Dual functional anti-oxidant and SPF enhancing lignin-based copolymers as additives for personal and healthcare products†
Dan Kai‡
*a,
Yun Khim Chua‡b,
Lu Jianga,
Cally Owha,
Siew Yin Chanac and
Xian Jun Loh*ade
aInstitute of Materials Research and Engineering (IMRE), A*STAR, 2 Fusionopolis Way, #08-03 Innovis, Singapore 138634. E-mail: kaid@imre.a-star.edu.sg; lohxj@imre.a-star.edu.sg
bSchool of Materials Science and Engineering, Nanyang Technological University, Block N4.1, Nanyang Avenue, Singapore 639798
cSchool of Science, Monash University Malaysia, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia
dDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore 117576
eSingapore Eye Research Institute, 11 Third Hospital Avenue, Singapore 168751
First published on 2nd September 2016
Abstract
The desire for protection against UV exposure has resulted in the development of an increasing number of sunscreen agents. Lignin-based polymers have potential to serve as promising sunscreen agents as they have good UV absorption and antioxidant properties contributing towards the reduction of UV-induced skin damage. In this study, a series of lignin–poly(ethylene glycol) methacrylate (PEGMA) copolymers were synthesized via atom transfer radical polymerization (ATRP) to enhance the dispersion efficiency of lignin in the commercial creams. These copolymers showed tunability in both molecular weight (10–25 kDa) and particle size (100–200 nm), as well as excellent antioxidant properties. Different amounts of such copolymers were blended into commercial creams to investigate their sunscreen performance. Results indicated that adding lignin–PEGMA copolymers into sunblock creams improved their SPF values from 15.36 ± 2.44 to 38.53 ± 0.26. In summary, such lignin-based polymers with good UV protection and antioxidant properties offer a green alternative for developing the next generation of sunscreen creams.
1 Introduction
The sun is a major source of UV radiation, which is required by our bodies to generate vitamin D for the strengthening of muscles, bones and our immune systems. Yet, prolonged UV exposure may cause adverse effects, such as damage to the eyes, skin and immune system.1 UV radiation can be classified into three main categories according to its wavelength – UVA, UVB and UVC. UVC has the shortest wavelength, which ranges from 100 nm to 290 nm, and is incapable of penetrating the Earth's ozone layer. UVB, however, has a longer wavelength that ranges from 290 nm to 320 nm and is consequently not only able to pass through the ozone layer, but also penetrate into the epidermis layer of the skin. With a wavelength range of 320 nm to 400 nm, UVA is capable of penetrating into the epidermis, dermis and subcutaneous tissue layers of the skin.2 When UV radiation penetrates through our skin, its primary target is the molecular oxygen in the mid-lower levels of the epidermis. This results in the formation of various free radical compounds, including reactive oxygen species (ROS), hydroxyl radicals, lipid alkyl radicals, lipid alkoxyl radicals, lipid peroxyl radicals, superoxide anion radicals, ascorbyl radicals, as well as tocopheroxyl radicals.3,4 The presence of these free radicals in the skin can lead to various forms of skin damage, such as skin aging, pigment darkening and even skin cancer.5,6 To prevent the proliferation of free radicals, a large group of natural and synthetic antioxidants, including vitamin C, vitamin E, isoflavones and polyphenols, were added into the formulation of facial creams.Since the exposure of skin to UV radiation is inevitable during daytime, it is advisable to apply a protective layer onto the skin surface regularly – a sun block cream. Nowadays, there is a wide range of sun block creams that are available on the market. These sun-creams can be classified into two main categories – physical sun block creams and chemical sun block creams. These two categories of sun block creams mainly differ in their mechanism of protection. Physical sun block creams, which mainly contain zinc oxide (ZnO2) and titanium dioxide (TiO2) as active ingredients, protect the skin through a reflection mechanism. On the other hand, chemical sun block creams function by absorbing UV radiation. Compared to physical sun block creams, chemical sun block creams do offer certain advantages, such as greater ease of application onto the skin, as well as greater comfort. However, the synthetic chemicals may have negative effects on skin tissue. Hence, recently, there has been increasing attention placed on the use of natural compounds in sunblock applications, due to their good UV radiation protection properties and antioxidant activities.
