The efficacy of interactive analogical models in the instruction of bond energy curves in undergraduate chemistry†
Vijay M.
Shahani
* and
Jodie
Jenkinson
Biomedical Communications, Department of Biology, University of Toronto, Ontario, Canada. E-mail: vijay.shahani@utoronto.ca
First published on 7th March 2016
Abstract
We explored analogies used for introducing students to the concept of potential energy wells. Two analogy systems were developed, a spring system and a novel system consisting of electrostatic spheres. These two, distinct analogies were housed within an interactive tool that allowed students to manipulate the analogous systems and witness changes to potential energy curves in real time. A pre-test/post-test evaluation provided insight into the impact the formulation of an analogy system can have on understanding. Students modified written descriptions to include new details in accordance to the structure-mapping theory of analogies. However, students failed to correct visual descriptions of energy wells. The failure of participants to apply key concepts after using the interactive and animated analogy systems highlights the importance of designing for education.
Introduction
The presentation of an analogy is a common teaching strategy that relates familiar concepts/systems to ones that are new and unfamiliar to the learner. Given their utility as an educational tool, instructional analogies have been investigated in multiple studies (Harrison and Treagust, 1993; Dagher, 1995; Glynn and Takahashi, 1998; Del Re, 2000; Coll et al., 2005). Well developed and validated analogies have demonstrated a number of educational advantages, including: (1) providing a base level of understanding for novel concepts (Glynn and Takahashi, 1998; Orgill et al., 2015), (2) providing a visual model upon which to approach abstract concepts (Treagust et al., 1996; Yaner and Goel, 2006; Nersessian and Chandrasekharan, 2009), and (3) providing a foundation to motivate students and spark further inquiry (Treagust et al., 1996; Glynn and Takahashi, 1998; Choi and Chang, 2004).According to the structure-mapping theory proposed by Gentner (1983), a system can serve as an analogy if it shares certain fundamental characteristics with an unfamiliar system. For an analogy to be successful, a familiar concept, referred to as the “analog”, must map its higher-order relations to an unfamiliar object or the “target” (higher-order relations include causal, mathematical or function relations). Therefore, an analog transfers concepts best when the characteristics it shares with the target are fundamental higher order relations rather than superficial attributes. Building on this notion is the positive, neutral, and negative aspects of an analogy (Nakiboglu and Taber, 2013). Positive aspects are consistent between the two models and supports understanding, negative aspects are incorrect or contradictory between the two systems, and neutral aspects are additional non-mappable details from one system to the other (i.e. extraneous information not critical to understanding). While deemed beneficial, there are cases where analogies can impede learning (Harrison and Treagust, 2006). This is especially true when students transfer negative aspects from an analog to the target leading to misconceptions. Students must be taught to recognize that eventually an analog will fail and they must develop their own internal representation of the target. Failing to update naïve conceptions with more robust mathematical and theoretical models can hamper knowledge progression.
Well-constructed analogies readily lend themselves to instruction within the sciences (Novick and Holyoak, 1991; Del Re, 2000; Aubusson and Fogwill, 2006). Chemistry in particular contains many abstract principles that are often made accessible through the use of metaphor, analogy or mental models (Thiele and Treagust, 1994; Gould, 1999; Hajkova et al., 2013; Orgill et al., 2015). For students, analogies help to make chemistry concepts that are complex and non-intuitive accessible. However, mapping a chemical concept through a single analogy is not sufficient to create proper understanding (Spiro et al., 1989). The educator must stress to students that the initial insights obtained from analogies must be challenged and refined to truly comprehend chemical concepts (Treagust et al., 1998; Harrison and Treagust, 2006).
Contemporary instructors have access to both interactive and animation technologies to produce learning tools capable of conveying concepts that are challenging to communicate (Wu et al., 2001; Yang et al., 2004; Jones, 2013). Animations have been shown to increase student understanding of atomic phenomenon; for example, the inclusion of animated atomic motion when describing the states of matter led to a significant increase in student comprehension (Ardac and Akaygun, 2004). An added benefit to using interactive educational media is its ability to allow students to learn through exploration (Barak and Dori, 2005; Frailich et al., 2009). Unfortunately, the dynamic visuals found within interactive learning tools can sometimes impede learning due to cognitive overload. (Sweller, 1994). Cognitive load theory posits that there are limitations in working memory when processing several channels of information at once that can prevent the conversion of short term into long term memory. In particular, there are three major loads: one that is germane to the subject matter, another that is intrinsic to the learning experience and one that is extrinsic to the learning experience. Students faced with complex visual stimuli may be unable to retain key information because of the extraneous burden often found in visual stimuli.
Additionally, a critical skill for students to have when studying chemistry is representational competence, which is the ability to navigate the multiple external representations found within chemistry (e.g. graphs, reaction mechanisms, equations) (Gilbert and Treagust, 2009; Johnstone, 2009; Nersessian and Chandrasekharan, 2009; Pande and Chandrasekharan, 2014). While multiple representations have been deemed beneficial for producing deeper understanding (Ainsworth, 1999), there is a need to carefully link these representations to observe their added benefits. Though there are challenges, interactive technologies may be useful for the creation of new and unique analogies for the instruction of difficult chemical concepts.
Background
Use of analogy in chemistry
Analogies are abundant in the instruction of abstract concepts within the discipline of chemistry (Thiele and Treagust, 1994). Fisher's lock and key for explaining protein-ligand binding, planetary orbits to introduce electron motion, and roller coasters exemplifying the concept of potential energy are a few examples of the many analogies available to educators. These very accessible analogies are useful to naïve students who are trying to comprehend new concepts. While analogies provide a foundation for understanding these concepts, instructional analogies must be supplemented over time to instill proper understanding. Exemplifying this is Fischer's lock and key, which is further refined with induced-fit model to better conceptualize protein-ligand binding. Failure to correct these early models can lead to confusion when more complex concepts are introduced (Harrison and Treagust, 2006).Chemistry is also abundant with visual analogies. To illustrate chemical concepts, instructors generally use three levels of visual representation: symbolic (Lewis structures), submicro (atomic/molecular level), and macroscopic (chemical reactions) (Treagust et al., 2003; Gilbert and Treagust, 2009; Johnstone, 2009). Symbolic and sub-micro representations are akin to visual analogies since they assign familiar shapes to entities that cannot be observed. While technology has come a long way in its ability to capture images of molecules in real-time (e.g. the single-molecule, real-time transmission electron microscope imaging technique), there remains a large reliance on symbolic representations (Nakamura, 2013). However, these representations can unfortunately convey qualities that are not consistent with reality. For example, the line that symbolizes bonds in Lewis representations falsely implies rigid bonds. Without additional corrective descriptions, many visual analogies fall short of reality. Therefore, it becomes critical for students to discuss and augment analogical systems in order to best develop schema that are better reflections of the learned abstract concept (Harrison and Treagust, 2000).
