Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium(II)-catalyzed synthesis of dibenzothiophene derivatives via the cleavage of carbon–sulfur and carbon–hydrogen bonds†
Mamoru
Tobisu
*ab,
Yoshihiro
Masuya
a,
Katsuaki
Baba
a and
Naoto
Chatani
*a
aDepartment of Applied Chemistry, Faculty of Engineering, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: tobisu@chem.eng.osaka-u.ac.jp; chatani@chem.eng.osaka-u.ac.jp
bCenter for Atomic and Molecular Technologies, Graduate School of Engineering, Osaka University, Suita, Osaka 565-0871, Japan
First published on 21st January 2016
Abstract
A new process has been developed for the palladium(II)-catalyzed synthesis of dibenzothiophene derivatives via the cleavage of C–H and C–S bonds. In contrast to the existing methods for the synthesis of this scaffold by C–H functionalization, this new catalytic C–H/C–S coupling method does not require the presence of an external stoichiometric oxidant or reactive functionalities such as C–X or S–H, allowing its application to the synthesis of elaborate π-systems. Notably, the product-forming step of this reaction lies in an oxidative addition step rather than a reductive elimination step, making this reaction mechanistically uncommon.
Introduction
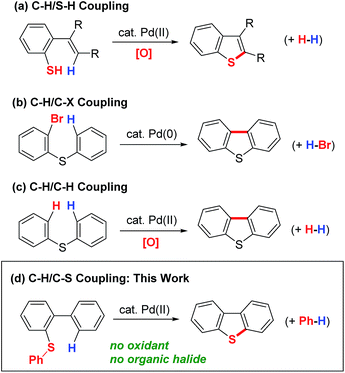
Thiophenes and their benzo-fused derivatives constitute a privileged class of scaffolds with numerous applications, in pharmaceuticals1 and advanced molecular materials.2 Although a wide range of methods are currently available for the synthesis of thiophene derivatives,3 recent research efforts have focused on the development of catalytic C–H functionalization reactions4 in the hope of achieving increasingly facile processes for the construction of elaborate thiophene derivatives. In this context, three different classes of catalytic reaction have been reported to date for the synthesis of thiophene derivatives. The first class involves a C–S bond-forming ring closure, which occurs via an oxidative C–H/S–H coupling reaction (Scheme 1a).5,6 However, the inherent instability and toxicity of thiophenol-based substrates have limited the practical application of this method. The second method is based on an intramolecular C–H/C–X coupling reaction under Pd(0)/Pd(II) catalysis (Scheme 1b).7 However, the starting halogenated biaryl sulfides (or sulfoxides) required for this strategy can only be synthesized by SNAr,7a Grignard,7a RLi7b or SEAr-type C–H palladation7c reactions, which has limited the structural diversity of the thiophene derivatives that can be accessed by this protocol. The third of these three different methods for the synthesis of dibenzothiophene derivatives involves the intramolecular oxidative C–H/C–H coupling of simple diaryl sulfides (Scheme 1c).6,8 This approach is particularly interesting in the sense that it allows for the direct conversion of less functionalized substrates into the desired products. Despite this advantage, the application of this process has been limited by its requirement for the use of a large excess of a silver oxidant (2–4 equiv.). Herein, we report a unique C–H/C–S coupling strategy for the catalytic synthesis of fused thiophene derivatives (Scheme 1d). The notable features of the reaction are as follows: (1) it does not require any reactive functionalities, such as C–X or S–H bonds; (2) it does not require an external oxidant, such as a silver salt; and (3) the reaction proceeds through the cleavage of inert C–H and C–S bonds.9 Although strong acid-mediated reaction of biphenyl sulfoxides was reported to form dibenzothiophenes through formal C–H and S–Me cleavage, its mechanism involves classical Friedel–Crafts type C–H functionalization and S–Me cleavage via the SN2 mechanism.10Results and discussion
