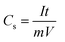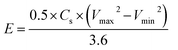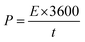DOI:
10.1039/C6RA22196C
(Paper)
RSC Adv., 2016,
6, 102961-102967
Flower-like ZnO@MnCo2O4 nanosheet structures on nickel foam as novel electrode material for high-performance supercapacitors
Received
5th September 2016
, Accepted 25th October 2016
First published on 25th October 2016
Abstract
Hierarchical flower-like ZnO@MnCo2O4 nanosheets and dandelion-like MnCo2O4 were synthesized on nickel foam using a facile and cost-effective hydrothermal approach combined with a short post-annealing treatment. The surface properties, crystalline phase, and electrochemical properties of the flower-like and dandelion-like electrodes were studied by field emission scanning electron microscopy, X-ray diffraction, cyclic voltammetry, galvanostatic charge–discharge cycling, and electrochemical impedance spectroscopy. The flower-like ZnO@MnCo2O4 nanosheet electrode exhibited high specific capacitance of 631.2 F g−1 and a high energy density of 56.10 W h kg−1 at a current density of 1 A g−1, which is higher than that of the dandelion-like MnCo2O4 electrode (452.5 F g−1). Impressively, as an electrode material for supercapacitors, the flower-like ZnO@MnCo2O4 also shows exceptional cycling performance over 1000 charge/discharge cycles, indicating good long-term cycling stability. These results highlight the unique ZnO@MnCo2O4 electrode as a promising electrode for energy storage applications in supercapacitors.
1 Introduction
Currently, numerous energy storage technologies have been developed to meet the needs of the rapid growth of portable electronic devices, hybrid motor vehicles, and certain energy-consuming products. Rechargeable batteries and electrochemical capacitors have become a tremendous approach for storing electrical energy with high energy density. On the other hand, the low power density, slow charging–discharging cycles, and shorter life span have limited their use. By overcoming those limitations, supercapacitors (SCs), also called ultracapacitors or electrochemical capacitors (ECs), have attracted considerable interest by offering superiority in the fields of longer cycle life, operating at ultrafast charge–discharge capability processes, the higher power density, delivers a considerable amount of energy at high power, high reliability, and environmental friendly by storing a huge volume of charge and then releasing the energy non-faradaically when required.1–5
The SCs can be divided into two types according to the principle of electrochemical storage response, electrical double layer capacitors (EDLCs) and pseudocapacitors. Generally, pseudocapacitors have a higher energy density and specific capacitance than EDLCs because of their fast and strong reversible redox reactions.4,6–8 The performance of SCs relied mostly on properties, such as high surface area, high mesoporosity, and good electrical conductivity of the electrode materials. The most widely used active electrode materials are carbon,9 conducting polymers,10 and transition metal oxides.11,12 Among them, transition metal oxides have been studied widely as the active electrode materials for supercapacitors owing to the higher specific capacitance obtained from the faradaic electrochemical reactions occurring on the surface of the electrode materials.
Recently, many studies have confirmed that complex oxides containing two or more different metal ions show improved electrochemical performance because of their synergetic effects during the charge–discharge process.13–16 In this regard, cobalt manganese composite materials (MnCo2O4) have attracted particular interest because cobalt has a higher oxidation potential, whereas manganese can transport more electrons and bring in higher capacity. On the other hand, the electrochemical performance of MnCo2O4, such as reversible capacity, cyclic stability or rate performance is currently unsatisfactory.17–19 Therefore, MnCo2O4 electrodes with excellent performances are now highly desirable.
The rational design and synthesis of advanced electrode materials, such as NiCo2O4@MnO2,20 ZnCo2O4@MnO2,21 ZnCo2O4@Ni,22 Co3O4@MnO2,23 TiO2@α-Fe2O3,24 ZnO@Ni3S2,25 and ZnO@Ni(OH)2 (ref. 26) has attracted considerable attention. ZnO was selected as the ideal core material among various core/shell heterostructures, which can function as a strong mechanical support and electron transport pathway owing to its good chemical stability and high electrical conductivity. Therefore, to take the advantage of ZnO and MnCo2O4 materials, unique ZnO@MnCo2O4 heterostructures were synthesized on nickel foam using a facile and cost-effective hydrothermal method. To the best of the authors' knowledge, there are no reports available on ZnO@MnCo2O4-based electrode materials for SCs. As a result, the flower-like ZnO@MnCo2O4 nanosheet electrode exhibits a higher specific capacitance of 631.2 F g−1 at a current density of 1 A g−1, good rate capability and cycling stability than the dandelion-like MnCo2O4 electrode.
2 Experimental section
2.1 Materials
Unless specified otherwise, all chemicals, such as nickel (Ni) foam, Manganese(II) nitrate tetrahydrate [Mn(NO3)2·4H2O], cobalt(II) nitrate hexahydrate [Co(NO3)2·6H2O], ammonium fluoride (NH4F), urea (CH4N2O), and zinc nitrate hexahydrate [Zn(NO3)2·6H2O], used in the synthesis of the supercapacitor were purchased from Sigma-Aldrich. These chemicals were of analytical grade and used as received.
2.2 Synthesis of MnCo2O4 and ZnO@MnCo2O4 electrodes
A simple fabrication technique was proposed for growing MnCo2O4 nanostructures on Ni foam (2 × 1 × 0.1 cm) by a hydrothermal method and was followed by heating. The current collector used in this deposition process was Ni foam. Prior to synthesis, the Ni foam was etched ultrasonically with a 2 M HCl solution for 30 min to remove the surface oxide layer. The substrates were then cleaned sequentially with acetone, ethanol, and deionized water (DI) for 10 min, respectively. For the precursor solution, 0.351 g (0.02 M) of Mn(NO3)2·4H2O, 0.416 g (0.02 M) of Zn(NO3)2·6H2O, and 0.814 g (0.04 M) Co(NO3)2·6H2O were dissolved in 70 mL of DI water with magnetic stirring to form a homogeneous solution. Subsequently, 0.311 g (0.12 M) of NH4F was added to the above homogeneous mixture with constant stirring for 10 min. This was followed by the addition of 1.009 g (0.24 M) of CH4N2O to the above precursor solution followed by stirring. The precursor solution was then transferred to a 100 mL Teflon-lined stainless steel autoclave and the Ni foam samples were immersed in the reaction mixture. After that, the autoclave was sealed and heated to 100 °C for 5 h and then cooled to room temperature naturally. The Ni foam loaded with the precursor was washed with distilled water and dried in the oven. The final product was obtained by sintering the precursor at 300 °C for 2 h in air. The MnCo2O4 heterostructures on the Ni foam was synthesized under the same conditions except that Zn(NO3)2·6H2O was not added. The mass loadings of the ZnO@MnCo2O4 and MnCo2O4 heterostructures on the Ni foam were approximately 2.0 and 1.5 mg cm−2, respectively.
2.3 Materials characterizations
The crystalline nature and structure of the films were studied by X-ray diffraction (XRD, D8 ADVANCE) using Cu Kα radiation operated at 40 kV and 40 mA over the 2θ range, 20–80°. The surface morphology of the electrodes were investigated by field emission scanning electron microscopy (FE-SEM, S-2400, Hitachi) operated at 15 kV. X-ray photon spectroscopy (XPS, VG Scientific ESCALAB 250) is a surface-sensitive spectroscopic technique that measures the elemental composition of thin films. XPS was performed using 1486.6 eV radiation and an electron take-off angle of 90°.
2.4 Electrochemical characterization
All electrochemical analyses were performed using BioLogic-SP150 workstation at room temperature with a three-electrode system, where Pt mesh, saturated calomel electrode, and the MnCo2O4 (or ZnO@MnCo2O4) electrodes were used as the counter, reference, and working electrodes, respectively. A 3 M KOH aqueous solution was used as the electrolyte. Cyclic voltammetry (CV) was performed over the potential range, −0.2 to +0.4 V, at different scanning rates. The galvanostatic charge/discharge tests were conducted using chronopotentiometry; various constant currents were applied between −0.2 to +0.6 V. Electrochemical impedance spectroscopy (EIS) was also performed over the frequency region between 0.1 Hz to 500 kHz. The specific capacitance (Cs), energy densities (E, W h kg−1), and power densities (P, W kg−1) were calculated from the charge/discharge analysis using the following equations:| |
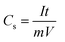 | (1) |
| |
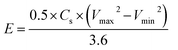 | (2) |
| |
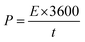 | (3) |
where I (A), t (s), V (V), and m (g) represent the discharge current, discharge time, potential window, and mass of the electrode materials, respectively.
3 Results and discussion
The morphologies of the MnCo2O4 and ZnO@MnCo2O4 were characterized by FESEM, as shown in Fig. 1. The MnCo2O4 material exhibited dandelion-like features, which is 5–8 μm in diameter with sharp nano-needles emanating from the ball center, to form a large amount of open-free space in the balls, as shown in Fig. 1(a1), (b1) and (c1). The tips of the nanoneedles were sharp, the length of the needles was 2.4–3.2 μm, and the width of the needle was 47–119 nm. Fig. 1(a2), (b2) and (c2) display a low and high magnification SEM image of the ZnO@MnCo2O4 material. The well-organized flower-like morphology of the ZnO@MnCo2O4 nanosheets were achieved. Fig. 1(a2, b2 and c2) shows SEM images of the flower-like ZnO@MnCo2O4 nanosheet heterostructures on Ni foam at different magnifications. The Ni foam was covered with ZnO@MnCo2O4 heterostructures on a large scale (Fig. 1(a2)). The ZnO@MnCo2O4 heterostructures displayed flower-like nanostructures, as observed from the high magnification SEM image (Fig. 1(b2)). The ZnO@MnCo2O4 nanosheets with diameters of 83–785 nm were grown on the Ni foam and self-assembled forming clusters (Fig. 1(c2)). These ZnO@MnCo2O4 nanosheets were highly porous, comprised of numerous nanocrystallites arranged together. The mesoporous structures can facilitate the transport of the electrolyte and ensure efficient contact between the active material and the electrolyte when used as a supercapacitor electrode.27,28
 |
| | Fig. 1 (a1, b1 and c1) Typical SEM images of the MnCo2O4 dandelion-like heterostructures on Ni foam at different magnifications. (a2, b2 and c2) Typical SEM images of the flower-like ZnO@MnCo2O4 nanosheet heterostructures on Ni foam at different magnifications. | |
The growth mechanism for such unique ZnO@MnCo2O4 heterostructures is discussed below: at the beginning of the hydrothermal reactions, Mn2+, Co2+ and Zn2+ ions can interact with F− ions to form MnF+, CoF+ and ZnF+ complexes in the homogeneous solution. When the reaction temperature increases to 100 °C, urea can provide OH− and carbonate ions simultaneously during the hydrolysis. Then, ZnF+ ions and OH− ions can easily combine into Zn(OH)F precipitate and firmly grow onto the substrate through the functional groups (e.g., hydroxyl groups) on the surface of nickel foam. When the ZnF+ ions are consumed to some degree, the MnF+ and CoF+ ions begin to precipitate into nanosheets on the Zn(OH)F core. The formation mechanism can be expressed as the following reactions:
| CO(NH2)2 + H2O → 2NH3 + CO2 |
| MnF+ + CO32− → MnCO3 + F− |
| CoF+ + CO32− → CoCO3 + F− |
| 2MnCO3 + 4CoCO3 + O2 → 2MnCO2O4 + 6CO2 |
XRD was conducted to examine the crystal structure and phase composition of MnCo2O4 and ZnO@MnCo2O4 heterostructures on nickel foam, as shown in Fig. 2. As shown in Fig. 2(a), all of the reflections could be indexed according to the standard data for MnCo2O4 (cubic, lattice parameter a = b = c = 8.269 Å, space group Fd3m, JCPDS card no. 23-1237),29,30 The characteristic reflections at 2θ = 30.5°, 36.0°, 57.9°, 63.6° and 75.3° corresponds to the (220), (311), (511), (440) and (533) phase structure of MnCo2O4, respectively. The XRD peaks in the Fig. 2(b) could be indexed to the (220), (311), (511), (440), and (533) planes of cubic MnCo2O4 (JCPDS no. 23-1237) and the (002) and (102) planes of the hexagonal ZnO (JCPDS no. 36-1451),31 from which all the diffraction peaks can be indexed and assigned to ZnO@MnCo2O4 without impurities.