Instructors commonly use analogies in succession in order to build a more complete picture of the concept being taught (Spiro et al., 1989). For example, to modify students' notion that bonds are rigid, as implied by Lewis structures and other static representations, one can use the analogy of two balls (representing the atoms) on a spring (representing the force between them) to provide a dynamic model. These two analogies synergize to give a more elaborate and complete understanding of atomic bonding. Additional analogies, like using “tug of war” for concepts like electronegativity and polar bonds, can scaffold and help to refine the mental model initialized by an earlier analogy.
The spring is an analogy and model readily used by physicists and chemists to describe and simplify difficult systems (Del Re, 2000). When using the spring analogy for atoms in a bond, the system's energy is often discussed. The spring is a simple harmonic oscillator and its energy can be represented by a symmetric, U-shaped energy well. Early descriptions of bonds as simple harmonic oscillators are necessarily replaced by the Morse potential to account for the anharmonicity found in actual bonds. Eventually, quantum descriptions can be made to better approximate the energy of bonded atoms. Energy plays a fundamental role in chemical phenomena and therefore, the origins and meaning behind energy well shapes found in energy diagrams and graphs are important concepts for students to understand.
Interactive energy well analogies
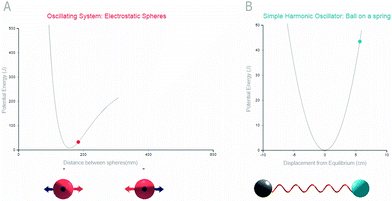
The Jablonski diagram has become a standard representation of the energy transitions associated with molecular excitation and emission events. The energy wells in the Jablonski diagram make up a significant component of the diagram and are important features to be understood by students. A traditional way to introduce students to the potential energy wells of bonds is through the analogous system of a spring and its corresponding U-shaped energy graph. Students are then introduced to a better approximation of bond energy given by the Morse potential. Unlike the symmetric energy wells of the spring system, analogies that map to the anharmonic Morse potential are not commonplace. In the present study we constructed a system featuring electrostatic spheres that possessed clearly defined regions of attraction and repulsion. This system was proposed to serve as an analogy for the Morse-potential and will be evaluated for its effectiveness.The proposed Morse-potential analogy was created within a digital, interactive platform (see Fig. 1A). The interactive analogy contained 3D representations of spheres that students were able to click and drag. Upon dragging the spheres, a Morse potential was drawn simultaneously with respect to the inter-sphere distance on a 2D graph. Arrows clearly demarked the magnitude and direction of the attractive, repulsive and net forces acting on the spheres. We also constructed an interactive digital model of the spring analogy (Fig. 1B). This model featured an interactive spring with two balls attached to its ends. Students could drag the balls in order to stretch and compress the spring and upon its release the spring system oscillated and dampened. Also, an energy potential was drawn in real time in direct relation to the spring's distance from equilibrium. Pulling the spring too far resulted in the spring breaking.
We wanted to investigate how well these analogies performed in the instruction of potential energy diagrams. In particular, we wanted to investigate the potential for hosting analogies, both traditional and newly developed, on interactive platforms. The research questions guiding this study were:
(1) Can analogies presented within an interactive digital platform foster changes to the learner's understanding?
(2) Would learners be successful in updating their mental models when using an analogy specifically constructed for use on an interactive platform, such as the electrostatic spheres featured in this study?
Methods
Participants and context
The study's participants were undergraduate students enrolled in a 3rd-year advanced analytical chemistry course taught during a single semester (N = 39) at the University of Toronto Mississauga. All students in the study were previously enrolled in a 2nd year analytical chemistry class and were taught the Jablonski diagram as part of that course's curriculum. The Jablonski diagram was reviewed at the beginning of the 3rd year course. Students were randomly assigned to either a “spring analogy” (N = 19) treatment group or an “electrostatic sphere analogy” (N = 20) treatment group. Students enrolled in particular study sessions, and each session was assigned a treatment type to provide equal groupings between students. Student age, academic background, study habits, and familiarity with technology were all considered when selecting the treatments for groupings. No statistical difference was observed between ages, years of study, and number of related chemistry courses completed between the two groups (ESI,† Appendix SA, Table SA1). Each group interacted with an analogy that was intended to instruct students about the energy of a molecular bond. The study was conducted over a one-hour period, where students completed a pre-test, interacted with the tool, and then completed post-test.Pre-experimental and post-experimental procedures
Students volunteered to participate in the study and were informed that their participation was voluntary and would help in the development of a study tool. Importantly, it was emphasized that the course instructor was not involved in the study and that participation would have no impact on their academic grade. Students were surveyed in order to collect background information on age, gender, study habits, previous chemistry courses completed and comfort level with digital devices. Participants logged into an online system to complete the survey and were provided the information necessary to obtain informed consent. Students were assigned random numbers to ensure their anonymity throughout the course of the experiment. Following the use of the interactive tool students were asked to complete a post-use survey which used an ordinal scale to rate their experience with the interactive tool.Experiment
To begin, all participants completed a pre-test featuring six multiple-choice questions about molecular excitation. They were also asked to draw a Jablonski diagram depicting fluorescence and to provide an explanation for the shape of drawn energy curves. Students were then prompted to interact with their assigned interactive analogy. One analogy featured the traditionally used “ball on a spring” model for conveying simple harmonic motion. The animated, three-dimensional spring along with its corresponding potential energy graph can be observed in Fig. 1A. A brief description on the significance of simple harmonic motion and molecular bonds was included with the metaphor. The second representation featured a system of electrostatic spheres that introduced anharmonic oscillatory motion. The electrostatic spheres were also 3D, interactive, and presented with a graph drawn in real time (Fig. 1B). The sphere module featured a text description that was marginally modified from the spring analogy's text to ensure consistency between the two treatments.Following tool usage, participants were asked to complete a post-test consisting of six questions that followed the same format as the pre-test. Students completed four multiple choice questions and were asked to draw a Jablonski diagram showing phosphorescence and were asked to explain the energy well shape. The total time required to complete the experiment was one hour.
Data analysis
The pre- and post-tests' multiple choice assessments were restricted to molecular excitation and related topics (ESI,† Appendix SB). Questions consisted of choosing the correct definition from a set of responses; for example, selecting types of molecular relaxation or choosing the time scale for a particular molecular event (i.e. molecular vibration). The questions were selected to orient students to the topic of molecular emission without priming their focus on the concept of energy wells. The questions tested concepts that were representative of a base level of understanding and didn't require a high degree of problem solving skills. Thus, these questions served as a measure of equivalency between the two groups. Non-parametric methods were used to detect differences in performance between the treatment groups as well as to compare total participant performance before and after intervention. Nonparametric analyses were used to address data that were not normally distributed (as assessed by a Shapiro-Wilk test) and to account for the small sample size of the test groups.Analysis of student diagrams was conducted by evaluating the distinguishing features of the energy well graph. These components are listed in Table 1 along with the evaluation criteria for these features. Diagrams were given an average mark based on the quality of the different components. Features were also examined independently, with focus placed on the well-shape featured in the Jablonski diagram. Responses from two students who drew Jablonski diagrams using only energy levels demarked by lines (i.e. did not include energy curves), were excluded from consideration.
| Component | High quality (3) | Medium quality (2) | Low quality (1) |
|---|---|---|---|
| Axis |
– Both y and x axis present
– y axis is labelled potential energy and x is labelled internuclear distance |
– Both y and x axis present
– Incorrect labels for a single axis |
– Missing one or more axes
– Missing or incorrect labels for both axes |
| Energy curve shape |
– Clear energy minimum
– Distinct vertical asymptote at low internuclear distances – Unambiguous horizontal asymptote as x value approaches infinity |
– Clear energy minimum
– Vertical asymptote indistinct (i.e. features curly end) – Horizontal asymptote has indistinct curly end |
– Missing energy minimum
– No indication of vertical asymptote – Missing or ambiguous horizontal asymptote |
| Vibrational states |
– Horizontal lines representing different vibration states present
– Lines are properly spaced and end at the horizontal asymptote |
– Horizontal lines representing different vibration states are present
– Lines aren't properly spaced and end incorrectly |
– Horizontal lines absent
– Horizontal lines extend beyond the confines of the energy curve |
| Electron transitions |
– Excited state depicted
– Excitation event demarked and correct states occupied – Relaxation events clearly marked |
– Excited state depicted
– Excitation event attempted though not necessarily correct – Relaxation events marked but not necessarily correct |
– No excited state presented
– Excitation event not indicated – Relaxation event not indicated |
Analysis of written descriptions was initiated by separating student responses into idea units. An idea unit is generated by dividing a student's written responses into singular thoughts or ideas. In this way, a single sentence could be broken down into multiple idea units each addressing a single fact (ESI,† Appendix SA, Fig. SA1). Idea units were further designated as either explanatory or descriptive in nature. As per Lowe's (1999) definition, a descriptive idea unit outlines a feature on the graph without giving the underlying cause for that shape (e.g. the curve had a vertical asymptote as distance decreased). For an idea unit to be considered explanatory, it must establish a link between an element of the graph and a rationalization for this observed feature (e.g. the vertical asymptote was established due to large repulsive forces at short distances). Idea units that did not discuss the well shape were excluded from consideration. Additionally, idea units were evaluated for their correctness.
Two neutral inter-raters assigned idea units as either explanatory or descriptive in nature. Inter-raters were trained with a sample set of idea units before categorizing idea units independent of one another. The reliability of the sorted responses was established using Cohen's kappa coefficient, a statistical measure of inter-rater agreement that accounts for random chance. Relatedly, marking idea units as correct or incorrect was performed by the authors after establishing an objective criterion. Furthermore, the number of idea units generated by students before and after intervention were evaluated for significance. Changes in the proportion of correct idea units after intervention was also investigated.
Results
Establishing equivalency between test groups
Two measures were used to ensure that the two test groups were equivalent. First, a sorting procedure outlined in the methods section ensured that the two test groups had equally distributed characteristics. Another measure took into account student performance on the multiple-choice section of the pre- and post-test. A Mann–Whitney test showed that student performance on both the pre-test and post-test multiple choice questions were indistinguishable between the two groups. As well, the observed increase in performance following intervention with the analogy was the same for both groups. The difference in performance on the post-test compared to the pre-test can be attributed to a difference in the difficulty of the questions rather than any influence by the analogy systems tested. Thus, measured characteristics and multiple choice test performance established equivalency between the two treatment groups.In the following section the results from the multiple choice section of the pre- and post-tests will be outlined. Next, representative examples of high, medium and low quality diagram responses are presented. This is followed by diagrammatical results presented for each treatment group. Lastly, non-parametric analysis of the number of idea units and proportion of correct idea units will precede the presentation of representative coded idea units.
Post-test multiple choice results
The results from both treatments (N = 39) were collected and analysed in identical fashion. The multiple-choice questions were marked for each of the groups and results are summarized in Table 2. A Mann–Whitney test determined no significant difference in student achievement on the pre-test multiple choice section between the groups (Mann–Whitney p = 0.50, two-tailed). Similarly, both groups have similar levels of success on the post-test multiple choice questions (Mann–Whitney p = 0.40, two-tailed). The total collection of participants showed an observed increase in average of 11.1% between the pre- and post-tests. To assess if any significant difference existed between the two groups increased performance, pre-test marks were subtracted from their paired post-test grade and compared. The observed increase in performance were indistinguishable between the two groups at 9.2% and 13.2% for the sphere and spring groups respectively (Mann–Whitney p = 0.63, two-tailed).| Group | N | Median (/6) | Sum of ranks | U | p | |
|---|---|---|---|---|---|---|
| Pre-test | Sphere | 20 | 4 | 423 | 167 | 0.50 |
| Spring | 19 | 4 | 357 | |||
| Post-test | Sphere | 20 | 5 | 429 | 161 | 0.40 |
| Spring | 19 | 4 | 351 | |||
| (Post-test–Pre-test) | Sphere | 20 | 0.5 | 383.5 | 173.5 | 0.63 |
| Spring | 19 | 1 | 396.5 | |||
Pre-test representative examples of energy wells
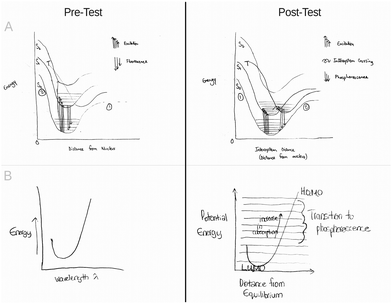
Students were asked to produce a Jablonski diagram depicting a fluorescence event. Student responses varied greatly in quality. Examples of drawings deemed high, average, and low quality prior to intervention with the analogy are available in Fig. 2. Of the 39 participants, 36 students drew versions of the Jablonski diagram that featured an energy well (1 student left the question blank and two students provided energy lines rather than curves). Fewer than 25 percent of the students (N = 8) drew well shapes that were deemed high quality. The majority of students produced energy wells that were symmetric or featured curly ends in place of vertical asymptotes and horizontal asymptotes (N = 26). Two students produced graphic representations of energy curves that were deemed low quality. A summary table of the quality of student drawings is provided in Table 3.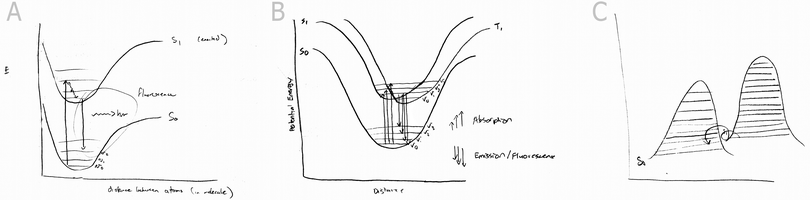 | ||
