The investigation of the electrochemically supercapacitive performances of mesoporous CuCo2S4†
Yirong Zhuab,
Xiaobo Jic,
Han Chena,
Liujiang Xia,
Wenqiang Gonga and
Yong Liu*b
aCollege of Metallurgy and Materials Engineering, Hunan University of Technology, Zhuzhou, 412007, China. E-mail: zhuyirong2004@163.com; Fax: +86-731-22183470; Tel: +86-731-22183470
bState Key Lab of Powder Metallurgy, Central South University, Changsha, 410083, China. E-mail: yonliu@csu.edu.cn; Fax: +86-731-88710855; Tel: +86-731-88876630
cCollege of Chemistry and Chemical Engineering, Central South University, Changsha, 410083, China. E-mail: xji@csu.edu.cn; Fax: +86-731-88879616; Tel: +86-731-88879616
First published on 1st September 2016
Abstract
Ternary metal sulfides have been regarded as a promising class of electrode materials for high-performance supercapacitors since they can offer higher electronic conductivity and higher electrochemical activity than single-component metal sulfides. In this work, mesoporous CuCo2S4 nanoparticles have been prepared by a solvothermal route and utilized as supercapacitor electrode materials. The as-fabricated mesoporous CuCo2S4 nanoparticles exhibit a specific capacitance of 752 F g−1 at a current density of 2 A g−1, excellent rate capability with 47.9% of capacity retention from 2 to 100 A g−1, and good cycling stability with 98.1% of initial capacity retention and 90.1% of activated maximum capacity retention at a current density of 3 A g−1 for 5000 cycles. This superior electrochemical performance can be ascribed to the synergetic effect of the large specific surface area, superior mesoporous structure and high electronic conductivity. These results above render the as-fabricated mesoporous CuCo2S4 nanoparticles promising electrode materials for supercapacitors. Additionally, this facile and cost-effective preparation method can be extended to the fabrication of other ternary or even quaternary metal sulfides in the development of high-performance supercapacitors and other energy storage systems.
1. Introduction
With the increasing tension of oil resources and the worsening of environmental pollution, it is urgent to explore new energy sources for sustainable development and advanced energy storage technology.1–3 Supercapacitors are a new type of energy storage device that can bridge the gap between conventional capacitors and batteries, and they have characteristics of high power density, long cycle life, and fast charging and discharging capability, and being maintenance free, economic and environmentally friendly, etc.4,5 In view of these advantages, supercapacitors have shown a wide application prospect in military, transportation, new energy, consumer electronics and instruments, which have become some of the research focuses in the field of new energy in the world.6,7Generally, the properties of supercapacitors are influenced by the electrode material, electrolyte and assembly technology. Among them, the vital factor is electrode material, and therefore it has become a research focus for scholars.8,9 Currently, the study of the electrode materials is mainly focused on improving the electrochemical performances of the existing materials and the development of new high-performance electrode materials. According to the storage mechanism, the electrode materials can be divided into two types, carbon materials and pseudocapacitive materials.10 The carbon materials such as activated carbon have realized commercial production, but they usually suffer from low specific capacitance. While pseudocapacitive materials exhibit higher specific capacitances than carbon materials, so they have become research hotspots in recent years. Among these pseudocapacitive materials, metal sulfides as a new type of electrode material have recently attracted wide attention due to their excellent electrochemical performances, especially ternary metal sulfides.11,12 Compared to the single-component metal sulfides, ternary metal sulfides show richer redox reactions and higher electronic conductivity, resulting in the enhancement of the electrochemical performances. Moreover, it has been recently reported that ternary metal sulfides exhibit superior electrochemical performances than ternary metal oxides because they not only own similar rich redox reactions but also have higher electronic conductivity. Chen et al.13 firstly reported the urchin-like NiCo2S4 as excellent electrode materials, which manifested higher specific capacitance and rate capability than those of NiCo2O4, Co9S8 and NiS counterparts. After that, a large quantity of literatures about NiCo2S4 with different morphologies were reported to utilize as promising candidate electrode materials for supercapacitors.14–18 However, to the best of our knowledge, CuCo2S4 is rarely reported as supercapacitor electrode materials. Very recently, CuCo2S4 nanoparticles have been firstly synthesized via solvothermal route using different solvents, and the as-obtained CuCo2S4 synthesized in glycerol displays remarkable electrochemical performances with an ultrahigh specific capacitance of 5030 F g−1 at a current density of 20 A g−1 in a polysulfide electrolyte, which is superior to those of previously reported ternary sulfides, suggesting the potential applications for supercapacitors.19
In this work, mesoporous CuCo2S4 nanoparticles were prepared by using a facile solvothermal method. The electrochemical properties of the as-obtained mesoporous CuCo2S4 nanoparticles were evaluated as electrode materials for supercapacitors by using cyclic voltammograms, galvanostatic charge–discharge and electrochemical impedance spectroscopy in a three-electrode system, revealing that the samples exhibited superior electrochemical performances with high specific capacitance, outstanding rate capability and good cycling stability.
2. Experimental
2.1 Materials and chemicals
All the reagents used in the experiments were analytical grade and were used without any further treatment. Cu(AC)2·H2O (analytical grade), Co(AC)2·4H2O (analytical grade), CS(NH2)2 (analytical grade) and ethylene glycol (analytical grade) were purchased from Sinopharm Chemical Reagent Beijing Co., Ltd.2.2 Materials synthesis
Mesoporous CuCo2S4 nanoparticles were prepared via a facile solvothemal method. 0.0389 g of Cu(AC)2·H2O and 0.0971 g of Co(AC)2·4H2O were dissolved in 40 mL of ethylene glycol and stirred for 60 min. Then, 0.0891 g of thiourea was introduced into the mixed solution under strong stirring. After stirring for 30 min, the solution was transferred to a Teflon-lined stainless steel autoclave and heated at 180 °C for 24 h. After cooling, the black precipitate was washed with ethanol for several times, and dried in vacuum at 80 °C for 12 h to obtain the products.2.3 Materials characterization
Different analytical techniques were used to characterize the as-obtained sample. The X-ray powder diffraction (XRD) was carried out on a Rigaku D/max 2550 VB+ 18 kW X-ray diffractometer. Field emission scanning electron microscopy (FESEM) and energy dispersive spectroscopy (EDS) images were obtained from a MIRA3 scanning electron microscope. Transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) were measured by a JEM-2100F microscope operating at 200 kV. The Brunauer–Emmett–Teller (BET, BELSORP-MINIII) specific surface area and the pore size distribution were acquired based on the N2 adsorption/desorption measurement.2.4 Electrochemical characterization
Electrochemical experiments were conducted by using a standard three-electrode system. The CuCo2S4 electrode, platinum plate and Hg/HgO electrode were used as working electrode, counter electrode and reference electrode, respectively. 2 M KOH solution was used as the electrolyte and glassy fibrous material was employed as the separator. The working electrode was fabricated by first mixing CuCo2S4, super P and polyvinylidene difluoride (PVDF) with a mass ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and then the prepared mixture were coated onto the pretreated foam nickel. The pretreated process was the same with our previous work.20–22 Finally, the obtained working electrode was dried in vacuum at 100 °C for 12 h to evaporate the solvent, and pressed at 10 MPa for 30 s. All the electrochemical measurements including cyclic voltammetry (CV), galvanostatic current charge–discharge and electrochemical impedance spectroscopy (EIS) were performed by a CHI 660D electrochemical workstation. EIS measurement was conducted by applying an AC voltage with 5 mV amplitude in a frequency range from 0.1 Hz to 100 kHz.
10, and then the prepared mixture were coated onto the pretreated foam nickel. The pretreated process was the same with our previous work.20–22 Finally, the obtained working electrode was dried in vacuum at 100 °C for 12 h to evaporate the solvent, and pressed at 10 MPa for 30 s. All the electrochemical measurements including cyclic voltammetry (CV), galvanostatic current charge–discharge and electrochemical impedance spectroscopy (EIS) were performed by a CHI 660D electrochemical workstation. EIS measurement was conducted by applying an AC voltage with 5 mV amplitude in a frequency range from 0.1 Hz to 100 kHz.
3. Results and discussion
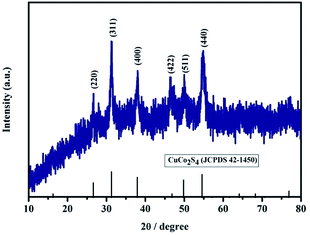
The structure and phase purity of the as-prepared CuCo2S4 sample were characterized by XRD, as shown in Fig. 1. All diffraction patterns can be indexed to the carrollite structure phase of CuCo2S4 (JCPDS card no. 42-1450), and the main diffraction peaks at 26.6°, 31.3°, 38.0°, 47.0°, 49.9° and 54.8° correspond to (220), (311), (400), (422), (511) and (440) planes of the carrollite CuCo2S4 phase, respectively. No obvious characteristic peaks from other crystallized phases can be observed, suggesting the formation of pure CuCo2S4 product.The morphology and structural features of the as-prepared CuCo2S4 sample were investigated by FESEM, TEM, HRTEM, SAED and EDS. As displayed in Fig. 2a and b, the FESEM images of the CuCo2S4 sample show the morphology of nanoparticles, and the sizes appear to be fairly uniform. The TEM images (Fig. 2c and d) further reveal that the obtained nanoparticles are basically uniform, and the particle sizes are less than 100 nm. The HRTEM image of the CuCo2S4 sample is shown in Fig. 2e, and the measured lattice fringes with an interplanar distance of 0.54 and 0.33 nm correspond to the spacing of the (111) and (220) planes of CuCo2S4, respectively. As demonstrated in the inset of Fig. 2e, the SAED pattern illustrates the polycrystalline nature of the mesoporous nanoparticles. Additionally, EDS was further utilized to investigate the composition of the CuCo2S4 sample (Fig. 2f). The EDS spectrum confirms the existence of Cu, Co and S elements in the CuCo2S4 sample, and the element molar ratio of Cu, Co and S is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.07
2.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.95, which is very close to the 1
3.95, which is very close to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 stoichiometric ratio of CuCo2S4, further verifying the formation of pure CuCo2S4.
4 stoichiometric ratio of CuCo2S4, further verifying the formation of pure CuCo2S4.
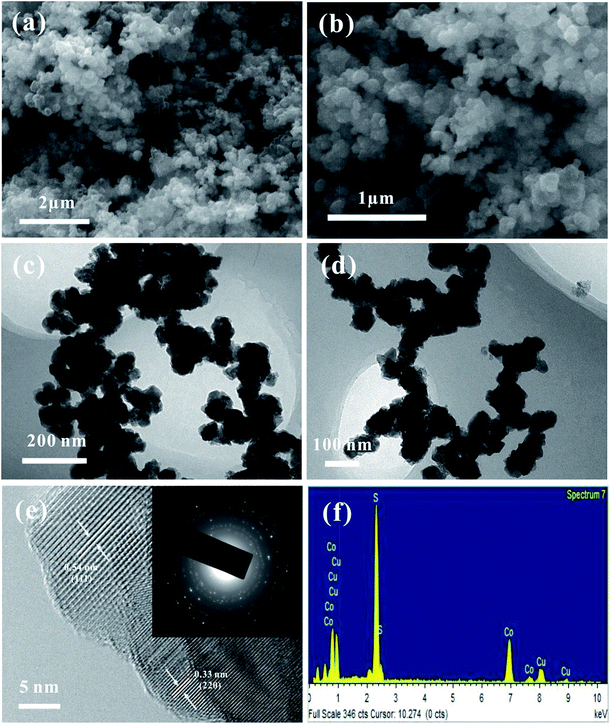 | ||
| Fig. 2 FESEM images (a and b), TEM images (c and d), HRTEM image (e) and the corresponding SAED pattern (the inset in (e)) and (f) EDS spectrum of the CuCo2S4 sample. | ||
The specific surface area and porosity of the as-prepared CuCo2S4 sample were investigated by Brunauer–Emmett–Teller (BET) nitrogen adsorption/desorption isotherms and Barrett–Joyner–Halenda (BJH) pore size distribution method. As demonstrated in Fig. 3, the adsorption/desorption curves belong to a type-IV isotherm with a H3 hysteresis loop, indicating the characteristic of mesoporous structure of the as-prepared sample.23 Furthermore, the BET specific surface area of the CuCo2S4 sample is calculated to be 45.35 m2 g−1 from the nitrogen adsorption/desorption isotherm. The BJH analysis (inset of Fig. 3) further confirms the existing of the mesoporous structure of the CuCo2S4 sample, and the pore size distribution is relatively narrow and mainly centered in the range of 3–5 nm, which is known to be optimal pore size range for supercapacitor applications.24–26 The mesoporous structure gives rise to a high porosity of 0.135 cm3 g−1. It is well accepted that the mesoporous materials with high specific surface area and superior porosity structure can offer rich electroactive sites and short diffusion paths for charge transports,27,28 which can greatly enhance the electrochemical performance of CuCo2S4.
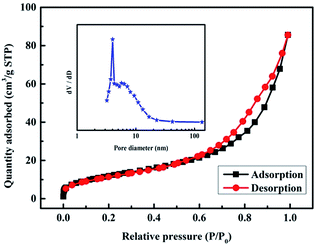 | ||
| Fig. 3 Nitrogen adsorption and desorption isotherm of the CuCo2S4 sample and the inset shows the corresponding BJH pore size distribution curve. | ||
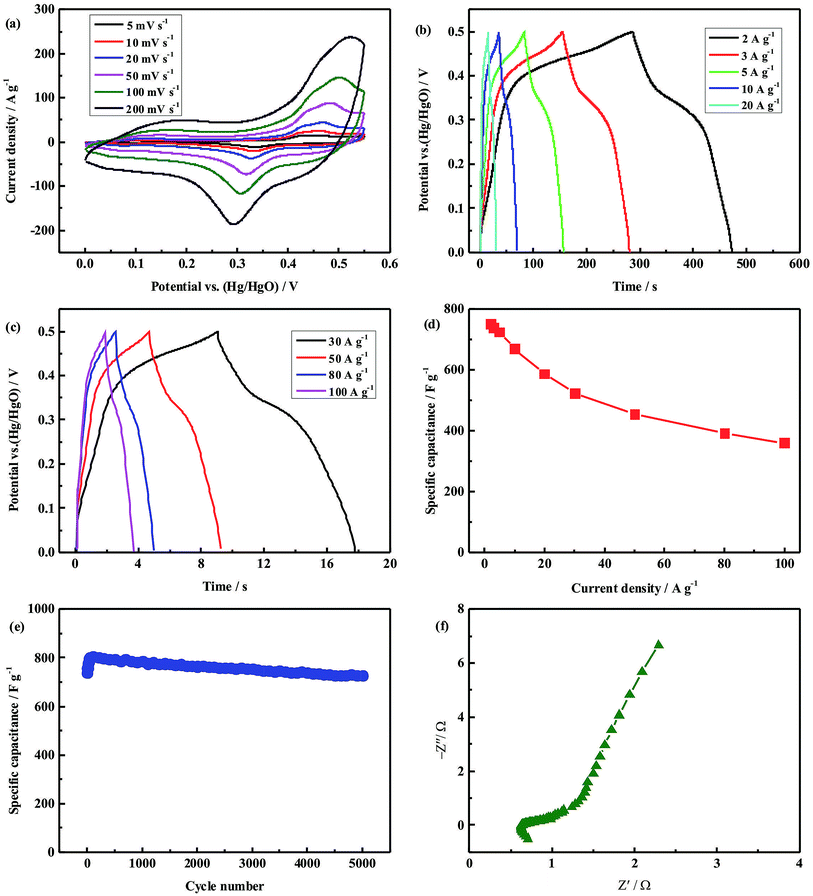
The electrochemical properties of the mesoporous CuCo2S4 nanaoparticles as supercapacitor electrodes were evaluated in a three-electrode system using 2 M KOH solution as the electrolyte. Fig. 4a shows the CV curves of the CuCo2S4 electrode at scanning rates of 5–200 mV s−1 in a potential range of 0–0.55 V. The shape of the CV curves indicates the pseudocapacitive characteristics arising from faradaic reactions of the Co4+/Co3+ and Cu2+/Cu+ redox pairs.19,29 Moreover, with the increase in scan rates from 5 to 200 mV s−1, the corresponding peak currents enhance while the shape of the CV curves remains almost unchanged, and only the position of the anodic or cathodic peak shifts slightly, suggesting the low resistance and good electrochemical reversibility of the CuCo2S4 electrode.
In order to estimate the supercapacitive performance of the mesoporous CuCo2S4 nanaoparticles, galvanostatic charge–discharge measurement was performed at different current densities of 2–100 A g−1 in a potential range of 0–0.5 V, as shown in Fig. 4b and c. The nonlinear discharge curves and the voltage plateaus indicate the characteristic of pseudocapacitance behaviors of the CuCo2S4 electrodes, which is in agreement with the peaks observed from the CV curves. The specific capacitance (Cm) of the CuCo2S4 electrode can be calculated by using the following formula:30 Cm = (I × Δt)/(ΔV × m), where I is the discharge current, Δt represents the discharge time, ΔV refers to the discharge potential window, and m denotes the mass of the active electrode material. The calculated specific capacitance as a function of current density is plotted in Fig. 4d. The specific capacitance of the CuCo2S4 electrode is up to 752 F g−1 at a current density of 2 A g−1. With a huge 50-fold increase in current density from 2 to 100 A g−1, the specific capacitance still remains at 360 F g−1, and 47.9% of the initial capacitance is retained in the CuCo2S4 electrode, indicating its ultrahigh rate capability. The outstanding rate performance of the mesoporous CuCo2S4 nanoparticles can be attributed to the synergetic effect of the high specific surface area, superior porosity structure and high conductivity, which not only can endow more active sites for electrochemical reaction and more efficient penetration of electrolyte into the electrode materials, but also can provide short path length for rapid transport of ions and electrons even at high rates, resulting in the enhancement of the rate capability.
To further demonstrate the potential application of the mesoporous CuCo2S4 nanoparticles, the cycling performance was conducted by the repeated charge–discharge measurements at a constant current density of 3 A g−1, as displayed in Fig. 4e. The specific capacitance of the CuCo2S4 electrode increases in the first 100 cycles, and this may be ascribed to full activation arising from the gradual pore opening during insertion/extraction of OH− ions through the mesopore channels and possibly micropore channels of the sample.31 Such phenomena have also been reported by many other researchers.19,25,32–34 After 5000 cycles, the initial capacity retention rate is as high as 98.1%, and the activated maximum capacity retention rate still remains at 90.1%. The good cycling stability can be attributed to the unique mesoporous structure with superior pore size distribution and abundant porosity, which can alleviate the volume changes during insertion/extraction processes of OH− ions while cycling. These electrochemical properties of the mesoporous CuCo2S4 nanoparticles above are comparable or even superior to the previously reported ternary or binary metal sulfides/oxides nanostructures, shown in the Electronic supplementary information (ESI†).
EIS measurement was performed to provide further insight into the electrochemical performance of the CuCo2S4 electrode. As shown in Fig. 4f, Nyquist plots are composed of an arc in the high frequency region and a linear line in the low frequency region. In the high frequency region, the x-intercept of the Nyquist plot represents the internal resistance (Rs), and the semicircle diameter of the Nyquist plot corresponds to the charge transfer resistance (Rct). From the Nyquist plot, we can clearly observe that the CuCo2S4 electrode exhibits a small x-intercept (only 0.626 Ω) and negligible semicircle, suggesting a very small internal resistance and charge transfer resistance. Such low resistance can reasonably illustrate the above excellent rate capability of the CuCo2S4 electrode because the resistance is an important factor in determining the rate performance for supercapacitor electrode materials.35 Additionally, we can see that the slope of the linear section at low frequency region is more than 45°, indicating that the electrolyte ions are easier to diffuse to the active materials for efficient redox reactions during the faradaic charge storage process, resulting in more ideal capacitor behavior,36 which further verifies that the CuCo2S4 electrode can retain superior capacitive performances.
4. Conclusions
In summary, mesoporous CuCo2S4 nanoparticles have been successfully fabricated by a facile solvothermal method. Benefiting from the combined advantages of the large specific surface area, superior mesoporous structure and high electronic conductivity, the as-obtained mesoporous CuCo2S4 nanoparticles manifest superior electrochemical performances with a high specific capacitance (752 F g−1 at a current density of 2 A g−1), remarkable rate capability (47.9% capacity retention rate from 2 to 100 A g−1) and good cycling stability (98.1% of initial capacity retention rate and 90.1% of activated maximum capacity retention rate at a current density of 3 A g−1 for 5000 cycles, respectively). The desirable electrochemical properties coupled with the facile and cost-effective preparation method will render the mesoporous CuCo2S4 nanoparticles as attractive electrode materials for promising application in supercapacitors.Acknowledgements
The work is financially supported by National Natural Science Foundation of China (21601057), Scientific Research Fund of Hunan Provincial Education Department (16C0462), Program for the New Century Excellent Talents in University (NCET-11-0513), Distinguished Young Scientists of Hunan Province (13JJ1004), and Grants from the Project of Innovation-driven Plan in Central South University (2016CX020).Notes and references
- J. Chmiola, C. Largeot, P. L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480–483 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- I. Hadjipaschalis, A. Poullikkas and V. Efthimiou, Renewable Sustainable Energy Rev., 2009, 13, 1513–1522 CrossRef.
- J. Chen, A. I. Minett, Y. Liu, C. Lynam, P. Sherrell, C. Wang and G. G. Wallace, Adv. Mater., 2008, 20, 566–570 CrossRef CAS.
- L. L. Zhang, R. Zhou and X. S. Zhao, J. Mater. Chem., 2010, 20, 5983–5992 RSC.
- W. Chen, R. B. Rakhi, L. B. Hu, X. Xie, Y. Cui and H. N. Alshareef, Nano Lett., 2011, 11, 5165–5172 CrossRef CAS PubMed.
- S. Vijayakumar, S. H. Lee and K. S. Ryu, Electrochim. Acta, 2015, 182, 979–986 CrossRef CAS.
- A. L. M. Reddy, S. R. Gowda, M. M. Shaijumon and P. M. Ajayan, Adv. Mater., 2012, 24, 5045–5064 CrossRef CAS PubMed.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Energy Environ. Sci., 2015, 8, 702–730 CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- M. R. Gao, Y. F. Xu, J. Jiang and S. H. Yu, Chem. Soc. Rev., 2013, 42, 2986–3017 RSC.
- X. Y. Yu, L. Yu and X. W. Lou, Adv. Energy Mater., 2016, 6 DOI:10.1002/aenm.201501333.
- H. Chen, J. Jiang, L. Zhang, H. Wan, T. Qi and D. Xia, Nanoscale, 2013, 5, 8879–8883 RSC.
- L. F. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. G. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694–6701 CrossRef CAS PubMed.
- L. Yu, L. Zhang, H. B. Wu and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 3711–3714 CrossRef CAS PubMed.
- C. Xia and H. N. Alshareef, Chem. Mater., 2015, 27, 4661–4668 CrossRef CAS.
- Y. R. Zhu, Z. B. Wu, M. J. Jing, X. M. Yang, W. X. Song and X. B. Ji, J. Power Sources, 2015, 273, 584–590 CrossRef CAS.
- Y. Zhang, M. Ma, J. Yang, C. Sun, H. Su, W. Huang and X. Dong, Nanoscale, 2014, 6, 9824–9830 RSC.
- J. Tang, Y. Ge, J. Shen and M. Ye, Chem. Commun., 2016, 52, 1509–1512 RSC.
- Y. R. Zhu, J. F. Wang, Z. B. Wu, M. J. Jing, H. S. Hou, X. N. Jia and X. B. Ji, J. Power Sources, 2015, 287, 307–315 CrossRef CAS.
- Y. R. Zhu, Z. B. Wu, M. J. Jing, H. S. Hou, Y. C. Yang, Y. Zhang, X. M. Yang, W. X. Song, X. N. Jia and X. B. Ji, J. Mater. Chem. A, 2015, 3, 866–877 CAS.
- Y. Zhu, X. Ji, Z. Wu, W. Song, H. Hou, Z. Wu, X. He, Q. Chen and C. E. Banks, J. Power Sources, 2014, 267, 888–900 CrossRef CAS.
- J. Cheng, H. Yan, Y. Lu, K. Qiu, X. Hou, J. Xu, L. Han, X. Liu, J.-K. Kime and Y. Luo, J. Mater. Chem. A, 2015, 3, 9769–9776 CAS.
- C. Yuan, L. Yang, L. Hou, L. Shen, X. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883–7887 CAS.
- T. Y. Wei, C. H. Chen, H. C. Chien, S. Y. Lu and C. C. Hu, Adv. Mater., 2010, 22, 347–351 CrossRef CAS PubMed.
- L. Wang, X. Liu, X. Wang, X. Yang and L. Lu, Curr. Appl. Phys., 2010, 10, 1422–1426 CrossRef.
- L. Shen, Q. Che, H. Li and X. Zhang, Adv. Funct. Mater., 2014, 24, 2630–2637 CrossRef CAS.
- A. Pendashteh, S. E. Moosavifard, M. S. Rahmanifar, Y. Wang, M. F. El-Kady, R. B. Kaner and M. F. Mousavi, Chem. Mater., 2015, 27, 3919–3926 CrossRef CAS.
- S. E. Moosavifard, S. Fani and M. Rahmanian, Chem. Commun., 2016, 52, 4517–4520 RSC.
- J. W. Lee, A. S. Hall, J. D. Kim and T. E. Mallouk, Chem. Mater., 2012, 24, 1158–1164 CrossRef CAS.
- S. K. Meher and G. R. Rao, J. Phys. Chem. C, 2011, 115, 15646–15654 CAS.
- C. Wang, X. Zhang, D. Zhang, C. Yao and Y. Ma, Electrochim. Acta, 2012, 63, 220–227 CrossRef CAS.
- H. Wang, C. M. B. Holt, Z. Li, X. Tan, B. S. Amirkhiz, Z. Xu, B. C. Olsen, T. Stephenson and D. Mitlin, Nano Res., 2012, 5, 605–617 CrossRef CAS.
- A. Pendashteh, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, Chem. Commun., 2014, 50, 1972–1975 RSC.
- R. B. Rakhi, W. Chen, D. Cha and H. N. Alshareef, J. Mater. Chem., 2011, 21, 16197–16204 RSC.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Comparison of the electrochemical performances of the as-prepared mesoporous CuCo2S4 nanoparticles with previously reported ternary and binary metal sulfides/oxides nanostructures. See DOI: 10.1039/c6ra20120b |
| This journal is © The Royal Society of Chemistry 2016 |