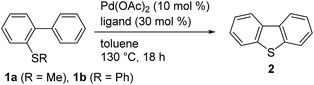
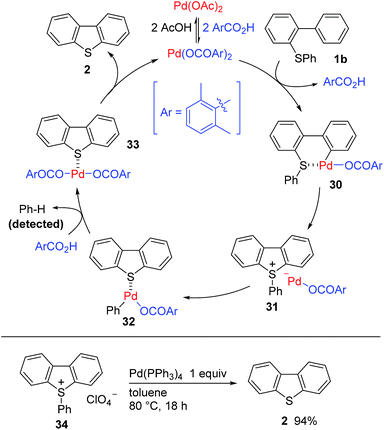
We initially selected biphenyl sulfide 1 as a test substrate to develop a catalytic synthesis of dibenzothiophene 2via the activation of its C–H and C–S bonds. It was envisioned that the required C–H activation of the 2′-position in 1 could be achieved by a sulfur-directed cyclometallation process.11,12 With this in mind, we focused our initial effort on the development of suitable conditions for this unprecedented cyclization process involving C–S activation.9 After several experiments, we found that Pd(OAc)2 performed as a potential catalyst for the cyclization of 1a (Table 1, entry 1). Changing the leaving group on the sulfur atom from Me (1a) to Ph (1b) led to an increase in the yield of 3 from 5 to 15% (entry 2). Further improvements in yield were accomplished by adding a carboxylic acid ligand,13 with 2,6-Me2C6H3CO2H (3) being optimal (entry 5).| Entry | Substrate | Ligand | NMR yield of 2 [%] |
|---|---|---|---|
| a Reaction conditions: 1 (0.30 mmol), Pd(OAc)2 (0.030 mmol), and ligand (0.090 mmol) in toluene (1.0 mL) at 130 °C for 18 h. b Pd(OAc)2 (0.045 mmol), and 3 (0.135 mmol) were used. c Isolated yield. | |||
| 1 | 1a | None | 5 |
| 2 | 1b | None | 15 |
| 3 | 1b | PivOH | 57 |
| 4 | 1b | 2,6-Me2C6H3CO2H (3) | 66 |
| 5b | 1b | 3 | 87 (79)c |
| 6b | 1a | 3 | 7 |
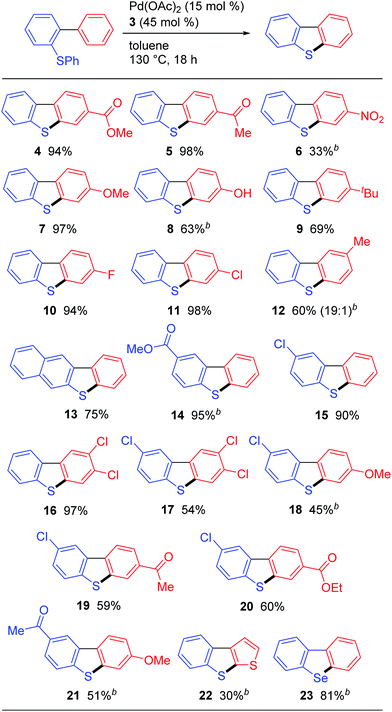
With the optimized conditions in hand, we proceeded to evaluate the scope of this palladium-catalyzed C–H/C–S coupling reaction (Fig. 1). Pleasingly, these conditions allowed for the successful activation of the C–H bonds in both electron-deficient (i.e., 4, 5 and 6) and electron-rich (i.e., 7, 8 and 9) aromatic rings to give the corresponding C–H/C–S coupling products. Notably, a phenolic OH, which would be incompatible with strong oxidants, was well-tolerated under these conditions, therefore highlighting one of the main advantages of our newly developed protocol over the existing oxidative methods (Scheme 1). Dibenzothiophenes bearing halogen atoms such as F and Cl can also be synthesized (compounds 10, 11, and 15). When the hydrogen atoms at the 2′- and 6′-positions of the substrate were non-equivalent, the cyclization proceeded at the least hindered C–H bond, as evidenced by the regioselective formation of 12. Unsymmetrical polysubstituted dibenzothiophenes are readily accessed by this method (compounds 16–20). This method can also be used for the synthesis of the benzo[b]thieno[3,2-d]thiophene ring systems 22, albeit in a lower yield than the corresponding dibenzothiophene derivatives likely because of the large angle strain associated with this target. Furthermore, this method provided facile access to dibenzoselenophene (23),14 thereby demonstrating that these palladium-catalyzed conditions can be used to activate a C–Se bond.15
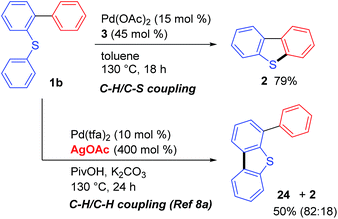
Interestingly, the course of the cyclization reaction of 1b could be regulated by the addition of a silver oxidant. As detailed above, dibenzothiophene (2) was obtained as the sole product when the palladium-catalyzed reaction of 1b was conducted in the absence of an external oxidant (Scheme 2, C–H/C–S coupling). In contrast, the addition of a silver salt (4.0 equiv. to 1b) led to the formation of 24 as the major product via a C–H/C–H coupling reaction, which was consistent with the results reported by Zhou.8a Although both of these reactions are likely to be initiated by a Pd(II)-mediated sulfur-directed C–H activation process,11,12 the position of the bond formation is clearly dependent on whether or not an oxidant is present in the reaction mixture.
Given the numerous successful applications of fused thiophene scaffolds across a wide range of fields,1,2 it would be useful for the development of new functional molecules if our C–H/C–S coupling procedure could be used for the late-stage introduction of benzothiophene moieties to existing π-systems with characteristic properties. Pleasingly, we were able to accomplish this process using boronic acid 25 as an effective elaborating reagent (Scheme 3). Thus, it is possible to extend a wide variety of π-systems by fusing a benzothiophene ring through the Suzuki–Miyaura reaction with 25, followed by ring closure via our C–H/C–S coupling. This whole process consists of two robust palladium-catalyzed reactions that do not require the use of any strong nucleophiles or oxidants, and therefore allows for the rapid modification of functionalized aromatic systems. For example, a bromophenyl group in 2,5-diaryloxadiazole motif can successfully participate in our two-step protocol to form benzothiophene-fused derivative 26. Similarly, this protocol was found to be applicable to the π-extension of a range of useful compounds, such as anthraquinone 27, amino acid 28 and BODIPY 29 derivatives.
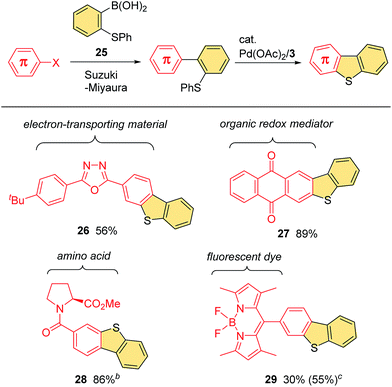 | ||
| Scheme 3 Boronic acid 25 as a versatile reagent for the incorporation of a fused benzothiophene ring to various π-systemsa. aConditions for the Suzuki–Miyaura reaction: see ESI.† Conditions for the cyclization: see footnote b in Fig. 1. Isolated yields for the cyclization step are shown. bSee footnote a in Fig. 1 for the conditions used for the cyclization. cNMR yield. | ||
Our current mechanistic proposal for the palladium-catalyzed C–H/C–S coupling is outlined in Scheme 4. Pd(OAc)2 would undergo ligand exchange with 3 to generate Pd(OCOAr)2,16 which would react with 1b to form palladacycle 30via a sulfur-directed cyclometallation process.10 We hypothesized that the unusual C–S bond cleavage process would proceed through sulfonium intermediate 31. Thus, a C–S bond-forming reductive elimination from 3017 would provide ion pair 31 consisting of a dibenzosulfonium cation and an anionic Pd(0) fragment. The oxidative addition of the Ph–S bond in dibenzosulfonium18 to the Pd(0) center would lead to the cleavage of the C–S bond to give complex 32. The cleaved phenyl group would be released as benzene following the protonolysis of 32 with 3, which would be generated during the initial cyclometallation process, leading to the regeneration of Pd(OCOAr)2. Several experiments were conducted to support this mechanistic proposal. For example, the treatment of the independently synthesized sulfonium salt 34 with Pd(0) complex provided 2, indicating the intermediacy of the sulfonium species in our catalytic reaction.19 Furthermore, we were able to confirm that benzene was generated (73% by GC) during the palladium-catalyzed reaction of 5, which was consistent with our proposal. There were no significant differences between the initial reaction rates for the independent reactions of 1b and deuterated 1b, which indicated that the C–H bond cleavage (i.e., 1b → 30) was not involved in the turnover-limiting step.19 Although no appreciable amounts of side product were observed in this C–H/C–S coupling, relatively high levels of catalyst loading (10–30 mol%) were currently required to obtain a high conversion. This requirement for a high catalyst loading could be attributed to the reluctance of the product 2 to dissociate from the Pd(II) center (i.e., 33 → 2). In fact, the addition of 2 to the palladium-catalyzed C–H/C–S coupling reaction led to a decrease in the yield of the cyclized product by 30%.19 A characteristic feature of the mechanism of the C–H/C–S coupling is that the product is released by an oxidative addition step (31 → 32), which occurs after the reductive elimination step (30 → 31), and allows for the regeneration of the Pd(II) species without the addition of an external oxidant. This mechanistic scenario is therefore unusual compared with the most common Pd(II)-catalyzed processes, which typically end up with a product-forming reductive elimination step to generate a Pd(0) species and consequently require an external oxidant to regenerate Pd(II).20
Conclusions
In summary, we have developed a new C–H/C–S coupling strategy for the catalytic synthesis of dibenzothiophene derivatives. In contrast to previously reported methods for the synthesis of benzothiophene derivatives via C–H functionalization, our newly developed method does not require reactive functionalities such as Ar–X or S–H, or the addition of an external stoichiometric oxidant. This C–H/C–S coupling procedure is characterized by its unique mechanism, with the product being formed by an oxidative addition step, rather than a reductive elimination. Further studies towards the application of this mechanistic feature to the synthesis of other heterocycles are currently underway in our laboratories.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from MEXT, Japan and ACT-C from JST, Japan. We also thank the Instrumental Analysis Center, Faculty of Engineering, Osaka University, for their assistance with HRMS.Notes and references
- Selected examples: (a) J. Wrobel, J. Sredy, C. Moxham, A. Dietrich, Z. Li, D. R. Sawicki, L. Seestaller, L. Wu, A. Katz, D. Sullivan, C. Tio and Z.-Y. Zhang, J. Med. Chem., 1999, 42, 3199 CrossRef CAS PubMed; (b) B. N. Balasubramanian, D. R. St. Laurent, M. G. Saulnier, B. H. Long, C. Bachand, F. Beaulieu, W. Clarke, M. Deshpande, J. Eummer, C. R. Fairchild, D. B. Frennesson, R. Kramer, F. Y. Lee, M. Mahler, A. Martel, B. N. Naidu, W. C. Rose, J. Russell, E. Ruediger, C. Solomon, K. M. Stoffan, H. Wong, K. Zimmermann and D. M. Vyas, J. Med. Chem., 2004, 47, 1609 CrossRef CAS PubMed; (c) S. Martin-Santamaria, J.-J. Rodriguez, S. de Pascual-Teresa, S. Gordon, M. Bengtsson, I. Garrido-Laguna, B. Rubio-Viqueira, P. P. Lopez-Casas, M. Hidalgo, B. de Pascual-Teresa and A. Ramos, Org. Biomol. Chem., 2008, 6, 3486 RSC; (d) M. R. V. Finlay and R. J. Griffin, Bioorg. Med. Chem. Lett., 2012, 22, 5352 CrossRef CAS PubMed.
- Selected reviews: (a) A. Mishra, C.-Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141 CrossRef CAS PubMed; (b) K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347 CrossRef CAS PubMed; (c) K. Takimiya, I. Osaka and M. Nakano, Chem. Mater., 2014, 26, 587 CrossRef CAS; (d) V. Malytskyi, J.-J. Simon, L. Patrone and J.-M. Raimundo, RSC Adv., 2015, 5, 354 RSC; (e) S. C. Rasmussen, S. J. Evenson and C. B. McCausland, Chem. Commun., 2015, 51, 4528 RSC.
- M. D. Andrews, Sci. Synth., 2001, 10, 211 CAS.
- Selected recent reviews: (a) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (b) K. M. Engle, T.-S. Mei, M. Wasa and J.-Q. Yu, Acc. Chem. Res., 2012, 45, 788 CrossRef CAS PubMed; (c) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (d) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369 CrossRef CAS PubMed; (e) M. Miura, T. Satoh and K. Hirano, Bull. Chem. Soc. Jpn., 2014, 87, 751 CrossRef CAS; (f) F. Kakiuchi, T. Kochi and S. Murai, Synlett, 2014, 25, 2390 CrossRef CAS; (g) S. De Sarkar, W. Liu, S. I. Kozhushkov and L. Ackermann, Adv. Synth. Catal., 2014, 356, 1461 CrossRef CAS; (h) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (i) B. Su, Z.-C. Cao and Z.-J. Shi, Acc. Chem. Res., 2015, 48, 886 CrossRef CAS PubMed; (j) C. Cheng and J. F. Hartwig, Chem. Rev., 2015, 115, 8946 CrossRef CAS PubMed.
- (a) K. Inamoto, Y. Arai, K. Hiroya and T. Doi, Chem. Commun., 2008, 5529 RSC; (b) A. Acharya, S. V. Kumar and H. Ila, Chem.–Eur. J., 2015, 21, 17116 CrossRef CAS PubMed.
- An elegant catalytic synthesis of dibenzo[b,d]thiophene-1-carbaldehyde via C–H/C–H and C–H/S–H coupling cascade: R. Samanta and A. P. Antonchick, Angew. Chem., Int. Ed., 2011, 50, 5217 CrossRef CAS PubMed.
- (a) T. Wesch, A. Berthelot-Bréhier, F. R. Leroux and F. Colobert, Org. Lett., 2013, 15, 2490 CrossRef CAS PubMed; (b) T. Mori, T. Nishimura, T. Yamamoto, I. Doi, E. Miyazaki, I. Osaka and K. Takimiya, J. Am. Chem. Soc., 2013, 135, 13900 CrossRef CAS PubMed; (c) P. Saravanan and P. Anbarasan, Org. Lett., 2014, 16, 848 CrossRef CAS PubMed.
- (a) R. Che, Z. Wu, Z. Li, H. Xiang and X. Zhou, Chem.–Eur. J., 2014, 20, 7258 CrossRef CAS PubMed; (b) P. Oechsle and J. Paradies, Org. Lett., 2014, 16, 4086 CrossRef CAS PubMed; (c) K. Saito, P. K. Chikkade, M. Kanai and Y. Kuninobu, Chem.–Eur. J., 2015, 21, 8365 CrossRef CAS PubMed; (d) Q. Huang, S. Fu, S. Ke, H. Xiao, X. Zhang and S. Lin, Eur. J. Org. Chem., 2015, 6602 CrossRef CAS. For the application of C–H/C–H coupling to the substrates bearing a preinstalled thiophene ring, see: (e) H. Kaida, T. Satoh, K. Hirano and M. Miura, Chem. Lett., 2015, 44, 1125 CrossRef CAS.
- Recent reviews on C–S cleavage: (a) L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599 RSC; (b) S. G. Modha, V. P. Mehta and E. V. Van der Eycken, Chem. Soc. Rev., 2013, 42, 5042 RSC; (c) F. Pan and Z.-J. Shi, ACS Catal., 2014, 4, 280 CrossRef CAS.
- (a) H. Sirringhaus, R. H. Friend, C. Wang, J. Leuninger and K. Müllen, J. Mater. Chem., 1999, 9, 2095 RSC; (b) V. B. Pandya, M. R. Jain, B. V. Chaugule, J. S. Patel, B. M. Parmer, J. K. Joshi and P. R. Patel, Synth. Commun., 2012, 42, 497 CrossRef CAS.
- Stoichiometric cyclometallation reactions assisted by a thioether-based directing group: J. Dupont, N. Beydoun and M. Pfeffer, J. Chem. Soc., Dalton Trans., 1989, 1715 RSC.
- Catalytic C–H functionalization reactions using a thioether-based directing group: (a) M. Yu, Y. Xie, C. Xie and Y. Zhang, Org. Lett., 2012, 14, 2164 CrossRef CAS; (b) J. Yao, M. Yu and Y. Zhang, Adv. Synth. Catal., 2012, 354, 3205 CrossRef CAS; (c) X.-S. Zhang, Q.-L. Zhu, Y.-F. Zhang, Y.-B. Li and Z.-J. Shi, Chem.–Eur. J., 2013, 19, 11898 CrossRef CAS PubMed; (d) B. Xu, W. Liu and C. Kuang, Eur. J. Org. Chem., 2014, 2576 CrossRef CAS; (e) X.-S. Zhang, Y.-F. Zhang, K. Chen and Z.-J. Shi, Org. Chem. Front., 2014, 1, 1096 RSC; (f) Y. Unoh, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2015, 17, 704 CrossRef CAS PubMed; (g) B. Wang, C. Lin, Y. Liu, Z. Fan, Z. Liu and Y. Zhang, Org. Chem. Front., 2015, 2, 973 RSC; (h) P. Villuendas and E. P. Urriolabeitia, Org. Lett., 2015, 17, 3178 CrossRef CAS; (i) X.-S. Zhang, Y.-F. Zhang, Z.-W. Li, F.-X. Luo and Z.-J. Shi, Angew. Chem., Int. Ed., 2015, 54, 5478 CrossRef CAS PubMed.
- Effect of a carboxylic acid ligand on palladium-catalyzed C–H activation reactions: K. M. Engle, D.-H. Wang and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 14137 CrossRef CAS PubMed and references therein.
- The method for the synthesis of dibenzoselenophenes depends on the reaction between 2,2′-dilithiobiphenyl with metallic selenium. We were aware of two Lewis acid-mediated methods for their synthesis: (a) S. Trosien, P. Böttger and S. R. Waldvogel, Org. Lett., 2014, 16, 402 CrossRef CAS PubMed; (b) S. M. Rafiq, R. Sivasakthikumaran and A. K. Mohanakrishnan, Org. Lett., 2014, 16, 2720 CrossRef CAS PubMed.
- Catalytic cleavage of Ar–Se has not been reported to the best of our knowledge. Catalytic cleavage of vinylic C–Se bonds: (a) M. Toyofuku, S.-i. Fujiwara, T. Shin-ike, H. Kuniyasu and N. Kambe, J. Am. Chem. Soc., 2008, 130, 10504 CrossRef CAS PubMed; (b) S.-i. Fujiwara, M. Okuyama, S. Tsuda, T. Iwasaki, H. Kuniyasu and N. Kambe, Tetrahedron, 2012, 68, 10523 CrossRef CAS.
- (a) D. P. Bancroft, F. A. Cotton, L. R. Falvello and W. Schwotzer, Polyhedron, 1988, 7, 615 CrossRef CAS; (b) Y. Mizuta, Y. Obora, Y. Shimizu and Y. Ishii, ChemCatChem, 2012, 4, 187 CrossRef CAS.
- A related stoichiometric reaction: J. Spencer, M. Pfeffer, A. DeCian and J. Fischer, J. Org. Chem., 1995, 60, 1005 CrossRef CAS.
- A catalytic reaction in which oxidative addition of a C–S bond of sulfonium salts to palladium(0) is proposed to be involved: D. Vasu, H. Yorimitsu and A. Osuka, Angew. Chem., Int. Ed., 2015, 54, 7162 CrossRef CAS PubMed.
- See ESI† for details.
- A review on Pd(II)-catalyzed C–H functionalization reactions: X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for all new compounds. See DOI: 10.1039/c5sc04890g |
| This journal is © The Royal Society of Chemistry 2016 |