| Fig. 2 Representative examples of student drawn Jablonski diagrams. (A) A high quality example, (B) an average quality response and (C) a low quality response. | ||
| Quality | Sphere group | Spring group | ||||||
|---|---|---|---|---|---|---|---|---|
| Well shape | Overall | Well Shape | Overall | |||||
| Pre-test (n) | Post-test (n) | Pre-test (n) | Post-test (n) | Pre-test (n) | Post-test (n) | Pre-test (n) | Post-test (n) | |
| High | 4 | 5 | 4 | 9 | 4 | 4 | 6 | 6 |
| Medium | 13 | 12 | 12 | 6 | 13 | 13 | 10 | 11 |
| Low | 1 | 1 | 2 | 3 | 1 | 1 | 2 | 1 |
Comparison of spring analogy pre-test and post-test diagrams
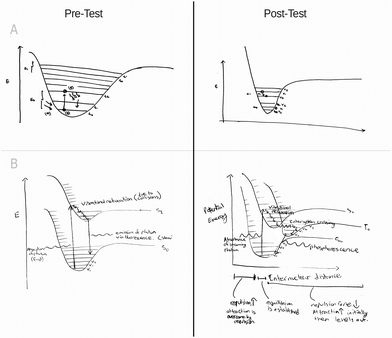
Students in the spring analogy group (N = 18) produced Jablonski diagrams that varied in quality, with the majority of students producing diagrams of average quality prior to intervention (N = 10). None of the diagrams drawn by the students showed any modification to the energy well shape after interacting with the spring system. Several students (N = 5) provided additional details to their Jablonski diagrams in the post-test, namely the inclusion of missing axes (N = 2), and corrections to the axes labels (N = 5). Students also altered their written descriptions after interacting with the analogy systems. The extent of these written modifications is addressed in the ‘Coded Responses’ section. Samples of student drawings before and after intervention with the spring analogy are found in Fig. 3.Comparison of sphere analogy pre-test and post-test diagrams
Similar to the spring analogy, the majority of sphere group's (N = 18) drawings from the pre-test were evaluated as average in quality (N = 12) and are summarized in Table 3. While several students (N = 3) made very slight modifications to their energy well shape, it is unclear if these changes were intentional or due to normal variation.A single student showed a marked difference in the drawn well-shape post treatment (Fig. 4A). In addition to modifications to the illustration, this particular student's written response also improved. As observed with the spring analogy, students added missing axes (N = 1) and added/improved axes labels (N = 5).
Coded responses
Students provided a diverse set of explanations for the shape of the energy wells they drew. A single student from each treatment group wrote a response that correctly explained the energy well shape prior to interaction with the tool. Most students provided incomplete explanations and a surprising amount of students initially left the question blank (N = 11). Student responses were deconstructed into idea units as described in the methods section. A summary of common idea units along with a complete categorization of idea units can be found in the ESI† (Appendix SC, Tables SC1 and SC2). Included in the idea unit summary table is a prototypical response, and a participant example of a correct and an incorrect idea unit.Total idea units
The total number of idea units before and after intervention was compared within groups and globally for all participants (Table 4). When the idea units were pooled for both groups, the entire population of students showed a significant increase in the number of idea units produced after intervention (Wilcoxon Signed-Rank p = 0.0015). When calculated within groups, it was found that the number of new idea units for the spring analogy was significantly higher when compared to earlier responses (Wilcoxon Signed-Rank p = 0.0058). Similarly, the sphere group showed an increase in idea units, however this increase could not be distinguished from random variability (Wilcoxon Signed-Rank p = 0.10).| Grouping | N | N unequal | Mean | z-Score | T | T-crit | p |
|---|---|---|---|---|---|---|---|
| Number of idea units produced | |||||||
| All participants | 29 | 23 |
Pre = 1.8
Post = 3.0 |
−3.16 | 35 | 73.6 | 0.0016 |
| Sphere | 13 | 9 |
Pre = 2.1
Post = 2.9 |
−1.62 | 9 | 5.7 | 0.10 |
| Spring | 16 | 14 |
Pre = 1.5
Post = 3.1 |
−2.75 | 9 | 21 | 0.0058 |
| Percentage of correct idea units produced | |||||||
| All participants | 29 | 20 |
Pre = 41.1%
Post = 72.64% |
−2.96 | 27 | 52.8 | 0.0031 |
| Sphere | 13 | 9 |
Pre = 41.7%
Post = 82.7% |
−2.53 | 1.5 | 5.7 | 0.011 |
| Spring | 16 |
Pre = 40.6%
Post = 64.5% |
−1.58 | 15.5 | 10.7 | 0.11 | |
Correct idea units
Next, we examined the number of correct idea units prior and post intervention (Table 4). Both groups were examined individually before and after intervention and each was analysed using the Wilcoxon Signed-Rank test. The sphere group's total number of correct idea units after intervention was significantly higher than pre-intervention (Wilcoxon Signed-Rank p = 0.011). In contrast, the proportion of correct idea units after intervention in the spring analogy treatment was indistinguishable from ordinary variability (Wilcoxon Signed-Rank p = 0.11).Idea unit categorization
When comparing the categorization of the idea units as either descriptive or explanatory the two inter-raters had 74% proportionate agreement (i.e. proportion of matched responses) and a Cohen's kappa coefficient of 0.47. In cases where only two categorizations are present, Cohen's kappa is anticipated to be below 0.5 even when observer accuracy is 90%, suggesting that the calculated Cohen's kappa of 0.47 is reflective of good agreement between inter-raters (Bakeman et al., 1997). The results from the spring analogy were consistent between the two inter-raters, with the majority of responses coded as descriptive in nature both before and after intervention. For the sphere analogy, approximately equal proportions of explanatory and descriptive idea units were recorded prior to intervention. However, the inter-raters varied in their assessment of the sphere group's post-intervention response, ascribing opposing ratios of descriptive/explanatory ideas. This contradiction makes it difficult to provide any definitive assertions about the affect the analogy had based on the categorization of the idea units. Comparisons of some representative idea units from each of the analogy groups are found in Table 5. The idea units presented are from the post-test and feature only explanatory units, categorized according to the part of the energy curve being described.| Spring analogy | Sphere analogy | |
|---|---|---|
| Vertical asymptote | “It cannot come too close to the nucleus” | “As distance between the two decreases the repulsion forces acting on the system increase drastically” |
| “The highest potential occurs when they are close due to nuclei repulsion (like bond compression).” | “When the atoms are too close, they repel each other (electronic repulsion)” | |
| Absolute minimum | “The bottom of the E well indicates when the electron is at an equilibrium between its potential and kinetic energy” | “At the bottom where the atoms are distance away from each other so that the attractive and repulsive forces cancel out, it will be in equilibrium and energy is low (stable).” |
| “The lowest energy point of the well is the point where there is equal repulsion and attraction” | “Shape of energy well is due to the distance between adjacent nuclei” | |
| Horizontal asymptote | “As the distance increases, this breaks as the atoms are too far away to be attractive to each other” | “It plateaus eventually as the two atoms/species no longer interact in space therefore no change in energy” |
| “If it stretches too far away from the nucleus, it flies off (like a spring breaking)” | “If molecules move very far from each other at one point the energy will flatten since bond will break” | |
| General well shape | “The electronic state is in a shape of a “U” because of the simple harmonic motion of the electron” | “You see a very steep energy slope initially, that rises again (smaller slope) then plateau's” |
| “the bottom of the E well indicates when the electron is at an equilibrium between its potential and kinetic energy” | “The energy well is the simple harmonic oscillation between 2 atoms due to competing attraction and repulsive forces” | |
A commonly observed addition to participants' idea units post-interaction with the spring analogy, was the inclusion of concepts like “potential energy” and “kinetic energy”. Additionally, mention of spring-like behaviour or oscillatory motion, particularly bond stretching and compression, was observed after treatment with the spring model. Alternatively, student responses following treatment with the sphere analogy primarily address attractive and repulsive forces. The sphere group also made fewer references to the oscillatory nature of the system or bond stretching and compression, favouring instead distances between atoms and the forces that dominate at these distances.
