Combination of gelatin and tranexamic acid offers improved haemostasis and safe use on internal hemorrhage control†
Umran Aydemir Sezerab,
Zeynep Kocerc,
Basak Arud,
Gulderen Yanıkkaya Demireld,
Mehmet Gulmeze,
Ali Aktekine,
Selvinaz Ozkaraf and
Serdar Sezer *c
*c
aMaterials Institute, TUBITAK Marmara Research Center, 41470 Kocaeli, Turkey
bSakarya University, Department of Electrical and Electronics Engineering, 54187 Sakarya, Turkey
cInstitute of Chemical Technology, TUBITAK Marmara Research Center, 41470 Kocaeli, Turkey. E-mail: serdar.sezer@tubitak.gov.tr; Fax: +90 262 641 2309; Tel: +90 262 677 3800
dYeditepe University, School of Medicine, Department of Immunology, 34755, Istanbul, Turkey
eHaydarpasa Numune Education and Research Hospital, Department of Surgery, 34688 Istanbul, Turkey
fHaydarpasa Numune Education and Research Hospital, Department of Pathology, 34688 Istanbul, Turkey
First published on 28th September 2016
Abstract
Effective hemorrhage control with materials developed by emerging technologies is important in preventing massive bleeding in hospitals, accident sites and battlefields. Various materials have been developed for the treatment of hemorrhage. Gelatin is one, which is commonly used in combination with thrombin. It is generally used in internal bleeding cases owing to its safety, and superior biocompatibility and biodegradability. On the other hand, thrombin the main component that provides rapid haemostasis has drawbacks such as serious disease transmission from donors and high cost. Chitosan is another haemostatic material component with low cost and superior efficiency. However, chitosan-based haemostatic materials are not recommended for internal use. In this paper, we examine the use of a combination of gelatin and tranexamic acid microparticles in internal bleeding control, on the basis of comparative studies both in in vitro and in in vivo conditions. This composite haemostatic material provided rapid haemostasis and biocompatibility, and provided a safe use in internal applications.
1. Introduction
The conversion of plasma protein and fibrinogen into fibrin monomers through spontaneous polymerization leads to the creation of an insoluble network in blood, which provides coagulation. Effective hemorrhage control with materials developed by emerging technologies is an area that focuses on preventing massive bleeding in hospitals, accident sites and battlefields. Currently, various materials are available for the treatment of hemorrhage. Celox®, Feracryl® and QuikClot® are commercial products used as efficient haemostatic materials.1–3 However, these materials are recommended for external applications.1,4 There is a great need for effective haemostatic biomaterials which can be applied into a wound, which are biocompatible and which induce rapid haemostasis with safe use for internal applications. Gelatin a denatured collagen with proven haemostatic effectiveness in surgical applications, is generally used in combination with thrombin to provide rapid coagulation.1,5 It is generally used in internal bleeding owing to its safe use in terms of superior biocompatibility and biodegradability.6,7 On the other hand, the main component, thrombin, that provides rapid haemostasis has some drawbacks such as potential serious disease transmission from donors8 in addition to high cost and storage difficulties. On the other hand, gelatin, when used alone, does not provide haemostasis as rapid as combination with thrombin.Currently, many approaches have been reported about the incorporation of functional materials into hemostats in order to enhance their therapeutic efficiencies. Recently, Chan et al. developed PolySTAT-modified chitosan gauze for external use and achieved improved haemostasis through chitosan gauze.4 Peptide incorporation leads to rapid and safe haemostasis,9,10 however, storage difficulty and the high cost of peptide restrict its use. As part of another approach, diaminopropionic acid was incorporated to graphene sponge which provided remarkably improved haemostasis.11 Behrens et al. also developed hydrogel particles with a high swelling capacity. These hydrogel particles provided rapid blood aggregation; however, they seem to be suitable for external use only due to their non-degradable nature.12
Tranexamic acid (TXA) is a widely used approved drug that initiates coagulation; and performance of its local use has been well reported.13,14 Li et al. reported haemostatic materials composed of chitosan/alginate and TXA; and they reported significantly reduced bleeding time with rabbit liver transection bleeding model.15 TXA was firstly introduced to treat heavy menstrual bleeding; however recently, researchers have also been focused on the topical use of TXA for efficient haemostasis recently.16–21 These reports evaluated haemostatic performance rather than investigating coagulation mechanisms. In this paper, we examine a topically applied haemostatic material that combines gelatin and TXA. The material is aimed to be capable of promoting rapid haemostasis through swelling and inhibition of fibrinolysis owing to the presence of TXA.13 Thus, the combination of TXA and gelatin would offer improved haemostasis through fast absorption of blood by gelatin and preservation of the fibrin matrix at coagulation site by TXA.
We first evaluated the best gelatin candidate for haemostatic material among different gelatin sources with varying characteristics. Then, TXA was combined with the selected gelatin by two different and practical methods. In vitro and in vivo studies were conducted to examine material–blood interaction in terms of haemostasis efficiency and safety of use in internal injuries. This paper reports on the preparation and characterization of the particles, including platelet activation and recalcification, to determine the extent of coagulation, and temporal swelling to investigate behaviors in body conditions. In vivo efficacy studies were conducted in a rat liver punch biopsy model and histopathological analyses of the incision sites were examined in order to assess the biocompatibility and efficacy of the material in in vivo conditions.
2. Experimental
2.1. Materials
Gelatin was obtained from Seljel (with 150, 200 and 250 bloom values, Turkey) and Rousselot (250 LB 8 and 275H 30, France). Celox® (Medtrade Products Ltd, UK, Ref: FG08830521) was obtained from Amazon (http://www.amazon.com). Tranexamic acid, glutaraldehyde (25% solution), phosphate-buffered saline (PBS) tablets and acetone were purchased from Sigma Aldrich (USA). Vegetable oil was purchased from a national supplier (Turkey). For flow cytometric analysis; anti-CD42B, anti-CD62P, anti-CD41 and anti-CD63 antibodies were purchased from Biolegend, San Diego, USA. Tubes containing sodium citrate as anticoagulant (BD Vacutainer Plus Citrate Tubes, 2.7 mL) were purchased from BD Biosciences, USA. Calcium chloride dihydrate, Dulbecco's Phosphate-Buffered-Saline (DPBS), paraformaldehyde, Corning®, Co® 96 well tissue culture plates and calcium chloride were all purchased from Sigma Aldrich, Germany.2.2. Preparation of gelatin microparticles
The absorption performances were examined with various gelatins having different characteristics by measuring swelling ratios. Gelatin aqueous solutions (10% w/v, Seljel with 150, 200, 250 bloom values, Rousselot 250 LB 8 and 275H 30) were prepared at 50 °C and these solutions were added into oil by continuous mixing. Glutaraldehyde (GA) solution (2% v/v) was added dropwise to the medium as cross-linker agent. The mixture was washed with acetone, then filtered and washed with acetone again for several times.2.3. Absorption performance study
1 g of gelatin microparticles from different gelatin sources were placed in filter paper bags and the bags were immersed in PBS. At predetermined time periods (2, 4, 6, 10, 30 min), the bags were removed from PBS and kept out until the last drop of PBS fell down. The bags were weighed and immersed in PBS again for the next time period. Prior to experiments, the weight (W) of the empty paper bag was also recorded after it was soaked in the PBS and reached equilibrium. The experiments were triplicated for each group and the measurements were made according to the equation below:The values were averaged. Table 1 summarizes the properties of the gelatin sources used.
| Brand name | Characteristics |
|---|---|
| Rousselot 250LB 8 | From lime bovine bones, 250 bloom, 8 mesh |
| Rousselot 275H 30 | From bovine hides, 275 bloom, 30 mesh |
| 150 Bloom Seljel | From bovine, 150 bloom, 20 mesh |
| 200 Bloom Seljel | From bovine, 200 bloom, 20 mesh |
| 250 Bloom Seljel | From bovine, 250 bloom, 20 mesh |
2.4. Preparation and characterization of gelatin–TXA microparticles
The gelatin–TXA composite microspheres were prepared with water-in-oil emulsion process, through two different methods. In the first method, a certain amount of TXA powder was added into the gelatin solution (10% w/v) and mixed for 15 min. This suspension was added into oil by continuous mixing. GA solution (2% v/v) was added to the medium as cross-linker agent; and after cross-linking, the mixture was filtered and washed with acetone for several times. The obtained microparticles, which were referred as Gel–TXA–Add, were kept at room temperature for drying. In the second method, microparticles were prepared without the addition of TXA through the same process as described above. TXA was loaded to the dried microparticles with vacuum-cycle pressure and dried again. The obtained particles were referred as Gel–TXA–Abs.2.5. Characterization of samples
The morphology of the prepared samples was characterized by scanning electron microscope (SEM) analysis through a JSM-6400 electron microscope (JEOL, USA). The samples were sputter-coated with Au–Pd thin film prior to the SEM investigations. Fourier transform infrared (FTIR) spectra were obtained with a Perkin Elmer FTIR spectrometer (USA). The samples were analyzed with an ATR apparatus over a 600–4000 cm−1 range, with 4 cm−1 resolution. The swelling ratio upon absorption of the liquid was detected by a Malvern Mastersizer (UK). Both Gel–TXA–Add and Gel–TXA–Abs samples were characterized in ethanol and PBS in order to determine non-swollen and swollen particle size distribution.2.6. Quantification of whole blood clotting time
All human experiments were carried out in accordance with World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects published in 2013 and a copy of declaration was signed by all researchers. Local scientific committee of Yeditepe University has approved the research protocol (İstanbul, Turkey). Informed consent forms were signed by all of the healthy volunteers. Two milliliters of blood taken from each of five healthy adult female volunteers was drawn into sodium citrate tubes. 0.0030 g powder samples were placed into a Co® 96 well tissue culture plate in triplicates. 50 μL blood and 5 μL 0.1 M CaCl2 was added to each well, and thrombus formation was observed in time.222.7. Quantification of platelet activation
Platelet activation was analyzed through with a Beckman Coulter FC500 flow cytometer (USA) on four healthy adult female volunteers' platelet samples. Platelet gate was set on logarithmic side scatter/forward scatter scatter gram. 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were counted per tube in this gate. 0.0060 g powder samples were placed in 2 mL plastic vials and suspended in 300 μm DPBS. 100 μL of drawn whole blood was added to the suspension; and after 5 minutes of incubation, 400 μL 1% paraformaldehyde solution was added on samples for fixation. After incubating at 4 °C for 2 hours, samples were filtered through 40 μm strainer and stained with anti-CD42B, anti-CD62P, anti-CD41 and anti CD-63 antibodies.23,24 Stained samples were incubated in the dark at room temperature for 15 minutes. Platelets were identified by logarithmic forward and side scatter signals. The fluorescence of each antibody was measured by mean channel intensity; and positive percentage values were reported. List mode data analysis was made with CXP Analysis Software (Beckman Coulter, USA).
000 cells were counted per tube in this gate. 0.0060 g powder samples were placed in 2 mL plastic vials and suspended in 300 μm DPBS. 100 μL of drawn whole blood was added to the suspension; and after 5 minutes of incubation, 400 μL 1% paraformaldehyde solution was added on samples for fixation. After incubating at 4 °C for 2 hours, samples were filtered through 40 μm strainer and stained with anti-CD42B, anti-CD62P, anti-CD41 and anti CD-63 antibodies.23,24 Stained samples were incubated in the dark at room temperature for 15 minutes. Platelets were identified by logarithmic forward and side scatter signals. The fluorescence of each antibody was measured by mean channel intensity; and positive percentage values were reported. List mode data analysis was made with CXP Analysis Software (Beckman Coulter, USA).
2.8. Preparation of platelet-poor plasma (PPP)
Three healthy adult female volunteers gave blood for the experiment. After discarding the first two milliliters of blood from each volunteer to avoid thromboplastin contamination, the next two milliliters of blood from each volunteer was put into blood collection tubes containing sodium citrate as the anticoagulant. The tubes were spun at 2000g for 15 minutes; and supernatant was collected for further analysis.2.9. Measurement of plasma recalcification profiles
Plasma recalcification times were determined by the method described previously.25 Powder was placed in a Co® 96 well tissue culture plate; and 100 μL PPP was added to each well. The positive control was CaCl2 added PPP, whereas the negative control was PPP with tissue culture plastic. Following the addition of PPP, 100 μL of 0.025 M CaCl2 was added to each well except for the negative control. The plate was immediately placed in an Epoch Microplate Reader (BioTek Instruments, Inc., USA) and recalcification profiles were monitored by measuring the absorbance at 405 nm for 5 minutes, measuring once each minute. Samples were placed in duplicate and the wells were averaged for calculating the mean absorbance.2.10. In vivo studies
In vivo haemostatic activity studies were conducted with a liver punch biopsy model.26 The surgical protocols were approved by the Medipol University Animal Research Ethical Committee (İstanbul, Turkey). All the animal experiments were performed in compliance with the animal experiments instructions of local ethics committee, Medipol University, İstanbul. Twenty one healthy adult Sprague-Dawley rats (male, weighing 280–340 g) were randomly divided into three groups. A cocktail of ketamine (60 mg kg−1) and xylazine (5 mg kg−1) was applied intravenously to induce general anesthesia. Liver punch biopsy models were generated on each animal by punching on the left lobe of liver with a 0.4 cm diameter biopsy puncher. This area was pulled with a clamp and cut with scissors to form a cavity in the liver. Haemostasis was provided with conventional sterilized gauze; and the waste materials in the wound were removed after 10 seconds. For the test groups (Gel–TXA–Abs and Celox®), one gram of dry haemostatic material was poured onto each wound, which was then compressed with pre-weighed gauzes immediately for 2 minutes with minimum pressure. After application, the gauze pieces were weighed again and recorded. If two observers decided haemostasis was not achieved in two minutes, then the gauze application was continued for 2 minutes more. For the control group, the cavitary lesion in the liver incision site was washed with 10 mL of saline solution only, before the gauze application without using any haemostatic material. After the haemostasis with the gauze application, the gauze pieces were weighed again and recorded. The masses resulting from blood absorption were averaged. Haemostasis time at which bleeding stopped was checked and noted at 2 minute intervals. At the end, the incision site was closed with 4/0 Prolene sutures.On day-15, the rats were decapitated after an injection of 60 mg kg−1 ketamine hydrochloride and 5 mg kg−1 xylazine. Adhesions on the cavitary liver were scored as follows: score 0, no adhesion; score 1, tissue adherence that would separate with gravity; score 2, tissue adherence separable with blunt dissection; score 3, tissue adherence separable with sharp dissection. For histological examination, the left lobe of liver was extracted and immersed into neutral formaldehyde solution.
2.11. Histopathological study
Liver tissue samples were embedded in paraffin blocks after routine tissue preparation procedures and cut in 5 μL slices with a microtome blade and stained with hematoxylin–eosin (H&E). Stained slides were evaluated by a blinded scientist and were graded with a semi-quantitative scoring system. Slides were examined under a light microscope (Olympus CX 41, Olympus, Germany).2.12. Statistical analysis
For assessment of the data obtained in the study, IBM SPSS Statistics 22 (IBM SPSS, Turkey) software was used for statistical analysis. Intergroup comparisons of parameters without normal distribution were made by Kruskal–Wallis test; and the group causing difference was determined by Mann–Whitney U test. Significance was evaluated at a level of p < 0.05.3. Results and discussion
3.1. Swelling study
Gelatin based haemostatic materials work mainly by the swelling of the gelatin particles during blood contact and tamponade bleeding in addition to mechanical compression.27,28 For instance, Gelfoam® can double in volume by swelling29 and the granules swelled by 10 to 20% upon contact with blood or body fluids.30 In this study, we aimed to select the best gelatin candidate for haemostatic action. For this purpose, gelatin microparticles were prepared with the same process as described in the microparticle preparation section with various gelatin types. The microparticles did not contain TXA.Afterwards, swelling study was conducted with 1 g of sample. Swelling values at different time periods can be seen in Fig. 1A. Both mesh size and bloom value affected the swelling capacity in PBS. The blooming test refers to gel strength depending on water absorption; so, the higher the bloom value, the higher the swelling capacity. The mesh size of the gelatin is another factor affecting gelatin characteristics. The bigger the particles, the longer it takes for them to become swollen. Thus, higher mesh size resulted in higher swelling ratio. At the end of 30 minutes, 200 bloom Seljel swelled the most. On the other hand, 250 bloom Seljel was the most swollen sample with a value about 60% in 2 minutes. All samples showed an increasing swelling ratio trend with time. Because the haemostatic action is desired to happen in the shortest time period possible, 250 bloom Seljel, which reached the highest swelling ratio at the end of 2 minutes, was used for further experiments.
3.2. Characterization of materials
In the FTIR spectrum of gelatin microparticles, the bands around 3600–3200 cm−1 indicate the O–H stretching modes, whereas the bands around 3070 and 2932 cm−1 indicate the N–H stretching modes of gelatin (Fig. 1B). The gelatin revealed a series of amide (1634, 1536, and 1235 cm−1) bands (Fig. 1B). The absorption peak around 1449 cm−1 indicated the aldimine peak of cross-linked gelatin.31 In Gel–TXA microparticles' spectrum, N–H stretching bands were observed in both TXA and gelatin, with N–H bending vibrations at 2980 cm−1 and 1600 cm−1 (Fig. 1B). The 2850 cm−1 band indicated the C–H stretching, while the band at 1650 cm−1 indicated the presence of carbonyl group. The characteristic absorption at 1450 cm−1, which is dominantly observed in TXA-containing materials, was attributed to the methylene group.32 Fig. 1C–E show morphological characteristics of Gel, Gel–TXA–Abs and Gel–TXA–Add through SEM images. While gel revealed smooth and fine particles, Gel–TXA–Add revealed larger particles during colonization (Fig. 1C and D). Gel–TXA–Abs had an irregularly shaped morphology (Fig. 1E). Because TXA was in aqueous solution, the addition of TXA onto Gel microparticles caused swelling and the sticking of some microparticles to each other; which was considered as the reason for the morphological distortions.3.3. In vitro coagulation studies
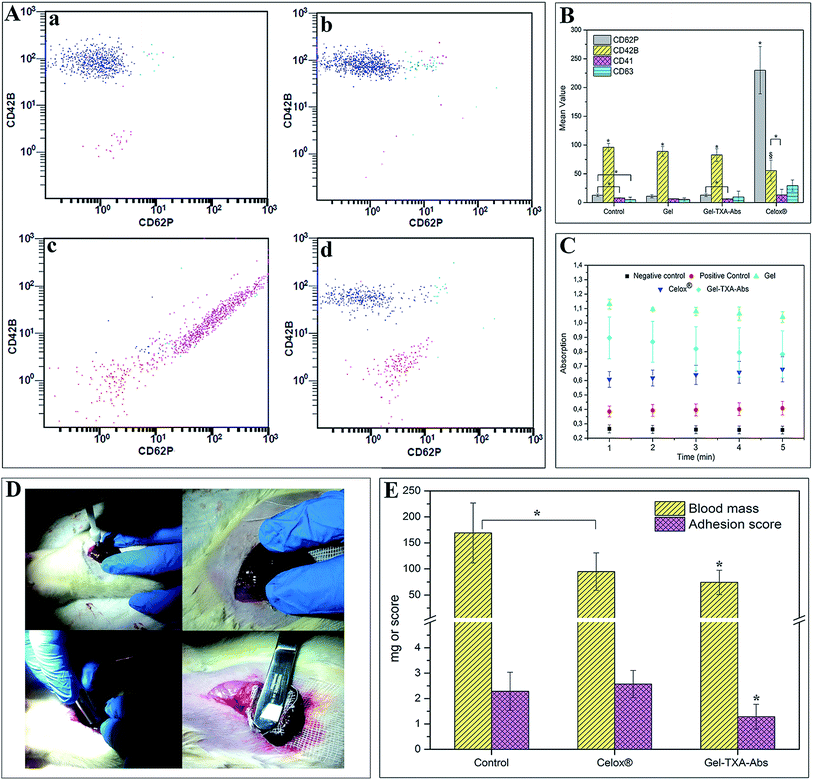
TXA is a powerful and safely used drug which is introduced for treating or preventing excessive blood loss from various medical conditions including hemophilia and heavy menstrual bleeding.21 In this research, TXA was combined with gelatin microparticles in order to increase the efficacy in coagulation. We employed two different methods which are mainly based on absorption and encapsulation. All materials used in in vitro haemostatic applications had a significantly reduced clotting time compared to the control group (p < 0.05) (Fig. 1F). Normal clotting time, representing the control group in this study, had an average value of 8.55 minutes. The microparticles locally formed hydrogel/blood aggregates in the in vitro coagulation study with whole human blood. The average haemostasis time for Celox®, Gel–TXA–Abs, Gel–TXA–Add and Gel groups were 1.43, 1.32, 2.27 and 2.22 minutes, respectively. All groups had a significantly lower haemostasis time compared to the control group, (p < 0.05). No significant difference was found between Celox® and Gel–TXA–Abs, as well as between Gel–TXA–Add and Gel groups (p > 0.05). On the other hand, the clotting periods of both Celox® and Gel–TXA–Abs were significantly shorter than those of Gel–TXA–Add and Gel (p < 0.05). The surface area, apart from the intrinsic characteristics of biomaterials, is an important factor that can influence the clotting time values.33,34 In Gel–TXA–Add sample, most of the TXA was incorporated into the gelatin matrix; while, in Gel–TXA–Abs, it was adsorbed onto the surface of matrix; which resulted in better haemostatic performance. The combination of TXA with gelatin led to a decrease in the observed in vitro clotting time. Contacting blood with materials containing TXA led to the formation of a darker colored-coagulant compared to Celox® and Gel samples (ESI Fig. 1†). This situation could result from the antifibrinolytic characteristics of tranexamic acid. Gelatin has tamponade capacity; it could absorb whole blood by swelling but could not stimulate clot formation under 2 minutes.35 It was found out that adding TXA to gelatin reduced the time period of blood clotting in any case; on the other hand, the Gel–TXA–Abs material which was developed with a method for placing larger TXA on surface area speeded up the clotting process.Whole blood in contact with gelatin begins clotting in 5–7 minutes.36 In our study, the Gel-treated group displayed a much higher coagulation performance. This result is attributed to comparedly small particle size which leads to a high surface-area-to-volume ratio. The larger the surface area for blood contact is, the more rapid the coagulation occurs. When gelatin was combined with TXA, a significant reduction was obtained in the time period of clotting. When we compare the decrease in the clotting time period observed with Gel–TXA–Abs to other groups, we find out that the improvement catches up the performance of Celox® and recorded values for other hemostats combining gelatin and thrombin.36,37A comparison of Gel–TXA–Abs with the commercial hemostat Celox® and the control group proved a decrease by 10.67% and 84.6%, respectively, in the time period of clot formation.
3.4. Swelling studies of microparticles
Swelling of gelatin hemostats is the leading factor for haemostatic efficiency; on the other hand this phenomenon restricts their use especially in neuronal sites.38 As per this situation, we conducted particle size distribution analyses in order to obtain data about the swelling ratios in body conditions, measuring the average particle sizes of both Gel and Gel–TXA–Abs (Fig. 1G and H). We found out that the average particle sizes for Gel were 72 and 133 μm in the presence of ethanol and PBS, respectively. On the other hand, the sizes of 50% of Gel–TXA–Abs particles were lower than 211 μm and 148 μm in ethanol and PBS, respectively. These results showed that Gel microparticles swelled by about 50% of their original sizes; which was consistent with the swelling ratio in the study (Fig. 1A). However, Gel–TXA–Abs indicated a reverse trend compared to Gel microparticles. Considering the fact that Gel–TXA–Abs had a colonized morphology, as seen in the SEM images previously, in PBS, the particles most probably separated from each other, which caused microparticles to be smaller in size. This result can be advantageous for use in sensitive areas like the nervous system in which swelling factor restricts gelatin use for haemostatic purposes.3.5. Platelet activation
Platelets play a major role in the regulation of haemostasis, as activated platelets adhere to the injured soft tissue. During coagulation, the level of platelet activation increases, and the expressions of several markers can be measured on the cell surface. Fig. 2 illustrates activation graphs with four different markers of sample from a randomly selected volunteer. The results of the other three volunteers were also consistent with each other (see the ESI, Fig. 2 and 3†). Fig. 2 shows that only Celox®-treated platelets are strongly activated while there is minimal or no activation with Gel or Gel–TXA–Abs. Regarding platelet activation study, Fig. 3A and B show the activation graphs and mean values of marker activation in Gel, Gel–TXA–Abs, Celox® and control groups, respectively. As can be observed in Fig. 3A, the platelets treated with Celox® have high percentages of positivity with all of the platelet markers; thus, activation occurs; noting only small decrease in CD42B levels; which is an indicator of conformational changes in CD42 complex. CD42B is a component of the GPIb-V-IX complex on platelets which binds von Willebrand factor, leading to platelet adhesion and platelet plug formation at injury sites.39 Fig. 3B shows that Celox® provides almost 5 times higher platelet activation than the other materials.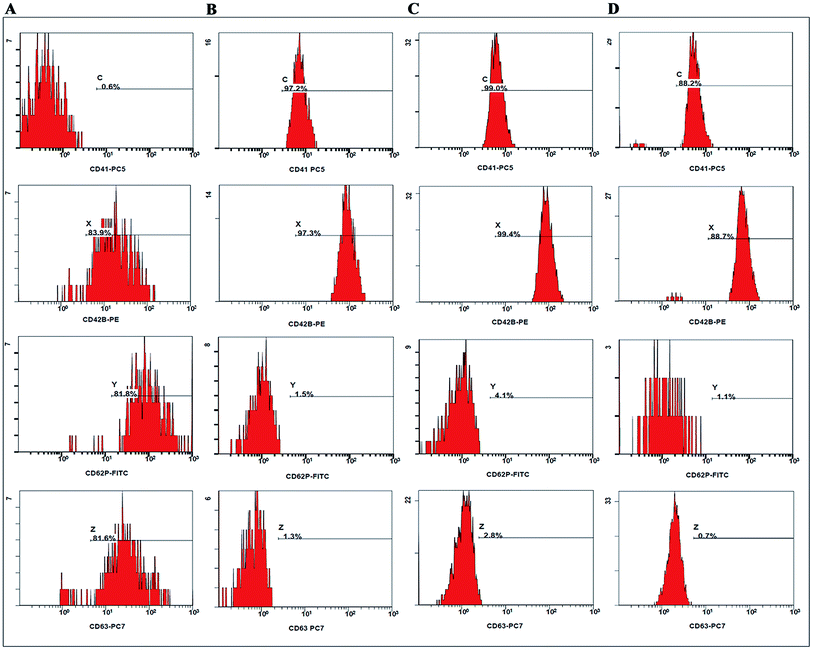 | ||
| Fig. 2 Quasi-activation graphs of CD41, CD63, CD62P, and CD42B after the contact with blood from a randomly selected volunteer. The groups are separated vertically: (A) Celox®, (B) control (without contact with any material), (C) Gel and (D) Gel–TXA–Abs groups. For the activation graphs of other volunteers, see the Fig. 2 and 3 in the ESI.† | ||
In our study, all groups of materials indicated significantly higher CD42B activation than the control group did; which represents normal coagulation (p < 0.05) (Fig. 3B). However, the CD42B activation in Celox®-treated group was lower than the other groups (p > 0.05). As reported in the literature, positively charged materials can initiate the coagulation cascade by charge interactions, such as the interactions with negatively charged GPIb-V receptors.40–42 The interaction of positively charged TXA with negatively charged GPIb-V receptors could contribute to a higher increase in the CD42B activation compared to the Celox® group. CD62P is one of the most abundant proteins, which is exposed on the cell surface within seconds after platelet activation.43 In the control group, the CD62P marker indicated a significant increase compared to CD41 and CD63 (p < 0.05). In Gel–TXA–Abs, the activation of CD62P marker was higher than CD41, significantly (p < 0.05). While the CD62P marker displayed a significant increase in Gel–TXA–Abs and Celox® groups; it didn't in the Gel group. Besides, CD63 and CD41 play a crucial role in coagulation. Among the groups, there was no significant difference in terms of CD63 compared to CD62P and CD41 markers (p > 0.05). Celox® provided a significant CD62P activation compared to CD42B, CD41 and CD63 markers (p < 0.05). Its CD42B activation was also higher than its CD41 activation, significantly (p < 0.05). On the other hand, no significant difference was observed for the CD63 marker compared to CD42B and CD41 (p > 0.05).
Despite their essential roles in blood coagulation, these markers did not display any significant differences in Gel–TXA–Abs in this study. On the other hand, when we compared the markers by material groups, only the CD42B activation indicated a significant increase in Celox®-treated group rather than in the control, Gel and Gel–TXA–Abs groups (p < 0.05). Other markers did not indicate any significant differences (p > 0.05).
3.6. Plasma recalcification
The plasma recalcification profile obtained by the contact with PPP is used for determination of materials' performances in the intrinsic pathway of coagulation cascade.25,44 Increased absorbance values indicate the accumulation of fibrin which causes turbidity in plasma. The measurements could not be performed in a longer term due to rapid coagulation. In this study, the shift of the curve was used as an indicator of the clotting time. A rightward shift of the curve implies a slower clotting, while a leftward shift implies a faster one.25,44 In our study, Gel–TXA–Abs produced a leftward shift in the kinetic profile suggesting a faster clotting time than Gel. The slope of the linear portion (Fig. 3C) of the curve indicated the clotting rate. Our observations showed that there was no statistical difference (p > 0.05) between Gel–TXA–Abs and Celox®, indicating a similar rate of clotting for both surfaces (Fig. 3C). The results of plasma recalcification profile analyzed together suggest that Gel is less coagulative than Gel–TXA–Abs and Celox®, significantly. Celox® is also found having less haemostatic performance than Gel–TXA–Abs; however, the results were not significant. Further studies, including activation and in vivo rat model studies, were conducted with the Gel–TXA–Abs group, the control group (without using any material) and the Celox® group which served as another control group (as commercial product).3.7. In vivo study
In vivo haemostatic performance and biocompatibility of Gel–TXA–Abs was investigated by liver punch biopsy model (Fig. 3D). Recently, this model is reported to provide better results for blood loss than the tail-cut model by Morgan et al. does.45 Liver laceration and spleen transection models indicate higher blood loss than the puncture biopsy model does. However, these models are unfavorable due to the difficulty of performing laceration or transection in the same dimensions at all samples. The size of the wound area is crucial for blood loss analysis; and the punch model guarantees the same wound dimensions, which minimizes the deviations. Seven animals for each group were evaluated in terms of blood loss and post-operative effects. Gel–TXA–Abs and Celox® samples were applied on wound pockets of liver, respectively (n = 7). A control group (without using any material) was generated by liver punch biopsy model, which was washed with saline (n = 7) only. Hemorrhage was stopped with gauze in this group. Haemostasis was obtained in 4 minutes (2 + 2 minutes) on one animal from each group.Fig. 3E shows blood loss after haemostasis and adhesion scores on day-15 after in vivo study. Blood loss in both the Celox® and Gel–TXA–Abs group was significantly lower than that in the control group (p < 0.05). Moreover, Gel–TXA–Abs indicated lower blood loss than the Celox® group (p < 0.05). The haemostasis results in in vivo conditions were consistent with the in vitro blood coagulation study. Most of the time, adhesion formation comes from severe inflammation response towards implanted material.46 In this study, the effects of haemostatic materials in internal applications were evaluated by observation on the 15th day after the application. Interestingly, soft tissue adhesions – to cavitary liver lesion – were observed in the control group most probably due to harsh application of the punch model. At day-15, the operation sites were reopened and adhesion was observed on the liver incision sites of all the animals treated with Celox®. There was also adhesion observed in the control group, which, however, was milder than that of the Celox®-treated group; and there was no significant difference between the control and Celox® groups. On the other hand, adhesion score of the Gel–TXA–Abs group was significantly lower than that of both the control and Celox® groups (p < 0.05).
These results indicated that Gel–TXA–Abs behaved like an adhesion barrier rather than causing inflammation and adhesion formation. The tissues and liver appeared macroscopically unchanged in the Gel–TXA–Abs-treated group. At explantation, no residue of Gel–TXA–Abs was observed, while Celox® was still present, accompanied by soft tissue adhesion to the lesion site.
3.8. Histopathological study
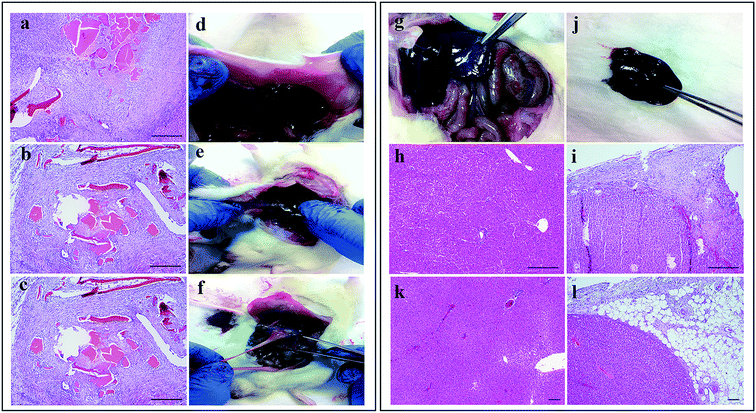
Further studies on the effects of haemostatic materials in long-term were conducted by histological observations on the liver samples on day-15 after in vivo studies. In the Celox®-treated group, both the tissue necrosis and fibrinoid changes on vascular wall were significantly higher – with a score of 85.7% – than in the Gel–TXA–Abs and control groups with 28.6% and 0% scores, respectively (p < 0.05). On the other hand, there was no significant difference between the Gel–TXA–Abs and control groups in terms of tissue necrosis (p > 0.05). Chronic inflammation in Celox®-treated group was significantly higher than those in the Gel–TXA–Abs and control groups (p < 0.05). Actually, there was no chronic inflammation observed in both the Gel–TXA–Abs and control groups, while the score of Celox®-treated group was 71.4%. There was also a significant difference in terms of vascularization. Either in the Gel–TXA–Abs-treated group or in the control group, no vascularization was observed; on the other hand, the score in the Celox®-treated group was significantly high (p < 0.05). No significant difference was found among the groups in terms of acute inflammation, fibrosis, giant cell, erythrocytes, concession, hydropic degeneration and spotty necrosis (p > 0.05).Fig. 4a–c illustrates the microscopic observation of the application areas on the Celox®-treated samples. In liver parenchyma, chronic and acute inflammation elements and tissue necrosis were remarkably observed at the application area (Fig. 4a). Moreover, vascular proliferation and fibrinoid changes were observed at the vascular wall around the material (Fig. 4b and c). The results were consistent with the macroscopic observations of the surgery areas on day-15 when adhesions and band formation were observed at liver; abdominal wall and visceral side (Fig. 4d–f). On the other hand, neither the control group nor the Gel–TXA–Abs group indicated any adhesions (Fig. 4g and j). Regular structures and mild inflammation were observed around fatty tissues – which are compatible with the post-operative stage – in the liver parenchyma of the control group and the Gel–TXA–Abs-treated group (Fig. 4h, i, k and l).
To conclude, Celox® induced an acute inflammation including cellular infiltration and chronic inflammatory reaction at the surrounding tissue in the host. The inflammatory response against Celox® was substantially stronger than that against Gel–TXA–Abs. Moreover, in the Celox® group, a dense fibrous tissue formation was detected around the surgical area at day-15. Gelatin has a collagen-like structure with intensively amino acid content and can accelerate regeneration of damaged tissues. In the same way, combination of TXA with gelatin offers a safe haemostatic biomaterial with insignificant inflammatory response. These results, together, proved that the Gel–TXA–Abs microparticles offer a strong haemostatic potential for in vivo applications and are suitable for internal use with no need for the removal of the material.
4. Conclusions
In conclusion, gelatin microparticles containing tranexamic acid form an efficient haemostatic material that can improve in vitro and in vivo coagulation. The most important bleeding preventive factor of gelatin is its liquid (blood) absorption capacity which depends on the characteristics of gelatin and time periods allowed. Addition of tranexamic acid to gelatin significantly improves the haemostatic performance. Observations in presence of both platelet-rich-plasma (PRP) and platelet-poor plasma (PPP) prove that the material has haemostatic efficiency in the both plasma types. Studies on the long-term effects of in vivo application of Gel–TXA–Abs indicate that the material is safe for internal application and causes no tissue damage when compared with both the commercial materials and the control group which is not subjected to any material. Moreover, the elimination of adhesion formation makes Gel–TXA–Abs a favorable material for internal use. Owing to these unique features, the newly developed micron-sized gelatin haemostatic material can be used as an efficient hemostat in the treatment of wounds including the internal ones, with no need for the removal of the material.Acknowledgements
We gratefully acknowledge the financial support by The Scientific and Technological Research Council of Turkey (as part of the Project BIYOTEG-5130028).References
- F. J. di Lena, J. Mater. Chem. B, 2014, 2, 3567–3577 RSC.
- A. M. Behrens, M. J. Sikorski, T. Li, Z. J. Wu, B. P. Griffith and P. Kofinas, Acta Biomater., 2014, 10, 701–708 CrossRef CAS PubMed.
- Y.-J. Zhang, B. Gao and X.-W. Liu, Int. J. Clin. Exp. Med., 2015, 8, 10–19 Search PubMed.
- L. W. Chan, C. H. Kim, X. Wang, S. H. Pun, N. J. White and T. H. Kim, Acta Biomater., 2016, 31, 178–185 CrossRef CAS PubMed.
- R. Watrowski, Arch. Gynecol. Obstet., 2014, 290, 411–415 CrossRef CAS PubMed.
- L. K. Krishnan, M. Mohanty, P. R. Umashankar and A. V. Lal, Biomaterials, 2004, 25, 5557–5563 CrossRef CAS PubMed.
- P. Cappabianca, F. Esposito, I. Esposito, L. M. Cavallo and C. A. Leone, Acta Neurochir., 2009, 151, 69–77 CrossRef PubMed.
- W. D. Spotnitz, ISRN Surg., 2014, 1–28 CrossRef PubMed.
- B. B. Hsu, W. Conway, C. M. Tschabrunn, M. Mehta, M. B. Perez-Cuevas, S. Zhang and P. T. Hammond, ACS Nano, 2015, 9, 9394–9406 CrossRef CAS PubMed.
- A. Saini, K. Serrano, K. Koss and L. D. Unsworth, Acta Biomater., 2016, 31, 71–79 CrossRef CAS PubMed.
- K. Quan, G. Li, L. Tao, Q. Xie, Q. Yuan and X. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 7666–7673 CAS.
- A. M. Behrens, M. J. Sikorski and P. Kofinas, J. Biomed. Mater. Res., Part A, 2014, 102, 4182–4194 CrossRef PubMed.
- C. J. Dunn and K. L. Goa, Drugs, 1999, 57, 1005–1032 CrossRef CAS PubMed.
- W. Ng, A. Jerath and M. Wąsowicz, Anaesthesiol. Intensive. Ther., 2015, 47, 339–350 CrossRef PubMed.
- D. Li, P. Li, J. Zang and J. Liu, J. Biomed. Biotechnol., 2012, 981321 Search PubMed.
- M. Aoki, Y. Okawa, Y. Goto, S. Ogawa and H. Baba, Asian Cardiovasc. Thorac. Ann., 2012, 20, 658–662 CrossRef PubMed.
- K. Ker, D. Beecher and I. Roberts, Cochrane Database Syst. Rev., 2013, 23, 1–8 Search PubMed.
- J. N. Patel, J. M. Spanyer, L. S. Smith, J. Huang, M. R. Yakkanti and A. L. Malkani, J. Arthroplasty., 2014, 29, 1528–2231 CrossRef PubMed.
- J. Wong, A. Abrishami, H. El Beheiry, N. N. Mahomed, J. R. Davey, R. Gandhi, K. A. Syed, S. M. O. Hasan, Y. D. Silva and F. Chung, J. Bone Jt. Surg., Am. Vol., 2010, 92, 2503–2513 Search PubMed.
- J. G. Martin, K. B. Cassatt, K. A. Kincaid-Cinnamon, D. S. Westendorf, A. S. Garton and J. H. Lemke, J. Arthroplasty, 2014, 29, 889–894 CrossRef PubMed.
- L. Tengborn, M. Blombäck and E. Berntorpa, Thromb. Res., 2015, 135, 231–242 CrossRef CAS PubMed.
- A. K. Gaharwar, R. K. Avery, A. Assmann, A. Paul, G. H. McKinley, A. Khademhosseini and B. D. Olsen, ACS Nano, 2014, 8, 9833–9842 CrossRef CAS PubMed.
- S. J. Shattil, M. Cunningham and J. A. Hoxie, Blood, 1987, 70, 307–315 CAS.
- N. Q. Shafi, Ann. Clin. Lab. Sci., 1986, 16, 365–372 CAS.
- N. Alexandre, E. Costa, S. Coimbra, A. Silva, A. Lopes, M. Rodrigues, M. Santos, A. C. Maurrosu, J. D. Santos and A. L. Luun, J. Biomed. Mater. Res., Part A, 2015, 103, 1366–1379 CrossRef PubMed.
- D. Csukas, R. Urbanics, A. Moritz and R. Ellis-Behnke, Nanomedicine, 2015, 11, 2025–2031 CAS.
- F. Richter, D. Schnorr, S. Deger, I. Tuuer, J. Roigas, A. Wille and S. Loening, Urology, 2003, 61(1), 73–77 CrossRef PubMed.
- H. H. I. Yao, M. K. H. Hong and K. J. Drummond, J. Clin. Neurosci., 2013, 20, 349–356 CrossRef CAS PubMed.
- C. Schonauer, E. Tessitore, G. Barbagallo, V. Albanese and A. Moraci, Eur. Spine. J., 2004, 13, S89–S96 CrossRef PubMed.
- M. C. Oz, J. F. Rondinone and N. S. Shargill, J. Cardiovasc. Surg., 2003, 18, 486–493 Search PubMed.
- U. Aydemir Sezer, E. A. Aksoy, C. Durucan and N. Hasirci, Polym. Compos., 2012, 33, 1644–1651 CrossRef.
- M. S. Arayne, N. Sultana, F. A. Siddiqui, A. Z. Mirza and M. H. Zuberi, J. Mol. Struct., 2008, 891, 475–480 CrossRef CAS.
- C. Sperling, M. Fischer, M. F. Maitz and C. Werner, Biomaterials, 2009, 30, 4447–4456 CrossRef CAS PubMed.
- J. M. Anderson, A. Rodriguez and D. T. Chang, Semin. Immunol., 2008, 20, 86–100 CrossRef CAS PubMed.
- R. A. de la Torre, S. L. Bachman, A. A. Wheeler, K. N. Bartow and J. S. Scott, Surgery, 2007, 142, S39–S45 CrossRef PubMed.
- J. P. Bertram, C. A. Williams, R. Robinson, S. S. Segal, N. T. Flynn and E. B. Lavik, Sci. Transl. Med., 2009, 1, 11ra22 Search PubMed.
- W. R. Wagner, J. M. Pachence, J. Ristich and P. C. Johnson, J. Surg. Res., 1996, 66, 100–108 CrossRef CAS PubMed.
- J. M. Buchowski, K. H. Bridwell, L. G. Lenke and C. R. Good, Spine, 2009, 34, E473–E477 CrossRef PubMed.
- A. D. Michelson, M. R. Barnard, L. A. Krueger, A. L. Frelinger and M. I. Furman, Methods, 2000, 21, 259–270 CrossRef CAS PubMed.
- A. Radomski, P. Jurasz, D. Alonso-Escolano, M. Drews, M. Morandi, T. Malinski and M. W. Radomski, Br. J. Pharmacol., 2005, 146, 882–893 CrossRef CAS PubMed.
- Y. Okamoto, R. Yano, K. Miyatake, I. Tomohiro, Y. Shigemasa and S. Minami, Carbohydr. Polym., 2003, 53, 337–342 CrossRef CAS.
- S. Kamath, A. D. Blann and G. Y. Lip, Eur. Heart J., 2001, 22(17), 1561–1571 CrossRef CAS PubMed.
- J. F. van Velzen, B. A. Laros-van Gorkom, G. A. Pop and W. L. van Heerde, Thromb. Res., 2012, 130, 92–98 CrossRef CAS PubMed.
- D. Motlagh, J. Yang, K. Y. Lui, A. R. Webb and G. A. Ameer, Biomaterials, 2006, 27, 4315–4324 CrossRef CAS PubMed.
- C. E. Morgan, V. S. Prakash, J. M. Vercammen, T. Pritts and M. R. Kibbe, JAMA Surg., 2015, 150, 316–324 CrossRef PubMed.
- G. S. diZerega, Peritoneal Surgery, ed. A. H. DeCherney, M. P. Diamond, H. Ellis, V. Gomel, A. F. Haney, L. Holmdahl, J. A. Rock, K. E. Rodgersr and J. N. Thompson, Springer-Verlag, New York, US, 2000 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16790j |
| This journal is © The Royal Society of Chemistry 2016 |