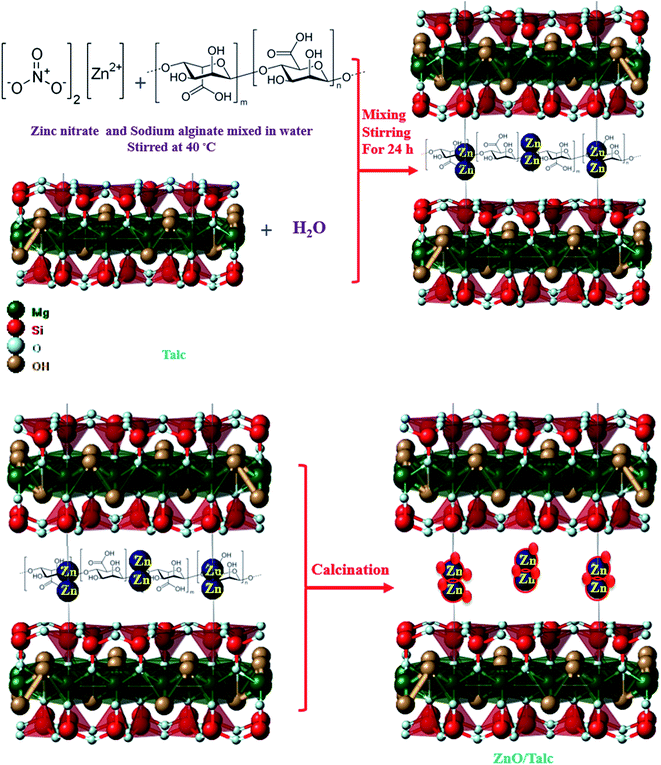
Synthesis of zinc oxide/talc nanocomposite for enhanced lead adsorption from aqueous solutions
Hannatu Abubakar Sania,
Mansor B. Ahmada and
Tawfik A. Saleh*b
aDepartment of Chemistry, Faculty of Science, Universiti Putra Malaysia, Malaysia. E-mail: mansorahmad@gmail.com
bChemistry Department, King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia. E-mail: tawfik@kfupm.edu.sa; tawfikas@hotmail.com
First published on 7th November 2016
Abstract
In this study, talc was modified with zinc oxide nanoparticles to form a ZnO/talc nanocomposite. The nanocomposite was characterized using X-ray powder diffraction, Fourier transform infrared spectroscopy, nitrogen adsorption/desorption, field emission scanning electron microscopy. The characterization revealed that ZnO nanoparticles were well incorporated with the talc. The adsorption efficiency of the prepared nanocomposite was evaluated for Pb(II) removal from aqueous solution. The related parameters such as agitating time, dosage and pH, were optimized. Adsorption characteristics of the talc, and ZnO/talc nanocomposite were compared and the results showed that ZnO/talc nanocomposite had the highest adsorption capacity. The kinetic sorption data were found to fit the pseudo-second-order kinetic model. The experimental isotherm data of lead adsorption were examined using the Freundlich and the Langmuir models. The maximum lead adsorption capacity of the adsorbent was determined as 48.3 mg g−1. The mechanism of adsorption was found to be controlled by electrostatic attraction on the nanocomposite. The overall results indicated the prepared nanocomposite can be employed as an alternative for Pb(II) removal from wastewater.
1. Introduction
The level of lead production and consumption has increased worldwide due to a number of agricultural and industrial activities. Lead is a toxic heavy metal that cannot degrade and can easily accumulate in the human body. Lead is commonly used for various industrial applications such as battery industries, paints, fuels, pigment, photographic materials, metallurgical, coatings and automotive industries.1 Drinking water and ingestion are the main source through which lead get into the body. Lead causes kidney disease, cancer, anemia, mental retardation and damage central nervous system.2,3 The maximum contaminant level allowed by US Environmental Protection Agency is 0.015 mg L−1 and the maximum contaminant level goal is zero.4 So, waters containing potentially lead metal need to be checked and treated before the discharge. Lead can pollute the environment from artificial sources as well as by natural geochemical processes.5Lead ions from various medium have been removed using different techniques and materials from different researchers. Electrodialysis process was used for removal of lead.6,7 The removal of lead by natural and pretreated clinoptilolite was reported. Magnetic magnetite (Fe3O4) nanoparticle was used for adsorption of lead(II) from water.8 A PSf/Fe3O4–talc membrane was also used for removal of lead.9 Two methods of electrochemical and chemical coagulation were combined to remove lead ions from wastewater.10 A new low-cost adsorbent from natural zeolite–kaolin–bentonite was used for lead removal.11 An improved chitosan bead was employed for lead removal.12 Adsorption is the most effective and cost-effective process for removal of heavy metal from waste water and serves as a substitute due to its advantage of adsorbent regeneration, high percentage removal, recovery of metal, reduction in the quantities of materials discharge.13 Zinc oxide is a unique material in nanotechnology because it exhibits unique physical and chemical properties such as high chemical stability, high electrochemical coupling coefficient, a broad range of radiation absorption and high photostability, which have to make it as a great potential in many applications.
Talc mineral is hydrated magnesium silicate with the chemical formula Mg3Si4O10(OH)2. It is the softest mineral. Talc is white clear material that is translucent to opaque with a density of 2.5–2.8. Talc does not dissolve in water. The talc layers are made of octahedral centered sheets coordinated Mg(OH)2 in a trioctahedral arrangement sandwiched between coordinated silicate sheets with tetrahedral structure.14 Talc is applicable in many industries such as paper, plastic, rubber, food, pharmaceuticals, cosmetics, and ceramics. Important properties that make talc be used for such applications are natural abundance, low-cost, high surface area, chemical inertness, high thermal stability, good lubricity, low electrical conductivity and distinctive pore structure.15 The adsorption of uranyl ions onto talc from aqueous solutions and heavy metals rapid adsorption by Fe3O4/talc nanocomposite was evaluated.6,17 Talc powder was employed for the removal of hexavalent chromium from aqueous solutions.18 Şener and Özyilmaz use sonicated talc for naphthalene adsorption.19
In this study, talc modified with ZnO nanoparticles was prepared by the heat-based method to form ZnO/talc nanocomposite. The obtained nanocomposite was characterized and its adsorption efficiency was explored for the removal of Pb2+. The ZnO/talc nanocomposite showed enhanced adsorption efficiency compared to ZnO and talc alone. The adsorption performance was evaluated in terms of kinetic properties and adsorption isotherms.
2. Materials and methods
2.1. Materials
Pb(NO3)2 and Zn(NO3)2·5H2O were purchased from Bendosen. Talc and sodium alginate were purchased from Sigma-Aldrich. All the chemicals were of analytical grade.2.2. Preparation of ZnO/talc nanocomposite
Zinc oxide nanoparticles were prepared by the reaction between zinc nitrate and sodium alginate. The aqueous solution of the prepared zinc nitrate was mixed with a solution of sodium alginate and vigorously stirred at 40 °C for 30 min. The nanocomposite was prepared by addition of the aqueous mixture of zinc nitrate and solution of sodium alginate into the aqueous suspension of talc and stirred for 24 h Fig. 1. The resulting solid phase was separated by decantation, washed several times with distilled water, and then dried at 105 °C for 24 h. The resulting powder was calcined at 500 °C for 1 h.2.3. Characterization
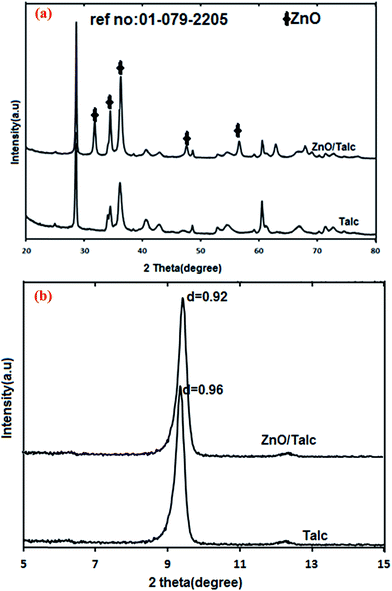
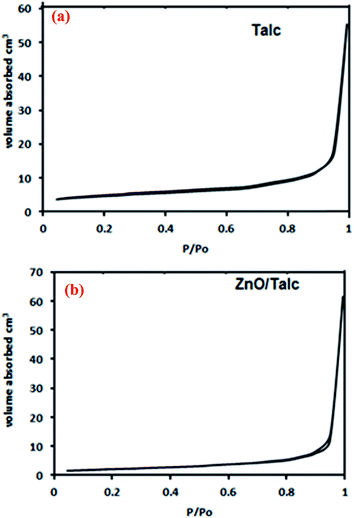
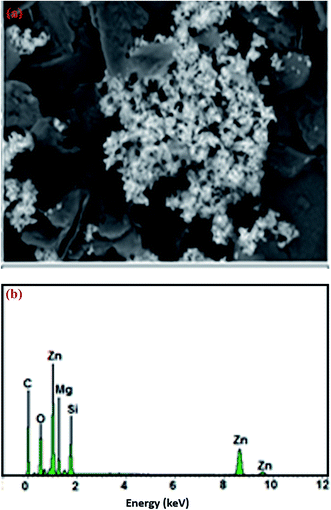
The X-ray powder diffraction (XRD) patterns were measured using X-ray diffractometer ShimadzuXRD-6000 instrument Cu Kα radiation (λ = 1.5406 Å, 30 kV, 30 mA) over a 2θ range of 5–80° with a resolution of 0.02°.Field emission scanning electron microscopy morphology (FESEM) and the surfaces of samples were studied using field emission scanning electron microscopy spectroscopy (EDX) (JEOL JSM-7600F (SEM)) equipped with energy dispersive spectrometer (EDS) for elemental analysis. Fourier transform infrared spectroscopy (FTIR) was measured by Perkin Elmer 2000. The measurement was carried out in the spectral range 4000–400 cm−1 using the KBr disc technique. Nitrogen adsorption/desorption was used to measure surface area and average adsorption pore width of the nanocomposite by Brunauer–Emmett–Teller (BET)-nitrogen gas analysis using a TriStar II Plus 3020 Automatic Physisorption.
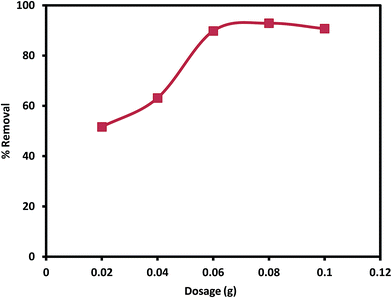
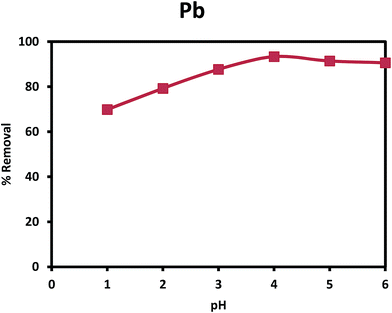
2.4. Adsorption experiments
The percentage removal and adsorption capacity were calculated and used to test the efficacy of the nanocomposite for removal of Pb(II) from aqueous solution. Calculation of % removal and adsorption capacity are shown below
 | (1) |
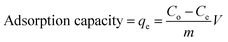 | (2) |
3. Results and discussion
3.1. Characterization
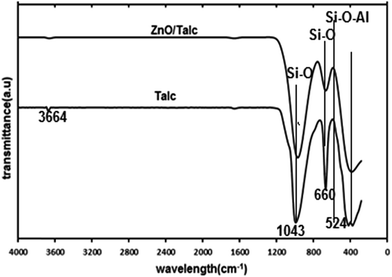
FTIR study was used to analyze the minerals and functional groups that present in the talc and modified ZnO/talc. The FT-IR spectra of talc and ZnO/talc samples are presented in Fig. 2 and spectra were recorded in the range from 400 to 4000 cm−1. The band at 1043 cm−1 is attributed to Si–O in-plane stretching and bands at 524 and 617 cm−1 are due to Si–O–Al bending and stretching vibrations, respectively.20,21 The band at 464 cm−1 is due to Si–O–Si bending vibrations. The bands at 3660 to 3665 cm−1 correspond to the stretching and bending vibrations for the hydroxyl groups of water molecules present in the talc. The bands in ZnO/talc move to lower wave numbers that signify the interaction of ZnO nanoparticles with the talc this confirmed the presence of ZnO nanoparticles present in the talc matrix.3.2. Adsorption studies
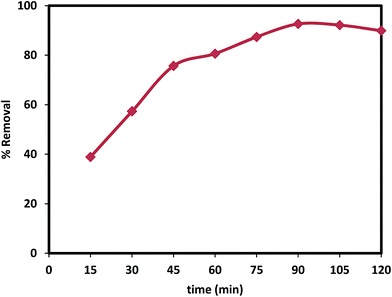
3.3. Kinetics of adsorption
Adsorption kinetic is used to predict the rate at which lead ions is removed from the aqueous solutions. The adsorption data of Pb(II) at different time intervals are fit for to pseudo-first-order kinetic model and pseudo-second-order kinetic model. Pseudo first order kinetic model describe mechanisms of metal species adsorption by an adsorbent and can be expressed as
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (3) |
Pseudo-second-order kinetic model
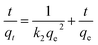 | (4) |
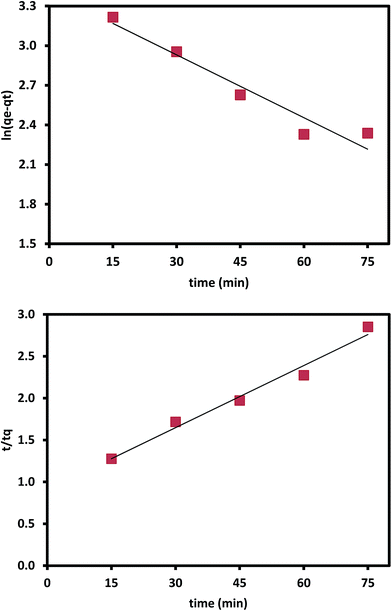 | ||
| Fig. 9 Pseudo first order and second reaction kinetics for the adsorption of Pb2+ on ZnO/talc nanocomposite. | ||
| Pseudo-first order | Pseudo-second order | |||||
|---|---|---|---|---|---|---|
| k1 (min−1) | qe | R2 | k2 (g mg−1 min−1) | qe | R2 | |
| Pb | 1.59 × 10−2 | 30.16 | 0.946 | 6.7 × 10−4 | 40.0 | 0.999 |
The theoretical adsorption capacity value (40.0 mg g−1) for pseudo second-order kinetics is in good agreement with the experimental adsorption capacity value (38.8 mg g−1). The regression coefficient R2 of second order is higher than that of the first order and hence the data best fitted to pseudo-second-order than pseudo-first-order and hence confirming chemisorption as the rate limiting the reaction, Fig. 9.
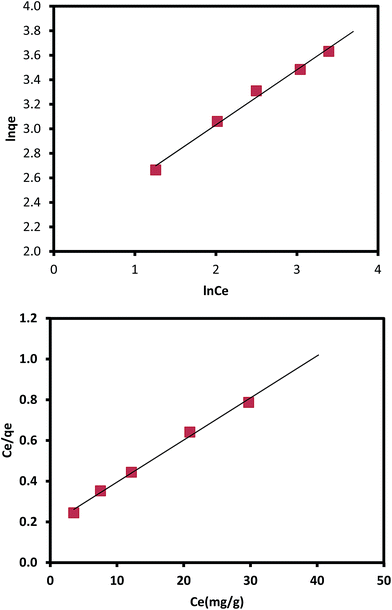
3.4. Isotherm of adsorption
Isotherm of adsorption gives information on mechanisms of adsorption, properties of the surface and affinity of an adsorbent towards heavy metal ions.30,31 The adsorption data were analyzed using Langmuir and Freundlich adsorption isotherm models and are presented in Fig. 10.
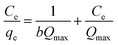 | (5) |
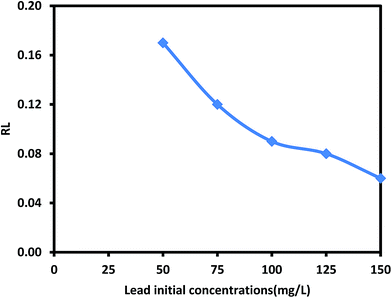
The separation factor RL was calculated and presented in Fig. 11 to confirm the adsorption process favorability. The adsorption of lead Pb(II) prepared ZnO/talc nanocomposite is favorable with value of RL between 0 and 1
| RL = 1/(1 + bCe) | (6) |
| qe = KfCen | (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe against ln
qe against ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce values of Kf and 1/n can be calculated. The value of the 1/n determines the adsorption is favorable.33
Ce values of Kf and 1/n can be calculated. The value of the 1/n determines the adsorption is favorable.33The adsorption data best fitted Langmuir adsorption isotherm since regression coefficient (R2) obtained as is higher than from the Freundlich hence implying monolayer adsorption. The constants of Freundlich and Langmuir isotherms and correlation coefficients calculated from the adsorption data are given in Table 2.
| Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|
| Qmax (mg g−1) | b | R2 | Kf | n | R2 | |
| Pb | 48.3 | 0.097 | 0.99 | 0.75 | 2.22 | 0.98 |
The results obtained from this study were compared with other reported materials, Table 3. The capacity data indicated that the reported ZnO/talc nanocomposite has comparable efficiency to those reported materials. In addition, the ZnO/talc nanocomposite has the advantages of being a cost-effective and simple method of preparation, thus, easiness of scaling up the production process.
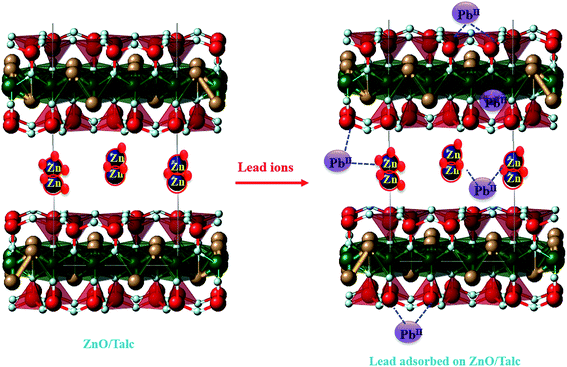
3.5. Mechanism of adsorption
Adsorption mechanism of lead ions onto the nanocomposite can be explained by the electrostatic attraction between the negatively charged sites on the surface of nanocomposite and the positively charged lead ions. The proposed mechanisms for the adsorption of Pb(II) onto ZnO/talc is illustrated in Fig. 12. The lead ions could be attracted by the negative sites on the zinc oxide nanoparticles and on the talc negative sites. The electrons on the surface can promote the formation of hydroxyl that increases the adsorption rate of metals from aqueous solution due to more interactions.34,40,41 Another mean of attraction is between the lead ions and the hydroxyl groups that are attached to the edge surface of the talc sheets.164. Conclusions
In this work, ZnO/talc nanocomposite prepared from talc and zinc oxide nanoparticles was effectively used for removal of lead ions from aqueous solutions. The structure and morphology were confirmed by PXRD and FTIR. The ZnO/talc exhibited good adsorption efficiency for the lead ion removal from aqueous solutions comparing with the talc and ZnO alone. The sorption performances were affected by parameters such as agitating time, dosage and pH. The adsorption kinetics was fitted to pseudo-first order and pseudo-second-order rate models, revealing different lead adsorption mechanisms on the nanocomposite, which is attributed to the presence of reactive sites that functionalize the adsorbent surface. The application of the isotherm models to experimental results showed that the adsorption equilibrium data fitted very well to the Langmuir isotherm in the studied concentration range. The maximum adsorption capacity was found 48.3 mg of lead per g of the nanocomposite.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to acknowledge the financial support from Universiti Putra Malaysia (UPM, Serdang, Malaysia).References
- A. Ahmad, M. Rafatullah, O. Sulaiman, M. H. Ibrahim, Y. Y. Chii and B. M. Siddique, Removal of Cu(II) and Pb(II) ions from aqueous solutions by adsorption on sawdust of Meranti wood, Desalination, 2009, 247, 636–646 CrossRef CAS.
- F. Fu and Q. Wang, Removal of heavy metal ions from wastewaters, a review, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- A. Kumar, G. K. Mishra, P. K. Rai, C. Rajagopal, P. N. Nagar and A. K. Meena, Removal of heavy metal ions from aqueous solutions using carbon aerogel as an adsorbent, J. Hazard. Mater., 2005, 122, 161–170 CrossRef PubMed.
- Us Epa O., National Primary Drinking Water Regulations, Drink Water Contam, 2013, pp. 141–142 Search PubMed.
- M. W. Wan, C. C. Kan, B. D. Rogel and M. L. P. Dalida, Adsorption of copper(II) and lead(II) ions from aqueous solution on chitosan-coated sand, Carbohydr. Polym., 2010, 80, 891–899 CrossRef CAS.
- C. V. Gherasim, J. Křivčík and P. Mikulášek, Investigation of batch electrodialysis process for removal of lead ions from aqueous solutions, Chem. Eng. J., 2014, 256, 324–334 CrossRef CAS.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, Chemical treatment technologies for waste-water recycling—an overview, RSC Adv., 2012, 2(16), 6380–6388 RSC.
- S. Rajput, C. U. Pittman, D. Mohan and M. Kumari, Heavy metals [chromium(VI) and lead(II)] removal from water using mesoporous magnetite (Fe3O4) nanospheres, J. Colloid Interface Sci., 2015, 442, 120–132 CrossRef PubMed.
- P. Moradihamedani, K. Kalantari, A. H. Abdullah and N. A. Morad, High efficient removal of lead(II) and nickel(II) from aqueous solution by novel polysulfone/Fe3O4–talc nanocomposite mixed matrix membrane, Desalin. Water Treat., 2016, 57(23), 10730–10744 CrossRef.
- K. A. Vo, X. J. Xu, T. G. Li, R. H. Peng, S. L. Liu and X. L. Yue, Research on a new electrochemical method combined with chemical coagulation in removal of lead, zinc, and copper from wastewater, Desalin. Water Treat., 2016, 57, 15343–15352 CrossRef CAS.
- A. Salem and R. Akbari Sene, Removal of lead from solution by combination of natural zeolite–kaolin–bentonite as a new low-cost adsorbent, Chem. Eng. J., 2011, 174, 619–628 CrossRef CAS.
- Y. Lu, J. He and G. Luo, An improved synthesis of chitosan bead for Pb(II) adsorption, Chem. Eng. J., 2013, 226, 271–278 CrossRef CAS.
- T. A. Saleh, Mercury sorption by silica/carbon nanotubes and silica/activated carbon: a comparison study, J. Water Supply: Res. Technol.--AQUA, 2015, 64(8), 892–903 CrossRef.
- K. Shameli, M. B. Ahmad, W. M. Z. W. Yunus, N. A. Ibrahim and M. Darroudi, Synthesis and characterization of silver/talc nanocomposites using the wet chemical reduction method, Int. J. Nanomed., 2010, 5, 743–751 CrossRef CAS PubMed.
- S. F. Li, S. C. Yang, S. L. Zhao, P. Li and J. H. Zhang, Microwave and acid-modified talc for the adsorption of methylene blue in aqueous solution, J. Serb. Chem. Soc., 2015, 80, 563–574 CrossRef CAS.
- M. Sprynskyy, T. Kowalkowski, H. Tutu, E. M. Cukrowska and B. Buszewski, Adsorption performance of talc for uranium removal from aqueous solution, Chem. Eng. J., 2011, 171, 1185–1193 CrossRef CAS.
- K. Kalantari, M. B. Ahmad, H. R. F. Masoumi, K. Shameli, M. Basri and R. Khandanlou, Rapid adsorption of heavy metals by Fe3O4/talc nanocomposite and optimization study using response surface methodology, Int. J. Mol. Sci., 2014, 15, 12913–12927 CrossRef CAS PubMed.
- M. E. Ossman, M. S. Mansour, M. A. Fattah, N. Taha and Y. Kiros, Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions, Bulg. Chem. Commun., 2014, 46, 629–639 Search PubMed.
- S. Şener and A. Özyilmaz, Adsorption of naphthalene onto sonicated talc from aqueous solutions, Ultrason. Sonochem., 2010, 17, 932–938 CrossRef PubMed.
- V. C. Farmer and J. D. Russel, Infrared absorption spectroscopy in clay studies, Clay Miner., 1967, 15, 121–141 CAS.
- M. A. Prado, G. Dias, C. Carone, R. Ligabue, A. Dumas, C. Le Roux and S. Einloft, Synthetic Ni–talc as filler for producing polyurethane nanocomposites, J. Appl. Polym. Sci., 2015, 132(16) DOI:10.1002/app.41854.
- Y. S. Feng, S. M. Zhou, Y. Li, C. C. Li and L. D. Zhang, Synthesis and characterization of tin oxide nanoparticles dispersed in monolithic mesoporous silica, Solid State Sci., 2003, 5, 729–733 CrossRef CAS.
- T. A. Saleh, A. Sarı and M. Tuzen, Effective adsorption of antimony(III) from aqueous solutions by polyamide–graphene composite as a novel adsorbent, Chem. Eng. J., 2017, 307, 230–238 CrossRef CAS.
- M. Mahdavi, M. B. Ahmad, M. J. Haron, Y. Gharayebi, K. Shameli and B. Nadi, Fabrication and Characterization of SiO2/(3-Aminopropyl)triethoxysilane-Coated Magnetite Nanoparticles for Lead(II) Removal from Aqueous Solution, J. Inorg. Organomet. Polym. Mater., 2013, 23, 599–607 CrossRef CAS.
- T. A. Saleh, A strategy for integrating basic concepts of nanotechnology to enhance undergraduate nano-education: statistical evaluation of an application study, J. Nano Educ., 2013, 4(1–2), 1–7 CrossRef.
- J. Kong, Q. Yue, S. Sun, B. Gao, Y. Kan and Q. Li, Adsorption of Pb(II) from aqueous solution using keratin waste–hide waste: equilibrium, kinetic and thermodynamic modeling studies, Chem. Eng. J., 2014, 241, 393–400 CrossRef CAS.
- G. I. Danmaliki and T. A. Saleh, Effects of bimetallic Ce/Fe nanoparticles on the desulfurization of thiophenes using activated carbon, Chem. Eng. J., 2017, 307, 914–927 CrossRef CAS.
- L. E. Liu, J. Liu, H. Li and H. Zhang, Equilibrium, kinetic, and thermodynamic studies of lead(II) biosorption on sesame leaf, BioResources, 2012, 7, 3555–3572 CAS.
- F. A. Dawodu and K. G. Akpomie, Simultaneous adsorption of Ni(II) and Mn(II) ions from aqueous solution unto a Nigerian kaolinite clay, J. Mater. Res. Technol., 2014, 3, 129–141 CrossRef CAS.
- T. A. Saleh, A. Sari and M. Tuzen, Chitosan-modified vermiculite for As(III) adsorption from aqueous solution: equilibrium, thermodynamic and kinetic studies, J. Mol. Liq., 2016, 219, 937–945 CrossRef CAS.
- T. A. Saleh and G. I. Danmaliki, Adsorptive desulfurization of dibenzothiophene from fuels by rubber tyres-derived carbons: Kinetics and isotherms evaluation, Process Saf. Environ. Prot., 2016, 102, 9–19 CrossRef CAS.
- T. A. Saleh and V. K. Gupta, Nanomaterial and polymer membranes: synthesis, characterization, and applications, Elsevier, Amsterdam, 2016, ISBN-13: 978-0128047033 Search PubMed.
- V. M. Boddu, K. Abburi, J. L. Talbott and E. D. Smith, Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent, Environ. Sci. Technol., 2003, 37, 4449–4456 CrossRef CAS PubMed.
- Z. Özlem-Kocabaş-Atakli and Y. Yürüm, Synthesis and characterization of anatase nanoadsorbent and application in removal of lead, copper and arsenic from water, Chem. Eng. J., 2013, 225, 625–635 CrossRef.
- A. K. Meena, G. K. Mishra, P. K. Rai, C. Rajagopal and P. N. Nagar, Removal of Heavy Metal Ions from Aqueous Solutions Using Carbon Aerogel as an Adsorbent, J. Hazard. Mater., 2005, 122, 161–170 CrossRef CAS PubMed.
- A. A. Alswat, M. B. Ahmad and T. A. Saleh, Zeolite modified with copper oxide and iron oxide for lead and arsenic adsorption from aqueous solutions, J. Water Supply: Res. Technol.--AQUA, 2016, 65(6), 465–479 CrossRef.
- J. H. Potgieter, S. S. Potgieter-Vermaak and P. D. Kalibantonga, Heavy Metals Removal from Solution by Palygorskite Clay, Miner. Eng., 2006, 19, 463–470 CrossRef CAS.
- T. A. Saleh, Nanocomposite of carbon nanotubes/silica nanoparticles and their use for adsorption of Pb(II): from surface properties to sorption mechanism, Desalin. Water Treat., 2016, 57(23), 10730–10744 CrossRef CAS.
- T. A. Saleh, V. K. Gupta and A. A. Al-Saadi, Adsorption of lead ions from aqueous solution using porous carbon derived from rubber tires: experimental and computational study, J. Colloid Interface Sci., 2013, 396, 264–269 CrossRef CAS PubMed.
- M. M. Al-Shalalfeh, T. A. Saleh and A. A. Al-Saadi, Silver colloid and film substrates in surface-enhanced Raman scattering for 2-thiouracil detection, RSC Adv., 2016, 6(79), 75282–75292 RSC.
- A. M. Alansi, W. Z. Alkayali, M. H. Al-qunaibit, T. F. Qahtan and T. A. Saleh, Synthesis of exfoliated polystyrene/anionic clay MgAl-layered double hydroxide: structural and thermal properties, RSC Adv., 2015, 5(87), 71441–71448 RSC.
| This journal is © The Royal Society of Chemistry 2016 |