Discussion
The success of educational analogies hosted on interactive media
In order to evaluate the ability of hosted interactive analogies to engender improved student understanding, responses from the pre-test and post-test were investigated. Both written and graphical responses were evaluated for changes following intervention. Improvements in either type of student responses would be indicative of an interactive analogy impacting student understanding.Written descriptions were altered following intervention with either of the two analogies systems. When combining the two treatment groups, students wrote significantly more about the energy wells following intervention as measured by the overall increase in idea units. While the number of idea units following intervention gives an idea of student confidence, a better indicator of the quality of student responses is determined through an objective grading of idea units as correct or incorrect. With respect to the number of correct ideas about energy wells, the sphere analogy promoted increased understanding whereas the spring analogy failed to significantly improve student performance. Since we are examining analogies holistically, when these results were pooled, there was an overall improvement in student responses, supporting the notion that interactive analogies can have a positive impact on student understanding.
It is clear that the two analogies transformed students' understanding in different ways. This observed differences may lie in the information presented in the analogies themselves. The spring analogy relies on the description of oscillatory motion, potential energy, and a restorative force when the spring is pulled/compressed from equilibrium. While these underlying forces are correct for the spring system, they are not the same forces present between two atoms in a bond. Hence, students described the energy curve in terms of a restorative force, which is incorrect. Also, students seemed inclined to include concepts such as kinetic energy even though these weren't factored into the potential energy of the molecular system (seemingly because of the motion featured within the animation). Interestingly, students related the spring breaking to the bond breaking at large internuclear distances; however, most students failed to indicate that the atoms are sufficiently far away to prevent electrostatic and orbital interaction. Results suggest that the spring analogy promoted the transfer of ideas about bonding that were not always consistent with the phenomena described and thus did not contribute to improved performance post-treatment, rather than an outright failure to transfer information.
Students from the sphere analogy group were introduced to attractive and repulsive forces between the spheres as the source of the anharmonic oscillatory motion present in the Morse potential. Many students modified their responses after using the sphere analogy to incorporate these forces as the underlying cause of their drawn energy well. Mention of the repulsion between nuclei at small distances was common in student responses. Students also mentioned an attractive force between atoms at certain distances, and the lack of attractive/repulsive forces at large distances to account for bond breaking. Interestingly, while the electrostatic spheres showed some oscillatory motion, many student answers failed to mention the vibrational properties of molecular bonds. Some misconceptions did occur, for example, a student suggested that an attractive force exists between atom nuclei, which is fundamentally incorrect. However, the majority of students were able to successfully integrate the repulsive and attractive forces into their description of the energy wells. The success of students following intervention with the sphere analogy suggests the constructed system served participants well as an analogy for molecular bonds.
While the quality of students' written responses suggest that there is value in using interactive analogies, students' graphic representation of the Jablonski diagram (i.e. drawings of energy well shape) offers a less compelling argument. With the exception of a single student, participants failed to correct the well shape of the Jablonski diagram after engaging with interactive analogies. This global failure to update the visual representation of the concept of energy wells is intriguing, particularly when changes in many written descriptions suggested that students were connecting the interactive analogies with the Jablonski diagram. This finding is the topic of following discussion.
The observation that the majority of student's modified their written descriptions of energy wells indicated that the interactive analogies were influencing student understanding. The failure of students to update their graphical representations of energy wells may be due to some intrinsic aspects of interactive analogies. These aspects will be discussed, along with methods to improve the topical interactive analogies.
Success of a novel interactive analogy: electrostatic spheres
Comparing the performance of the spring analogy group with that of the electrostatic sphere's group can indicate how successfully the novel interactive analogy aided knowledge building. As previously mentioned, there was an observed increase in the number of ideas generated post-intervention for both groups, suggesting that students were supplementing their responses with information they acquired by interacting with the analogy systems. It is interesting that when the groups were examined in isolation, only the spring group showed a definitive increase in idea units. While trending close to significance (p-value = 0.10), the observed increase within the sphere group could not be distinguished from normal variance.If the number of idea units was the sole indicator of increased understanding, it would appear that the sphere analogy had little impact on student performance. However, an indicator that the two analogies differed in performance is the proportion of idea units that were evaluated to be correct. As previously discussed, while the number of idea units didn't necessarily increase for the sphere group, the proportion of correct idea units generated post-intervention did increase. The opposite was observed for the spring analogy group, where the number of idea units increased but the quality of responses after treatment were the same prior to intervention.
In contrast to the written responses, few students in the electrostatic spheres group improved their graphic representation of energy wells after intervention. Only a single student from the electrostatic spheres group showed clear adaptation of the correct energy well shape after using the interactive analogy. Other students from the sphere group updated peripheral graph details (i.e. labels and axes) after intervention. Therefore, it is likely that properties of interactive analogies restrict transfer of visual information to students. To summarize, given the noticeable improvements to written descriptions and few examples of positive influence on visual representations, it is proposed that creating novel educational analogies strictly for use on interactive platforms, as was undertaken with the electrostatic spheres analogy, is an educational strategy worth pursuing.