Lignin is the most abundant aromatic polymer on earth. Owing to the global economic and environmental pollution issues in the recent years, as well as the abundance and renewability of lignin, there has been a growing research interest in its promising applications.7–9 Advanced lignin-based functional materials have been developed and engineered.10–13 Gupta et al. synthesized polyacrylamide and poly(acrylic acid) grafted lignin copolymers via reversible addition-fragmentation chain-transfer polymerization, and the DLS results indicated that such polymer-grafted lignin copolymers displayed both isolated (15 to 90 nm) and aggregated (120 to 370 nm) forms in water.14 Recently, lignin-g-polycaprolactone-g-poly(lactide) copolymer particles were developed via ring-opening polymerization and used as a filler for PLA/lignin composites.15 The lignin particles exhibited aggregations in chloroform and showed large hydrodynamic radii values (∼4 μm). The copolymer particle sizes were significantly reduced after adding poly(L-lactide) into the solution, indicating that a stereocomplex was formed between the two polymers. As lignin has complex chemical structures, comprising of high carbon content, UV chromophoric groups and aromatic rings of hydroxyl and methoxyl groups, it will be a suitable candidate for applications as UV protective agents, antioxidants, reinforcing fillers and flame retardants.5,6 Lignin contains many UV chromophoric groups (phenolics, hydroxyl groups, double bonds, and carbonyl groups), which are responsible for its absorption capability in the UV/visible regions.7 Liu et al. developed a UV-absorbent film by grafting poly(n-butyl acrylate-co-methyl methacrylate) onto bio-butanol lignin. The results showed that the grafted copolymer film had excellent UV absorption properties (96.2%) and the ability to retain exceptional UV absorption capabilities even after continuous UV irradiation for 75 minutes.16 Therefore, the incorporation of lignin into sun block creams seems to be a rational approach to enhance their UV protection properties while further providing antioxidant properties.
In this study, the effects of lignin and lignin copolymers on commercial creams were investigated. Poly(ethylene glycol) methacrylate (PEGMA) was grafted onto lignin via atom transfer radical polymerization (ATRP) to enhance the miscibility of lignin in creams, as poly(ethylene glycol) is one of the main ingredients in cream formulation. Different lignin–PEGMA copolymers were synthesized by varying the feed ratio of lignin to PEGMA and the molecular weight of the PEGMA monomers. Such lignin–PEGMA copolymers were incorporated into the commercial creams at various percentages for investigation of their improvement in UV protection properties in terms of sun protection factor (SPF). Antioxidant properties of such copolymers and the viscosities of lignin sun block creams were also studied.
2 Experimental section
2.1 Materials
Sodium lignosulfonate (SL) was purchased from TCI (Japan). Other chemicals were purchased from Sigma-Aldrich Chemicals (Singapore). Alkali lignin (AL) was dried at 105 °C overnight before use. PEGMA monomers (with different molecular weights of 500 and 1100 Da) were purified by passing them through a column with inhibitor removers before use. The following commercial creams were purchased from OG department store (Singapore): Himalaya Herbals intensive moisturising cream (HC), Shiseido White Lucent brightening moisturising cream (SC), Shiseido White Lucent brightening protective cream SPF15 (SC-15), Shiseido Urban Environment UV protector extra mild SPF30 (SC-30) and Shiseido Urban Environment UV protection cream plus SPF50 (SC-50).2.2 Synthesis of lignin–PEGMA copolymers
The synthesis steps of lignin–PEGMA copolymers are shown in Fig. 1. 5 g of AL was dissolved in 50 ml of anhydrous N,N-dimethylacetamide (DMA) at room temperature under nitrogen atmosphere. Triethylamine (12.4 ml) was then added into lignin solution. 10 ml of anhydrous DMA containing 2-bromoisobutyryl bromide (BIBB, 9.2 ml) was added dropwise into lignin solution under rapid stirring over 2 h in ice-water bath. The reaction mixture was left to stir for 1 day at room temperature. After that, the reaction mixture was centrifuged and the supernatant was precipitated with 1000 ml of ethyl ether. The lignin macroinitiator (lignin-Br) was obtained and dried under vacuum. The number of initiator sites on lignin was determined by 1H nuclear magnetic resonance (NMR).Lignin-Br and PEGMA monomer with different feed ratios (as shown in Table 1), 1,1,4,7,10,10-hexamethyltriethylenetetramine (HMTETA) and 20 ml of degassed acetone were prepared in a reaction flask. The mixture was stirred at room temperature until the solids were dissolved. After this, the reaction mixture was purged with nitrogen gas for 15 minutes. A trace amount of copper(I) bromide was then added and the reaction mixture was purged again with nitrogen gas for another 10 min. The reaction mixture was left to stir at room temperature for 12 h. The final reaction mixture was exposed to air and then diluted with THF before it was passed through a short basic Al2O3 column to remove the copper catalyst. The resulting solution was precipitated with 1000 ml hexane twice. The lignin–PEGMA copolymers were collected by centrifugation and dried under vacuum at 40 °C.
