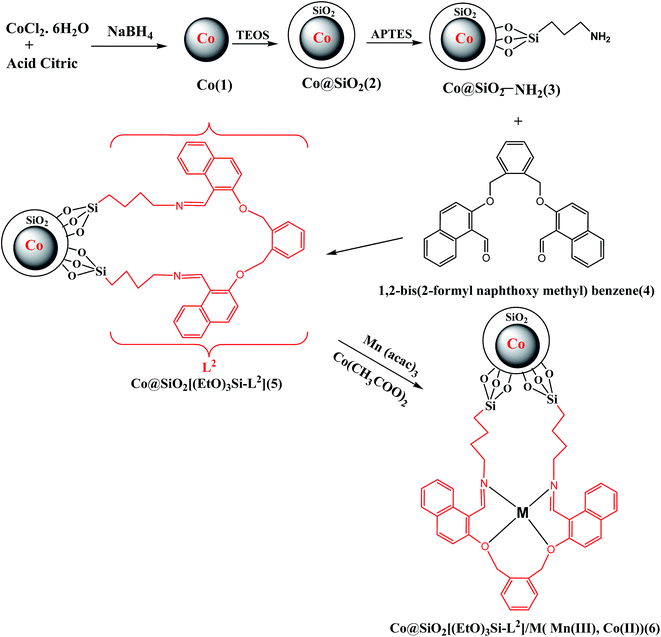
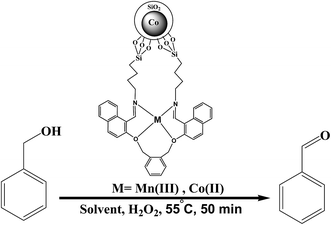
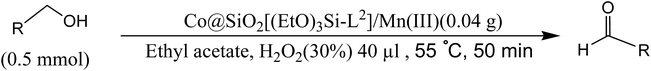Novel Schiff base Mn(III) and Co(II) complexes supported on Co nanoparticles: efficient and recyclable magnetic nanocatalysts for alcohol oxidation
Hassan Keypour*a,
Shokoufeh Ghahri Saremia,
Hojat Veisib and
Reza Azadbakhtb
aFaculty of Chemistry, Bu-Ali Sina University, Hamedan 65174, Iran. E-mail: haskey1@yahoo.com
bDepartment of Chemistry, Payame Noor University, Tehran, Iran
First published on 9th August 2016
Abstract
In this study, efficient and highly selective heterogeneous catalysts were developed by immobilization of manganese and cobalt Schiff base complexes on Co magnetite nanoparticles (MNPs). Firstly, Co@SiO2 core–shell nanoparticles were synthesized through a one-pot reaction. Secondly, the Co@SiO2 was amino-functionalized using 3-aminopropyl triethoxysilane and then Schiff base ligand Co@SiO2[(EtO)3Si–L2] was obtained by the reaction of the amino-functionalized nanoparticles with the dialdehyde of 1,2-bis(2-formyl naphthoxy methyl)benzene. Finally, Co@SiO2[(EtO)3Si–L2]/M (M = Mn(III) and Co(II)) were successfully synthesized. These surface-modified nanoparticles were characterized using various techniques such as TEM, XRD, TGA, SEM, VSM, XPS, EDX and FT-IR. The catalytic activities of the prepared catalysts were investigated by employing them in the oxidation of various aromatic alcohols with an environmentally friendly oxidant under mild conditions. The catalysts can be readily recovered and reused in at least 5 consecutive cycles without significant leaching and loss their catalytic activity.
Introduction
One of the most stimulating features of nanotechnology is its potential use for in almost any field. The “nano” in nanocatalysis refers to very small particles in the nanoscale range of size. Because nanoparticles have a large surface-to-volume ratio relative to bulk materials, they offer an attractive alternative to conventional catalysts.1–3 Thus, nanocatalysts benefit from several advantages over conventional catalyst systems; however, isolation and recovery of these tiny nanocatalysts from the reaction mixture is not easy. This limitation hampers the economics and sustainability of these nanocatalytic protocols. To overcome this issue, the use of magnetic nanoparticles has emerged as a viable solution; their insoluble and paramagnetic nature enables easy and efficient separation of the catalysts from the reaction mixture with an external magnet.4–11Oxidation reactions are vitally important to the chemical industry because of their potential for generating a variety of fine chemicals and drugs. Conventionally, oxidation has been achieved noncatalytically using stoichiometric quantities of oxidants (such as chromium and manganese oxides) in the presence of corrosive mineral acids, which in turn produces huge amounts of toxic waste. Because of growing environmental concerns, continuous efforts have been made to develop oxidation protocols using green chemistry principles with a focus on the development of easily recoverable and reusable heterogeneous catalysts. In light of these concerns, the use of magnetic nanomaterials as a solid support for heterogeneously catalyzed oxidation reactions has proven to be highly effective.12–16
Schiff base transition metal complexes have been extensively studied because of their potential use as catalysts in a wide range of reactions.17,18 These complexes have been extensively used for hydrogenation of organic substrates,19 epoxidation of olefins,20 conversion of epoxides into halohydrins,21,22 asymmetric ring opening of terminal epoxides23 and oxidation reactions.24,25
In recent years, various supports have been reported for the immobilization of Mn(III) complexes. These supports are include activated carbon26,27 and silica.28–32 Therefore, here we report the further chemically modification of the surface tethered amino groups with Schiff-base ligands through reaction between amine and aldehyde groups of 1,2-bis(2-formyl naphthoxy methyl)benzene to form corresponding imines. Also, we studied the catalytic behavior of Co(II) and Mn(III) complexes of the Schiff base ligand as heterogeneous catalysts in the oxidation of various aromatic alcohols with environmentally friendly oxidant.
Experimental
Materials and equipment's
The reagents and solvents used in this work were obtained from Fluka or Merck and used without further purification. Nanostructures were characterized using a Holland Philips PW-1840 with monochromated Cu Kα radiation and λ 1.54 Å X-ray powder diffraction (XRD) diffractometer, at a scanning speed of 2° min−1 from 5° to 80° (2θ). Magnetic measurement of materials was investigated with a vibrating sample magnetometer (VSM) at room temperature. The particle size and morphology surface were investigated by scanning electron microscopy (SEM) of a Holland Philips XL30 microscope with an accelerating voltage of 25 kV. The FT-IR measurements were performed using KBr disc on a Shimadzu Fourier Transform Infrared spectra (FT-IR) 8400. Thermogravimetric Analysis (TGA) was taken on a Mettler Toledo TGA SDTA 85-e instrument at 30–800 °C with 10 °C min−1. Transmission electron microscopy (TEM) images were obtained on an EM10C (Zeiss) transmission electron microscope at an accelerating voltage of 80 kV. XPS (small area X-ray photoelectron spectroscopy) data were recorded with the PHI-5702 Multi-Technique System.Preparation of the magnetic Co@SiO2 nanoparticles
Step-by-step synthesis of the nanocatalyst (6) is shown in Scheme 1. In order to preparation of the magnetic Co@SiO2 nanoparticles (2) firstly, cobalt nanoparticles (1) were synthesized according to the procedure previously reported in literature.33 In a ordinary method, 0.07 g citric acid was added to 60 ml aqueous solution contain 1 g cobalt chloride hexahydrate under N2 atmosphere and this solution is stirred for 2 min. Then in a beaker, 3 g sodium borhydride was dissolved in 100 ml water and drop wise added to the stock solution under nitrogen atmosphere. With the first drop of NaBH4, the color of the solution was quickly changed to black.Secondly, cobalt magnetic core was protected and coated with SiO2 shell to obtain core–shell nanoparticles Co@SiO2. The solution containing Co nanoparticles was left be stirred for 30 minutes. Then 200 ml ethanolic solution of 200 ml of 3-aminopropyl-triethoxysilane (APTES) and 800 ml tetraethoxysilane (TEOS) was added to the stock solution under nitrogen atmosphere. The nitrogen atmosphere was cut off and the solution was left be strongly stirred for 24 h. Finally, a puffy black precipitate with strong magnetic property was obtained. This precipitate was collected using a magnet and washed for several times with deionized water and ethanol. Then the precipitate Co@SiO2 was dried under vacuum at room temperature.
Preparation of Co@SiO2[(EtO)3Si–L2] (5)
3 g of magnetic nanoparticles Co@SiO2 was distributed and sonicated in 200 ml toluene for 50 min. 4.2 ml (17.93 mmol) of APTES was added dropwise to the suspended solid under vigorous mechanical stirring. The mixture was refluxed at 120 °C for 24 h. Eventually, the reaction mixture was cooled down to room temperature. The resulting amine functionalized MNPs were magnetically separated by external magnet and washed for several times with deionized water and ethanol. Then the precipitate was left to be dried at 55 °C in the oven.In this step, in order to Schiff base reaction with Co@SiO2–NH2 (3), dialdehyde-1,2-bis(2-formyl naphthoxy methyl) benzene (4) was synthesized.
300 ml acetonitrile solution includes 2.62 g (10 mmol) 1,2-bis(bromomethyl)benzene, 3.44 g (20 mmol) 2-hydroxy-1-naphthaldehyde and 2.79 g (20 mmol) potassium carbonate were refluxed for 48 h. Then the solution was allowed to cool and the product formed as colourless crystals (3.3 g, yield: 72%). Anal. calcd for C30H22O4: C, 80.70; H, 4.97. Found: C, 80.84; H, 5.03. FT-IR (KBr), cm−1: 1682 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). 1H NMR (CDCl3, 500 MHz) δ (ppm): 5.34 (s, 4H, O–CH2–Ar), 7.21–9.1 (m, 12H, Ar), 10.95 (s, 2H, CH
O). 1H NMR (CDCl3, 500 MHz) δ (ppm): 5.34 (s, 4H, O–CH2–Ar), 7.21–9.1 (m, 12H, Ar), 10.95 (s, 2H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Typically procedure for the preparation of the Co@SiO2[(EtO)3Si–L2] (5), explained as follow:
1 g of Co@SiO2–NH2 was distributed and sonicated in 80 ml of dry methanol for 20 min, 3.12 g (7 mmol) of 1,2-bis(2-formyl naphthoxy methyl) benzene were added and the mixture was stirred and heated at 50 °C for 24 h. After cooling the reaction mixture to room temperature, the magnetic Co@SiO2[(EtO)3Si–L2] nanoparticles were collected using a magnet and washed for several times with dry methanol and dried under a vacuum for 20 h.
Preparation of Co@SiO2[(EtO)3Si–L2]/M (M = Co(II) and Mn(III)) (6)
The Co@SiO2[(EtO)3Si–L2] (300 mg) were dispersed in ethanol (30 ml) by ultrasonic bath for 30 min. Subsequently, a solution of a ethanolic solution (10 ml) of Mn(acac)3 (1 mmol) was added to dispersion of Co@SiO2[(EtO)3Si–L2] and the mixture was stirred and refluxed for 12 hours. Then, the Co@SiO2[(EtO)3Si–L2]/Mn(III) was separated by magnetic decantation and washed by ethanol, water and acetone respectively to remove the unattached substrates. All the above steps are done for preparation of Co@SiO2[(EtO)3Si–L2]/Co with Co(CH3COO)2·4H2O instead of Mn(acac)3.The Mn content in Co@SiO2[(EtO)3Si–L2]/Mn(III) catalyst was determined 3.91 wt% (amount of Mn = 0.7 mmol g−1) by atomic absorption spectroscopic (AAS) analysis. For the Co@SiO2[(EtO)3Si–L2]/Co(II) catalyst, percent cobalt was determined 11.35 wt%. This amount of cobalt is related to the cobalt magnetic core and the cobalt ions in hybrid. The prepared catalysts were characterized by FTIR, XRD, SEM, EDX, TEM, XPS and VSM.
General procedure for alcohol oxidation
In a typical reaction, 0.02 g of the catalyst Co@SiO2[(EtO)3Si–L2]/M was placed in a 25 ml reaction flask. 1 mmol of the benzyl alcohol in 3 ml of ethyl acetate and then 30 μl H2O2 (30%) was added in flask. The mixture was stirred for 50 min at 50 °C. The reaction was monitored by thin layer chromatography (TLC).Results and discussion
Characterization of the catalysts
Fig. 1 shows the FT-IR absorption spectrum of Co@SiO2–NH2 (a), Co@SiO2[(EtO)3Si–L2] (b) and Co@SiO2[(EtO)3Si–L2]/Mn(III) (c). The FT-IR spectrum for the pure Co@SiO2–NH2 (curve a) shows a stretching vibration at 3475 and 1627 cm−1 which incorporates the contributions from both symmetrical and asymmetrical modes of the O–H bonds which are attached to the surface cobalt atoms. The peaks at 2901 and 2872 cm−1 is attributed to the symmetric and asymmetric vibration of methylene (–CH2) of the propyl in the pendant APTES which confirms that APTES molecules have bonded to the surface of the silica-coated MNPs. The main peak at 1082 cm−1 indicates the formation of silica layer on magnetic cobalt NPs. The strong IR absorption band at 472 cm−1 comes from vibrations of Co–O bonds.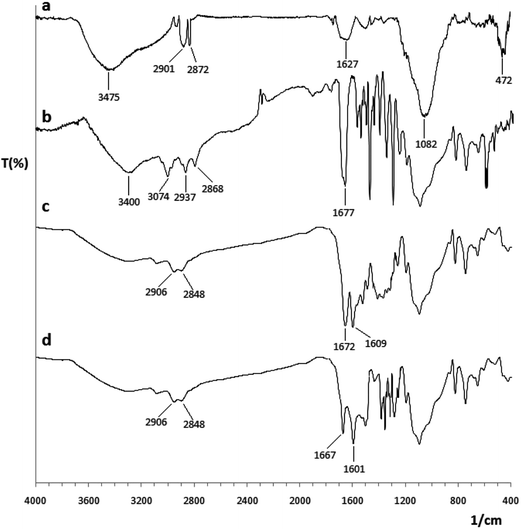 | ||
| Fig. 1 FT-IR spectra of (a) Co@SiO2–NH2, (b) Co@SiO2[(EtO)3Si–L2], (c) Co@SiO2[(EtO)3Si–L2]/Mn(III) and (d) Co@SiO2[(EtO)3Si–L2]/Co(II). | ||
The characteristic vibrations of imine group at 1677 cm−1 in the FT-IR spectra (curve b) of the grafted material confirmed the presence of the imine group on the surface of the magnetic cobalt NPs. Shifting stretching vibration of the carbonyl group (1682 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)) in the ligand 4 to 1677 cm−1 represents a Schiff base reaction and the formation of the imine bond. Vibrations in the range of 1480–1600 cm−1 are attributed to the aromatic ring.
O)) in the ligand 4 to 1677 cm−1 represents a Schiff base reaction and the formation of the imine bond. Vibrations in the range of 1480–1600 cm−1 are attributed to the aromatic ring.
The IR spectra of Co@SiO2[(EtO)3Si–L2]/Mn(III) complex (curve c) show important bands from the Schiff base ligand. The free ligand exhibits a –(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretch at 1677 cm−1 while in the complexes, this band shifts to lower frequency and appears at 1672 cm−1 due to the coordination of the nitrogen with the Mn(III). This band in the complex Co@SiO2[(EtO)3Si–L2]/Co(II) (curve d) appears at 1667 cm−1.
N) stretch at 1677 cm−1 while in the complexes, this band shifts to lower frequency and appears at 1672 cm−1 due to the coordination of the nitrogen with the Mn(III). This band in the complex Co@SiO2[(EtO)3Si–L2]/Co(II) (curve d) appears at 1667 cm−1.
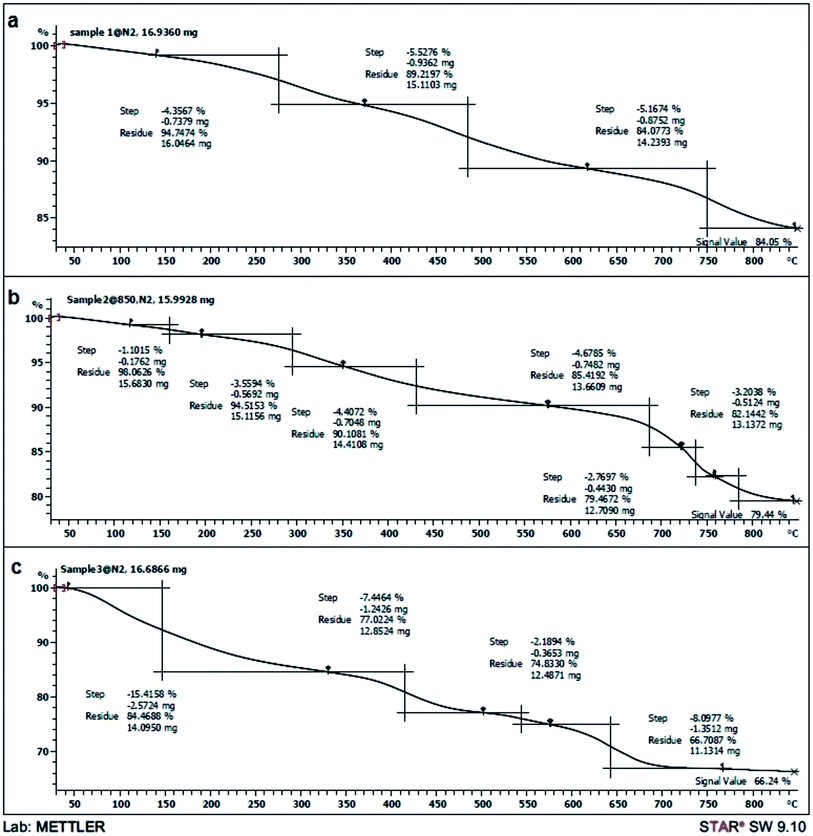
Fig. 2 illustrates the TGA curves, depicting the variations of residual masses of the samples with a heating rate of 10 °C min−1 between 30 and 800 °C temperature. The magnetite, metal ions (cobalt, manganese and silicon) and organic materials of the samples were completely converted into metals, metal oxides and burned to generate gas product at elevated temperatures, respectively. The thermal analysis of Co@SiO2 (curve a) shows initially a negligible weight loss (4.35%) in the region of 90–250 °C which confirms the loss of hydrogen bonded water molecule present at the surface of nano cobalt. A second small weight loss (5.52%) occurred in the temperature range 280–480 °C which is probably due to the removal of trapped water molecules from the lattice. Further weight loss of 5.16% is observed in the range 500–750 °C, the decomposition event can be attributed to conversion SiO2 to SiO. Curve (b) that related to Co@SiO2–NH2, is similar to curve “a” shows that the small weights loss (1.10%) in the ranges of 40–150 °C and (3.55%) 150–280 °C are probably due to the evaporation of physically adsorbed water and trapped water molecules from the lattice, respectively. A weight loss is detected in the range of 300–450 °C and 500–750 °C which are predominantly attributed to the decomposition of organic substances in magnetic Co nanoparticles. Curve (c) that related to Co@SiO2[(EtO)3Si–L2]/Mn(III), shows residue 66.24%. It is estimated that the weight loss of [(EtO)3Si–L2] coated on the Co nanoparticles is about 43.76%. These results prove the attachment of [(EtO)3Si–L2H] moiety on to the surface of Co nanoparticle.
Fig. 3 shows the X-ray diffraction patterns of Co, Co@SiO2[(EtO)3Si–L2], Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) in sections (a), (b), (c) and (d), respectively.
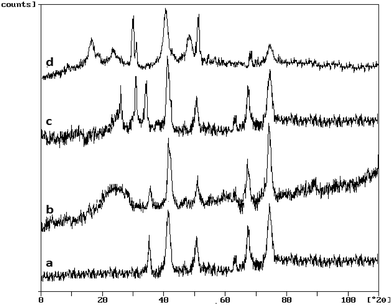 | ||
| Fig. 3 The X-ray diffraction patterns of (a) Co@SiO2, (b) Co@SiO2–NH2, (c) Co@SiO2[(EtO)3Si–L2]/Mn(III) and (d) Co@SiO2[(EtO)3Si–L2]/Co(II). | ||
For magnetic cobalt NPs, diffraction peaks (curve a) with 2θ at 44.2°, 51.5° and 75.9° which corresponds to (111), (200) and (220) Bragg reflection, respectively were observed, indicative of a fcc metallic cobalt structure of the magnetite (card 15-0806 in the JCPDS file). The same set of characteristic peaks were also observed for curves (b–d) indicating the stability of the crystalline phase of Co nanoparticles during [(EtO)3Si–L2] coating and Mn(III) and Co(II) complexation. The XRD patterns of Co@SiO2[(EtO)3Si–L2], Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) (curves b–d) shows an obvious diffusion peak at 2θ = 15–25° that appeared because due to the existence of amorphous silica. XRD pattern of Co@SiO2[(EtO)3Si–L2]/Mn(III) complex (curve c) is shown three major peaks in the range of 20–40°. XRD pattern of Co@SiO2[(EtO)3Si–L2]/Co(II) (curve d) can be assigned to the diffraction from the three major peaks with 2θ° at 19, 30 and 40 reflection indexes of (100), (200) and (220), respectively (card 25-0250 in the JCPDS file).
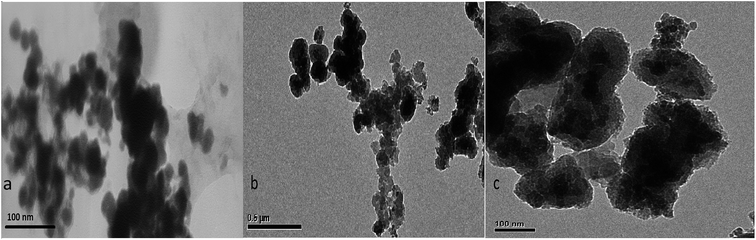
The TEM micrographs of Co nanoparticles (a), Co@SiO2[(EtO)3Si–L2]/Mn(III) (b) and Co@SiO2[(EtO)3Si–L2]/Co(II) (c) are shown in Fig. 4. We can see that the Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) are nearly in core–shell structures, with black color shows Co nanoparticles and ashy color is the Schiff base complex Mn(III) and complex Co(II) shell. This indicates the successful coating on the surface of the magnetic particles. In the transmission electron microscopy images, the diameter of the cobalt nanoparticles was 20–25 nm.
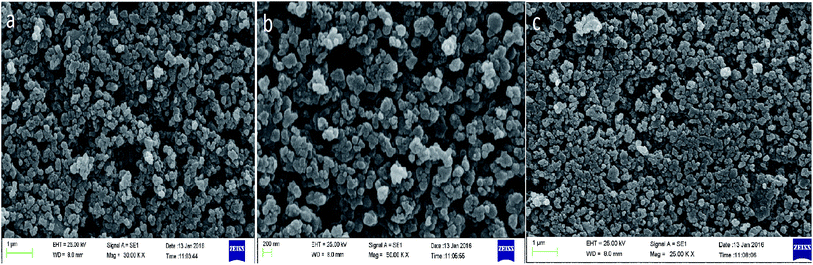
The morphology of the Co nanoparticles, Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) are clearly seen from the SEM image shown in Fig. 5. Curve (a) shows the SEM images of the magnetite nanoparticles. Curves (b) and (c) show that the catalysts were made up of uniform nanometer-sized particles. It can be seen that the particles are not fully spherical. In addition, some particle aggregations are observed, which is likely to be caused by the magneto static interactions between particles.
Presence of Co atoms in Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) were confirmed using energy dispersive X-ray spectroscopy (EDS), which was recorded at random points on the surface for qualitative analysis (Fig. 6). The EDX spectrum of Co@SiO2[(EtO)3Si–L2]/Mn(III) also showed the signals of cobalt, manganese and silicon atoms, which also indicated their presence in the composite in curve (b).
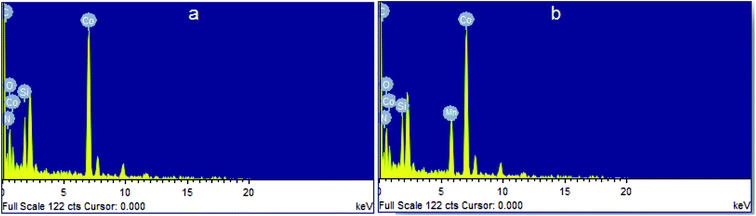 | ||
| Fig. 6 Energy-dispersive X-ray spectroscopy (EDX) of (a) Co@SiO2[(EtO)3Si–L2]/Co(II) and (b) Co@SiO2[(EtO)3Si–L2]/Mn(III). | ||
The magnetic properties of Co nanoparticles, Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) were characterized by a vibrating sample magnetometer (VSM) with an applied field of −8000 Oe < H < 8000 Oe at 300 K. These nanoparticles show high permeability in magnetization and their magnetization is sufficient for magnetic separation with an external magnetic field. Fig. 7 shows the typical room temperature magnetization curves of bare Co nanoparticles (a), Co@SiO2[(EtO)3Si–L2]/Mn(III) (b), and Co@SiO2[(EtO)3Si–L2]/Co(II) (c). All the samples show a typical super paramagnetic behavior. The saturation magnetization of Co NPs at room temperature by measuring the magnetization curve (a) is 39.78 emu g−1; however, the Ms of Co@SiO2[(EtO)3Si–L2]/Mn(III) (curve b) and Co@SiO2[(EtO)3Si–L2]/Co(II) prepared in this study are 36.78 and 36.43 emu g−1, respectively, which are lower than of the bare Co nanoparticles. The reduction of measured saturation magnetization can be mainly attributed to a SiO2[(EtO)3Si–L2] coating formation on the surface of Co NPs. Even with this reduction in the saturation magnetization, the catalysts can still be separated from the reaction medium rapidly and easily with a magnetic field.
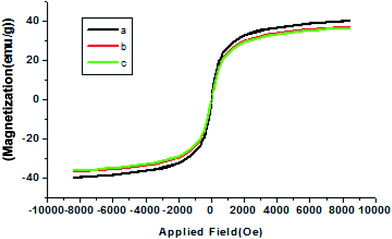 | ||
| Fig. 7 Room temperature magnetization curves of (a) Co nanoparticles, (b) Co@SiO2[(EtO)3Si–L2]/Mn(III) and (c) Co@SiO2[(EtO)3Si–L2]/Co(II). | ||
Catalytic activities of the synthesized catalysts
After structural characterization of the prepared Co@SiO2[(EtO)3Si–L2]/M (M = Mn(III), Co(II)) nanocatalyst, its catalytic activity was investigated as an active and stable magnetically separable nanocatalyst in the oxidation of alcohols (Scheme 2).We employed the oxidation reaction of benzyl alcohol to benzaldehyde as a model reaction. At first test was run with catalysts at the following conditions reaction: benzyl alcohol (0.5 mmol), hydrogen peroxide 30% (40 μl, 0.293 mmol), catalyst 0.02 g, reaction time 45 min, reaction temperature 40 °C, various solvents without optimized amounts and the results show that catalysts are highly active (Table 1). Importantly, these catalysts have different activities in different solvents. Ethyl acetate was the best choice as solvent and this solvent is used in other reactions. The yield of the reactions was determined by as follows: after the end of reaction, the nanocatalyst was separated from the reaction mixture with a magnet. Then the organic phase was separated by water/ethyl acetate extraction method and the solvent was dried. The mixture was stirred in warm n-hexane several times. Thus, the aldehyde was dissolved in n-hexane, and isolated from remaining alcohol and then was weighted. On the basis weight of the obtained aldehyde, the reaction yield can be calculated. In order to optimize the reaction time and temperature, the reaction progress was monitored by thin layer chromatography technique (TLC) in a regular alternative time and temperature.
| Entry | Catalyst | Solvent | Selectivity (%) | Isolated yield |
|---|---|---|---|---|
| a Reaction condition: benzyl alcohol (0.5 mmol), hydrogen peroxide 30% (40 μl, 0.293 mmol), catalyst 0.03 g, reaction time: 45 min, reaction temperature 40 °C. | ||||
| 1 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | THF | 100 | 66 |
| 2 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | MeCN | 100 | 71 |
| 3 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | DMSO | 100 | 54 |
| 4 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | DMF | 100 | 56 |
| 5 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | CH2Cl2 | 100 | 51 |
| 6 | Co@SiO2[(EtO)3Si–L2]/Mn(III) | Ethyl acetate | 100 | 74 |
| 7 | Co@SiO2[(EtO)3Si–L2]/Co(II) | THF | 100 | 53 |
| 8 | Co@SiO2[(EtO)3Si–L2]/Co(II) | MeCN | 100 | 64 |
| 9 | Co@SiO2[(EtO)3Si–L2]/Co(II) | DMSO | 100 | 48 |
| 10 | Co@SiO2[(EtO)3Si–L2]/Co(II) | DMF | 100 | 50 |
| 11 | Co@SiO2[(EtO)3Si–L2]/Co(II) | CH2Cl2 | 100 | 45 |
| 12 | Co@SiO2[(EtO)3Si–L2]/Co(II) | Ethyl acetate | 100 | 67 |
In this case the reactions was examined by two catalysts Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) in ethyl acetate as solvent (Table 1, entries 6 and 12). Finally, at a constant temperature 40 °C, 50 minutes was obtained and applied as the optimum time reaction. Also, reaction temperature 55 °C was obtained as optimum temperature at a constant time 45 min (Fig. 8).
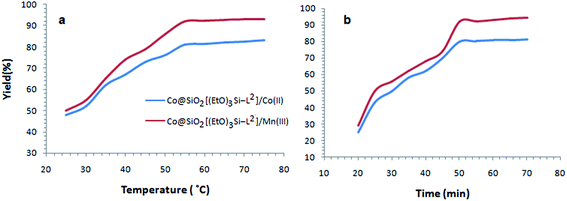 | ||
| Fig. 8 Effect of temperature (a) and time (b) on the oxidation of benzyl alcohol catalyzed by Co@SiO2[(EtO)3Si–L2]/Mn(III) (red) and Co@SiO2[(EtO)3Si–L2]/Co(II) (blue). | ||
After optimizing the solvent, time and temperature reaction, the other reaction conditions such as catalyst amount and oxidant amount were optimized. For this purpose, a series of reactions was designed and done (Table 2).
| Entry | Co@SiO2[(EtO)3Si–L2]/Mn(III) (g) | H2O2 30% (μl) | Selectivity (%) | Isolated yield |
|---|---|---|---|---|
| a Reaction condition: benzyl alcohol (0.5 mmol), reaction time: 50 min, reaction temperature 55 °C. | ||||
| 1 | 0.02 | 30 | 100 | 64 |
| 2 | 0.02 | 40 | 100 | 91 |
| 3 | 0.02 | 50 | 100 | 92 |
| 4 | 0.02 | 60 | 100 | 93 |
| 5 | 0.03 | 30 | 100 | 79 |
| 6 | 0.03 | 40 | 100 | 92 |
| 7 | 0.03 | 50 | 100 | 94 |
| 8 | 0.03 | 60 | 100 | 94 |
| 9 | 0.04 | 30 | 100 | 89 |
| 10 | 0.04 | 40 | 100 | 98 |
| 11 | 0.04 | 50 | 100 | 98 |
| 12 | 0.04 | 60 | 100 | 98 |
| 13 | Not used | 60 | 100 | 8 |
The amounts of Co@SiO2[(EtO)3Si–L2]/Mn(III) catalyst was used to ranging from 0.02–0.04 (g). The evaluation results are shown in Table 2 indicates that increase the amount of catalyst, increase reaction yield. Also increases the amount of H2O2 (30%) as oxidizing agent to 40 μl, increases the reaction yield. Optimized amounts of used catalyst and oxidizing agent were determined 0.04 g and 40 μl, respectively (Table 2, entry 10).
The number of available alcohols were used for the oxidation reaction in the presence of the catalyst Co@SiO2[(EtO)3Si–L2]/Mn(III) (Table 3). The optimal reaction conditions were applied and the selectivity was 100% in any case of alcohol. The results in Table 3 show that the catalytic oxidation reactions of alcohols have done with efficiency and high yield. The different functional groups on the benzene ring did not seem to have influence on the yield of the reaction.
| Entry | Alcohols | Products | Yield (%) | Bp/mp (°C) | |
|---|---|---|---|---|---|
| Found | Lit. | ||||
| 1 | 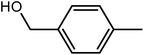 |
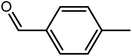 |
95 | 204–206 | 205 (ref. 34) |
| 2 | 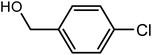 |
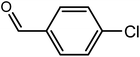 |
94 | 47–48 | 48 (ref. 35) |
| 3 | 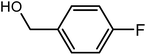 |
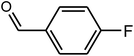 |
99 | 178–180 | 180 (ref. 34) |
| 4 | 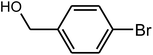 |
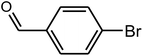 |
96 | 56–58 | 55–58 (ref. 36) |
| 5 | 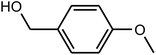 |
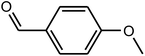 |
97 | 246–248 | 248 (ref. 37) |
| 6 | 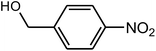 |
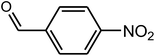 |
94 | 104–105 | 106 (ref. 35) |
| 7 | 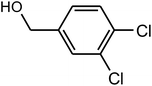 |
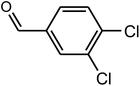 |
90 | 40–42 | 43 (ref. 38) |
| 8 | 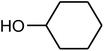 |
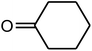 |
91 | 154 | 155 (ref. 39) |
| 9 |  |
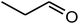 |
89 | 48 | 46–50 (ref. 39) |
| 10 | 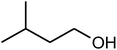 |
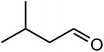 |
92 | 94 | 93 (ref. 39) |
In order to investigate the recyclability of the catalyst, reuse experiments of the catalyst were carried out. The oxidation of benzyl alcohol (0.5 mmol) were studied under reaction time 50 min, reaction temperature 55 °C, catalyst amount 0.04 g and hydrogen peroxide 40 μl (Fig. 9). In order to regenerate the catalyst, after each cycle, it was separated by a magnet, and washed several times with deionized water and ethanol. Then, it was dried in oven at 50 °C and used in the next run. The results show that this catalyst can be reused five times without any significant loss in activity performance. The leaching of Mn into the reaction mixture from the recovered complex supported is analyzed using ICP-MS.
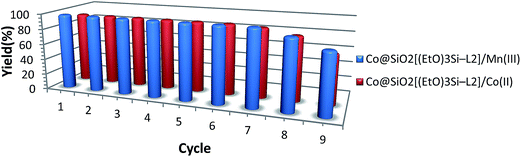 | ||
| Fig. 9 Recycling of the two synthesized magnetic nanocatalysts for oxidation of benzyl alcohol to benzaldehyde. | ||
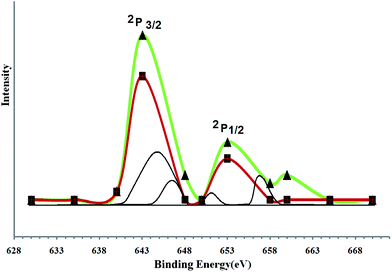
Typical XPS spectra of Co@SiO2[(EtO)3Si–L2]/Mn(III) catalyst used is shown in Fig. 10. The XP spectroscopic analysis usually employed to detect the chemical state of observed element.40,41 In this work, was studied of oxidation state of immobilized manganese on the surface of Co@SiO2[(EtO)3Si–L2]. The XPS spectrum of Mn catalyst produced a Mn 2p3/2 and a Mn 2p1/2 peak at 643.3 eV and 653.1 eV, and is depicted in Fig. 10. These results indicated that most of the Mn ions on the surface of the heterogeneous catalyst were in +3 oxidation state (due to the binding energy (BE) 643.3 eV which are attributed to Mn(III) state of manganese), and they are in accordance with earlier literature data.42–44 This result confirms that during the oxidation of alcohols, parts of the Mn3+ in catalyst are converted to Mn2+.
Conclusion
In conclusion, we have successfully prepared Co@SiO2[(EtO)3Si–L2]/Mn(III) and Co@SiO2[(EtO)3Si–L2]/Co(II) catalysts through surface modification of silica coated magnetic cobalt nanoparticles with [(EtO)3Si–L2H] Schiff base and then interaction with Mn(acac)3 and Co(CH3COO)2·4H2O respectively. The catalysts were proven to be reusable easily for five times after recovery by separation and drying without significant catalytic deactivation which were typically happened due to the leaching of active species or degradation of the structure. These complexes are good heterogeneous catalysts for oxidation of the variety of alcohols.Acknowledgements
We are appreciates the Bu-Ali Sina University and Payame Noor University for their partial support on this project.References
- M. Benaglia, Recoverable and Recyclable Catalysts, John Wiley & Sons, Chichester, 2009 Search PubMed.
- Nanoparticles and Catalysis, ed. D. Astruc, Wiley-VCH, Weinheim, 2008 Search PubMed.
- G. A. Somorjai, H. Frei and J. Y. Park, J. Am. Chem. Soc., 2009, 131, 16589 CrossRef CAS PubMed.
- A. Hu, G. T. Yee and W. Lin, J. Am. Chem. Soc., 2005, 127, 12486 CrossRef CAS PubMed.
- K. K. Senapati, C. B. Orgohain and P. Phukan, J. Mol. Catal. A: Chem., 2011, 33, 924 Search PubMed.
- (a) C. W. Lim and I. S. Lee, Nano Today, 2010, 5, 412 CrossRef CAS; (b) H. Veisi, A. Sedrpoushan, B. Maleki, M. Hekmati, M. Heidari and S. Hemmati, Appl. Organomet. Chem., 2015, 29, 834 CrossRef CAS; (c) H. Veisi, A. Sedrpoushan and S. Hemmati, Appl. Organomet. Chem., 2015, 29, 825 CrossRef CAS.
- H. Gu, R. Zheng, X. Zhang and B. Xu, J. Am. Chem. Soc., 2004, 126, 5664 CrossRef CAS PubMed.
- J. Gao, B. Zhang, Y. Gao, Y. Pan, X. Zhang and B. Xu, J. Am. Chem. Soc., 2007, 129, 11928 CrossRef CAS PubMed.
- (a) T. J. Yoon, K. N. Yu, E. Kim, J. S. Kim, B. G. Kim, S. H. Yun, B. H. Sohn, M. H. Cho, J. K. Lee and S. B. Park, Small, 2006, 2, 209 CrossRef CAS PubMed; (b) M. Pirhayati, H. Veisi and A. Kakanejadifard, RSC Adv., 2016, 6, 27252 RSC; (c) B. Abbas Khakiani, K. Pourshamsian and H. Veisi, Appl. Organomet. Chem., 2015, 29, 259 CrossRef CAS.
- S. Y. Yu, H. J. Zhang, J. B. Yu, C. Wang, L. N. Sun and W. D. Shi, Langmuir, 2007, 23, 7836 CrossRef CAS PubMed.
- X. Yu, J. Wan, Y. Shan, K. Chen and X. Han, Chem. Mater., 2009, 21, 4892 CrossRef CAS.
- M. V. Barmatova, I. D. Ivanchikova, O. A. Kholdeeva, A. N. Shmakov, V. I. Zaikovskii and M. S. Mel'gunov, J. Mater. Chem., 2009, 19, 7332 RSC.
- A.-K. Tucker-Schwartz and R.-L. Garrell, Chem.–Eur. J., 2010, 16, 12718 CrossRef CAS PubMed.
- Ya. Ma, B. Yue, L. Yu, X. Wang, Z. Hu, Y. Fan and Y. Chen, J. Phys. Chem. C, 2008, 112, 472 CAS.
- M. J. Jacinto, H. C. F. Santos, R. F. Jardim, R. Landers and L. M. Rossi, Appl. Catal., A., 2009, 360, 177 CrossRef CAS.
- R. L. Oliveira, P. K. Kiyohara and L. M. Rossi, Green Chem., 2010, 12, 144 RSC.
- L. Canali and D. C. Sherrington, Chem. Soc. Rev., 1999, 28, 85 RSC.
- T. Katsuki, Coord. Chem. Rev., 1995, 140, 189 CrossRef CAS.
- R. Skoda-Foldes, L. Koll Jr and A. Arcadi, J. Mol. Catal., 1995, 101, 37 CrossRef.
- B. S. Lane and K. Burgess, Chem. Rev., 2003, 103, 2457 CrossRef CAS PubMed.
- G. Righi and C. Bonini, Synthesis, 1994, 3, 225 Search PubMed.
- G. X. Zheng, J. J. Eisch, Z. R. Lui and X. Ma, J. Org. Chem., 1992, 57, 5140 CrossRef.
- L. P. C. Nielson, C. P. Stevenson, D. G. Backmond and E. N. Jacobsen, J. Am. Chem. Soc., 2004, 126, 1360 CrossRef PubMed.
- J. Lopez, S. Liang and X. R. Bu, Tetrahedron Lett., 1998, 39, 4199 CrossRef CAS.
- A. M. Daly, C. T. Dalton, M. F. Renehan and D. G. Gilheany, Tetrahedron Lett., 1999, 40, 3617 CrossRef CAS.
- A. R. Silva, C. Freire and B. De Castro, Carbon, 2004, 42, 3027–3030 CrossRef CAS.
- A. R. Silva, V. Budarin, J. H. Clark, C. Freire and B. de Castro, Carbon, 2007, 45, 1951–1964 CrossRef CAS.
- H. Zhang, Y. M. Wang, L. Zhang, G. Gerrtsen, H. C. L. Abbenhuis, R. A. van Santen and C. Li, J. Catal., 2008, 256, 226–236 CrossRef CAS.
- H. Zhang, Y. Zhang and C. Li, J. Catal., 2006, 238, 369–381 CrossRef CAS.
- R. I. Kureshy, I. Ahmad, N. H. Khan, S. H. R. Abdi, K. Pathak and R. V. Jasra, J. Catal., 2006, 238, 134–141 CrossRef CAS.
- R. I. Kureshy, I. Ahmad, N. H. Khan, S. H. R. Abdi, K. Pathak and R. V. Jasra, Tetrahedron: Asymmetry, 2005, 16, 3562–3569 CrossRef CAS.
- X. Tang, Y. Tang, G. Xu, S. Wei and Y. Sun, Catal. Commun., 2008, 317–320 CrossRef CAS.
- M. Aslam, S. Li and V. P. Dravid, J. Am. Ceram. Soc., 2007, 90(3), 950–956 CrossRef CAS.
- A. J. Bosco, C. Lawrence, J. R. Radhakrishnan, A. Arul and R. Vasudevan, J. Phys. Org. Chem., 2015, 28, 591–595 CrossRef CAS.
- B. L. Gadilohar, H. S. Kumbhar and G. S. Shankarling, Ind. Eng. Chem. Res., 2014, 53(49), 19010–19018 CrossRef CAS.
- G. Zhang, X. Wen, Y. Wang, W. Mo and C. Ding, J. Org. Chem., 2011, 76, 4665–4668 CrossRef CAS PubMed.
- K. Asadolah, M. M. Heravi, R. Hekmatshoar and S. Majedi, Molecules, 2007, 12, 958–964 CrossRef CAS PubMed.
- M. Kerfanto, Bull. Soc. Chim. Fr., 1965, 3544–3549 CAS.
- (a) R. Ghorbani-Vaghei, H. Veisi and M. Amiri, J. Chin. Chem. Soc., 2007, 54, 1257 CrossRef CAS; (b) R. Ghorbani-Vaghei, M. Amiri, M. Chegny and H. Veisi, S. Afr. J. Chem., 2007, 60, 58 CAS.
- D. Briggs and M. P. Seah, Practical Surface Analysis: Auger and X-Ray Photoelectron Spectroscopy, Wiley, New York, 2nd edn, 1990, vol. 1 Search PubMed.
- J. C. Vickerman, Surface Analysis: The Principal Techniques, Wiley, New York, 1997 Search PubMed.
- Z. Ozaydin, S. Yasyerli and G. Dogu, Ind. Eng. Chem. Res., 2008, 47, 1035–1042 CrossRef CAS.
- J. Blake Aronson, C. F. Blanford and A. Stein, J. Phys. Chem. B, 2000, 104, 449–459 CrossRef.
- H. Y. L. Sylvia, Y. Y. Wang and C. C. Wan, J. Colloid Interface Sci., 2007, 310, 190–195 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2016 |