Barriers to using interactive analogies
A major shortcoming of the two interactive analogy systems was their common inability to foster improvement in students' graphical representation of energy wells. To better employ interactive analogies in the classroom, it is critically important to understand why students were unable to modify their understanding after using these tools. Therefore, we propose several explanations for the observed inadequacies of interactive analogies and will follow that discussion with exploration into possible improvements.First, the unchanged graphic representation of the energy wells may be due students failing to connect the energy curves found in the analogy systems to those in Jablonski diagram. Perhaps transferring the well shapes from the interactive analogy to the Jablonski diagram may have been too great a cognitive leap, even when considering students' familiarity with the topic. This could be due to poor understanding of the Jablonski diagram and failure to recognize energy wells as features of the diagram (Cook, 2006). Notably, students' written responses integrated details from the interactive analogy to support their explanations of the well shape from the Jablonski diagram. However, student responses that contained descriptions from the interactive analogy may not be an indicator of increased conceptualization but rather could be rote memorization of text-based descriptions. Thus, the human ability to recall recently observed facts may be factoring into student's written responses. Unfortunately, students were not assessed at an interval to test long-term acquisition of the information presented. Additionally, the intervention took place within an hour, it is possible that this time span is too short to engender meaningful conceptualization of the underlying target system from the analogy system. Consequently, it is possible that students failed to understand what was presented in the analogy and failure to correct the well-shape was a symptom of this superficial understanding.
Another likely explanation recognizes the challenge of linking graphics to concepts. Students interacted with the analogy and witnessed the drawing of an energy well curve, but may have struggled to correlate that shape with the underlying concepts. For example, students linked shorter internuclear distances with repulsion and increased potential energy, but often failed to expand this relationship to the formation of a vertical asymptote. In support of this idea is the observation that students often have trouble interpreting the real-world meaning of graphs well into university (Berg and Phillips, 1994). It is possible that students were unable to conceptualize the well shape (i.e. understand the real world implications of the shape) and thus were unable to reproduce it within the Jablonski diagram. In this way, students might have failed to see the significance in the well shape and dismissed it as an unnecessary detail, suggesting a fundamental misunderstanding of graphical representations (Schorr, 2003).
The previous explanations focused on general challenges with graphical representations, but failed to mention the interactive/animation component of the investigated analogy systems. Cognitive load is an important consideration when designing instructional tools – this is particularly true for animated or interactive media – and may have factored into the students' ability to retain information (Sweller, 1994). While the interactive analogies attempted to reduce cognitive load by including multiple representations (i.e. 3D model and 2D graph), properties of the interactive analogy may have detracted from focusing on well shape. Specifically, the motion of the spring or spheres could have distracted focus away from the static energy curves (Simola et al., 2011). It has been shown that moving objects are highly salient and can detract focus away from other static representations in an animation (Franconeri and Simons, 2003; Lowe, 2003). Furthermore, the energy well was linked to the oscillatory motion of the spring/sphere by a tracer dot; thus, the tracer dot may have been the point of fixation for students rather than the more meaningful, underlying well shape. Hence, students were likely unable to retain critical details, like well shape, because of the distracting motion found in the interactive analogy.
A final related topic is the consideration of when to implement an analogy when teaching. Prompting this commentary was the discovery of a single student who provided inferior answers following treatment with the analogy. After interacting with the sphere analogy the student sacrificed accurate details and relationships in favour of a simplified explanation. For example, details about the “electronic repulsion from the inner molecular orbitals” of two atoms were replaced in favour of the generic statement, “when atoms are too close, they repel each other”. Previous studies have suggested that analogies are best employed when introducing new, basic concepts to novice learners, but may cause confusion for the intermediate student who is trying to consolidate prior knowledge using the analogy (Spiro et al., 1989). This may have also been evidenced by the poorer performance of students interacting with the spring analogy, these students may have passed a level of study where this introductory analogy would bring about better understanding and instead lead to confusion. An alternative explanation for why the student provided less robust answers following intervention is that it could be an attempt to anticipate the answer the instructor desired most, rather than decreased understanding of the phenomenon (Crisp et al., 2008). As other studies have indicated, choosing when to deliver analogy models is an important consideration when employing them in an educational setting.
In summary, student's inability to rectify drawings of energy wells suggests that students may have overlooked the graphs presented in the analogy, failed to understand the significance of these graphs, or were unable to recall the graph shape due to the visual treatment provided. It is clear that addressing these issues would be critical for developing a successful interactive analogy system. This topic is explored the next section.
Addressing problems with interactive analogies
We identified several potential problems with the interactive analogies explored herein. The integration of strategies that mitigate the identified problems can improve interactive analogies' utility as teaching tools. These strategies include implementable features that better support the interpretation of a graph's real-world meaning, connects multiple external representations, and reduces cognitive load.It was proposed that students had trouble understanding the real-world meaning of the energy well shape (i.e. not grasping that pushing nuclei resulted in a vertical asymptote). Interaction with the visual representation of the analogy system (i.e. pulling the spring or moving electrostatic spheres), simultaneously moves the tracer dot and outlines the energy well curve. When designing the interactive analogies, it was hoped that the simultaneous movement and drawing of the graphs would provide a sufficient link between the two representations. To lower cognitive load, this relationship could have been emphasized with a line connecting the physical representation to the graph/energy well. Furthermore, studies have shown that superimposing information on graphics rather than placing them in figure captions can lower cognitive load (Leahy and Sweller, 2004). Consequently, the authors recommend placing labels on different portions of the graph that activate upon interaction events; for example, pop-up information can be displayed when the student moves the system into the energy minimum. The pop-up information can include labels of the energy well, the relationship between the forces acting on the system and related expressions (e.g. 1/r12 for the repulsive force term). In this fashion, students can relate the portions of the graph directly to their underlying causes.
An additional layer of information can be built into the interaction of the system through the input-device (e.g. mouse). In an upgraded and not yet assessed version of the interactive analogies, moving the spring system away from the equilibrium position required increased movement of the mouse to stretch or compress the spring. In a similar fashion, the electrostatic spheres possessed dampened input from the mouse when moving the spheres away from the equilibrium distance. Thus, the user perceives resistance to motion that mimics the sensation of moving against an increasing, opposing force. Hence, students can “feel” the forces involved in the system and better appreciate the features of the well. Feeling the forces further connects the underlying real world meaning of the energy curve.
Students may have had difficulty observing the static energy curve due to the highly salient motion of the spring or electrostatic sphere representations and the moving tracer dot. To compensate for this distraction, the authors suggest the incorporation of a pause feature. The pause feature will stop the ongoing motion of the system, however, student's can still manipulate the positions of the spring/spheres and observe the corresponding changes in the energy. This feature, coupled with the pop-up labels or contextual information, will provide meaningful associations between graph and real-world connections. An additional recommendation would be to emphasize the very important energy well shape by increasing the curve's line weight, adding a bold colour or a pulsation effect. As one of the more important concepts, the salience of the curve shape should be substantial compared to the other features of the analogy system.
Lastly, there were observed differences in the concepts conveyed between the two analogy systems, which hints at how these analogy systems may be effectively used on an interactive platform. The spring analogy fostered ideas about the oscillatory behaviour of bonded atoms and provided the concept of restorative forces for the bond's energy. The springs were also very accessible and provided a good foundation for simple, symmetric energy curves. The electrostatic sphere analogy promoted the concept of repulsive and attractive forces between atoms, and how these forces change with respect to distance in a more complex fashion than the symmetric energy curves of springs. Perhaps, rather then presenting these two systems as competing analogies, they would be best used in combination. Given the spring system's prevalence in education, the spring system can serve as a primer to potential energy curves. The electrostatic sphere's can be used as a complementary analogy to further refine student's understanding. Using the interactive technology available, more refined and specific analogy systems can be produced for abstract concepts, which, when used in sequence, can help accommodate students' understanding of the system. Eventually, these interactive analogy systems can build towards an interactive model that is no longer analogous, but rather a direct representation of the phenomenon being explained. In this way, educators can build the pathway to understanding by using carefully constructed and complementary analogy systems and circumvent issues with poorly timed use of analogies by having a pre-determined sequence. Analogies that build upon one another towards a complete representation of a target system has been previously described in the literature as “bridging analogies” (Clement, 1993). Consequently, bridging analogies would be efficiently implemented on an interactive platform.
Conclusions
It was anticipated that interactive platforms would support the use of educational, interactive analogies. Specifically, knowledge transfer would be supported by an interactive technology since it provides students the opportunity to manipulate the analogy systems and observe the consequences of changes in real time. Two analogy systems were prepared within an interactive interface that featured two external representations (i.e. the graph/energy well itself and a graphical representation of the system). To evaluate the properties of the interactive analogies, student volunteers were grouped according to an analogy system and were tested before and after intervention with their respective interactive analogy. The results of the study highlighted that the sphere analogy transferred concepts of repulsion and attraction and the spring analogy conveyed the oscillatory nature of the molecular bond, which indicates that both these systems were successful to a degree. However, it was observed that students did not correct drawings of energy curves in Jablonski diagrams following intervention. Student's inability to draw correct energy diagrams after using the analogical system might be an indicator of cognitive overload, where thematically relevant details on the graph shapes were overlooked for more salient features. Thus, while interactive analogies are promising teaching tools, these prototypic examples need refinement before they can be employed effectively.Exploration into the construction of novel, interactive analogy prompted the creation of the electrostatic spheres model as means to instruct on the Morse potential. The electrostatic spheres analogy leveraged available technology to make it interactive, animated and possess multiple levels of representation. The analogy was capable of transferring concepts like the attractive and repulsive forces found between the atoms in a molecular bond, and students were observed using these concepts to explain features of the Morse potential. Hence, within its interactive framework, the novel analogy successfully transferred concepts consistent with the system studied, emphasizing that original and specific interactive, digital analogies can be built for conveying abstract concepts where conventional or traditional analogies are unavailable.
Both interactive analogy systems failed to improve energy well visualizations produced by students. It was suggested that cognitive load may have played a role in this observed shortfall when employing interactive analogies. The authors provided some recommendations on how to design an interactive analogy to better present multiple levels of representation and molecular motion. Specifically, contextual labels should be provided, distracting motion should be played and paused as needed, and information hierarchy should be preserved (i.e. important concepts are highly salient). If implemented, these features together can reduce cognitive load and aid student comprehension. The authors also proposed that interactive analogies can be assembled and disseminated in a fashion that allows each analogy to build upon its predecessor, to facilitate learning. Eventually, these bridging analogies can build towards a complete, interactive representation of the concept being explored. Technology has provided many teaching opportunities, with novel interactive analogies being a tool to be explored and refined in order to support learning in the classroom.
Acknowledgements
The authors would like to thank Dr Ulrich Krull for his guidance and input into the analogy systems. A special thank you to Dr Paul Piunno for both his and his students' participation in the study. A special thank you to Robert Lancefield and Andrea Gauthier for serving as inter-raters. Additional thanks go to Daniel Ball and Harry Soor for their valuable input. The authors would also like to thank the funding agencies Canadian Institutes of Health Research and the Social Sciences and Humanities Research Council for their financial support.Notes and references
- Ainsworth S., (1999), The functions of multiple representations, Comput. Educ., 33(2), 131–152.
- Ardac D. and Akaygun S., (2004), Effectiveness of multimedia-based instruction that emphasizes molecular representations on students' understanding of chemical change, J. Res. Sci. Teach., 41(4), 317–337, DOI: http://10.1002/tea.20005.
- Aubusson P.J. and Fogwill S., (2006), Metaphor and Analogy in Science Education, Dordrecht: Springer, DOI: http://10.1007/1-4020-3830-5.
- Bakeman R., McArthur D., Quera V. and Robinson B. F., (1997), Detecting sequential patterns and determining their reliability with fallible observers, Psychol. Methods, 2(4), 357.
- Barak M. and Dori Y. J., (2005), Enhancing undergraduate students' chemistry understanding through project-based learning in an IT environment, Sci. Educ., 89(1), 117–139, DOI: http://10.1002/sce.20027.
- Berg C. A. and Phillips D. G., (1994), An investigation of the relationship between logical thinking structures and the ability to construct and interpret line graphs, J. Res. Sci. Teach., 31(4), 323–344.
- Choi K. and Chang H., (2004), The effects of using the electric circuit model in science education to facilitate learning electricity-related concepts, J. Korean Phys. Soc., 44(6), 1341–1348, Retrieved from http://www.scopus.com/inward/record.url?eid=2-s2.0-3042811907&partnerID=tZOtx3y1.
- Clement J., (1993), Using bridging analogies and anchoring intuitions to deal with students' preconceptions in physics, J. Res. Sci. Teach., 30(10), 1241–1257, DOI: http://10.1002/tea.3660301007.
- Coll R. K., France B. and Taylor I., (2005), The role of models/and analogies in science education: implications from research, Int. J. Sci. Educ., 27(2), 183–198, DOI: http://10.1080/0950069042000276712.
- Cook M. P., (2006), Visual representations in science education: the influence of prior knowledge and cognitive load theory on instructional design principles, Sci. Educ., 90(6), 1073–1091, DOI: http://10.1002/sce.20164.
- Crisp V., Sweiry E., Ahmed A. and Pollitt A., (2008), Tales of the expected: the influence of students' expectations on question validity and implications for writing exam questions, Educ. Res., 50(1), 95–115, DOI: http://10.1080/00131880801920445.
- Dagher Z. R., (1995), Review of studies on the effectiveness of instructional analogies in science education, Sci. Educ., 79(3), 295–312, DOI: http://10.1002/sce.3730790305.
- Del Re G., (2000), Models and analogies in science, HYLE, 6(1), 5–15.
- Frailich M., Kesner M. and Hofstein A., (2009), Enhancing Students' Understanding of the Concept of Chemical Bonding by Using Activities Provided on an Interactive Website, J. Res. Sci. Teach., 46(3), 289–310, DOI: http://10.1002/tea.20278.
- Franconeri S. L. and Simons D. J., (2003), Moving and looming stimuli capture attention, Percept. Psychophys., 65(7), 999–1010, Retrieved from http://www.scopus.com/inward/record.url?eid=2-s2.0-0346961120&partnerID=tZOtx3y1.
- Gentner D., (1983), Structure-Mapping: A Theoretical Framework for Analogy, Cognitive Sci., 7(2), 155–170, DOI: http://10.1016/S0364-0213(83)80009-3.
- Gilbert J. K. and Treagust D. F., (2009a), Introduction: macro, submicro and symbolic representations and the relationship between them: key models in chemical education, in Multiple representations in chemical education, Springer, pp. 1–8.
- Gilbert J. K. and Treagust D. F., (2009b), Multiple representations in chemical education (vol. 2). Springer.
- Glynn S. M. and Takahashi T., (1998), Learning from analogy-enhanced science text, J. Res. Sci. Teach., 35(10), 1129–1149.
- Gould E. S., (1999), Phosphate Buffers and Telephone Poles - A Useful Analogy with Limitations, J. Chem. Educ., 76(11), 1511, DOI: http://10.1021/ed076p1511.
- Hajkova Z., Fejfar A. and Smejkal P., (2013), Two Simple Classroom Demonstrations for Scanning Probe Microscopy Based on a Macroscopic Analogy, J. Chem. Educ., 90(3), 361–363, DOI: http://10.1021/ed3004947.
- Harrison A. G. and Treagust D. F., (1993), Teaching with analogies: a case study in grade-10 optics, J. Res. Sci. Teach., 30(10), 1291–1307, DOI: http://10.1002/tea.3660301010.
- Harrison A. G. and Treagust D. F., (2000), Learning about atoms, molecules, and chemical bonds: a case study of multiple-model use in grade 11 chemistry, Sci. Educ., 84(3), 352–381.
- Harrison A. G. and Treagust D. F., (2006), Teaching and learning with analogies, in Metaphor and analogy in science education, Springer, pp. 11–24.
- Johnstone A. H., (2009), Multiple Representations in Chemical Education, International Journal of Science Education (vol. 31), Dordrecht: Springer, DOI: http://10.1080/09500690903211393.
- Jones L. L., (2013), How Multimedia-Based Learning and Molecular Visualization Change the Landscape of Chemical Education Research, J. Chem. Educ., 90(12), 1571–1576, DOI: http://10.1021/ed4001206.
- Leahy W. and Sweller J., (2004), Cognitive load and the imagination effect, Appl. Cognitive Psych., 18(7), 857–875, DOI: http://10.1002/acp.1061.
- Lowe R. K., (1999), Extracting information from an animation during complex visual learning, Eur. J. Psychol. Educ., 14(2), 225–244.
- Lowe R. K., (2003), Animation and learning: selective processing of information in dynamic graphics, Learn. Instr., 13(2), 157–176, DOI: http://10.1016/S0959-47520200018-X.
- Nakamura E., (2013), Movies of molecular motions and reactions: the single-molecule, real-time transmission electron microscope imaging technique, Angew. Chem.,Int. Ed., 52(1), 236–252, DOI: http://10.1002/anie.201205693.
- Nakiboglu C. and Taber K. S., (2013), The Atom as a Tiny Solar System: Turkish High School Students' Understanding of the Atom in Relation to a Common Teaching Analogy, in Concepts of matter in science education, Springer, pp. 169–198.
- Nersessian N. J. and Chandrasekharan S., (2009), Hybrid analogies in conceptual innovation in science, Cogn. Syst. Res., 10(3), 178–188, DOI: http://10.1016/j.cogsys.2008.09.009.
- Novick L. R. and Holyoak K. J., (1991), Mathematical Problem Solving by Analogy, J. Exp. Psychol.-Learn. Mem. Cogn., 17(3), 398–415.
- Orgill M., Bussey T. J. and Bodner G. M., (2015), Biochemistry instructors' perceptions of analogies and their classroom use, Chem. Educ. Res. Pract., 16(4), 731–746.
- Pande P. and Chandrasekharan S., (2014), Integration of multiple external representations in chemistry: a requirements-gathering study, in Workshop Proceedings of the 22nd International Conference on Computers in Education, ICCE, Asia-Pacific Society for Computers in Education, pp. 732–737.
- Schorr R. Y., (2003), Motion, speed, and other ideas that “should be put in books,” The Journal of Mathematical Behavior, 22(4), 465–477, DOI: http://10.1016/j.jmathb.2003.09.005.
- Simola J., Kuisma J., Oörni A., Uusitalo L. and Hyönä, J., (2011), The impact of salient advertisements on reading and attention on web pages, J. Exp. Psychol.-Appl., 17(2), 174–90, DOI: http://10.1037/a0024042.
- Spiro R. J., Feltovich P. J., Coulson R. and Anderson D. K., (1989), Multiple analogies for complex concepts: antidotes for analogy-induced misconceptionin advanced knowledge acquisition, in Vosniadou S. and Ortony A. (ed.) Similarity and Analogical Reasoninglarity and Analogical Reasoning, Cambridge: Cambridge Univ Press, pp. 498–530, DOI: http://10.1017/CBO9780511529863.023.
- Sweller J., (1994), Cognitive load theory, learning difficulty, and instructional design, Learn. Instr., 4(4), 295–312, DOI: http://10.1016/0959-4752(94)90003-5.
- Thiele R. B. and Treagust D. F., (1994), The nature and extent of analogies in secondary chemistry textbooks, Instr. Sci., 22(1), 61–74, DOI: http://10.1007/BF00889523.
- Treagust D., Chittleborough G. and Mamiala T., (2003), The role of submicroscopic and symbolic representations in chemical explanations, Int. J. Sci. Educ., 25(11), 1353–1368, DOI: http://10.1080/0950069032000070306.
- Treagust D. F., Harrison A. G., Venville G. J. and Dagher Z., (1996), Using an analogical teaching approach to engender conceptual change, Int. J. Sci. Educ., 18(2), 213–229.
- Treagust D. F., Harrison A. G. and Venville G. J., (1998), Teaching science effectively with analogies: an approach for preservice and inservice teacher education, J. Sci. Teacher Educ., 9(2), 85–101.
- Wu H.-K., Krajcik J. S. and Soloway E., (2001), Promoting understanding of chemical representations: students' use of a visualization tool in the classroom, J. Res. Sci. Teach., 38(7), 821–842.
- Yaner P. W. and Goel A. K., (2006), Visual analogy: viewing analogical retrieval and mapping as constraint satisfaction problems, Appl. Intell., 25(1), 91–105, DOI: http://10.1007/s10489-006-8868-x.
- Yang E.-M., Greenbowe T. J. and Andre T., (2004), The effective use of an interactive software program to reduce students' misconceptions about batteries, J. Chem. Educ., 81(4), 587.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5rp00194c |
| This journal is © The Royal Society of Chemistry 2016 |