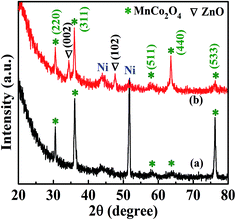 |
| | Fig. 2 XRD pattern of the (a) MnCo2O4 and (b) ZnO@MnCo2O4 heterostructures on nickel foam. | |
To investigate the chemical composition and oxidation states of the metals in MnCo2O4 and ZnO@MnCo2O4, the samples were analyzed by XPS and the results are shown in Fig. 3. The MnCo2O4 survey spectrum indicated the presence of Ni, Co, Mn, O, and C; Zn was also observed in ZnO@MnCo2O4. The Mn 2p spectrum (Fig. 3(b)) consists of Mn 2p3/2 and Mn 2p1/2 at 642 and 654 eV, respectively, together with a spin-energy separation of approximately 12 eV, indicating the presence of Mn2+ in the structure. On the other hand, the Co 2p spectrum featured two main spin–orbit lines of Co 2p3/2 at 780.5 eV and Co 2p1/2 at 795.5 eV with a separation of 15 eV corresponding to Co2+. The Zn 2p core level spectrum (Fig. 3(d)) in ZnO@MnCo2O4 showed two peaks with binding energies of 1045 eV and 1021.7 eV corresponding to Zn 2p3/2 and Zn 2p3/2, respectively, and the binding energy peaks of Zn 2p were separated by 23.3 eV. These results clearly show that Mn2+, Co2+, and Zn2+ make up the chemical composition of the prepared ZnO@MnCo2O4 heterostructures.
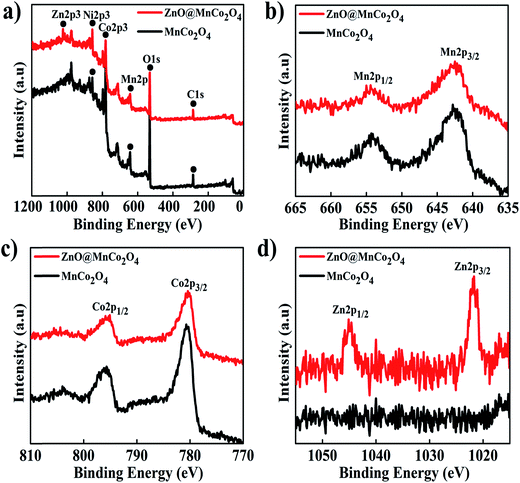 |
| | Fig. 3 XPS spectrum of MnCo2O4 and ZnO@MnCo2O4 on nickel foam: (a) survey spectrum (b) Mn 2p, (c) Co 2p (bottom-left), and (d) Zn 2p. | |
To clearly demonstrate the morphological superiority, the electrochemical properties of the MnCo2O4 and ZnO@MnCo2O4 electrodes were evaluated further, as shown in Fig. 4. Cyclic voltammetry was performed on the as-prepared MnCo2O4 and ZnO@MnCo2O4 in a three-electrode configuration with a Pt plate counter electrode and a SCE reference electrode in a 3 M KOH aqueous electrolyte. Fig. 4 denotes the CV curves of the MnCo2O4 and ZnO@MnCo2O4 electrodes at various scan rates of 2–100 mV s−1 over the potential range, −0.2 to +0.4 V. As the scan rate was increased from 2 to 100 mV s−1, the peak current increased and the CV curves at various scan rates exhibited a similar shape, which reveals its good electrochemical reversibility and high power characteristics. The CV curve integral area of the ZnO@MnCo2O4 electrode was much larger than of the MnCo2O4 electrode, indicating that a larger capacitance of the ZnO@MnCo2O4 electrode is obtained.
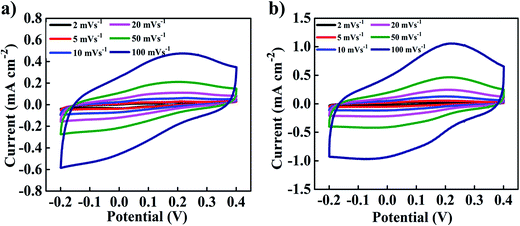 |
| | Fig. 4 CV curves of the (a) MnCo2O4 and (b) ZnO@MnCo2O4 electrodes measured in 3.0 M KOH at different scan rates (2–100 mV s−1). | |
To further examine the electrochemical performance of two electrodes, galvanostatic charge/discharge measurements were conducted at various current densities with an electrochemical window of −0.2 to +0.6 V vs. Ag/AgCl, as shown in Fig. 5(a) and (b). The specific capacitance of the MnCo2O4 and ZnO@MnCo2O4 electrodes can be calculated based on the charge and discharge curves, and the results are presented in Fig. 5(c). The MnCo2O4 electrode exhibited specific capacitance values of 452.5, 412.5, 356.2, 287.5, 218.7, and 150 F g−1 at different current densities of 1, 2, 3, 5, 7, and 10 A g−1, respectively. On the other hand, the ZnO@MnCo2O4 electrode exhibited higher specific capacitance values of 631.2, 525, 480, 362.5, 288.7, and 200 F g−1 at the same current densities. The improved specific capacitance of ZnO@MnCo2O4 was attributed to its high specific surface area because the effective surface area of ZnO@MnCo2O4 flower-like structure in contact with the electrolyte is increased. The energy density and power density are two main parameters for supercapacitors in practical applications. Based on the as-extracted Cs values, the energy density and power density of the MnCo2O4 and the ZnO@MnCo2O4 electrodes can be calculated and were used in the Ragone plot shown in Fig. 5(d). The maximum energy density and power density for the ZnO@MnCo2O4 electrode were found to be 56.10 W h kg−1 and 406 W kg−1, which is considerably higher than that of the MnCo2O4 electrode (approximately 40.22 W h kg−1 and 399 W kg−1) at a current density of 1 A g−1.
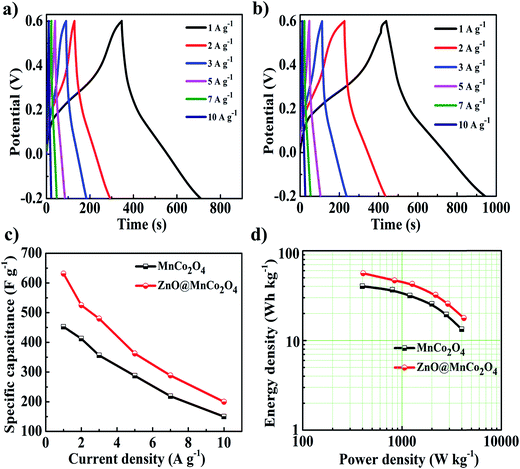 |
| | Fig. 5 Charge and discharge curves of the (a) MnCo2O4 and (b) ZnO@MnCo2O4 electrodes at different current densities; (c) the calculated specific capacitance as a function of the current density and (d) Ragone plot of the MnCo2O4 and ZnO@MnCo2O4 electrodes. | |
The long-term cycling stability of the electrodes is also an important requirement for practical supercapacitor applications. Fig. 6 shows the cycling performance of the MnCo2O4 and ZnO@MnCo2O4 electrodes at a current density of 1.0 A g−1 for 1000 cycles. In the first cycle, the MnCo2O4 electrode exhibited a specific capacitance of 453 F g−1 that decreased gradually to 343 F g−1 after 1000 cycles, leading to an overall capacitance loss of ∼24.0%. Interestingly, the specific capacitance decreased slightly with increasing cycling number and 93.5% of initial capacitance remained after 1000 cycles in ZnO@MnCo2O4 electrode. These results suggest that the porous ZnO@MnCo2O4 electrode also exhibited better cycling stability than the MnCo2O4 electrode, which is due to the electrolyte gradually penetrating the inner region of the ZnO@MnCo2O4 electrode, and an increasing number of sites become activated, resulting in an increase in capacitance.32
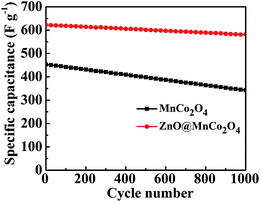 |
| | Fig. 6 Cycling performance of the MnCo2O4 and ZnO@MnCo2O4 electrodes at a current density of 1 A g−1 for 1000 cycles. | |
To further examine the electrochemical behavior of the MnCo2O4 and ZnO@MnCo2O4 electrode-based supercapacitor, EIS was carried out at a bias of 0 V and frequencies ranging from 100 mHz to 500 kHz. Fig. 7 compares the Nyquist plots of both the dandelion-like MnCo2O4 structures and the flower-like ZnO@MnCo2O4 nanosheets. In the low-frequency region, the impedance plot increased sharply and tended to become vertical lines, which are the characteristic of pure capacitive behavior.33 The impedance spectra are composed of a spike at the low frequency region and a semicircle at the high frequency region. At the high frequency, the intercept of the curve at the Z′-axis represents the equivalent series resistance (ESR, which includes the combined resistance of the ionic resistance of the electrolyte, the intrinsic resistance of the substrate, and the contact resistance at the active material/current collector interface) and the semicircle diameter represents the charge transfer resistance (Rct) at the electrode/electrolyte interface. The ESR value of the ZnO@MnCo2O4 electrode was smaller than that of the MnCo2O4 electrode, highlighting the good conductivity of the electrolyte and a much smaller bulk resistance of the flower-like ZnO@MnCo2O4 heterostructures. The Rct for the MnCo2O4 and ZnO@MnCo2O4 electrodes was 5.88 and 5.01 Ω, respectively. The small Rct of the ZnO@MnCo2O4 electrodes revealed fast ion diffusion and electron transport. The small ESR and Rct of the ZnO@MnCo2O4 electrodes play crucial roles in enhancing the performance of supercapacitors. Therefore, the unique morphology, high conductivity and excellent dispersion state synergistically improve the capacity and cycling performance of the ZnO@MnCo2O4 electrode.
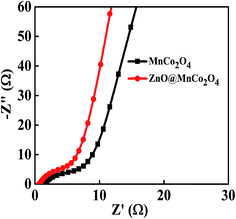 |
| | Fig. 7 Impedance Nyquist plots of the MnCo2O4 and ZnO@MnCo2O4 electrodes. | |
4 Conclusions
Flower-like ZnO@MnCo2O4 nanosheets were synthesized successfully through a facile and cost-effective hydrothermal method for high-performance supercapacitors. The supercapacitor electrode made from the ZnO@MnCo2O4 flower-like sheets demonstrated excellent electrochemical performance in terms of the specific capacitance (631.2 F g−1 at 1 A g−1), energy density (56.10 W h kg−1) and cyclic stability (6.4% loss after 1000 cycles), which are much better than dandelion-like MnCo2O4 electrodes. The above results show that the flower-like ZnO@MnCo2O4 nanosheet is a promising energy storage electrode material for the next generation high performance supercapacitors as well as for applications in other fields, such as solar cells, chemical and electrochemical sensing, catalysis, and field emission.
Notes
The authors declare no competing financial interest. The samples were analyzed by scanning electron microscope (SEM, Hitachi S-4200) with a 4k × 4k CCD camera (Ultra Scan 400SP, Gatan corp.) at the Busan KBSI, South Korea.
Acknowledgements
This research was supported by Basic Research Laboratory through the National Research Foundations of Korea funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A4A1041584).
References
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Y. J. Chen, B. H. Qu, L. L. Hu, Z. Xu, Q. H. Li and T. H. Wang, Nanoscale, 2013, 5, 9812–9820 RSC.
- D. P. Dubal, S. V. Patil, A. D. Jagadale and C. D. Lokhande, J. Alloys Compd., 2011, 509, 8183–8188 CrossRef CAS.
- J. P. Zheng and T. R. Jow, J. Electrochem. Soc., 1995, 142, L6–L8 CrossRef CAS.
- Y. Chen, X. Zhang, D. Zhang and Y. Ma, J. Alloys Compd., 2012, 511, 251–256 CrossRef CAS.
- Y. Zhai, Y. Dou, D. Zhao, P. F. Fulvio, R. T. Mayes and S. Dai, Adv. Mater., 2011, 23, 4828–4850 CrossRef CAS PubMed.
- J. Zhang and X. S. Zhao, J. Phys. Chem. C, 2012, 116, 5420–5426 CAS.
- M. Wu, J. Gao, S. Zhang and A. Chen, J. Power Sources, 2006, 159, 365–369 CrossRef CAS.
- M. Nakayama, T. Kanaya and R. Inoue, Electrochem. Commun., 2007, 9, 1154–1158 CrossRef CAS.
- H. B. Wu, H. Pang and X. W. Lou, Energy Environ. Sci., 2013, 6, 3619–3626 CAS.
- L. J. Wang, B. Liu, S. H. Ran, L. M. Wang, L. N. Gao, F. Y. Qu, D. Chen and G. Z. Shen, J. Mater. Chem. A, 2013, 1, 2139–2143 CAS.
- J. F. Li, S. L. Xiong, X. W. Lia and Y. T. Qian, Nanoscale, 2013, 5, 2045–2054 RSC.
- Z. Y. Wang, Z. C. Wang, W. T. Liu, W. Xiao and X. W. Lou, Energy Environ. Sci., 2013, 6, 87–91 CAS.
- J. F. Li, J. Z. Wang, X. Liang, Z. J. Zhang, H. K. Liu, Y. T. Qian and S. L. Xiong, ACS Appl. Mater. Interfaces, 2014, 6, 24–30 CAS.
- S. G. Mohamed, T. F. Hung, C. J. Chen, C. K. Chen, S. F. Hu and R. S. Liu, RSC Adv., 2014, 4, 17230–17235 RSC.
- T. Y. Ma, Y. Zheng, S. Dai, M. Jaroniecb and S. Z. Qiao, J. Mater. Chem. A, 2014, 2, 8676–8682 CAS.
- L. Yu, G. Q. Zhang, C. Z. Yuan and X. W. Lou, Chem. Commun., 2013, 49, 137–139 RSC.
- W. Q. Ma, H. H. Nan, Z. X. Gu, B. Y. Geng and X. J. Zhang, J. Mater. Chem. A, 2015, 3, 5442–5448 CAS.
- Z. P. Sun, W. Ai, J. L. Liu, X. Y. Qi, Y. L. Wang, J. H. Zhu, H. Zhang and T. Yu, Nanoscale, 2014, 12, 6563–6568 RSC.
- J. P. Liu, J. Jiang, C. W. Cheng, H. X. Li, J. X. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076 CrossRef CAS PubMed.
- Y. S. Luo, J. S. Luo, J. Jiang, W. W. Zhou, H. P. Yang, X. Y. Qi, H. Zhang, H. J. Fan, D. Y. W. Yu, C. M. Li and T. Yu, Energy Environ. Sci., 2012, 5, 6559–6566 CAS.
- Z. Xing, Q. Chu, X. Ren, C. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, J. Power Sources, 2014, 245, 463–467 CrossRef CAS.
- Z. Pu, Q. Liu, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. Sun, Electrochim. Acta, 2013, 109, 252–255 CrossRef CAS.
- G. Zhang and X. W. Lou, Adv. Mater., 2013, 25, 976–979 CrossRef CAS PubMed.
- C. Yuan, L. Yang, L. Hou, L. Shen, X. Zhang and X. W. Lou, Energy Environ. Sci., 2012, 5, 7883–7887 CAS.
- A. K. Mondal, D. W. Su, S. Q. Chen, A. Ung, H. S. Kim and G. X. Wang, Chem.–Eur. J., 2015, 21, 1526–1536 CrossRef CAS PubMed.
- M. Y. Qiu, S. H. Zhan, H. B. Yu, D. D. Zhu and S. Q. Wang, Nanoscale, 2015, 7, 2568–2577 RSC.
- G. R. Li, Z. L. Wang, F. L. Zheng, Y. N. Ou and Y. X. Tong, J. Mater. Chem., 2011, 21, 4217–4221 RSC.
- X. Y. Liu, Y. Q. Zhang, X. H. Xia, S. J. Shi, Y. Lu, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2013, 239, 157–163 CrossRef CAS.
- P. H. Yang, Y. L. Chen, X. Yu, P. F. Qiang, K. Wang, X. Cai, S. Z. Tan, P. Y. Liu, J. H. Song and W. J. Mai, Nano Energy, 2014, 10, 108–116 CrossRef CAS.
Footnote |
| † These authors have made an equal contribution to this work. |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.