a simple one-pot hydrothermal method. In this composite, the rGO nanosheets function as the substrate upon which to deposit the NiMoO4 nanowires. Besides, the rGO improves not only the electrical conductivity of the hybrids, but also the effective utilization of the active material. As a result, the hybrid composite possesses a huge specific capacitance and superior cycling stability, providing with a prospective candidate for high-performance pseudocapacitors.
2 Experimental
2.1 Synthesis of NiMoO4 nanowires on graphene
Graphene oxide (GO) was synthesized from natural graphite flake by a modified Hummer's method.45 The synthesis of NiMoO4 nanowires on rGO was carried out through one-pot hydrothermal process. In a typical synthesis, 10 mg of GO was dispersed in 10 mL deionized water under ultrasonication and then 0.25 g Ni(CH3COO)2·4H2O was dissolved into the GO suspension under vigorous magnetic stirring at room temperature. After stirring for 1 h, 0.2 g (NH4)6Mo7O2·4H2O was added to the solution and the mixture was stirred for another 1 h in a water bath of 65 °C. Then the mixture was transferred into a 25 mL Teflon-lined stainless steel autoclave and kept in 160 °C for 10 h. The product was washed with deionized water and ethanol for several times and finally dried in a vacuum at 60 °C for 24 h. The as-obtained samples were denoted as NiMoO4/rGO. In addition, according to the same method without adding GO, the pure NiMoO4 nanowires was prepared to be as a contrast. And for comparison, rGO was also synthesized with the same process without the additions of Ni(CH3COO)2·4H2O and (NH4)6Mo7O2·4H2O. Meanwhile, the mechanical mixture sample of NiMoO4 nanowires and rGO (simplified as NiMoO4 + rGO) was also synthesized at same composition ratio with the NiMoO4/rGO composite prepared by one-pot hydrothermal process.2.2 Characterizations
X-ray diffraction (XRD) patterns were recorded on a Bruker D8 advance diffractometer, using Cu-Kα radiation at 40 kV and 40 mA. Raman measurements were performed on a Horiba Jobin Yvon LabRAM HR800 confocal micro-Raman spectrometer with incident laser light of 532 nm. X-ray photoelectron spectroscopy (XPS) was carried out by using Thermo Scientific ESCALAB 250XI spectrometer. Nitrogen adsorption/desorption isotherms were measured on ASAP 2020 analyser. The nanostructured samples were observed by using a field emission scanning electron microscope (SEM, JEOL JSM-7001F) and a field emission transmission electron microscope (TEM, FEI Tecnai G20).2.3 Electrochemical measurements
The electrochemical measurements were performed using a three-electrode system in 2 M KOH aqueous electrolyte at room temperature. The fabrication of working electrode was carried out as follows. The as-synthesized electroactive material was mixed with carbon black and polytetrafluoroethylene (PTFE) in a mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 in ethanol to produce a homogeneous paste. Then the paste was coated onto a piece of nickel foam (≈1 cm2), and then dried and pressed at a pressure of 10 MPa to give the working electrode. The mass loading of the active materials on Ni foam was around 1.0 mg cm−2 for pure NiMoO4 and NiMoO4 + rGO mechanical mixture, and 0.8 mg cm−2 for hybrid NiMoO4/rGO composite. A platinum foil (1.5 × 1.5 cm2) and a saturated calomel electrode (SCE) served as the counter and reference electrodes respectively. Cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS) measurements were carried out on the Autolab PGSTAT302N electrochemical workstation. Impedance spectroscopy tests were performed at open circuit potential with a potential amplitude of 5 mV in the frequency range from 100 kHz to 0.01 Hz. The specific capacitance of the electrode were calculated according to the following equations:
5 in ethanol to produce a homogeneous paste. Then the paste was coated onto a piece of nickel foam (≈1 cm2), and then dried and pressed at a pressure of 10 MPa to give the working electrode. The mass loading of the active materials on Ni foam was around 1.0 mg cm−2 for pure NiMoO4 and NiMoO4 + rGO mechanical mixture, and 0.8 mg cm−2 for hybrid NiMoO4/rGO composite. A platinum foil (1.5 × 1.5 cm2) and a saturated calomel electrode (SCE) served as the counter and reference electrodes respectively. Cyclic voltammetry (CV), galvanostatic charge–discharge and electrochemical impedance spectroscopy (EIS) measurements were carried out on the Autolab PGSTAT302N electrochemical workstation. Impedance spectroscopy tests were performed at open circuit potential with a potential amplitude of 5 mV in the frequency range from 100 kHz to 0.01 Hz. The specific capacitance of the electrode were calculated according to the following equations:
 | (1) |
3 Results and discussion
Fig. 1 briefly illustrates the fabrication of NiMoO4/rGO composite through a simple one-pot hydrothermal process. When the Ni(CH3COO)2·4H2O was mixed with the GO suspension, metal cation of Ni2+ was adsorbed on the surface of GO due to the electrostatic attraction between the Ni2+ and the negatively charged oxygen-containing functional groups of GO, such as C–OH and C–O–C. Followed by adding (NH4)6Mo7O2·4H2O, the mixture was transferred into an autoclave and heated at 160 °C for 10 h. The reduction of GO and the in situ formation of NiMoO4 were achieved simultaneously, causing the generation of NiMoO4/rGO.The morphologies of rGO, pure NiMoO4 and hybrid NiMoO4/rGO were investigated by SEM and TEM. In Fig. 2a, the rGO shows a sheet-like morphology with characteristic rough and crumpled architecture due to its high flexibility and strong interaction between nanosheet layers. For NiMoO4/rGO composite (Fig. 2c), the NiMoO4 nanowires are homogenously distributed in the interconnected rGO network compared to pure NiMoO4 sample (Fig. 2b). Meanwhile, as seen from the TEM image in Fig. 2e, the NiMoO4 nanowires are encapsulated within the rGO sheets, which can efficiently prevent the rGO nanosheets from agglomerating and restacking. Such a nanostructured surface morphology can provide more voids among the interconnected NiMoO4 nanowires, resulting in improving contact area and shortening diffusion path between the electrode material and the electrolyte.46 From the HRTEM image presented in Fig. 2f, the ambiguous interface can be observed between NiMoO4 and rGO. The distinguishable lattice spacing of 0.214 nm is attributed to the (121) plane of NiMoO4 in the hybrid structure,47 and the crinkled lattice fringes along the edge of NiMoO4 nanowire correspond to the rGO nanosheet layers.48
XRD was used to identify the as-prepared samples and the results are shown in Fig. 3a. For GO, the diffraction peak at 2θ = 10.6° is corresponded with the (002) interlayer spacing of 0.83 nm, indicating the deep oxidation of graphene and the introduction of the oxygen-containing functional group.49 For rGO, the peak at 10.6° disappears and a new broad diffraction peak at about 2θ = 25° appears, implying the GO is readily reduced after hydrothermal treatment at 160 °C for 10 h.50 The strong diffraction peaks of NiMoO4/rGO are observed at 2θ = 11.0, 27.2, 29.7 and 33.2°, which is in good accordance with the standard diffraction pattern of NiMoO4·xH2O (JCPDS card no. 13-0128).51 On the whole the peak intensities for NiMoO4 in hybrid NiMoO4/rGO are relatively weak compared with those in pure NiMoO4 nanowires. However, the peak intensity at 2θ = 27.2° in NiMoO4/rGO composite is similar with that in pure NiMoO4 nanowires, which is due to the broad diffraction peak at 2θ = 20–30° of rGO and verifies the existence of rGO in the hybrid composite. So the XRD analysis proves the successful preparation of NiMoO4/rGO.
 | ||
| Fig. 3 (a) XRD patterns and (b) Raman spectra of GO, rGO, pure NiMoO4 nanowires and hybrid NiMoO4/rGO composite. | ||
The Raman spectra of GO, rGO, pure NiMoO4 and hybrid NiMoO4/rGO are shown in Fig. 3b. The Raman spectrum of GO or rGO exhibits a G band at 1601 cm−1 which is related to the vibration of sp2-bonded carbon atoms in two degree hexagonal lattice, and a D band at 1357 cm−1 which corresponds to the defects and disorder in the hexagonal graphitic layer.52,53 For pure NiMoO4 nanowires, the Raman spectrum shows an intense peak at 945 cm−1 along with some medium intensity peaks appearing at 860, 825 and 357 cm−1, which can be attributed to the characteristic peaks of NiMoO4.54,55 The main peaks of rGO and NiMoO4 are all observed in the Raman spectrum of NiMoO4/rGO composite again verifying the successful synthesis of hybrid NiMoO4/rGO composite.
The intensity ratio of the D to G band (ID/IG) can be used to estimate the degree of defect in carbonaceous material.56 As shown in Fig. 3b, the ID/IG value of rGO (1.05) is higher than that of GO (0.98), indicating the rGO has a small size and a large quantity of edges which act as defects and lead to an increased D peak.57,58 In addition, compared with the pure rGO (1.05), a higher ID/IG ratio is obtained for the hybrid NiMoO4/rGO (1.09), suggesting that the rGO was greatly reduced and formed a high level disordered structure during the hydrothermal process in the presence of Ni2+ or NH4+. As we know, increasing the defect and disorder of rGO can provide more active sites for electron storage and enhance the binding energy with ions in electrolyte.59 So the as-synthesized NiMoO4/rGO composite is expected to have a promising application as an efficient electrode for high-performance pseudocapacitors.
Information on the chemical composition of the as-prepared hybrid is obtained from XPS measurement, just as shown in Fig. 4 and Table 1. The XPS survey spectrum of NiMoO4/rGO (Fig. 4b) indicates four dominant elements: C, O, Ni and Mo with atomic percentages of 32.24, 47.05, 10.59 and 9.94% respectively. The atomic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo is 1.06
Mo is 1.06![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is close to the stoichiometry of NiMoO4, further proving the formation of NiMoO4. As seen in Table 1, because the O in hybrid NiMoO4/rGO is from both MoO42− and rGO, the percentage of O in rGO is 7.29% (47.05% minus 39.76%). The ratio of C/O in hybrid NiMoO4/rGO (4.42) is higher than that in pure GO (2.10), indicating again the oxygen-containing functional groups were removed after the hydrothermal process.39 The mass amount of NiMoO4 in the hybrid NiMoO4/rGO composite calculated from the XPS data is 81.5%.
1, which is close to the stoichiometry of NiMoO4, further proving the formation of NiMoO4. As seen in Table 1, because the O in hybrid NiMoO4/rGO is from both MoO42− and rGO, the percentage of O in rGO is 7.29% (47.05% minus 39.76%). The ratio of C/O in hybrid NiMoO4/rGO (4.42) is higher than that in pure GO (2.10), indicating again the oxygen-containing functional groups were removed after the hydrothermal process.39 The mass amount of NiMoO4 in the hybrid NiMoO4/rGO composite calculated from the XPS data is 81.5%.
| Atomic (%) | C | O | Ni | Mo | C/O |
|---|---|---|---|---|---|
| Hybrid NiMoO4/rGO | 32.24 | 47.05 | 10.59 | 9.94 | — |
| NiMoO4 in hybrid | 39.76 | 10.59 | 9.94 | — | |
| rGO in hybrid | 32.24 | 7.29 | — | — | 4.42 |
| Pure GO | 67.79 | 32.21 | — | — | 2.10 |
The C 1s core level XPS spectra could be deconvoluted into three synthetic peaks, just as seen in Fig. 4c and d. The peaks centered at 284.6, 286.0 and 288.3 eV are assigned to the sp2 carbon atoms (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), hydroxyl/epoxy groups (C–O) and carbonyl group (C
C), hydroxyl/epoxy groups (C–O) and carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) respectively.57 The peak intensity of C–O in NiMoO4/rGO decreases dramatically compared with that in GO, which further indicates that the C–O–C and C–OH groups were selectively occupied by Ni2+ or Mo7O26− and then reduced during the one-pot hydrothermal synthesis.57
O) respectively.57 The peak intensity of C–O in NiMoO4/rGO decreases dramatically compared with that in GO, which further indicates that the C–O–C and C–OH groups were selectively occupied by Ni2+ or Mo7O26− and then reduced during the one-pot hydrothermal synthesis.57
For high resolution scan of Ni 2p (see Fig. 4e), there are two main peaks at 856.0 and 873.8 eV assigned to Ni 2p3/2 and Ni 2p1/2 respectively, with a spin energy separation of 17.8 eV. Meanwhile the satellite peaks (Ni 2p3/2 satellite at 862.3 eV and Ni 2p1/2 satellite at 880.5 eV) are also observed, which are associated with the Ni2+ oxidation state.58 The Mo 3d core level spectrum (Fig. 4f) shows two peak at 232.4 and 235.5 eV, corresponding to Mo 3d5/2 and Mo 3d3/2 respectively with a spin energy separation of 3.1 eV, which signifies a Mo6+ oxidation state.54 So the XPS results again proves the formation of NiMoO4/rGO composite.
The electrochemical performance of the as-prepared samples partially depends on their specific surface area and the porous structure, which were characterized by nitrogen adsorption/desorption measurement. As see in Fig. 5, a type IV isotherm plot with a distinct hysteresis loop for the pure NiMoO4 and hybrid NiMoO4/rGO occurs in the range of 0.6–1.0 p/p0, indicating the existence of mesopores in the materials. The corresponding pore size distribution derived from the adsorption branches of isotherms in the inset of Fig. 5, further confirms the mesoporous nature of the samples by the sharp peaks in the pore size range of 2–6 nm. Meanwhile, compared to the pure NiMoO4 (0.056 cm3 g−1), the hybrid NiMoO4/rGO composite shows a more Barrett–Joyner–Halenda (BJH) mesoporous volume of 0.072 cm3 g−1, which can be attributed to the presence of ultrathin rGO nanosheets. These ultrathin rGO nanosheets create voids between adjacent NiMoO4 nanowires that increase the surface area. So the calculated Brunauer–Emmett–Teller (BET) surface area of the NiMoO4/rGO was 58.1 m2 g−1, which was higher than that of pure NiMoO4 nanowires (32.1 m2 g−1). This mesoporous structure with higher specific surface area and more mesopore volume has potential applications in pseudocapacitors, as it provides more electroactive sites and larger interface of active electrode materials with electrolyte solution.60
 | ||
| Fig. 5 Nitrogen adsorption/desorption isotherm plots and Barrett–Joyner–Halenda (BJH) pore size distribution of (a) pure NiMoO4 nanowires and (b) hybrid NiMoO4/rGO composite. | ||
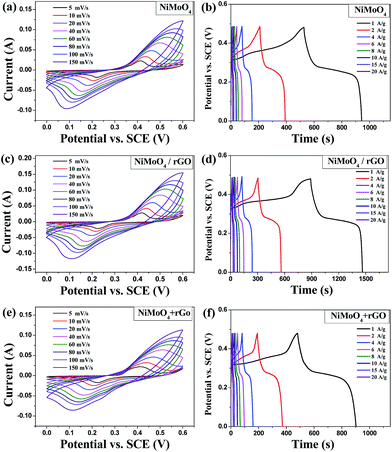
The electrochemical properties of as-synthesized materials as electrodes were explored in a three-electrode system. The typical cyclic voltammetry (CV) curves of pure NiMoO4 nanowires, hybrid NiMoO4/rGO composite and NiMoO4 + rGO mechanical mixture within the potential window of 0–0.6 V at various scan rates are shown in Fig. 6a, c and e. All are observed a couple of well-defined redox peaks, indicating the capacitance characteristics are mainly governed by faradaic redox reactions, which possibly corresponds to the reversible conversion between Ni3+/Ni2+.54 Besides, in contrast to the CV curve of pure NiMoO4 nanowires and NiMoO4 + rGO mechanical mixture, the hybrid NiMoO4/rGO electrode exhibits larger enclosed CV curve area at the same scan rate (as seen in Fig. 7a), indicating higher capacitance. This is in good accordance with the results obtained by the galvanostatic charge–discharge measurement in Fig. 6b, d and f. The specific capacitance of the electrodes calculated from the discharge curves are shown in Fig. 7b. The capacitance value of NiMoO4/rGO composite electrode is 1202 F g−1 at the current density of 1 A g−1, which is much higher than that of pure NiMoO4 (873 F g−1) and NiMoO4 + rGO mechanical mixture at the same current density. Meanwhile, the specific capacitance of pure NiMoO4 and hybrid NiMoO4/rGO decreases gradually with increasing the current density. However, the hybrid NiMoO4/rGO still exhibits excellent pseudocapacitance of 903, 775, 653 and 592 F g−1 at the high current densities of 6, 10, 15 and 20 A g−1 respectively. For comparison, the capacitance of pure NiMoO4 is only 493, 392, 320 and 267 F g−1 at the same high current densities of 6, 10, 15 and 20 A g−1 respectively. And the capacitances of NiMoO4 + rGO mechanical mixture were just similar with those of pure NiMoO4. Impressively, these results are remarkable as compared to the previous reported NiMoO4 and graphene based composites. For example, the specific capacitance of the graphene decorated with 1D NiMoO4·nH2O nanorods is 312 F g−1 at 5 A g−1;54 the mesoporous NiMoO4 nanorod/rGO composite prepared by a microwave solvothermal method exhibits much lower specific capacitance of 573 F g−1 at high current density of 10 A g−1 although its initial capacitance is 1274 F g−1 at 1 A g−1.61 The clearly enhanced capacitive performance of hybrid NiMoO4/rGO composite is closely related to the excellent electrical conductivity of the rGO sheets in the composite, which can act as the current collector to decrease the electrode resistance. Besides, the large surface area of rGO sheets facilitates the uniform growth of NiMoO4 nanowires, and on the other hand the well-dispersed NiMoO4 nanowires further prevent the agglomerating and restacking of rGO nanosheets. Therefore, the uniform nanostructure of as-prepared NiMoO4/rGO composite can provide a larger electrode–electrolyte contact area, improve the electrochemical utilization of active material, shorten the diffusion length for adsorbing ions, and accelerate the electron transfer during the charge–discharge process.40,62
Fig. 8a shows the cycling performance of the pure NiMoO4 nanowires, hybrid NiMoO4/rGO composite and NiMoO4 + rGO mechanical mixture at current density of 20 A g−1. The specific recharge capacitance of NiMoO4/rGO composite maintains 66.1% in contrast with 57.1% capacitance retention of pure NiMoO4 for 3000 cycles under same experimental condition, indicating that the rGO significantly improves the cyclic stability of NiMoO4 in hybrids. Meanwhile capacitance retention of synthesized NiMoO4/rGO composite was also higher than that of NiMoO4 + rGO mechanical mixture. In addition, for NiMoO4/rGO electrode, the specific capacitance decreases about 30% of the initial capacitance during the first 1000 cycles, which can be generally explained by the irreversible of the Faraday reactions or the dissolution of active materials from the hybrid nanostructures at the beginning of the cycle test.63 Then it only shows a slight decrease of 4% after the rest 2000 cycles, exhibiting relatively good stability. And from the SEM image of NiMoO4/rGO electrode material after cycling test in Fig. 2d, it showed that cycling test has little effect on the morphology of the composite, indicating that the structure of NiMoO4 nanowires on rGO nanosheets is still maintained.
 | ||
| Fig. 8 (a) Cycling performance at 20 A g−1 and (b) EIS spectra for pure NiMoO4 nanowires, hybrid NiMoO4/rGO composite and NiMoO4 + rGO mechanical mixture electrodes. | ||
To further understand the superior performance of the NiMoO4/rGO composite, the kinetic feature of the corresponding electrode was investigated using electrochemical impedance spectroscopy (EIS) in 2 M KOH solution. Fig. 8b shows the Nyquist plots extracted from the EIS measurements on pure NiMoO4 nanowires, hybrid NiMoO4/rGO composite and NiMoO4 + rGO mechanical mixture. It can be seen that all impedance spectra are composed of a semicircle at high frequency and a straight line at low frequency. At the high frequency region, the semicircle is corresponding to faradic resistance caused by the interfacial electron transport in electroactive material, and the diameter of the semicircle reflects the electron transfer resistance on the electrode surface.54,64 As shown in Fig. 8b, the NiMoO4/rGO composite exhibits much lower faradic resistance than pure NiMoO4 and NiMoO4 + rGO mechanical mixture, suggesting that the introduction of rGO by chemical hydrothermal method significantly improves the electrical conductivity of the composite. At the low frequency region, the slope of the line is associated with Warburg resistance resulted from ion diffusion in the electrolyte to the electrode interface.57 Compared with pure NiMoO4 and NiMoO4 + rGO mechanical mixture, the NiMoO4/rGO composite shows a more vertical curve in the low frequency region, indicating the fast ion diffusion in the electrolyte and adsorption onto the electrode surface. Therefore, within the as-prepared NiMoO4/rGO composite, the introduction of rGO by hydrothermal method can dramatically improve the energy storage performance of NiMoO4, by favouring the electron transfer of NiMoO4 and increasing the efficient access of electrolyte ions.
4 Conclusions
In summary, we have successfully synthesized hybrid NiMoO4/rGO composite using a facile one-pot hydrothermal process. In the composite, the NiMoO4 nanowires are homogenously distributed into the interconnected rGO network, and at the same time the rGO nanosheets are prevented from agglomerating and restacking by encapsulating the NiMoO4 nanowires into its sheet layers. Due to their special nanostructure and unique composition, the as-prepared NiMoO4/rGO composite shows a great enhancement in electrical conductivity, specific capacitance and cycling stability by comparison with the pure NiMoO4. Its maximum specific capacitance is 1202 F g−1 at 1 A g−1, and still as high as 775 F g−1 at 10 A g−1 and 592 F g−1 at 20 A g−1, which are comparable or superior to those so far reported for the graphene and NiMoO4 hybrids in alkaline electrolyte. The excellent electrochemical performance of the hybrid NiMoO4/rGO composite renders their application as a candidate electrode material for the high-performance pseudocapacitors of energy storage.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21576054), Guangdong Province Science and Technology Project (Grant No. 2016A020221033), Pearl River Nova Program of Guangzhou (Grant No. 2011J2200041), Guangdong Province Science and Technology Project (Grant No. 2012A030600006), Science and Technology Achievements Transformation Projects of Guangdong Higher Education Institutes (Grant No. cgzhzd1104).References
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- T. Lin, I. W. Chen, F. Liu, C. Yang, H. Bi, F. Xu and F. Huang, Science, 2015, 350, 1508–1513 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, M. D. Stoller, K. J. Ganesh, W. Cai, P. J. Ferreira, A. Pirkle, R. M. Wallace, K. A. Cychosz, M. Thommes, D. Su, E. A. Stach and R. S. Ruoff, Science, 2011, 332, 1537–1541 CrossRef CAS PubMed.
- Y. Zhang, Z. Zhen, Z. Zhang, J. Lao, J. Wei, K. Wang, F. Kang and H. Zhu, Electrochim. Acta, 2015, 157, 134–141 CrossRef CAS.
- S. Liu, S. H. Sun and X. Z. You, Nanoscale, 2014, 6, 2037–2045 RSC.
- M. Y. Ho, P. S. Khiew, D. Isa, T. K. Tan, W. S. Chiu and C. H. Chia, Nano, 2014, 9, 25 CrossRef.
- K. Dai, L. Lu, C. Liang, J. Dai, Q. Liu, Y. Zhang, G. Zhu and Z. Liu, Electrochim. Acta, 2014, 116, 111–117 CrossRef CAS.
- X. Xu, L. Pei, Y. Yang, J. Shen and M. Ye, J. Alloys Compd., 2016, 654, 23–31 CrossRef CAS.
- Z.-D. Ni, Y.-T. Song, H.-Q. Chen and L.-Y. Lin, Electrochim. Acta, 2016, 190, 313–321 CrossRef CAS.
- J. Jiang, A. Zhang, L. Li and L. Ai, J. Power Sources, 2015, 278, 445–451 CrossRef CAS.
- J. Zhao, J. Chen, S. Xu, M. Shao, Q. Zhang, F. Wei, J. Ma, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 2938–2946 CrossRef CAS.
- Y. Wimalasiri, R. Fan, X. S. Zhao and L. Zou, Electrochim. Acta, 2014, 134, 127–135 CrossRef CAS.
- L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li and T. Wang, Nano Energy, 2014, 3, 36–45 CrossRef CAS.
- Y. Ruan, J. Jiang, H. Wan, X. Ji, L. Miao, L. Peng, B. Zhang, L. Lv and J. Liu, J. Power Sources, 2016, 301, 122–130 CrossRef CAS.
- J.-J. Li, Y.-X. Hu, M.-C. Liu, L.-B. Kong, Y.-M. Hu, W. Han, Y.-C. Luo and L. Kang, J. Alloys Compd., 2016, 656, 138–145 CrossRef CAS.
- C. An, Y. Wang, Y. Wang, G. Liu, L. Li, F. Qiu, Y. Xu, L. Jiao and H. Yuan, RSC Adv., 2013, 3, 4628–4633 RSC.
- X. Wang, W. Li, D. Xiong and L. Liu, J. Mater. Chem. A, 2016, 4, 5639–5646 CAS.
- X. Lu, M. Yu, T. Zhai, G. Wang, S. Xie, T. Liu, C. Liang, Y. Tong and Y. Li, Nano Lett., 2013, 13, 2628–2633 CrossRef CAS PubMed.
- X. Lu, T. Liu, T. Zhai, G. Wang, M. Yu, S. Xie, Y. Ling, C. Liang, Y. Tong and Y. Li, Adv. Energy Mater., 2014, 4, 1300994 CrossRef.
- W. Li, C.-Y. Cao, C.-Q. Chen, Y. Zhao, W.-G. Song and L. Jiang, Chem. Commun., 2011, 47, 3619–3621 RSC.
- D. Guo, Y. Z. Luo, X. Z. Yu, Q. H. Li and T. H. Wang, Nano Energy, 2014, 8, 174–182 CrossRef CAS.
- Z. Yin, S. Zhang, Y. Chen, P. Gao, C. Zhu, P. Yang and L. Qi, J. Mater. Chem. A, 2015, 3, 739–745 CAS.
- K. Xiao, L. Xia, G. Liu, S. Wang, L.-X. Ding and H. Wang, J. Mater. Chem. A, 2015, 3, 6128–6135 CAS.
- D. Cai, B. Liu, D. Wang, L. Wang, Y. Liu, H. Li, Y. Wang, Q. Li and T. Wang, J. Mater. Chem. A, 2014, 2, 4954–4960 CAS.
- X. Xia, W. Lei, Q. Hao, W. Wang and X. Wang, Electrochim. Acta, 2013, 99, 253–261 CrossRef CAS.
- G. K. Veerasubramani, K. Krishnamoorthy, R. Sivaprakasam and S. J. Kim, Mater. Chem. Phys., 2014, 147, 836–842 CrossRef CAS.
- D. Ghosh, S. Giri, M. Moniruzzaman, T. Basu, M. Mandal and C. K. Das, Dalton Trans., 2014, 43, 11067–11076 RSC.
- B. Wang, S. Li, X. Wu, W. Tian, J. Liu and M. Yu, J. Mater. Chem. A, 2015, 3, 13691–13698 CAS.
- Z. Yang, X. Zhou, Z. Jin, Z. Liu, H. Nie, X. Chen and S. Huang, Adv. Mater., 2014, 26, 3156–3161 CrossRef CAS PubMed.
- X. Xia, Y. Zhang, Z. Fan, D. Chao, Q. Xiong, J. Tu, H. Zhang and H. J. Fan, Adv. Energy Mater., 2015, 5, 1401709 CrossRef.
- C. Yu, J. Yang, C. Zhao, X. Fan, G. Wang and J. Qiu, Nanoscale, 2014, 6, 3097–3104 RSC.
- J. Zhu, D. Yang, Z. Yin, Q. Yan and H. Zhang, Small, 2014, 10, 3480–3498 CrossRef CAS PubMed.
- V. Chabot, D. Higgins, A. P. Yu, X. C. Xiao, Z. W. Chen and J. J. Zhang, Energy Environ. Sci., 2014, 7, 1564–1596 CAS.
- M. Yu, Y. Huang, C. Li, Y. Zeng, W. Wang, Y. Li, P. Fang, X. Lu and Y. Tong, Adv. Funct. Mater., 2015, 25, 324–330 CrossRef CAS.
- H. Zhou, X. Yang, J. Lv, Q. Dang, L. Kang, Z. Lei, Z. Yang, Z. Hao and Z.-H. Liu, Electrochim. Acta, 2015, 154, 300–307 CrossRef CAS.
- Y. Yang, Y. Hao, J. yuan, L. Niu and F. Xia, Carbon, 2015, 84, 174–184 CrossRef CAS.
- X. Xu, F. Xia, L. Zhang and J. Gao, Sci. Adv. Mater., 2015, 7, 423–432 CrossRef CAS.
- L. Ma, X. Shen, H. Zhou, Z. Ji, K. Chen and G. Zhu, Chem. Eng. J., 2015, 262, 980–988 CrossRef CAS.
- Y. Liu, Z. Cheng, H. Sun, H. Arandiyan, J. Li and M. Ahmad, J. Power Sources, 2015, 273, 878–884 CrossRef CAS.
- M. Li, B. Dong, G. Gao and S. Ding, Mater. Lett., 2015, 155, 30–33 CrossRef CAS.
- X. Li, J. Shen, N. Li and M. Ye, Mater. Lett., 2015, 139, 81–85 CrossRef CAS.
- Q. Ke, Y. Liao, S. Yao, L. Song and X. Xiong, J. Mater. Sci., 2016, 51, 2008–2016 CrossRef CAS.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Chem. Mater., 1999, 11, 771–778 CrossRef CAS.
- X. A. Chen, X. Chen, F. Zhang, Z. Yang and S. Huang, J. Power Sources, 2013, 243, 555–561 CrossRef CAS.
- D. Cheng, Y. Yang, J. Xie, C. Fang, G. Zhang and J. Xiong, J. Mater. Chem. A, 2015, 3, 14348–14357 CAS.
- H. Quan, B. Cheng, Y. Xiao and S. Lei, Chem. Eng. J., 2016, 286, 165–173 CrossRef CAS.
- K. Krishnamoorthy, M. Veerapandian, K. Yun and S. J. Kim, Carbon, 2013, 53, 38–49 CrossRef CAS.
- Y. Xu, K. Sheng, C. Li and G. Shi, ACS Nano, 2010, 4, 4324–4330 CrossRef CAS PubMed.
- M. C. Liu, L. Kang, L. B. Kong, C. Lu, X. J. Ma, X. M. Li and Y. C. Luo, RSC Adv., 2013, 3, 6472–6478 RSC.
- J. I. Paredes, S. Villar-Rodil, P. Solis-Fernandez, A. Martinez-Alonso and J. M. Tascon, Langmuir, 2009, 25, 5957–5968 CrossRef CAS PubMed.
- L.-S. Zhang, W. Li, Z.-M. Cui and W.-G. Song, J. Phys. Chem. C, 2009, 113, 20594–20598 CAS.
- D. Ghosh, S. Giri and C. K. Das, Nanoscale, 2013, 5, 10428–10437 RSC.
- A. Maione and M. Devillers, J. Solid State Chem., 2004, 177, 2339–2349 CrossRef CAS.
- W. Li, L.-S. Zhang, Q. Wang, Y. Yu, Z. Chen, C.-Y. Cao and W.-G. Song, J. Mater. Chem., 2012, 22, 15342–15347 RSC.
- S. Min, C. Zhao, Z. Zhang, G. Chen, X. Qian and Z. Guo, J. Mater. Chem. A, 2015, 3, 3641–3650 CAS.
- B. Dong, H. Zhou, J. Liang, L. Zhang, G. Gao and S. Ding, Nanotechnology, 2014, 25, 435403 CrossRef PubMed.
- H. Liu, J. Zhang, D. Xu, B. Zhang, L. Shi, L. Huang and S. Tan, Appl. Surf. Sci., 2014, 317, 370–377 CrossRef CAS.
- Y. Li, H. Wang, J. Jian, Y. Fan, L. Yu, G. Cheng, J. Zhou and M. Sun, RSC Adv., 2016, 6, 13957–13963 RSC.
- T. Liu, H. Chai, D. Jia, Y. Su, T. Wang and W. Zhou, Electrochim. Acta, 2015, 180, 998–1006 CrossRef CAS.
- G. K. Veerasubramani, K. Krishnamoorthy and S. J. Kim, RSC Adv., 2015, 5, 16319–16327 RSC.
- H. Zhang, Y. Chen, W. Wang, G. Zhang, M. Zhuo, H. Zhang, T. Yang, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 8593–8600 CAS.
- X. Li, Y. Feng, M. Li, W. Li, H. Wei and D. Song, Adv. Funct. Mater., 2015, 25, 6858–6866 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |