Synthesis and characterization of Cu–MoS2 hybrids and their influence on the thermal behavior of polyvinyl chloride composites
Keqing Zhou*a,
Zhou Guib and
Yuan Hub
aFaculty of Engineering, China University of Geosciences (Wuhan), 388 Lumo Road, Wuhan, Hubei 430074, P. R. China. E-mail: zhoukq@cug.edu.cn
bState Key Laboratory of Fire Science, University of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China
First published on 12th September 2016
Abstract
In this work, Cu–MoS2 hybrids were synthesized by a facile wet chemical method. The structure and morphology of the synthesized hybrids were characterized by X-ray diffraction, X-ray photoelectron spectroscopy and transmission electron microscopy measurements. Subsequently, the hybrids were introduced into a polyvinyl chloride matrix to act as a thermal stabilizer. The hybrids were well dispersed in the matrix and no obvious aggregation was observed. The obtained nanocomposites exhibited significant improvements in thermal stability and inhibited the release of pyrolysis products effectively, which were mainly attributed to the enhancement of char formation, a physical barrier effect of the MoS2 nanosheets and an inhibition effect of the Cu nanoparticles.
1. Introduction
Poly(vinyl chloride) (PVC), as a typical commodity polymer, has been widely used in many fields due to its excellent processability, good mechanical properties, inherent flame retardancy and low production cost.1 However, the main drawback of PVC is its low thermal stability. The thermal degradation of PVC involves an autocatalytic dehydrochlorination reaction, which may produce undesirable discoloration and deterioration of mechanical properties.2,3 In addition, another challenge for PVC is how to efficiently inhibit the generation of black smoke upon combustion.4 Therefore, it is of great significance to explore appropriate thermal stabilizers which are environmentally friendly and highly efficient at restraining the thermal degradation of PVC.As is well known, the introduction of small amounts of two dimensional (2D) layered nanofillers can inhibit the thermal degradation of polymer materials effectively.5 This property enhancement is mainly attributed to the good barrier effect and low heat conductivity of 2D nanomaterials.6 Typical 2D layered nanofillers such as layered double hydroxides (LDHs) and montmorillonite (MMT) have been widely used to enhance the thermal stability of PVC composites in previous work.7 In addition, it has found that copper containing compounds might be the most effective class of smoke suppressants for PVC.8 Moreover, Liu et al. has investigated the influence of copper containing LDHs on thermal and smoke behavior of PVC. The results indicated that the combination of copper and LDHs can improve the thermal stability and smoke suppression property of PVC composites effectively.9
As an emerging 2D layered nanomaterial, the exfoliated MoS2 nanosheets exhibit good thermal insulating property and high thermal stability (initial thermal temperature is over 500 °C), which make it a promising candidate for thermal property application in polymers. In the past few years, some reports have demonstrated that incorporation of exfoliated MoS2 nanosheets at extremely low loading can endow polymer matrices with prominent thermal and mechanical properties.10–13 In addition, MoS2 or its derivatives have been reported as flame retardant nanoadditives to improve the flame retardancy of various polymers such as PVA, PS, PMMA, EP and TPEE.12,14–20 However, to the best of our knowledge, few researchers have investigated the effect of MoS2 on the thermal behavior of PVC. Additionally, it is worth noting that the excellent performances are only achieved when MoS2 nanosheets are uniformly dispersed in polymer matrix.19 But actually, the exfoliated MoS2 nanosheets are inclined to agglomerate and even restack in polymer matrices owing to their large specific surface area and van der Waals interactions.21
Recently, polymer nanocomposites based on hybrid nanomaterials which comprise two or more components with different properties, have been proven to be a more attractive strategy, because they usually offer unexpected properties. Moreover, it can be expected that the agglomeration of the nanoparticles and weak interfacial interaction problems in polymer nanocomposites could be solved simultaneously by using such functional hybrid nanofillers.22 Previous work in our group has reported that Cu/graphene hybrids can effectively inhibit the generation of smoke gas.23 Moreover, it is well known that the exfoliated MoS2 nanosheets have a large specific surface area and abundant active edges so that they can act as a perfect support for nanoparticle decoration.24 From the perspective of materials design, the decoration of nanoparticles not only suppresses the agglomeration and restacking of MoS2 nanosheets, but also endows MoS2 nanosheets with new physical and chemical properties. Inspired by this, it provides us a novel strategy to decorate copper nanoparticles on the surface of exfoliated MoS2 nanosheets, the well-dispersed Cu nanoparticles can serve as a nanospacer to inhibit or decrease of serious agglomeration and restacking of MoS2 nanosheets, which is beneficial for the uniform dispersion of MoS2 in the polymer matrix. Therefore, it is interesting to investigate how the combination of the two conceptions, which means the synthesis of copper nanoparticles decorated MoS2 hybrids, will actually affect the thermal behavior of PVC.
Herein, the Cu–MoS2 hybrids are synthesized by a facile and efficient wet chemical method and then incorporated into the PVC matrix through solvent blending. The thermal degradation behavior of PVC composites is investigated via TGA and TG-IR. It is anticipated that loading Cu nanoparticles on the MoS2 nanosheets can inhibit the emission of HCl and prevent the MoS2 nanosheets from restacking, thus resulting in enhanced thermal stability and reduced pyrolysis products of PVC. This work attempts to provide a promising strategy for constructing a new class of environmental friendly thermal stabilizers with efficient smoke suppression effect for PVC composites.
2. Materials and methods
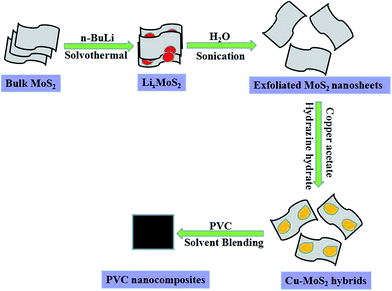
All reagents were analytical reagents and used as received without further purification. The schematic illustration for the fabrication of Cu–MoS2 hybrids was presented in Fig. 1. The typical process included two steps: (1) the preparation of exfoliated MoS2 nanosheets from bulk MoS2 through the hydrolysis and ultrasonication of LixMoS2 in deionized water14 and (2) chemical reduction of the copper acetate by hydrazine to generate Cu–MoS2 hybrids. Briefly, 0.2 g of LixMoS2 was hydrolyzed in 300 mL distilled water, and ultrasonicated at room temperature for 4 h to obtain a colloidal suspension of MoS2 layers. Then 1.5 mmol of copper acetate was dissolved in the above suspension at room temperature. Finally, excessive hydrazine hydrate was dripped into the solution slowly and further heated at 100 °C for 2 h. The as-prepared products were separated by centrifugation and washed with deionized water for several times and then dried in a vacuum at 40 °C. PVC composites with 0.5 wt% Cu–MoS2 hybrids were prepared by a solvent blending method.25XRD patterns were performed with a Japan Rigaku D/Max-Ra rotating anode X-ray diffractometer equipped with a Cu-Kα tube and Ni filter (λ = 0.1542 nm). X-ray photoelectron spectroscopy (XPS) spectra were performed on VG ESCALB MK-II electron spectrometer. TEM images were obtained on a JEOL JEM-2100F transmission electron microscope at an accelerating voltage of 200 keV. Scanning electron microscopy (SEM) (JSM-6800F, JEOL) was used to observe the fracture surface structure of PVC nanocomposites. Thermogravimetric analysis (TGA) was carried out using a Q5000 thermoanalyzer instrument (TA Instruments Inc., New Castle, DE) under N2 flow of 60 mL min−1. In each case, the samples were heated from room temperature to 800 °C at a linear heating rate 20 °C min−1. The thermogravimetric analysis/infrared spectrometry (TG-IR) of the samples was performed using a TGA Q5000 IR thermogravimetric analyzer that was interfaced to the Nicolet 6700 FTIR spectrophotometer.
3. Results and discussion
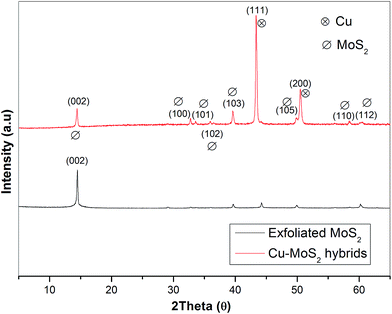
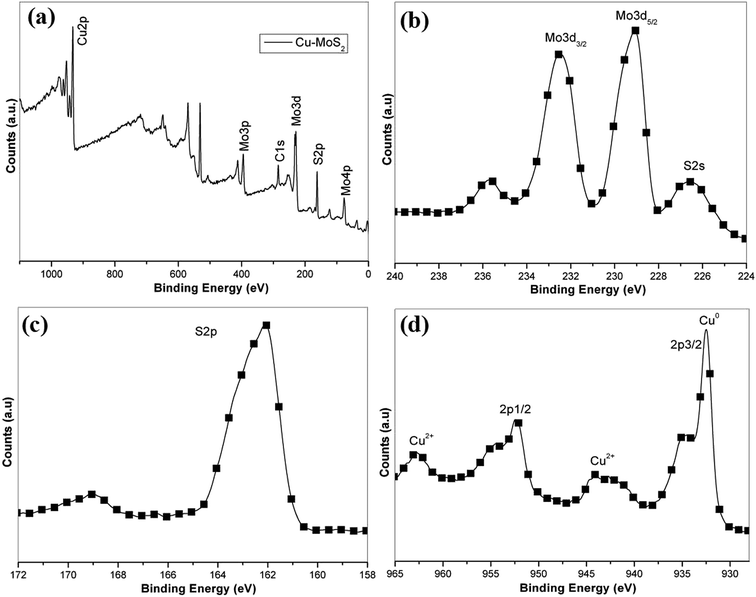
Fig. 2 shows the XRD patterns of the exfoliated MoS2 and Cu–MoS2 hybrids. It can be seen that the exfoliated MoS2 shows a characteristic reflection peak centered at 2θ = 14.4°, corresponding to the (002) plane. For the Cu–MoS2 hybrids, the diffraction patterns located at 2θ = 43.3° and 50.5° are attributed to the (111) and (200) crystalline planes of the face centered-cubic Cu, respectively (JCPDS 85-1326).23 In addition, the intensity of the diffraction peak for (002) (MoS2) in the Cu–MoS2 hybrids decreases, indicating that the face-to-face stacking is broken due to the deposition of Cu nanoparticles on the surface of MoS2 nanosheets.26XPS analysis (Fig. 3) is further performed to reveal the chemical composition of the obtained Cu–MoS2 hybrids. The entire XPS spectrum (Fig. 3a) shows the peaks of Cu, Mo, and S derived from the Cu–MoS2 hybrids. As shown in Fig. 3b, the two peaks at 229.1 eV and 232.5 eV can be attributed to the doublet of Mo 3d5/2 and Mo 3d3/2. The bonding energy for S 2p in Fig. 3c is 162.2 eV, which are in good agreement with the results of a previous report on MoS2 nanosheets.27 In Fig. 3d, the XPS peak corresponding to Cu 2p3/2 appeared at 932.1 eV which indicated the formation of Cu0 nanoparticles. However, the peaks around 943 and 963 eV are mainly attributed to the Cu2+ satellite peak which can be evidently observed in copper(II) oxide.28 The results suggested that the surface of Cu nanoparticles was partially oxidized. The XPS results further indicate the successful preparation of Cu–MoS2 hybrids.
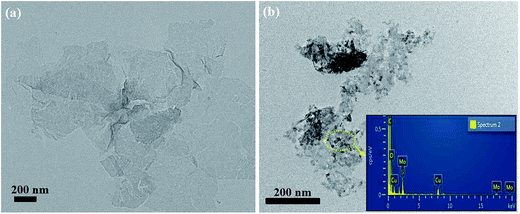
The morphology and microstructure of the exfoliated MoS2 nanosheets and Cu–MoS2 hybrids are characterized by TEM (Fig. 4a and b). As observed in Fig. 4a, the exfoliated MoS2 nanosheets exhibit a typical two dimensional nanoplatelet shape. As for the Cu–MoS2 hybrids (Fig. 4b), the Cu nanoparticles are decorated on the surface of MoS2 nanosheets successfully without obvious aggregation, which inhibit the restacking of MoS2 nanosheets effectively. The EDX spectra (inset in Fig. 4b) further confirm that the Cu–MoS2 hybrids are successfully fabricated.
To evaluate the dispersion state of Cu–MoS2 hybrids in PVC nanocomposites, the morphologies of the fractured surfaces are observed by SEM (Fig. 5). For pure PVC, it has a uniform surface morphology revealing a rather smooth surface (Fig. 5a and b). Compared to the pure PVC, the fractured surface of the PVC composites shows considerably different fractographic features with a relative rough surface, as shown in Fig. 5c and d. Moreover, it can be observed that the Cu–MoS2 hybrids are dispersed uniformly and embedded in the PVC matrix without obvious aggregation. The homogeneous distribution of Cu–MoS2 hybrids across PVC matrix can increase the interfacial area between the matrix and the nanosheets, which is important for the following property improvements.
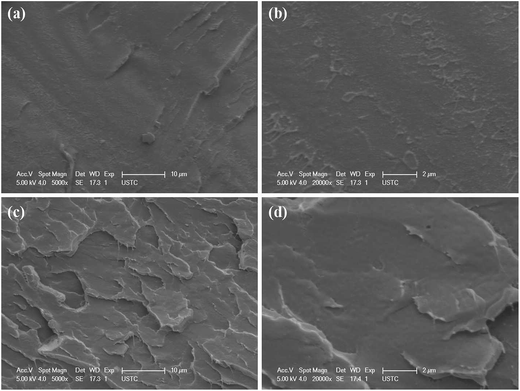 | ||
| Fig. 5 SEM images of the fractured surfaces for neat PVC (a and b) and PVC/0.5% Cu–MoS2 composites (c and d). | ||
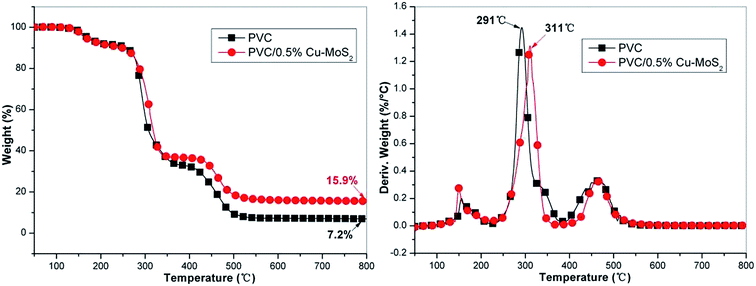
The thermal degradation behavior of PVC and its composites is investigated by TG and TG-IR. TG-DTG profiles of neat PVC and its composites under nitrogen atmosphere are plotted in Fig. 6. As can be observed, the PVC/Cu–MoS2 composites present similar degradation behaviors to that of neat PVC, consisting of three stages referred to the DTG profiles, corresponding to the vaporization of small molecules, dehydrochlorination of the polymer, resulting in the formation of conjugated double bonds that break during the third step, respectively.25 However, it is clearly observed that the addition of Cu–MoS2 hybrids can inhibit the emission of hydrogen chloride effectively in the second degradation stage (240–350 °C), which is good agreement with previous work.29 In comparison with those of neat PVC, the onset degradation temperature of the PVC composites with 0.5% Cu–MoS2 hybrids is almost the same. On the contrary, T−50% and Tmax of the PVC nanocomposites is enhanced obviously, indicating that the presence of Cu–MoS2 hybrids defers the thermal degradation of PVC. Moreover, it can be obtained from DTG profiles that the maximum mass loss rate (the peak of DTG curve) of the PVC composites with Cu–MoS2 hybrids is lower than that of neat PVC, suggesting that MoS2 nanosheets function as an effective mass transport barrier to inhibit the mass loss during the thermal degradation process.19 In addition, the incorporation of Cu–MoS2 hybrids exhibits higher char residues compared to neat PVC. When 0.5% Cu–MoS2 hybrids are added, the residues increase significantly by 120 wt% in comparison to the pure PVC, owning to the catalytic carbonization effect of the Cu–MoS2 hybrids. The formation of high char residues decreases the release of combustible gases and provides a protective shield of mass and heat transfer.
To further investigate the influence of Cu–MoS2 hybrids on the evolved gaseous volatiles during pyrolysis, the volatile components released from PVC and its composites are monitored by TG-IR. The gaseous decomposition products of the PVC materials are mainly composed of hydrocarbons (2950 cm−1), HCl (2798 cm−1), CO2 (2360 cm−1), aromatic compounds (690 cm−1) and water (4000–3500 and 1300–1800 cm−1).25 To investigate the differences between PVC and its composites, the Gram–Schmidt (GS) curves as well as pyrolysis products vs. time are shown in Fig. 7a–d. It can be clearly observed that the absorbance of total release of the decomposition products from PVC/0.5% Cu–MoS2 composites is much lower than that from virgin PVC. In order to provide a more clear comparison, the intensity of typical gaseous organic volatiles such as HCl, hydrocarbons and aromatic compounds of neat PVC and its composites are represented in Fig. 7b–d. It can be obviously observed that the addition of Cu–MoS2 hybrids reduces the evolution of all gaseous organic volatiles significantly which is mainly attributed to the enhancement of char formation and the physical barrier effect of the MoS2 nanosheets and inhibition effect of Cu nanoparticles. The reduced amount of the organic volatiles further leads to the inhibition of smoke.19 All in all, these results suggest that incorporating Cu–MoS2 hybrids into PVC would retard the thermal degradation of PVC molecular chains and inhibit the emission of toxic gaseous products, organic volatiles and smoke.
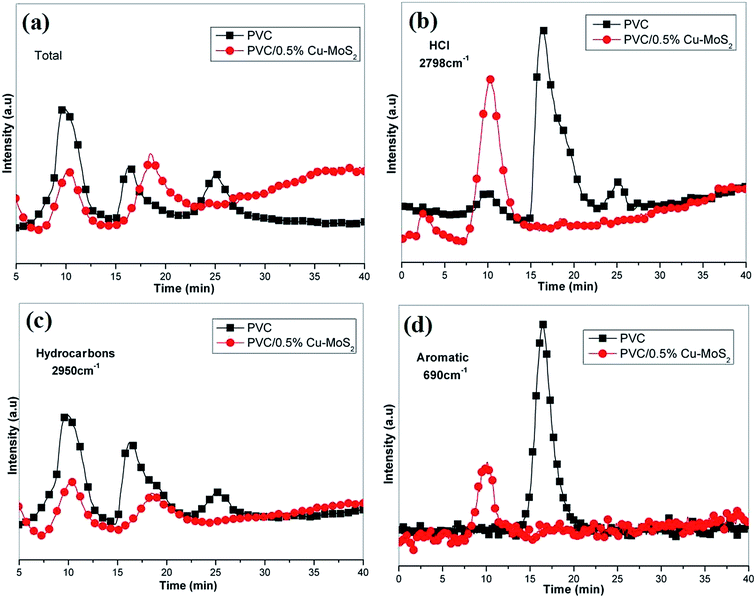 | ||
| Fig. 7 Gram–Schmidt curves (a) and intensity of characteristic peaks for pyrolysis products of neat PVC and PVC/0.5% Cu–MoS2 composites (b–d). | ||
4. Conclusion
In present study, Cu–MoS2 hybrids were synthesized by a facile wet chemical method and its structure was confirmed by XRD and XPS. A morphological study showed that Cu nanoparticles were homogeneously anchored on the MoS2 nanosheets, which effectively prevented the MoS2 nanosheets from restacking. SEM results demonstrated that the Cu–MoS2 hybrids were homogenously dispersed in PVC matrix without obvious aggregation structure. The thermal stability of the PVC composites was significantly improved and the release of combustible gas and toxic HCl was effectively restrained, which was mainly attributed to the inhibition effect of Cu nanoparticles, physical barrier effect and charring effect of Cu–MoS2 hybrids.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (CUG160607).References
- J. Liu, G. M. Chen and J. P. Yang, Polymer, 2008, 49, 3923–3927 CrossRef CAS.
- K. Yao, J. Gong, N. N. Tian, Y. C. Lin, X. Wen, Z. W. Jiang, H. Na and T. Tang, RSC Adv., 2015, 5, 31910–31919 RSC.
- Y. T. Pan and D. Y. Wang, RSC Adv., 2015, 5, 27837–27843 RSC.
- H. Q. Qu, C. H. Liu and W. H. Wu, et al., J. Therm. Anal. Calorim., 2014, 115, 1081–1087 CrossRef CAS.
- D. K. Chattopadhyay and D. C. Webster, Prog. Polym. Sci., 2009, 34, 1068–1133 CrossRef CAS.
- S. H. Jiang, X. F. Peng, Y. Hu and Z. Gui, Mater. Lett., 2015, 161, 561–564 CrossRef CAS.
- P. Kiliaris and C. D. Papaspyrides, Prog. Polym. Sci., 2010, 35, 902–958 CrossRef CAS.
- S. M. Grimes, H. Lateef, A. J. Jafari and L. Mehta, Polym. Degrad. Stab., 2006, 91, 3274–3280 CrossRef CAS.
- H. Zhu, W. S. Wang and T. X. Liu, J. Appl. Polym. Sci., 2011, 122, 273–281 CrossRef CAS.
- K. Q. Zhou, S. H. Jiang and Y. Q. Shi, et al., RSC Adv., 2014, 4, 40170–40180 RSC.
- S. K. Kim, J. J. Wie, Q. Mahmood and H. S. Park, Nanoscale, 2014, 6, 7430–7435 RSC.
- K. Q. Zhou, S. H. Jiang, C. L. Bao and L. Song, et al., RSC Adv., 2012, 2, 11695–11703 RSC.
- O. Eksik, J. Gao, S. A. Shojaee and A. Thomas, et al., ACS Nano, 2014, 8, 5282–5289 CrossRef CAS PubMed.
- K. Q. Zhou, W. Yang and G. Tang, et al., RSC Adv., 2013, 3, 25030–25040 RSC.
- M. Zvonimir, S. Ruchi and E. Manias, et al., Polym. Degrad. Stab., 2012, 97, 2481–2486 CrossRef.
- K. Q. Zhou, Q. J. Zhang and J. J. Liu, et al., RSC Adv., 2014, 4, 13205–13214 RSC.
- K. Q. Zhou, J. J. Liu and W. R. Zeng, et al., Compos. Sci. Technol., 2015, 107, 120–128 CrossRef CAS.
- K. Q. Zhou, J. J. Liu and Y. Q. Shi, et al., ACS Appl. Mater. Interfaces, 2015, 7, 6070–6081 CAS.
- D. Wang, L. Song, K. Q. Zhou, X. J. Yu, Y. Hu and J. Wang, J. Mater. Chem. A, 2015, 3, 14307–14317 CAS.
- W. Wu, M. L. Li and Y. H. Zhong, et al., RSC Adv., 2016, 6, 3267–3275 RSC.
- K. Q. Zhou, J. J. Liu, P. Y. Wen, Y. Hu and Z. Gui, Appl. Surf. Sci., 2014, 316, 237–244 CrossRef CAS.
- C. Zhang, W. W. Tjiu and T. X. Liu, et al., J. Phys. Chem. B, 2011, 115, 3392–3399 CrossRef CAS PubMed.
- Y. Q. Shi, X. D. Qian and K. Q. Zhou, et al., Ind. Eng. Chem. Res., 2013, 52, 13654–13660 CrossRef CAS.
- X. Huang and Z. Y. Zeng, et al., Nat. Commun., 2013, 4, 1444 CrossRef PubMed.
- X. M. Feng, W. Y. Xing and L. Song, et al., Chem. Eng. J., 2015, 260, 524–531 CrossRef CAS.
- X. M. Feng, W. Y. Xing, L. Song and Y. Hu, J. Mater. Chem. A, 2014, 2, 13299–13308 CAS.
- J. W. Huang, Y. Q. He, J. Jin and Y. R. Li, et al., Electrochim. Acta, 2014, 136, 41–46 CrossRef CAS.
- C. J. Wu, S. L. Cheng, Y. J. Sheng and H. K. Tsao, RSC Adv., 2015, 5, 53275–53279 RSC.
- J. Z. Xu and C. H. Liu, et al., Polym. Degrad. Stab., 2013, 98, 1506–1514 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |