Facile synthesis of a nickel vanadate/Ni composite and its electrochemical performance as an anode for lithium ion batteries†
Yang Lia,
Ling-Bin Kong *ab,
Mao-Cheng Liuab and
Long Kangab
*ab,
Mao-Cheng Liuab and
Long Kangab
aState Key Laboratory of Advanced Processing and Recycling of Non-ferrous Metals, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: konglb@lut.cn; Fax: +86-931-2976578; Tel: +86-931-2976579
bSchool of Materials Science and Engineering, Lanzhou University of Technology, Lanzhou 730050, P. R. China
First published on 13th September 2016
Abstract
Ni3V2O8/Ni composites were prepared by a simple one-step hydrothermal route and they showed superior electrochemical performance as anode materials for Li-ion batteries. They had a unique three-dimensional (3D) architecture, in which interlaced Ni3V2O8 nanosheets and nanoflakes were uniformly grown on Ni foam. The Ni3V2O8/Ni composites delivered a discharge capacity of 1626.1 in the initial cycle at a specific current of 200 mA g−1, maintaining 1286.8 mA h g−1 after 100 cycles. After 600 cycles at 1000 mA g−1, the discharge capacity was maintained at 941.3 mA h g−1, and even when the current was 10 A g−1, a discharge capacity of 477.7 mA h g−1 was achieved. The excellent lithium storage performance of the Ni3V2O8/Ni composites could be attributed to their unique 3D architecture, favorable electrochemical reconstruction in cycling and their intimate contact with the Ni foam substrates, which offered fast electrical and ionic transport, provided a sufficient electrode/electrolyte contact area and facilitated accommodation of strain during lithiation/delithiation cycling. In addition, details of the electrochemical reaction process of Ni3V2O8 were carefully investigated to discover the conversion and intercalation reaction routes. Such novel Ni3V2O8/Ni composites might provide insight for the use of metal vanadate as an energy storage material.
1 Introduction
Currently, lithium-ion batteries (LIBs) with their high energy density, long lifespan and low self-discharging rate, have been regarded as one of the most important means for modern electrochemical energy storage.1,2 However, graphitic carbon, the most commercially used anode material, limits the advancement of LIBs in large-size power tools due to its low theoretical capacity (372 mA h g−1), poor rate performance and safety issues. In this sense, it is highly desirable to continually research and develop new anode materials with high capacities and long cycle life to meet the growing demands of next-generation, high-power LIBs.3,4Due to their much higher theoretical capacities over the commercially used graphite, transition metal oxides (TMOs) such as Co3O4,5 CoO,6 NiO,7 Fe2O3,8 Fe3O4,9 CuO (ref. 10) and Cu2O (ref. 11) have been regarded as promising candidates as alternative anode materials for LIBs. In particular, nickel-based oxides have been extensively studied because of their high theoretical capacity, enhanced safety and low cost.12 However, low electrical conductivity, large volume variation and subsequent particle pulverization upon a charge–discharge process limit their rate capabilities and make them rather difficult to fully utilize the conversion reaction in practice. To address this problem, one attractive approach is to combine the active materials with an electronic substrate, which can enhance their electronic conductivity and structure stability, leading to improved electrochemical performance.13–15 Furthermore, the electrochemical performance of the prepared composite materials exhibited a close relationship with the electric substrates. Among them, Ni foam becomes an ideal electric substrate due to its three dimensional (3D) porous architecture and large surface area.16–19 Another strategy is to design binary metal oxides to improve their electrochemical performances. The introduction of the second metal oxides can synergistically enhance electrochemical performances compared with the single phase metal oxides, containing electrical-ionic conductivity, reversible capacity and mechanical stability.20,21 In this case, the binary metal oxides such as ZnCo2O4,22 Co2GeO4,23 NiCo2O4,24 NiMn2O4,25 and NiMoO4 (ref. 26) have shown attractive electrochemical performance as anode materials for LIBs. As important binary metal oxides, metal vanadium oxides stand out from the others due to their wide potential window originating from the multiple valence states of the vanadium element and highly natural abundance, which can be considered as promising anode for LIBs.27–32 For example, Yang et al. reported the preparation of Co3V2O8 multilayered nanosheets, which exhibited remarkable specific capacities (1114 mA h g−1) after 100 cycles.33 In that study, the Li storage mechanism of Co3V2O8 was also investigated where the conversion reaction of CoO into Co resided in the amorphous LixV2O5 matrix served as a reactive site. Yin et al. reported the preparation of MoV2O8 nanorods, which exhibited a high discharge capacity of 1325 mA h g−1 after 50 cycles at a current rate of 0.2 A g−1.34 Only very recently, Wang et al. demonstrated electrochemical performance of Ni3V2O8 as a new anode for LIBs.35 The resulting Ni3V2O8 nanostructure was capable of reaching a specific capacity of 969.72 mA h g−1 at 500 mA g−1 after 500 cycles, indicating a good prospect of Ni3V2O8 for LIB application. However, the synthesis processes of the Ni3V2O8 nanostructures in their studies require a complicated two-step process, making the development of Ni3V2O8 nanostructures costly and time-consuming. Furthermore, systemic evaluations on the electrochemical performances and charge/discharge mechanism of Ni3V2O8 have not been reported by now, which still remain largely unexplored.
In this work, we fabricated Ni3V2O8 by a simple one-step hydrothermal method, and the electrochemical performance of Ni3V2O8/Ni composite as a new sort of anode for LIBs was systemically studied. The composites exhibited superior electrochemical performance in terms of impressive high-rate capability and outstanding long-life cycling stabilities due to 3D macroporous structure, the favorable structure reconstruction in cycling and the use of Ni foam during the preparation, endowed Ni3V2O8 with potential application for high-performance LIBs. In addition, detailed charge/discharge process of the as-prepared Ni3V2O8/Ni composite was also investigated to explore the electrochemical reaction routes of Ni3V2O8, which could make contributions to the nickel vanadate applied for energy storage.
2 Experimental sections
2.1 Synthesis of materials
All chemicals used in this work were of analytical grade, which were directly used after purchase without any further purification. Deionized water was used throughout. The Ni3V2O8/Ni composites were synthesized via a hydrothermal approach. In a typical synthesis procedure, 3.38 mmol Ni(NO3)2·6H2O and 2.24 mmol Na3VO4·12H2O were dissolved into 80 mL deionized water at room temperature, then the obtained mixed solution was transferred into a 100 mL Teflon-lined autoclave with insertion of a piece of clean Ni foam, and maintained at 120 °C for 3 h. After that, the resultant samples were washed with distilled water and absolute ethanol several times, dried at 60 °C for 12 h to obtain Ni3V2O8/Ni composite. In order to demonstrate the superior electrochemical performance of the products, the sediment (Ni3V2O8 powders) in the solution was also collected for the comparison in electrochemical measurements.2.2 Materials characterization
The crystallographic structure of the products was characterized by X-ray diffractometer (XRD, Rigaku D/MAX 2400). The morphology was characterized by field-emission Scanning Electron Microscope (SEM, JEOL, JSM-6701F), and the microstructure was determined by Transmission Electron Microscope (TEM, JEOL, JEM-2010). Energy Dispersive Spectrometer (EDS) and Inductively Coupled Plasma (ICP-AES, IRIS Intrepid II XSP) were carried out to measure the element component of samples.2.3 Electrochemical measurements
The electrochemical properties were tested using CR-2032 coin-type cells. The Ni3V2O8/Ni composites directly acted as working electrode. The mass loading of the active materials was around 1–2 mg cm−2. The Ni3V2O8 powders electrode was fabricated by mixing the Ni3V2O8, acetylene black and a sodium alginate at a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. A Li foil was used as both the counter electrode and the reference electrode. The used electrolyte was 1 M LiPF6 in ethylene carbonate and dimethyl carbonate (1
10. A Li foil was used as both the counter electrode and the reference electrode. The used electrolyte was 1 M LiPF6 in ethylene carbonate and dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, by volume). The cells were cycled using a lithium-ion battery cycler (LAND CT2001A) in the voltage window 0.01–3 V (vs. Li+/Li) at different current rates. Cyclic voltammetry (CV; 0.01–3 V, 0.1 mV s−1) and electrochemical impedance spectroscopy (EIS; 100 kHz to 0.01 Hz, 5 mV s−1) of the cell was performed on an electrochemical workstation (CHI660C).
1, by volume). The cells were cycled using a lithium-ion battery cycler (LAND CT2001A) in the voltage window 0.01–3 V (vs. Li+/Li) at different current rates. Cyclic voltammetry (CV; 0.01–3 V, 0.1 mV s−1) and electrochemical impedance spectroscopy (EIS; 100 kHz to 0.01 Hz, 5 mV s−1) of the cell was performed on an electrochemical workstation (CHI660C).
3 Results and discussion
3.1 Synthesis and characterization
The XRD pattern of Ni3V2O8/Ni electrode was shown in Fig. 1a. Three diffraction peaks at 44.8, 52.2 and 76.8 were correlated to Ni (111), (200) and (220) faces, respectively (JCPDF card no. 03-1051). The characteristic peaks at 15.6, 18.9, 27.5, 30.0, 35.9, 58.9 and 64.0 degree were readily indexed to (020), (120), (211), (131), (122), (162) and (442) reflections of Ni3V2O8 phase (JCPDF card no. 74-1484). No additional diffraction peaks were detected, indicating the high purity of the samples. The EDS results indicated the presence of the Ni, V and O elements (Fig. 1b), and the Ni/V molar ratio was about 1.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which approximately corresponded to a Ni3V2O8 molar ratio of 1.5
1, which approximately corresponded to a Ni3V2O8 molar ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The elemental analyses of the Ni3V2O8/Ni samples were further determined using ICP technique, Ni/V atomic ratios of two samples were about 1.54
1. The elemental analyses of the Ni3V2O8/Ni samples were further determined using ICP technique, Ni/V atomic ratios of two samples were about 1.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as shown in Table S1,† which was consistent with EDS results.
1 as shown in Table S1,† which was consistent with EDS results.
 | ||
| Fig. 1 (a) The XRD patterns of Ni3V2O8/Ni composites. (b) The EDS spectrums of Ni3V2O8/Ni composites. | ||
The XPS analysis was further employed to investigate chemical composition and oxidation state of each element in the samples as shown in Fig. 2. The typical Ni 2p, V 2p and O 1s signals were observed in the full spectrum (Fig. 2a). Among them, the peak at 855.9 eV can be ascribed to the existence of Ni3+, while that at 872.9 eV can be assigned to be Ni2+. These results indicated that Ni2+ and Ni3+ cations existed together in Ni3V2O8 structures, which was in good agreement with previous reports.35,36 Similarly, the high-resolution V 2p spectrum showed two peaks with binding energies at 524.6, and 517.4 eV. These two peaks could correspond the V5+ phase (Fig. 2c).37,38 The high-resolution O 1s spectrum exhibited a strong peak around 530.5 eV, and this value matched well with the characteristic peak of O 1s (Fig. 2d).39
The morphology and microstructure of the as-fabricated Ni3V2O8/Ni electrodes were characterized by SEM and TEM. Fig. 3a showed a low magnification SEM image of the Ni3V2O8/Ni electrodes, indicating that Ni3V2O8 directly grew on the Ni foam inheriting the porous structure of the Ni foam (Fig. S1†). As shown in Fig. 3b, the Ni3V2O8 consisted of a large number of interlaced nanosheets, forming 3D macroporous structures. In addition, nanoflakes-like morphology were also observed, which derived from the accumulation of nanosheets (Fig. 3c). The mean diameter of these nanoflakes ranged from tens of nanometers to several microns. The high-magnification SEM and TEM images of sample further confirmed that Ni3V2O8 were composed of a large number of nanosheets with mean thickness about 100–200 nm (Fig. 3d and e). Fig. 3f was a HR-TEM image of the Ni3V2O8 nanosheets, which exhibited clear crystal grain boundaries and lattice fringes, and the interplanar spacing of a single particle was about 0.28 nm, corresponding to the (040) plane of the Ni3V2O8. As demonstrated above, the current method fabricated the Ni3V2O8/Ni composites successfully.
 | ||
| Fig. 3 (a–d) The SEM and (e and f) the TEM images of the Ni3V2O8/Ni composites at different magnification. | ||
3.2 Electrochemical performances of the electrodes
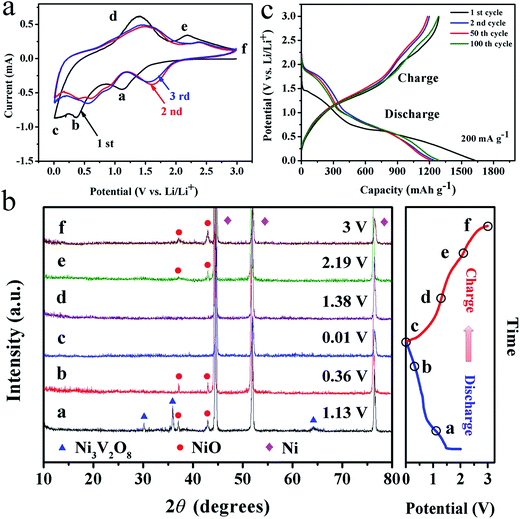
The electrochemical reaction of the Ni3V2O8/Ni as anodes for LIBs was examined by the CV measurement for the first three cycles at a scan rate of 0.1 mV s−1 in the range of 0.01–3.0 V (versus Li/Li+) at room temperature (Fig. 4a). In the first discharge curve, a reduction peak at around 1.13 V could be assigned to the transformation of Ni3V2O8 into NiO accompanied by the formation of LixV2O5.33,40,41 A strong peak at 0.36 V was detected corresponding to the further insertion of Li+ into the LixV2O5 to form Lix+yV2O5 and reduction reactions of NiO to Ni, companied with the irreversible formation of SEI film. During the subsequent cathodic scans, two reduction peaks in the voltage regions of 2.06–1.19 and 1.17–0.20 V were ascribed to insertion of Li+ into the LixV2O5 and the reduction process from NiO to Ni. The cyclic voltammogram for the first cycle was substantially different from those of the subsequent ones. This result revealed that drastic lithium driven, structure/textural rearrangement and modifications occurred on active. Two broader anodic peaks of the first cycle located at about 1.38 and 2.19 V can be described as the extraction of Li+ from the LixV2O5 and the oxidation of Ni to NiO companied with the decomposition of Li2O and the possible decomposition of the as-formed SEI. Interestingly, the area integrated within the current potential curves greatly increased along with scan number, leading to a much larger capacity.In order to further clarify the lithium storage mechanism of composites, a series of partially lithiated cells were selected in different states as denoted with letters “a” to “f” in Fig. 2a and b. The corresponding ex situ X-ray patterns were shown in Fig. 2b. When first discharging from the open circuit voltage to 1.13 V (a), the initial Ni3V2O8 phase gradually turned to the NiO phase (JCPDF card no. 44-1159) and amorphous LixV2O5. After discharging to 0.36 V (b), the Ni3V2O8 phases disappeared, suggesting the reduction of all the Ni3V2O8 into new phases. Meanwhile, the diffraction peak of NiO became weak (b) and gradually disappeared along with the further discharging to 0.01 V (c). This indicated that the reduction reactions of NiO to Ni companied with further insertion of Li+ into the LixV2O5 to form Lix+yV2O5 and the irreversible formation of SEI film. Owning to the amorphous characteristic of LixV2O5, the related XRD parameters could not be measured,33 and it was hard to find the metallic Ni and Li2O phase in XRD patterns, which might be due to the strong impacts of Ni foam and the low crystallization of Li2O. In the subsequent charge process, amorphous state of the electrode was preserved when charging to 1.38 V (d), which could contribute to the extraction of Li+ from the LixV2O5. The NiO phase reappeared when charging to 2.19 V (e), which corresponded to the oxidation of Ni into NiO. Then, the NiO phase showed increased intensity when further charging to 3 V (f), indicating enhanced phase transition with the increase of potential. Through these analyses, we could make preliminarily confirmation about the electrochemical reaction mechanism of the composite for LIBs as summarized by the following equations:
| Ni3V2O8 + xLi+ + xe− → 3NiO + LixV2O5 | (1) |
| LixV2O5 + yLi+ + ye− ↔ Lix+yV2O5 | (2) |
| NiO + 2Li+ + 2e− ↔ Ni + Li2O | (3) |
Fig. 4b presented the discharge–charge curves of the Ni3V2O8/Ni electrode at a current density of 200 mA g−1 in the range of 0.01–3.0 V. As seen, the charge and discharge curves showed similar profiles from the 2nd to the 100th cycle, indicating highly stable lithiation/delithiation process of the Ni3V2O8/Ni electrode. The initial discharge curve showed a clear flat plateau in sloping potential region from 1.45–0.64 V, which was relevant to the irreversible reactions of Ni3V2O8 and Li+ as eqn (1). The first discharge capacity were 1626.1 mA h g−1, and the potential plateau in sloping potential region from 0.65–0.01 V delivered the majority of the capacity, being associated with the reduction reactions of eqn (2) and (3). Moreover, this discharge process also contained the formation organic polymeric/gel-like layer of and SEI film by electrolyte decomposition. The initial charge capacities were 1287.3 mA h g−1, displaying a capacity loss of 338.8 mA h g−1 and a charging retention of 79.6%. The irreversible capacity could be ascribed to the irreversibility of eqn (1), the formation of a solid electrolyte interphase (SEI) and some undecomposed Li2O phase, which was commonly observed in metal oxide-based anodes.42–44
Fig. 5a depicted the cycling performances of Ni3V2O8/Ni and Ni3V2O8 powders at 200 mA g−1. We observed that both discharge and charge capacities attenuated slightly along with the increasing of the cycle number in the first few cycles, then increased slowly and finally reached stable value of 1286.7 and 1282.2 mA h g−1 after the 100 cycles. Moreover, Ni3V2O8/Ni electrode delivered coulombic efficiency (CE) of 79.6% in the initial cycle. However, the CE increased distinctly in the subsequent cycles and then gradually reached a stable, delivering a mean value of 99.6% after 100 cycles, indicating the ascendant oxidation reduction conversion capability. Furthermore, the resultant capacity of Ni3V2O8/Ni electrode was larger than that of individual Ni3V2O8 powders. As shown in Fig. 5a, Ni3V2O8 powders show initial capacity of 1359.9 mA h g−1, but dropped to 644.1 mA h g−1 after 100 cycles. Meanwhile, the cycling performance of Ni3V2O8/Ni electrodes for another intermediate current density of 300 and 500 mA g−1 were also examined as shown in Fig. 5b. Similarly, the specific capacities initially decreased and gradually increased onwards, after 100 cycles, the discharge capacities were 1147.2 and 1144.9 mA h g−1 at 300 and 500 mA g−1, respectively. The increasing capacity suggested a gradual participation in electrochemical reaction of active Ni3V2O8 along with cycling number. The delivered capacity of Ni3V2O8/Ni electrode was higher than those of the NiO nanosheet arrays,45 V2O5 nanorods46 at the same current density.
Besides the high capacity and good cycling stability, the Ni3V2O8/Ni electrodes also showed an outstanding rate performance, as shown in Fig. 5c. The Ni3V2O8/Ni electrodes delivered discharge capacity of 1193.1, 1152.9, 985.8, 786.4 and 611.6 mA h g−1, at the current densities of 0.2, 0.5, 1, 2 and 5 A g−1 respectively. Even at a high rate of 10 A g−1, a large capacity of 477.7 mA h g−1 was still reached. This value of capacity was higher than the theoretical capacity of commercial graphite (372 mA h g−1). Upon altering the current density back to 0.2 A g−1, the capacity of was recovered to 1178.1 mA h g−1. This result demonstrated clearly that the electrochemical performance of the Ni3V2O8/Ni electrodes were superior to the Ni3V2O8 powder electrode. The substantial enhancement of cycling ability and rate capability could be related to the unique structural feature of the Ni3V2O8 nanosheet and nanoflakes and their intimate contact with the underlying Ni foam. On the one hand, the Ni3V2O8 nanosheet and nanoflakes shortened the ionic diffusion length; while they constructed 3D porous architectures that promoted the permeability of electrolyte making the active materials was fully utilized, and accommodated the volume change of the Ni3V2O8 in cycling. On the other hand, the Ni foam provided the electrode with a large surface area, high porosity, and high mechanical flexibility, thus ensuring abundant electro-active site for Li-storage reaction and strong electrode integrity for long cycling and high-rate operations. In addition, the binder-free electrode without the inactive binders or conducting additives could further accelerate Li-ion and electron kinetics of the redox reactions.
To further understand the electrochemical performance, a long cycling performance of Ni3V2O8/Ni electrode at a high current density of 1000 mA g−1 had been investigated. The discharge/charge profiles of the 1st, 2nd, 100th, 200th, 400th and 600th cycle were exhibited in Fig. 5d. The reversible capacity has slightly decreased in the initial 200 cycles. Nevertheless, both the discharge and charge capacity gradually increased in the following hundreds cycles, which maintained of 941.3 and 939.4 mA h g−1 even after 600 cycled, and the CE of the composites was almost close to 99.9% during the 600 cycles, indicating the highly reversible Li-storage process in cycling. Despite the low cost and facility of fabrication method, the electrochemical performance of the Ni3V2O8/Ni composites was comparable and even better than Ni3V2O8 nanowire arrays,35 NiCo2O4 nanowire arrays,47 NiMoO4 nanostructure/graphene foam,26 and Co3V2O8 interconnected hollow microsphere,43 which endowed them with practical application in LIBs.
It was confirmed that the structure and morphology variation of electrode during the cycling process had crucial effects on the electrochemical performance.48–50 For investigating the causing of the superior electrochemical performance of the Ni3V2O8/Ni electrode, the structure and morphology variation of the Ni3V2O8/Ni electrode after 100 cycles with charge state was studied. Fig. 6a showed a low magnification SEM image of the Ni3V2O8/Ni electrode after cycling with charge state. A complete collapse of the original Ni3V2O8 nanosheets and nanoflake was observed. For further investigating the microstructure of the cycled Ni3V2O8/Ni electrode, a high magnification SEM image was exhibited in Fig. 6b. It was interesting to observe that the morphology of cycled Ni3V2O8/Ni electrode changed completely, forming a film-like architecture originated from the assembly of numbers nanoparticles, and many holes with size several tens of nanometers were observed. Fig. 6c showed a TEM image of the cycled electrode, revealing that film-like electrode consisted of a large number of nanoparticles with mean size of 20–40 nm. A HRTEM image of the cycled electrode showed clear lattice fringes with interplanar spacing of 0.21 nm (Fig. 6d), corresponding to the (012) planes of NiO. Such morphology variation could attribute to an electrochemical activation and electrochemical reconstruction, accompanied by the initial particle size reduction of Ni3V2O8 and subsequent reassembly of these nanoparticles into secondary architecture. The formation of film-like architecture could enhance the electrical contact between Ni3V2O8 and Ni foam, thus improving reaction kinetics of the Ni3V2O8/Ni electrode, which can account for the excellent electrochemical performance.48,50
 | ||
| Fig. 6 (a and b) The SEM and (c and d) TEM images of Ni3V2O8/Ni electrodes after 100 cycles with different magnification. | ||
Fig. 7a showed the electrochemical impedance spectroscopy (EIS) of Ni3V2O8/Ni at different discharge/charge cycles. The equivalent circuits were displayed in the inset of Fig. 7a, where Rs was the SEI film and/or contact resistance; Rct was the charge transfer resistance, and Cdl was the capacitance related to the double layer, while Zw represented Warburg impedance associated with the Li-ion diffusion process within electrodes.51 Table S2† listed the parameters of the equivalent circuit for the Ni3V2O8/Ni electrode after fitting the diameter of the semicircular curve. The results revealed that the Rs of Ni3V2O8/Ni electrode increased slightly upon cycling process, which might be ascribed to the enhanced formation of SEI film due to the morphology change of Ni3V2O8/Ni during the cycling process. The Rct of the Ni3V2O8/Ni electrode for the 5th cycle decreased drastically compared with that of the fresh electrode, indicating improved charge-transfer process. In addition, the Rct after 5 cycles and 100 cycles test displayed similar value, indicating highly stable charge transfer process of the electrode in cycling. These findings might be associated with the electrochemical reconstruction of electrode, which resulted in the formation of a symmetrical film-like architecture during the cycling process, being accordance with the SEM and TEM observation in Fig. 6. The symmetrical film-like architecture facilitated the electric contact between Ni3V2O8 and Ni foam, thus can enhance the charge transfer process of electrode. Fig. 7b showed the EIS spectra of the fresh Ni3V2O8/Ni and Ni3V2O8 powders electrode. The fresh Ni3V2O8/Ni electrode exhibited a lower Rct (60.4 Ω) than that of the Ni3V2O8 powder electrode (94.1 Ω), demonstrating the improvement in electronic conductivity of the Ni3V2O8/Ni electrode due to the presence of the Ni foam.
 | ||
| Fig. 7 EIS spectra of (a) the Ni3V2O8/Ni electrodes after different cycles with charge state and (b) fresh Ni3V2O8/Ni and Ni3V2O8 powders electrode. | ||
As discussed above, the excellent electrochemical properties of Ni3V2O8/Ni electrodes might resulted from the original structural merits of Ni3V2O8/Ni electrode, favorable structure reconstruction in cycling and the use of Ni foam substrates during the preparation. Firstly, the Ni3V2O8 nanosheets and nanoflakes structure enabled short electron and ion transport pathways to boost the electrochemical reaction kinetics. Moreover, the nanosheets and nanoflakes constructed a 3D macroporous structure that provided sufficient electrolyte–electrode contact area and buffered the volume variation caused by the Li-ion insertion/extraction. Besides, unique 3D macroporous structure of Ni3V2O8 induced the formation of a symmetrical film-like architecture during the cycling process. In this secondary structure, the small sized Ni3V2O8 nanoparticles well anchored on Ni foam, resulted in improved reaction kinetics and highly stable charge transfer process. In addition, the Ni foam substrates also played an important role in improving the electrode conductivity by maintaining good electrical and mechanical connections to the Ni3V2O8 for fast and stable charge transfer, while provided the electrode with high electro-active surface area and high mechanical flexibility.
4 Conclusions
In summary, the Ni3V2O8/Ni composites had been successfully fabricated by a simple hydrothermal route as anodes for LIBs. The electrochemical reaction process had been carefully studied, which indicated the common phenomenon in the conversion and intercalation reaction of metal vanadium oxide. The Ni3V2O8/Ni composites delivered discharge capacities of 1286.8 after 100 cycles at 200 mA g−1, and a high reversible capacity of about 941.3 mA h g−1 after 600 cycles at 1000 mA g−1. Even when the current was 10 A g−1, capacity of 477.7 mA h g−1 had been achieved. The superior electrochemical performance was attributed to the structural advantages of the Ni3V2O8/Ni composites (nanosheets and nanoflakes architectures, enlarged electro-active sites and effective relief of volumetric change strains), the favorable morphology variation in cycling and the presence of Ni foam substrates (large surface area, high electronic conductivity, good mechanical flexibility and fast mass and electron transport kinetics). The results suggested good electrochemical compatibility between Ni3V2O8 and Ni foam, which demonstrates great potential of Ni3V2O8 as promising electrode material for high-performance energy storage systems.Acknowledgements
The work was supported by the National Natural Science Foundation of China (no. 51362018) and the Foundation for Innovation Groups of Basic Research in Gansu Province (no. 1606RJIA322).References
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496–499 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- B. Scrosati and J. Garche, J. Power Sources, 2010, 195, 2419–2430 CrossRef CAS.
- Q. Zhang, E. Uchaker, S. L. Candelaria and G. Cao, Chem. Soc. Rev., 2013, 42, 3127–3171 RSC.
- D. Wang, Y. Yu, H. He, J. Wang, W. Zhou and H. D. Abruna, ACS Nano, 2015, 9, 1775–1781 CrossRef CAS PubMed.
- Y. Qi, N. Du, H. Zhang, X. Fan, Y. Yang and D. Yang, Nanoscale, 2012, 4, 991–996 RSC.
- C. Yuan, J. Li, L. Hou, L. Yang, L. Shen and X. Zhang, Electrochim. Acta, 2012, 78, 532–538 CrossRef CAS.
- Y. Song, S. Qin, Y. Zhang, W. Gao and J. Liu, J. Phys. Chem. C, 2010, 114, 21158–21164 CAS.
- S. Zhang, W. He, X. Zhang, G. Yang, J. Ma, X. Yang and X. Song, Electrochim. Acta, 2015, 174, 1175–1184 CrossRef CAS.
- L. Wang, H. Gong, C. Wang, D. Wang, K. Tang and Y. Qian, Nanoscale, 2012, 4, 6850–6855 RSC.
- Y. Yang, K. Wang, Z. Yang, Y. Zhang, H. Gu, W. Zhang, E. Li and C. Zhou, Thin Solid Films, 2016, 608, 79–87 CrossRef CAS.
- J. Liang, H. Hu, H. Park, C. Xiao, S. Ding, U. Paik and X. W. Lou, Energy Environ. Sci., 2015, 8, 1707–1711 CAS.
- X. Xie, T. Makaryan, M. Zhao, K. L. Van Aken, Y. Gogotsi and G. Wang, Adv. Energy Mater., 2016, 6, 1502161 CrossRef.
- S. Saadat, J. Zhu, D. H. Sim, H. H. Hng, R. Yazami and Q. Yan, J. Mater. Chem. A, 2013, 1, 8672 CAS.
- Y. Sun, X. Hu, J. C. Yu, Q. Li, W. Luo, L. Yuan, W. Zhang and Y. Huang, Energy Environ. Sci., 2011, 4, 2870 CAS.
- D. Kong, J. Luo, Y. Wang, W. Ren, T. Yu, Y. Luo, Y. Yang and C. Cheng, Adv. Funct. Mater., 2014, 24, 3815–3826 CrossRef CAS.
- G. Gao, H. B. Wu, S. Ding, L. M. Liu and X. W. Lou, Small, 2015, 11, 804–808 CrossRef CAS PubMed.
- X. Zhao, B. Liu, C. Hu and M. Cao, Chem.–Eur. J., 2014, 20, 467–473 CrossRef CAS PubMed.
- S. Ni, X. Lv, J. Ma, X. Yang and L. Zhang, Electrochim. Acta, 2014, 130, 800–804 CrossRef CAS.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- Y. Sharma, N. Sharma, G. V. Subba Rao and B. V. R. Chowdari, Adv. Funct. Mater., 2007, 17, 2855–2861 CrossRef CAS.
- Y. Wang, J. Ke, Y. Zhang and Y. Huang, J. Mater. Chem. A, 2015, 3, 24303–24308 CAS.
- S. Jin, G. Yang, H. Song, H. Cui and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 24932–24943 CAS.
- G. Chen, J. Yang, J. Tang and X. Zhou, RSC Adv., 2015, 5, 23067–23072 RSC.
- W. Kang, Y. Tang, W. Li, X. Yang, H. Xue, Q. Yang and C. S. Lee, Nanoscale, 2015, 7, 225–231 RSC.
- B. Wang, S. Li, X. Wu, W. Tian, J. Liu and M. Yu, J. Mater. Chem. A, 2015, 3, 13691–13698 CAS.
- E. Baudrin, S. Laruelle, S. Denis, M. Touboul and J. M. Tarascon, Solid State Ionics, 1999, 123, 139–153 CrossRef CAS.
- S. Denis, E. Baudrin, F. Orsini, G. Ouvrard, M. Touboul and J. M. Tarascon, J. Power Sources, 1999, 81–82, 79–84 CrossRef CAS.
- D. Hao Sim, X. Rui, J. Chen, H. Tan, T. M. Lim, R. Yazami, H. H. Hng and Q. Yan, RSC Adv., 2012, 2, 3630 RSC.
- Y. Cao, D. Fang, R. Liu, M. Jiang, H. Zhang, G. Li, Z. Luo, X. Liu, J. Xu, W. Xu and C. Xiong, ACS Appl. Mater. Interfaces, 2015, 7, 27685–27693 CAS.
- Y. Cao, D. Fang, C. Wang, L. Li, W. Xu, Z. Luo, X. Liu, C. Xiong and S. Liu, RSC Adv., 2015, 5, 42955–42960 RSC.
- D. Fang, L. Li, W. Xu, G. Li, Z. Luo, C. Liang, Y. Ji, J. Xu and C. Xiong, RSC Adv., 2014, 4, 25205 RSC.
- G. Yang, H. Cui, G. Yang and C. Wang, ACS Nano, 2014, 8, 4474–4487 CrossRef CAS PubMed.
- Z. Yin, Y. Xiao, X. Wang, W. Wang, D. Zhao and M. Cao, Nanoscale, 2016, 8, 508–516 RSC.
- C. Wang, D. Fang, H. Wang, Y. Cao, W. Xu, X. Liu, Z. Luo, G. Li, M. Jiang and C. Xiong, Sci. Rep., 2016, 6, 20826 CrossRef CAS PubMed.
- N. S. McIntyre and M. G. Cook, Anal. Chem., 1975, 47, 2208–2213 CrossRef CAS.
- M. C. Liu, L. B. Kong, L. Kang, X. Li, F. C. Walsh, M. Xing, C. Lu, X. J. Ma and Y. C. Luo, J. Mater. Chem. A, 2014, 2, 4919 CAS.
- F. Wu, S. Xiong, Y. Qian and S. H. Yu, Angew. Chem., Int. Ed., 2015, 54, 10787–10791 CrossRef CAS PubMed.
- M. Xing, L. B. Kong, M. C. Liu, L. Y. Liu, L. Kang and Y. C. Luo, J. Mater. Chem. A, 2014, 2, 18435–18443 CAS.
- L. H. Gan, D. Deng, Y. Zhang, G. Li, X. Wang, L. Jiang and C. R. Wang, J. Mater. Chem. A, 2014, 2, 2461 CAS.
- J. Ma, S. Ni, J. Zhang, X. Yang and L. Zhang, Phys. Chem. Chem. Phys., 2015, 17, 21442–21447 RSC.
- G. Gao, S. Lu, B. Dong, Y. Xiang, K. Xi and S. Ding, J. Mater. Chem. A, 2016, 4, 6264–6270 CAS.
- Y. Luo, X. Xu, X. Tian, Q. Wei, M. Yan, K. Zhao, X. Xu and L. Mai, J. Mater. Chem. A, 2016, 4, 5075–5080 CAS.
- Z. Zheng, Y. Cheng, X. Yan, R. Wang and P. Zhang, J. Mater. Chem. A, 2014, 2, 149–154 CAS.
- X. Wang, L. Qiao, X. Sun, X. Li, D. Hu, Q. Zhang and D. He, J. Mater. Chem. A, 2013, 1, 4173 CAS.
- A. M. Glushenkov, M. F. Hassan, V. I. Stukachev, Z. Guo, H. K. Liu, G. G. Kuvshinov and Y. Chen, J. Solid State Electrochem., 2010, 14, 1841–1846 CrossRef CAS.
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater., 2014, 24, 2630–2637 CrossRef CAS.
- Y. Sun, X. Hu, W. Luo, F. Xia and Y. Huang, Adv. Funct. Mater., 2013, 23, 2436–2444 CrossRef CAS.
- T. Kennedy, E. Mullane, H. Geaney, M. Osiak, C. O'Dwyer and K. M. Ryan, Nano Lett., 2014, 14, 716–723 CrossRef CAS PubMed.
- S. Ni, X. Lv, T. Li, X. Yang, L. Zhang and Y. Ren, Electrochim. Acta, 2013, 96, 253–260 CrossRef CAS.
- J. Y. Xiang, J. P. Tu, Y. Q. Qiao, X. L. Wang, J. Zhong, D. Zhang and C. D. Gu, J. Phys. Chem. C, 2011, 115, 2505–2513 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra19430c |
| This journal is © The Royal Society of Chemistry 2016 |