| Polymer code | Mn of PEGMA monomer | Feed ratio (lignin-Br![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGMA) PEGMA) |
Mna (kDa) | Mwa (kDa) | Mass% of lignina | Z-Averageb (nm) | PDIb |
|---|---|---|---|---|---|---|---|
| a Mn (number average molecular weight) and Mw (weight average molecular weight) were determined by GPC results. Mass% of lignin was calculated based on the molecular weight of lignin (5 kDa).b Z-Average size and polydispersity index (PDI) were determined by dynamic light scattering. | |||||||
| A1 | 1100 | 0.4 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 g 4 g |
16.7 | 17.3 | 29.9 | 102 | 0.467 |
| A2 | 1100 | 0.2 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 g 4 g |
24.4 | 24.8 | 20.5 | 156 | 0.656 |
| B1 | 500 | 0.4 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g 2 g |
10.1 | 12.8 | 49.5 | 205 | 0.533 |
| B2 | 500 | 0.2 g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 g 2 g |
14.3 | 19.1 | 35.0 | 196 | 0.427 |
2.3 Characterization of lignin–PEGMA copolymers
Lignin–PEGMA copolymers were characterized by 1H NMR (Bruker 400 MHz). Deuterated chloroform (CDCl3) was used as a solvent to dissolve the copolymers.The molecular weights and polydispersity indexes of the polymer samples were analyzed by gel permeation chromatography (GPC, a Shimadzu SCL-10A and LC-8A system equipped with two Phenogel 5 mm 50 and 1000 Å columns in series and a Shimadzu RID-10A refractive index detector). HPLC-grade tetrahydrofuran was used as an eluent. The flow rate of tetrahydrofuran eluent was 1.0 ml min−1 at 25 °C. The number average molecular weights (Mn), weight average molecular weights (Mw) and polydispersity index (PDI, Mw/Mn) were determined with a calibration based on linear poly(methyl methacrylate) standards.
Particle size analysis of the lignin–PEGMA copolymers was conducted through dynamic light scattering (DLS) technique with a Zetasizer device (Nano Z, Malvern, UK) with a red laser (wavelength of 633 nm). Each copolymer was dissolved in DI water (pH = 7) at a concentration of 1 mg ml−1. Z-Average sizes, polydispersity indexes (PDI), and zeta potentials were obtained.
The antioxidant activities of the lignin–PEGMA copolymers were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay.17,18 Each copolymer (5 mg, triplicate) was prepared in glass vials. 60 μM DPPH solution in MeOH was prepared, and 2 ml of the solution was added into each vials. The free radical scavenging activities (%) of lignin–PEGMA copolymers were measured by monitoring the decrease in absorbance of methanolic DPPH solutions at 517 nm compared to control solutions at each time point.
2.4 Preparation of lignin sun block creams
Lignin–PEGMA and lignins (AL and SL) in creams were prepared by simple magnetic stirring. For instance, the 10 wt% lignin–PEGMA cream was prepared by blending 0.2 g of lignin–PEGMA copolymer with 1.8 g cream. The prepared creams were left to stir at 1000 rpm for 48 h under room temperature condition (in dark).2.5 UV-visible spectrophotometry and SPF calculation
The sun block cream samples for UV transmittance measurement were prepared according to Qian's method.19 Briefly, 2 mg cm−2 of the cream was uniformly spread over the entire quartz slide. The quartz slide was left to dry for about 20 minutes before the test. Transmittance percentage over a wavelength range of 600 nm to 200 nm was measured by UV-visible spectrophotometer (UV-2501PC, SHIMADZU, Singapore). Each sample was prepared thrice for transmittance measurement.SPF can be calculated with the following formula:
| Eλ = 1.0 for wavelengths 250 nm < λ < 298 nm |
| Eλ = 100.094(298 − λ) for wavelengths 298 nm < λ < 328 nm |
| Eλ = 100.015(139 − λ) for wavelengths 328 nm < λ < 400 nm |
2.6 Rheological studies of lignin sun block creams
The viscosities of lignin sun block creams were evaluated at room temperature in a Discovery Hybrid Rheometer 3 (TA Instrument, USA) fitted with 40 mm parallel-plate geometry. The gap was set at a distance of 500 μm. The parameter of peak hold flow test was set at a constant shear rate of 200 s−1 for 100 seconds while the condition of the flow ramp test was set at increasing shear rate ranging from 1 s−1 to 1000 s−1.3 Results and discussion
3.1 Synthesis and characterization of lignin–PEGMA copolymers
The lignin–PEGMA copolymers were synthesized via ATRP reaction, as shown in Fig. 1. Lignin contains plenty of hydroxyl groups (both aliphatic and phenol) which reacted with BIBB to form the lignin-Br ATRP macroinitiator (1H NMR shown in Fig. S1†). After calculation, the lignin-Br macroinitiator had 2.3 mmol of initiator sites per gram of material. Next, ATRP of PEGMA using lignin-Br was carried out in the presence of CuBr as catalyst and HMTETA as ligand. Different amounts of PEGMA monomers with different molecular weights were grafted onto the macroinitiator to form copolymers composed of lignin core and PEGMA arms. The 1H NMR spectra of lignin–PEGMA copolymer is shown in Fig. 1. Characteristic peaks of PEGMA were shown at 3.3 and 3.7 ppm corresponding to methyl and methylene protons from PEGMA, while the signals associated with the lignin methoxyl groups (3.8 ppm) was also found in the spectrum.As shown in Table 1, the lignin copolymers displayed different molecular weights, which varied from 10.1 kDa for B1 to 24.4 kDa for A2, according to the feed ratio of lignin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEGMA and Mn of PEGMA. The copolymers with PEGMA of Mn = 1100 Da showed higher molecular weights than those with PEGMA of Mn = 500 Da. The content of lignin in the copolymers were calculated based on the molecular weight of lignin (5 kDa) and mass% of lignin varied from 20.5% for A1 to 49.5% for B1. Both NMR and GPC results demonstrate that PEGMA was successfully grafted on lignin cores, which might enhance the miscibility of such copolymers in creams.
PEGMA and Mn of PEGMA. The copolymers with PEGMA of Mn = 1100 Da showed higher molecular weights than those with PEGMA of Mn = 500 Da. The content of lignin in the copolymers were calculated based on the molecular weight of lignin (5 kDa) and mass% of lignin varied from 20.5% for A1 to 49.5% for B1. Both NMR and GPC results demonstrate that PEGMA was successfully grafted on lignin cores, which might enhance the miscibility of such copolymers in creams.
The particle sizes of lignin and lignin–PEGMA copolymers were determined by a Zetasizer Nano Z. AL did not dissolve in water (pH = 7), and the particles formed agglomerates of >2 μm in size, while water-soluble SL showed a smaller particle diameter of 321 nm and PDI of 0.626. Large agglomerates are typically not desired for cosmetic cream applications. As shown in Table 1, lignin–PEGMA copolymers exhibited smaller Z-average sizes compared to raw lignin. Both the chain length of PEGMA arms and the Mn of PEGMA affected the particle sizes of the copolymers. It was found that a longer PEGMA chain or a higher molecular weight of PEGMA resulted in a smaller copolymer size, indicating that the PEGMA side chains would favor the dispersion of the lignin copolymer in water, and thus hopefully in creams. Copolymer A1 with the most PEGMA exhibited the smallest particle size of 102 nm. We believe that the particle sizes of lignin–PEGMA copolymers may play an important role in UV protection and rheological properties of the lignin sun block creams. Furthermore, the good dispersion of the lignin copolymers could potentially enhance the absorption of the UV irradiation due to a higher surface area of exposure of lignin in the formulation.
Fig. 2 shows the UV-Vis absorption spectra of lignin–PEGMA copolymers and lignins. Lignin–PEGMA copolymers in distilled water have the similar UV-Vis absorption shape between the range 260–400 nm with the λmax ≈ 282 nm. AL has the stronger absorption than others between 300–360 nm, while SL has relatively weaker absorption in this area. Aromatic compounds normally have a multiple π → π* absorption band with medium intensity around 230–270 nm. For lignin, due to the existence of large amount of substituents on phenyl rings, including auxochrome –OH and –OCH3 groups, this π → π* absorption band is red-shifted (∼280 nm from UV-Vis spectra), has higher intensity and lower fine degree.20–22 PEGMA component only has the weak UV-Vis absorption between 270–300 nm due to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl groups. Therefore, lignin–PEGMA copolymers with higher mass% of lignin should have stronger UV absorption effect for better sunblock effect. It is also important to note that alkali lignin has poor solubility in water which results in poor absorption of UV in solution.
O carbonyl groups. Therefore, lignin–PEGMA copolymers with higher mass% of lignin should have stronger UV absorption effect for better sunblock effect. It is also important to note that alkali lignin has poor solubility in water which results in poor absorption of UV in solution.
 | ||
| Fig. 2 Normalized UV-Vis spectra of lignin–PEGMA copolymers (H2O as solvent), alkali lignin (AL, MeOH as solvent) and sodium lignosulfonate (SL, H2O as solvent). | ||
3.2 Antioxidant properties of lignin–PEGMA copolymers
Here, the antioxidant activities of lignin–PEGMA copolymers were evaluated by DPPH assay. As shown in Fig. 3, all four lignin copolymers showed good radical scavenging capabilities. In the first hour, A1 exhibited the highest free radical inhibition (62.8 ± 5.7%@0.5 h and 80.0 ± 4.4%@1 h). B1, which had the highest lignin%, displayed the highest free radical inhibition (∼98%) after 6 h of incubation. Due to the poor water solubility of alkali lignin, the antioxidant property of alkali lignin cannot be detected. The good antioxidant properties of these copolymers hence suggest that they would perform well in sun block creams to reduce free-radical damage and environmental stress on skin due to UV radiation.The proposed major radical termination reaction between lignin–PEG copolymer and UV induced radicals has been shown in Scheme 1. Actually both aliphatic and phenol hydroxyl groups on lignin were partially grafted with PEGMA,23 but here in order to emphasize the main possible radical termination reaction on more active phenol hydroxyl group, PEG chains were only drawn in the sites of aliphatic alcohols of lignin. In real applications, the radical termination processes are quite complicated and have many possibilities due to existence of diverse kinds of radicals.24 Here we just show some main possible radical termination reactions. Compared with –OH/–SH groups linked to alkyl chains, the proton of –OH group directly linked to phenyl ring is much easier to be attacked by radical R˙ due to electron delocalization. And there are three major possible radical termination ways: coupling reactions between two formed lignin radicals to give bigger structure (dimerization of the two aromatic moieties); combination with another radical R˙; second radical abstraction by ˙SH to give a nucleophile substituted structure with the potential for further reaction with another radical.25 It is worthy to note that some phenol groups on lignin were lost due to bromization and ATRP, and the radical termination capability of lignin–PEGMA may not as strong as unmodified lignin. Even though, those phenol groups of lignin were not fully modified, and the remained phenol groups could still function as active sites for the radical termination for antioxidant properties.
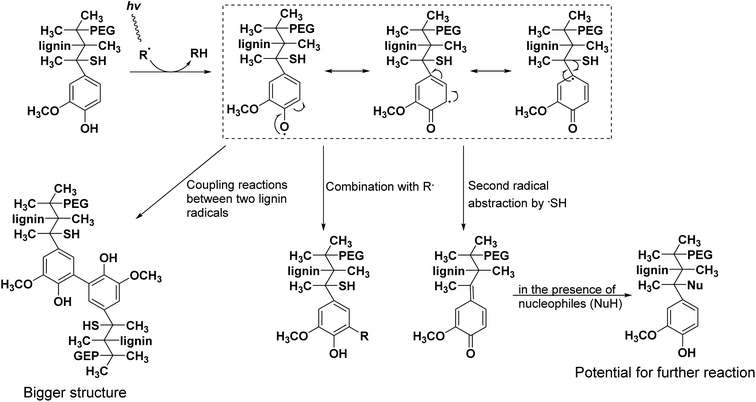 | ||
| Scheme 1 Proposed major radical termination reaction of lignin–PEGMA copolymers with UV induced radicals. | ||
3.3 Lignin copolymers in pure creams
Different amounts of A1 and SL were blended into HC As shown in Table 2 and Fig. 4, pure HC showed no absorbance of UVA and UVB, with an SPF of 1.10. Adding A1 into the cream barely increased its SPF, which only increased to 1.16 with 10% of A1. Compared to A1, SL was better able to improve the SPF of the pure cream, which increased to 1.42 with 10% SL. The problem with SL is that it affects the pH of the cream drastically, owing to its extraction process.| Cream code | Polymer | Concentration (wt% of lignin) | SPF |
|---|---|---|---|
| HC | N.A. | N.A. | 1.10 ± 0.01 |
| 2% A1HC | A1 | 0.6 | 1.13 ± 0.01 |
| 5% A1HC | A1 | 1.5 | 1.14 ± 0.01 |
| 10% A1HC | A1 | 3.0 | 1.16 ± 0.01 |
| 2% SLHC | SL | 2.0 | 1.34 ± 0.03 |
| 5% SLHC | SL | 5.0 | 1.42 ± 0.01 |
| 10% SLHC | SL | 10.0 | 1.42 ± 0.01 |
Table 3 and Fig. 5 exhibited the SPF values and UV transmittance of pure SC with different lignin–PEGMA copolymers. 5% of A1 and A2 displayed little enhancement of UV absorbance. Compared to A1 and A2, B1 and B2 with lower molecular weight of PEGMA showed higher SPF values. 5% B1SC showed SPF of 1.22 ± 0.01, which was the highest value of those of 5% lignin copolymers. Similar to HC, the creams with raw lignin exhibited higher SPF values. However, such lignin-contained creams were still not comparable with SC-15, which had an SPF value of 15.36 ± 2.44.
| Cream code | Polymer | Concentration (wt%) | SPF |
|---|---|---|---|
| SC | N.A. | N.A. | 1.04 ± 0.01 |
| 5% A1SC | A1 | 5 | 1.08 ± 0.03 |
| 5% A2SC | A2 | 5 | 1.07 ± 0.02 |
| 5% B1SC | B1 | 5 | 1.22 ± 0.01 |
| 10% B1SC | B1 | 10 | 2.62 ± 0.04 |
| 5% B2SC | B2 | 5 | 1.11 ± 0.05 |
| 10% B2SC | B2 | 10 | 1.63 ± 0.06 |
| 5% SLSC | SL | 5 | 1.34 ± 0.04 |
| 5% ALSC | AL | 5 | 2.45 ± 0.05 |
3.4 Lignin copolymers in SPF15 sun block creams
The above results indicated that a lignin–PEGMA copolymer itself was not enough to confer SPF upon pure creams. It might thus be a more viable option to use the copolymers as additive sun blockers for sunscreen enhancement. In this study, lignin copolymers were added into SC15 to investigate their sun block cream enhancement performances and results are shown in Table 4 and Fig. 6. As shown in Fig. 6A, the addition of lignin–PEGMA showed little improvement in UVB area (290 to 320 nm), but dramatically enhanced absorbance in UVA region. Similar to the results shown in the previous section, where the copolymers were added to pure creams, 5% of A1 and A2 exhibited little enhancement on SPF values. It was interesting to find that the SPF enhancement in SC15 was not so obvious with 5% B2 addition (16.08 ± 0.11 for 5% B2SC15), but the SPF value more than doubled (36.11 ± 0.17 for 10% B2SC15) when the polymer concentration reached 10%. Among all the copolymers, the addition of B1 resulted in the highest increase of SPF. 5% B1SC15 displayed the SPF of 18.09 ± 0.01, and the value of 10% B1SC15 increased up to 38.53 ± 0.26. As shown in Fig. 6B, 10% B1SC15 and 10% B2SC15 not only outperformed SC30, but also surpassed the performance of SC50, especially in the UVA area. It is still unclear why lignin (and its copolymers) could remarkably enhance the SPF value of the commercial sunblock creams. The active ingredients in such creams are octinoxate, ensulizole, titanium dioxide. Qian et al. proposed a mechanism to explain the synergistic effect between lignin and octinoxate.26 These two chemicals might form J-aggregation (one type of π–π* stacking), a larger conjugated structure which required lower energy for the π–π* transition. The formation of J-aggregation assisted to enhance the photo absorption in UVA zone.| Cream code | Polymer | Concentration (wt% of lignin) | SPF (enhancement%) |
|---|---|---|---|
| SC15 | N.A. | N.A. | 15.36 ± 2.44 |
| 5% A1SC15 | A1 | 1.5 | 16.02 ± 0.07 (↑4.3%) |
| 5% A2SC15 | A2 | 1.0 | 15.83 ± 0.06 (↑3.1%) |
| 5% B1SC15 | B1 | 2.5 | 18.09 ± 0.01 (↑17.8%) |
| 10% B1SC15 | B1 | 5 | 38.53 ± 0.26 (↑150.8%) |
| 5% B2SC15 | B2 | 1.75 | 16.08 ± 0.11 (↑4.7%) |
| 10% B2SC15 | B2 | 3.5 | 36.11 ± 0.17 (↑135.1%) |
| 5% SLSC15 | SL | 5 | 19.74 ± 0.25 (↑28.5%) |
| 5% ALSC15 | AL | 5 | 36.11 ± 0.17 (↑135.1%) |
It was reported that high amount of lignin in sun block creams might result in the negative sunscreen effect due to the poor dispersion of lignin as well as major adjustment of the pH of the formulation.19 In our study, grafting PEG onto lignin improved the compatibility and miscibility of such lignin copolymers in the sun block creams. Therefore, the high amount of lignin copolymers in the creams with their good dispersion will always lead to a better sunblock performance. Although AL exhibited better sunscreen performance, it turned the white cream into dark brown which is hardly acceptable for cosmetic creams. Conversely, the sun block creams with lignin–PEGMA copolymers were either white or light yellow in colour, which give a suitable tone to match human skin (Fig. S2†).
3.5 Rheology studies
Fig. 7 shows the viscosities (versus shear rate) of different lignin copolymers in SC and SC15. Both SC and SC15 displayed shear-thinning and pseudoplastic behaviors. This rheopectic property is favorable during the application of the creams, as it will assist in forming a uniform, impenetrable and protective gel film over skin surface to shield UV radiation.27 Unlike Newtonian fluids, the pseudoplastic cream can be broken down for easy spreading, and the applied layer can recover instantaneously to gain enough viscosity such that the cream will not run after application.28 | ||
| Fig. 7 Viscosity versus shear rate curves of (A) SC and (B) SC-15 blended with 5% different lignins and lignin–PEGMA copolymers. | ||
It is found that the addition of lignin copolymers retained the shear-thinning properties of the creams, even though they caused modifications to the viscosities. As shown in Fig. 7A, AL and SL remarkably reduced the viscosities of SC, probably due to their poor dispersion (that cause phase separation) in the cream. Blending the lignin–PEGMA copolymers into SC slightly increased the viscosities of the creams, and the resulting viscosities varied according to the chain length and molecular weight of PEGMA. Lower molecular weight PEGMA and a shorter chain length led to a higher viscosity. As shown in Fig. 7B, B1 increased the viscosity of SC15 sun block cream, but the sun block creams with other copolymers exhibited comparatively lower viscosities than that of SC15. This result might be attributed to some complicated interactions between lignin, PEGMA chain and active ingredients in the sun block cream. Further studies will be carried out to investigate the interesting rheological properties of the lignin-based sun block creams and the mechanisms behind them.
4 Conclusion
Lignin–PEGMA functional copolymers were synthesized to evaluate their potential as sunblock agents. It is found that such lignin copolymers were not sufficient to significantly improve sunscreen performance on their own, but 10% of the copolymer B1 was able to increase the SPF of SC15 from 15 up to 38+. The excellent antioxidant properties of the copolymer could also assist to attenuate oxidative stress on skin tissue induced by UV radiation. With further studies, such lignin-based green polymers may make a big contribution in the cosmetic industry.References
- D. A. J. Connolly and A. R. Wilcox, J. Sports Med. Phys. Fitness, 2000, 40, 35–40 CAS.
- D. Dondi, A. Albini and N. Serpone, Photochem. Photobiol. Sci., 2006, 5, 835–843 CAS.
- E. Damiani, R. Castagna and L. Greci, Free Radical Biol. Med., 2002, 33, 128–136 CrossRef CAS PubMed.
- N. Wada, T. Sakamoto and S. Matsugo, Antioxidants, 2015, 4, 603–646 CrossRef CAS PubMed.
- L. Borska, C. Andrys, J. Krejsek, V. Palicka, V. Vorisek, K. Hamakova, J. Kremlacek, P. Borsky and Z. Fiala, J. Dermatol. Sci., 2016, 81, 192–202 CrossRef CAS PubMed.
- A. K. Srivastav, S. F. Mujtaba, A. Dwivedi, S. K. Amar, S. Goyal, A. Verma, H. N. Kushwaha, R. K. Chaturvedi and R. S. Ray, J. Photochem. Photobiol., B, 2016, 156, 87–99 CrossRef CAS PubMed.
- D. Kai, M. J. Tan, P. L. Chee, Y. K. Chua, Y. L. Yap and X. J. Loh, Green Chem., 2016, 18, 1175–1200 RSC.
- S. Sen, S. Patil and D. S. Argyropoulos, Green Chem., 2015, 17, 4862–4887 RSC.
- S. Laurichesse and L. Averous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- J. Yu, J. F. Wang, C. P. Wang, Y. P. Liu, Y. Z. Xu, C. B. Tang and F. X. Chu, Macromol. Rapid Commun., 2015, 36, 398–404 CrossRef CAS PubMed.
- Y. S. Kim and J. F. Kadla, Biomacromolecules, 2010, 11, 981–988 CrossRef CAS PubMed.
- D. Kai, W. Ren, L. Tian, P. L. Chee, Y. Liu, S. Ramakrishna and X. J. Loh, ACS Sustainable Chem. Eng., 2016 DOI:10.1021/acssuschemeng.6b00478.
- D. Kai, S. Jiang, Z. W. Low and X. J. Loh, J. Mater. Chem. B, 2015, 3, 6194–6204 RSC.
- C. Gupta and N. R. Washburn, Langmuir, 2014, 30, 9303–9312 CrossRef CAS PubMed.
- Y. Sun, L. Yang, X. Lu and C. He, J. Mater. Chem. A, 2015, 3, 3699–3709 CAS.
- X. H. Liu, J. F. Wang, J. Yu, M. M. Zhang, C. P. Wang, Y. Z. Xu and F. X. Chu, Int. J. Biol. Macromol., 2013, 60, 309–315 CrossRef CAS PubMed.
- R. van Lith, E. K. Gregory, J. Yang, M. R. Kibbe and G. A. Ameer, Biomaterials, 2014, 35, 8113–8122 CrossRef CAS PubMed.
- N. Baheiraei, H. Yeganeh, J. Ai, R. Gharibi, M. Azami and F. Faghihi, Mater. Sci. Eng., C, 2014, 44, 24–37 CrossRef CAS PubMed.
- Y. Qian, X. Q. Qiu and S. P. Zhu, Green Chem., 2015, 17, 320–324 RSC.
- A. J. Stamm, J. Semb and E. E. Harris, J. Phys. Chem., 1931, 36, 1574–1584 CrossRef.
- T. Higuchi, Y. Ito, M. Shimada and I. Kawamura, Phytochemistry, 1967, 6, 1551–1556 CrossRef CAS.
- B. Xiao, X. F. Sun and R. C. Sun, Polym. Degrad. Stab., 2001, 74, 307–319 CrossRef CAS.
- M. Nahmany and A. Melman, Org. Biomol. Chem., 2004, 2, 1563–1572 CAS.
- V. Turkovic, S. Engmann, N. Tsierkezos, H. Hoppe, M. Madsen, H. G. Rubahn, U. Ritter and G. Gobsch, J. Phys. D: Appl. Phys., 2016, 49, 7 CrossRef.
- P. C. Eklund, O. K. Langvik, J. P. Warna, T. O. Salmi, S. M. Willfor and R. E. Sjoholm, Org. Biomol. Chem., 2005, 3, 3336–3347 CAS.
- Y. Qian, X. Qiu and S. Zhu, ACS Sustainable Chem. Eng., 2016, 4, 4029–4035 CrossRef CAS.
- J. Marto, L. F. Gouveia, B. G. Chiari, A. Paiva, V. Isaac, P. Pinto, P. Simoes, A. J. Almeida and H. M. Ribeiro, Ind. Crops Prod., 2016, 80, 93–100 CrossRef CAS.
- L. R. Gaspar and P. Campos, Int. J. Pharm., 2003, 250, 35–44 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra21433a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |






