Open Access Article
Open Access ArticleA fascinating multifaceted redox-active chelating ligand: introducing the N,N′-dimethyl-3,3′-biquinoxalinium “methylbiquinoxen” platform†
Nicolas
Leblanc
 *a,
Stephen
Sproules
*a,
Stephen
Sproules
 b,
Karin
Fink
a,
Lionel
Sanguinet
c,
Olivier
Alévêque
c,
Eric
Levillain
b,
Karin
Fink
a,
Lionel
Sanguinet
c,
Olivier
Alévêque
c,
Eric
Levillain
 c,
Patrick
Rosa
c,
Patrick
Rosa
 d and
Annie K.
Powell
d and
Annie K.
Powell
 *ae
*ae
aInstitut für Nanotechnologie, Karlsruher Institut für Technologie, D-76344 Eggenstein-Leopoldshafen, Germany. E-mail: nicolas.leblanc@partner.kit.edu
bWestCHEM, School of Chemistry, University of Glasgow, Glasgow, G12 8QQ, UK
cUniversité d'Angers, CNRS UMR 6200, MOLTECH-Anjou, 2 bd Lavoisier, 49045 Angers Cedex, France
dInstitut de Chimie de la Matière Condensée de Bordeaux CNRS, UPR 9048, 87 Avenue du Dr Albert Schweitzer, 33600 Pessac, France
eInstitut für Anorganische Chemie, Karlsruher Institut für Technologie, Engesserstraβe 15, D-76131, Karlsruhe, Germany. E-mail: annie.powell@kit.edu
First published on 24th February 2016
Abstract
To intimately combine a chelating ligand function with the numerous properties of a viologen-like redox-active centre would offer a rare possibility to design controllable multi-redox states, whose properties arise from strongly correlated phenomena between the organic ligand as well as with any metalloid coordinated centres. Such a concept previously proved to be feasible, however is not widely applicable owing to challenges in terms of synthesis, isolation, and aerial sensitivity of both the ligand and its metal complexes. Here we report the first stable example of such a redox-active molecule, N,N′-dimethyl-3,3′-biquinoxalinium2+/˙+/0 “methylbiquinoxen, MBqn2+/˙+/0”, which shows a rich redox chemistry and chelates a metal ion in the case of the metal complex [CdCl2(MBqn0)]. This goes beyond what is possible to achieve using viologens, which are limited by not providing chelation as well as having no accessible biradicaloid state, corresponding to the neutral direduced MBqn0 open-shell behaviour we observe here.
Introduction
Organic molecules with multiple oxidation levels accessed by electro-, photo-, or chemical means are of extreme importance in many biological, chemical and physical processes.1–6 Among these, viologens, or N,N′-disubstitued-4,4′-bipyridinium (V2+) species, have attracted most attention over the last 80 years. Acting as strong electron-acceptors, viologens undergo two reversible one electron reduction steps leading to a radical cation intermediate (V˙+) and neutral form (V0).7,8The interest in viologen derivatives is motivated, amongst other things, by the ease of synthesis and tunability, the high degree of control concerning their reversible oxidation–reduction process, the range of colours from colourless (V2+) to dark green/blue/purple (V˙+), the paramagnetic nature of V˙+, and their strong structural templating effects (V2+).9 These possibilities have led to their incorporation in a range of donor–acceptor complexes developed for various applications such as photo- and electrochromic displays,10,11 charge accumulation,12,13 fluorescence,14,15 supramolecular host–guest complexes and molecular machines4,16 as well as ferroelectrics.17
Research on viologens is mainly focused on two aspects. The first of these is the diversification of their properties achieved through incorporation of additional functions. The second aims to improve their longevity, in particular through improving the chemical and air stability of the radical intermediates. In this context, Kaim's group have replaced some carbon atoms with nitrogen atoms within the aromatic heterocycles, leading to a new type of redox-active ligand that intimately combines a viologen-like centre with the chelating function of 2,2′-bipyridine.18–20 Despite an improved π-electron deficiency and an interesting coordination function that provides strong electronic coupling between the coordinated site (typically a metal ion) and the organic redox centre, synthetic challenges and persistent chemical and air instability, render these conceptually interesting molecules currently not useful for applications.
In the context of the potential for radical-based systems and the challenges to be addressed, we report here on the synthesis and characterization of a new stable reversible organic redox-active chelating ligand N,N′-dimethyl-3,3′-biquinoxalinium (MBqn2+, 12+, Fig. 1 – grey), “methylbiquinoxen”. In particular the dication 12+ and its monoprotonated direduced form (MBqn0-H+, 10-H+, Fig. 1 – purple) are thermally stable (12+ and 10-H+ decomposes at T ≈ 500 K and T ≈ 440 K respectively, Fig. S2 and S4†), and neither moisture nor air-sensitive in the solid state even without complexation to a metal ion. As a consequence, BF4− salts of these can be prepared and handled as simple crystalline starting pro-ligands. A seemingly facile and reversible chemical reduction at room temperature leads to a doublet radical intermediate which is stable in solution under inert atmosphere (Fig. 1 – blue).
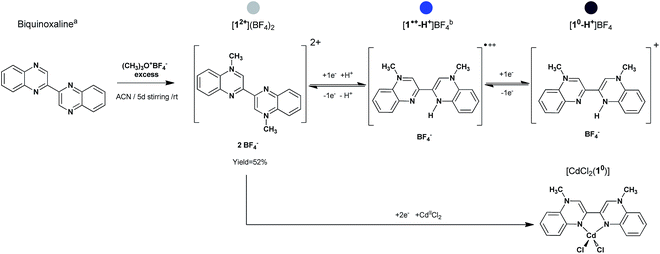 | ||
| Fig. 1 Path for the synthesis of [12+](BF4)2, [10-H+]BF4 and [CdCl2(10)]. a = from ref. 21; b = [1˙+-H+]BF4 not isolated in the solid state. The coloured stickers indicate the relative redox state of the methylbiquinoxen. Grey is for the dication, blue for the doublet radical, and purple for the direduced form. | ||
Using solvothermal conditions under inert atmosphere, the doubly reduced form MBqn0 (10) has been isolated in two crystalline forms, firstly as a cation in the monoprotonated species [10-H+]BF4 and secondly as a neutral biradicaloid chelating ligand in the metal complex [CdCl2(10)]. Noteworthy is that dissolution of [10-H+]BF4 (Fig. 1 – purple) in DMF under inert atmosphere generates the same doublet radical species in solution (Fig. 1 – blue), thus granting the methylbiquinoxen three different accessible redox states which are chemically reversible (vide infra).
Both 12+ and 10-H+ have been characterized by X-ray diffraction analysis (single crystal, powder), UV-Vis-NIR, IR and mass spectrometry, thermogravimetric analysis, cyclic voltammetry, spectroelectrochemistry measurements, as well as DFT and TDDFT (Time Dependent Density Functional Theory) calculation. Additional SQUID, NMR and EPR studies of 10-H+ in the solid state (SQUID) and dissolved in DMF (SQUID, NMR, EPR) support the presence of the doublet radical intermediate species in solution.
Results and discussion
Synthetic procedures
General procedure for synthesis of compounds [12+](BF4)2, [10-H+]BF4, and [CdCl2(10)] (Fig. 1). Compound [12+](BF4)2 was obtained from Meerwein alkylation by mixing biquinoxaline21 with an excess of trimethyloxonium tetrafluoroborate in acetonitrile. After 5 d stirring at room temperature, the resulting solid was isolated by filtration, washed with Et2O, and recrystallized from water to give pale yellow/off-white crystals in 52% yield. Crystals of the direduced monoprotonated species [10-H+]BF4 as well as of the metal complex [CdCl2(10)] were obtained from solvothermal reaction using the solvent (here methanol) as the reducing agent. In a Schlenk tube, equipped with a hermetic Teflon stopper, [12+](BF4)2 were mixed with a mixture of MeOH/DMF. The Schlenk was purged with nitrogen and inserted in a solvothermal oven to follow a heat/flat/cool temperature programme, resulting in a pure phase of dark purple crystals of [10-H+]BF4 in 68% yield. Phase purity of [12+](BF4)2 and [10-H+]BF4 was checked by XRPD (Fig. S1 and S3†). For the synthesis of the cadmium complex the same precedent procedure was followed by adding a stoichiometric amount of CdCl2 in the Schlenk tube. A violet crystal of [CdCl2(10)] was selected from a mixture of phases which always result from the reaction. In spite of many attempts it was only possible to generate the intermediate doublet radical species in solution, but not to isolate it as a crystalline salt.Crystallography
Single crystals suitable for X-ray diffraction analysis were isolated for the dication [12+](BF4)2 (Fig. 1 – grey) for the direduced monoprotonated form [10-H+]BF4 (Fig. 1 – purple) as well as for the metal complex [CdCl2(10)]. A summary of the crystallographic data and refinement results are listed Table 1. Datasets for both structures were collected at 180 K. They were refined in the space groups P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) for [12+](BF4)2 and [CdCl2(10)], and P21/n for [10-H+]BF4. All atoms including hydrogens were identified from Fourier difference maps. All atoms occupy general positions.
for [12+](BF4)2 and [CdCl2(10)], and P21/n for [10-H+]BF4. All atoms including hydrogens were identified from Fourier difference maps. All atoms occupy general positions.
| [12+](BF4)2 | [10-H+]BF4 | [CdCl2(10)] | |
|---|---|---|---|
| Fw (g mol−1) | 461.97 | 376.16 | 471.85 |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P21/n |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a, Å | 7.5470(8) | 7.1330(9) | 7.4683(5) |
| b, Å | 7.8860(7) | 20.0360(16) | 10.4739(7) |
| c, Å | 8.9920(9) | 11.5260(13) | 11.3249(8) |
| α, ° | 66.005(7) | 90 | 80.150(6) |
| β, ° | 85.305(8) | 99.454(9) | 82.274(5) |
| γ, ° | 83.508(8) | 90 | 81.982(6) |
| V, Å3 | 485.40(8) | 1624.9(3) | 858.70(10) |
| Z | 1 | 4 | 2 |
| T (K) | 180 | 180 | 180 |
| Obs. reflns (I > 2σ(I)) [Rint] | 1527 [0.021] | 2273 [0.042] | 3386 [0.028] |
| No. of parameters | 177 | 313 | 291 |
| R1(I > 2σ(I))/wR2 (all data) | 0.044/0.125 | 0.042/0.126 | 0.027/0.067 |
| CCDC number | 1043174 | 1043175 | 1437399 |
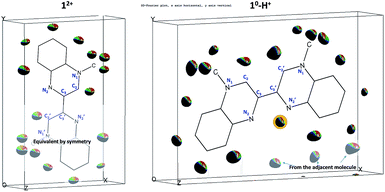
The crystallographic asymmetric units of the structures of [12+](BF4)2 and [10-H+]BF4 are shown in Fig. 2. The asymmetric unit of [12+](BF4)2 contains one independent half [MBqn2+] (12+) (N-methylquinoxalinium MQ+ type) and one independent ordered BF4−. On the other hand, the asymmetric unit of [10-H+]BF4 contains one independent [MBqn0-H+] (10-H+), as well as one independent ordered BF4− per compound.
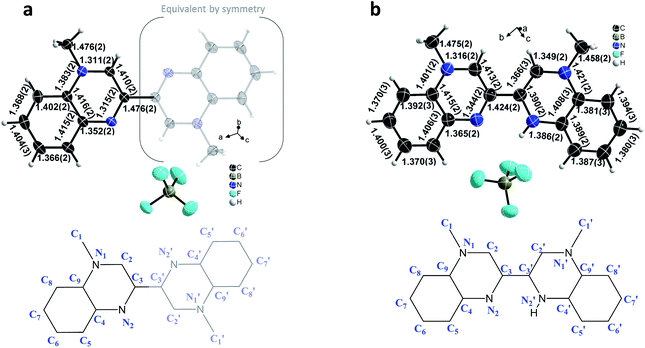 | ||
| Fig. 2 Crystallographic structures of 12+/10-H+: view of the asymmetric unit of [MBqn2+](BF4)2, [12+](BF4)2 (a) and [MBqn0-H+]BF4, [10-H+]BF4 (b). Salient C–N and C–C bond lengths are indicated. | ||
In the case of 10-H+, after several attempts with different crystals and from different batches, the refinement is such that equal and significant residual electron density peaks (0.4–0.6 e Å−3; background 0.18 e Å−3) are clearly and systematically located on all aromatic and methyl hydrogen sites, as for 12+, but with an additional peak close to the nitrogen on 2′ position (Fig. 3, full explanation in the ESI Tables S1–S3†).
Thus for 10-H+, because 17 H-atoms are clearly present in the molecule, the latter has to be doubly reduced in order to match the singly positive charge balanced by one BF4−. This can be also viewed as the monoprotonated dication (leading to a 16 + 1 = 17H atoms trication) which was further reduced by two electrons to give the so-called direduced monoprotonated cation 10-H+.
Complementary FAB and MALDI-TOF mass spectrometry measurement performed on crystals of [10-H+]BF4 are reproducible and in agreement with a single species, differing only by one H-atom either in solution (FAB) or in the solid state (MALDI) (Fig. S5 and S6†). In particular the MALDI spectra in SM matrix shows a clear difference in peak intensity between the 16 H-atoms and 17 H-atoms (m/z = 288.3 and 289.3) which can be assigned as originating from the molecular entities 12+ and 10-H+.
As a result of the rotational freedom around the central C3–C′3 bond, 12+ and 10-H+ could adopt any geometry between planar trans- (ϕ = 180°) and planar cis- (ϕ = 0°), where the dihedral angle is defined as ϕ = C2–C3–C′3–C′2. What we observe is that for 12+ the geometry is exactly planar trans, whereas for 10-H+ it is nearly planar cis (ϕ = 2.5°).
In the dication 12+, constituted of two equivalent MQ+ moieties, the structure reveals an aromatic π-conjugated system. The average C–C bond length in the benzene ring [1.392(3) Å] corresponds to the intermediate C–C distance of 1.39–1.40 Å observed in a benzene molecule with electron delocalization.22 In the pyrazinium moiety, the aromaticity is confirmed by the values of the C–C and C–N bond distances, in the range 1.410(2)-1.416(2) Å and 1.311(2)–1.383(2) Å respectively, that are comparable to those found in the literature.23 The pyrazinium structure is particularly clear on the 1,2,3,4 atomic positions, as shown by the two shortest distances N1–C2 [1.311(2) Å] and N2–C3 [1.315(2) Å], alternating with an intermediate length for C2–C3 [1.410(2) Å] (Fig. 2).
In contrast, in the direduced monoprotonated cation 10-H+ the different redox state of the species and the protonation of only one side of the molecule gives rises to an asymmetric environment. On one hand, the non-protonated MQ moiety remains essentially similar to 12+, where the bond lengths of both benzene ring and pyrazinium core are comparable (N1–C2 [1.316(2) Å], N2–C3 [1.344(2) Å], C2–C3 [1.413(2) Å]). On the other hand, in the protonated half molecule, although the benzene ring remains aromatic (C–C ∼ 1.39 Å), significant changes occur mostly within the pyrazinium core. The structure is such that the N′1–C′2, N′1–C′9 and N′2–C′3 bond lengths increase by 0.038 Å, 0.038 Å, and 0.075 Å respectively with a concomitant 0.044 Å decrease in the C′2–C′3 bond distance. The central C3–C′3 bond also changes dramatically with a net decrease by 0.052 Å, with 1.424(2) Å for 10-H+ and 1.476(2) Å for 12+.
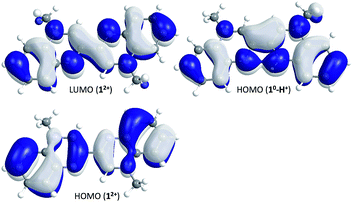
These structural features of 12+ and 10-H+ have been investigated by quantum chemical calculations using Turbomole24 (for details see Experimental section in ESI†). For both compounds geometrical parameters were fully optimized in the gas phase at the PBE0/def2-TZVPP level of theory (Tables S4, S5 and S8†), the PBE0 hybrid functional being known to provide the best estimates for such π-conjugated systems.25,26 The calculated bond lengths fit well with the experimental data with mean absolute errors (MAE) of 0.0068 Å (12+) and 0.0071 Å (10-H+), whereas the twist angle of the molecule differs only very slightly for 10-H+ (ϕexp = 2.5°, ϕcalc = 0°). For 12+ and 10-H+ the frequencies calculations, in addition with a scan of the relaxed potential energy surface along the twist angle (ϕ) for 12+, confirmed that the described structures correspond to the ground state of the molecules (Fig. S9 and S13, Tables S6 and S9†). The HOMO and LUMO of 12+, as well as the HOMO of 10-H+ are represented in Fig. 4.
As previously stated by A. di Matteo, “a reduction process increasing or decreasing the electron population, in the bonding or antibonding regions, strengthens or weakens the bonds, which become significantly shorter or longer”.25 Here, by populating the LUMO of 12+ (reduction process), a decrease and increase of the bond length is expected between atoms with bonding and antibonding characteristics respectively. This effect will be even more obvious between atoms where the HOMO and LUMO orbitals exhibit the opposite bonding and antibonding features. Thus focusing on the bipyrazinium core, the experimental parameters clearly show that the chemical reduction of 12+ will lead to a shortening of the C2–C3 (C′2–C′3) and C3–C′3 bond lengths and to an increase of the N1–C2 (N′1–C′2), N1–C9 (N′1–C′9) and N2–C3 (N′2–C′3) bond lengths, both having respectively bonding and antibonding characteristic in the LUMO orbital.
The HOMO of 10-H+ and the LUMO of 12+ both exhibit comparable bonds with bonding and antibonding characteristics, which is in line with the above reasoning and the observed bond lengths in 10-H+. This also shows that the trans–cis isomerization which exists between 12+ (trans) and 10-H+ (cis) has no strong influence on the way the π-orbitals overlap.
However, the N′2–H situation in 10-H+ creates a dissymmetry within the molecule leading to a strengthening of the bonding and antibonding features in the pyrazinium core of the protonated MQ. This results in a partial stiffening of the system, in the sense that the electron density can be rather described as localized and alternate single and double bonds, like in polyene-like structure such as dipyran or dithiopyran.27,28 The N′2–H situation also stabilizes the preferential cis-form in the solid. It creates a strong intramolecular hydrogen bond between the protonated and non-protonated amines (N2⋯H–N′2 ∼ 2.25 Å), which is also confirmed by the strong bonding characteristic in the N2⋯H–N′2 region (HOMO of 10-H+Fig. 4, detail of H-bonds Fig. S7†). This apparently stabilizes 10-H+ and might also act as a template by constraining the cis-form, i.e. the chelate function of the 2,2′-bipyridine.
Spectroscopy, electrochemistry, magnetic susceptibility, spectroelectrochemistry and TDDFT
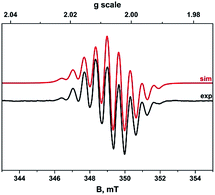
Because of the poor solubility of [10-H+]BF4 and in order to get comparative data, both EPR, UV-Vis-NIR, cyclic voltammetry, spectro-electrochemistry, NMR and SQUID experiments have been performed in DMF. Nevertheless, a complementary UV-Vis experiment has been performed in MeCN for [12+](BF4)2 in order to fully identify its electronic transitions below 400 nm.The absence of additional hyperfine structure from coupling to the methyl and aromatic protons of the organic radical confirms these couplings are less than the isotopic linewidth ∼2 × 10−4 cm−1, which is invariant irrespective of the measurement temperature. Nevertheless this does not show conclusively whether the paramagnetic species is an impurity or not.
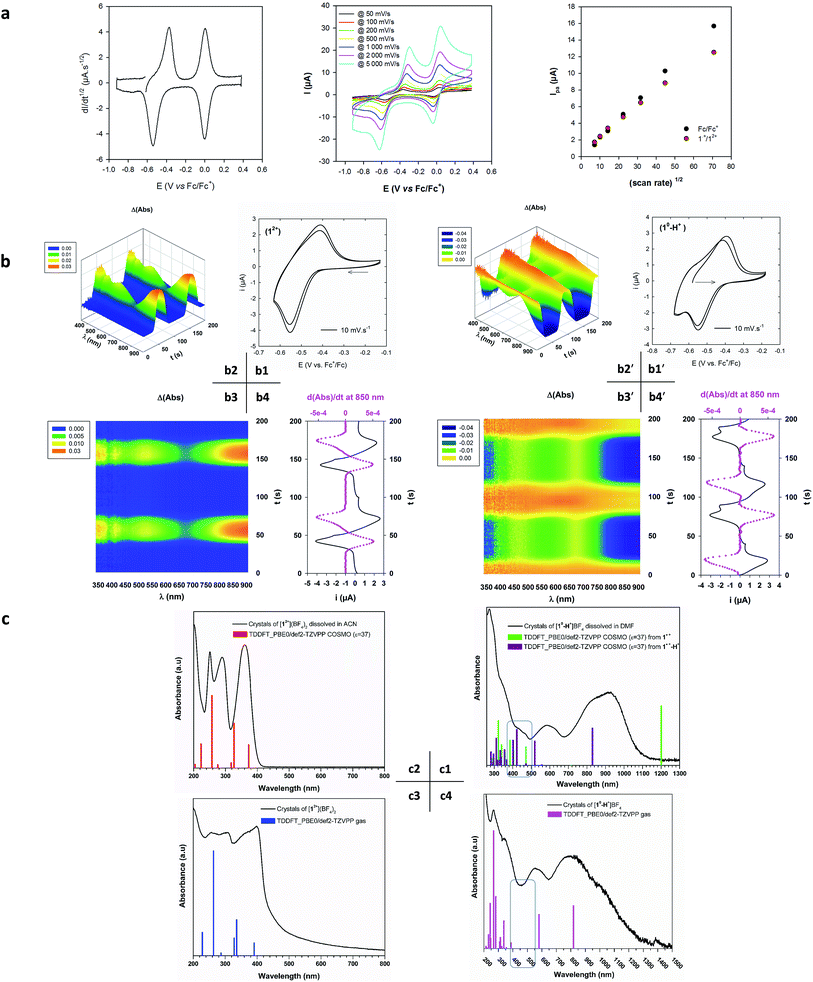
The deconvolution of the CV allows us to circumvent diffusion problems and to conclude that there is a single-electronic process by comparing with the current peak of the ferrocene/ferrocenium system. This rules out the presence of any impurity and points to the oxidation of the doubly reduced monoprotonated cation [10-H+]BF4 to give the doublet radical. Furthermore, the voltage separation between current peaks of ca. 200 mV is significantly higher than the theoretical value of 59 mV. This is in line with a redox process inducing a significant structural rearrangement such as a trans–cis isomerization.37
The magnetic susceptibility of [10-H+]BF4 (Fig. 1 – purple) was measured on a polycrystalline sample over the 4–380 K temperature range under an applied dc field of 1 T. As shown in Fig. S10,† in the solid state the product of magnetic susceptibility with temperature is very close to zero (<0.007 cm3 K mol−1), which confirms the diamagnetic nature of [10-H+]BF4 in the solid state.
Furthermore, crystals of [10-H+]BF4 dissolved in DMF-d7 under inert atmosphere exhibit a very strong diamagnetism at −5000 × 10−6 cm3 mol−1, even after correcting with the expected diamagnetic contribution calculated from Pascal constants. Given that we were expecting to see a paramagnetic signature, which would be expected for isolated paramagnetic species, this behaviour suggests the presence of superdiamagnetism. This has been previously observed in 3D inorganic compounds and was coined superdiamagnetism, in an obvious reference to the Meissner effect and the perfect diamagnetism observed in superconductors.38,39 More recently superdiamagnetism was induced at low temperatures by the application of a magnetic field in a one-dimensional PtII complex.40 To the best of our knowledge this has never been observed in a purely organic based compound. In our case the diamagnetic susceptibility reaches 6% of the 1/4π value expected for complete diamagnetism. This unexpected superdiamagnetism was confirmed using the Evans' 1H-NMR method41 at room temperature from the strong negative chemical shift of the reference tert-butyl alcohol signal at −27.3 Hz in DMSO-d6 and −25.5 Hz in DMF-d7 in the presence of our studied molecule (Fig. S11 and S12†). This is typical of an additional diamagnetic environment around the protons of the reference. The calculated magnetic susceptibilities, respectively χ = −962 × 10−6 cm3 mol−1 and χ = −3510 × 10−6 cm3 mol−1 are in line with the SQUID measurement, though the effect seems much weaker in DMSO.
This allows us to conclude from the CV, EPR, SQUID and NMR results that a single radical intermediate species is present in solution. Due to the perfect planarity of the molecule (no steric hindrance) and the fact that the spin density is located in π-orbitals, the apparent superdiamagnetic behaviour of such a radical in solution could be tentatively explained from the formation of extended stacks of radicals, in analogy to the unidimensional PtII complex.40 Strongly spin-paired π-dimers, as already observed in other π-radicals such as viologen and TTF,34,42–44 would only provide a “normal” diamagnetic behaviour.
However the question remains: what is the nature of the radical species in solution? In their studies on the prototype N,N′-dimethyl-3,3′-bipyrazinium dication,20 and the N-methylpyrazinium,45,46N-methylquinoxalinium47 and N-methylphenazinium48 cations, Kaim et al., Rouiller & Laviron, Eaton and Takagi et al. showed that under mild acidic or neutral conditions the chemical reduction of such kinds of cations is accompanied by protonation thus leading to 4-hydro-1-methyl-diazinium 7π electron radical cations in solution. We thus see two possibilities here. Firstly, the dication (12+) is reduced and isomerises through the addition of an electron to give the cis-radical cation (1˙+). Secondly, this cis-radical cation is formed and further protonated to give the cis-protonated radical cation (1˙+-H+).
In order to investigate this, additional spectroelectrochemical (SE), UV-Vis-NIR and TDDFT experiments were performed. The purpose was to identify a possible structure/optical signature relationship for the three different redox states. Optical spectra have been computed using TDDFT at the PBE0/def2-TZVPP level of theory in a closed shell (12+ and 10-H+) and unrestricted open-shell (1˙+ and 1˙+-H+) configuration. To reproduce the behaviour of the molecules in solution, the conductor-like screening model COSMO has been applied, with ε = 37 as a mean value for a mixture of DMF and MeCN.
In Fig. 6(b and b′), we see that both voltabsorptograms (Δ(Abs) vs. initial species) are perfect mirror images of each other, indicating that the two species are part of the same redox couple. Furthermore, the spectra measured during the SE experiments are quite similar to those obtained by dissolving crystals of [12+](BF4)2 and [10-H+]BF4 (Fig. 6c2 and 6c1). The results match perfectly those from the CV in terms of the exclusive presence of one reversible and stable 12+/1radical redox couple in solution.
The TDDFT spectrum corresponding to hypothesis (a) cis-1˙+ is shown with green lines in Fig. 6c1. This is clearly different from the experimental spectrum in that it exhibits a single broad transition at λmax = 1200 nm and no transitions between 500 and 1100 nm.
The TDDFT spectrum corresponding to hypothesis (b) cis-1˙+-H+ is shown with violet lines in Fig. 6c1. The main electronic transitions observed experimentally in the range 400 to 850 nm in the SE spectrum are perfectly reproduced theoretically. The lowest energy band λmax = 820 nm corresponds to the αSOMO → αLUMO transition (Fig. 6c1, Table S13†).
This leads us to conclude that the radical intermediate species present in solution is intrinsically composed of the cis-protonated radical dication 1˙+-H+ (Fig. 1 – blue). Furthermore the formation of extended stacks of radicals 1˙+-H+ in solution is substantiated by the presence of an additional band at lower energy (λ = 920 nm, Fig. 6c1), analogous to the low energy bands observed in aggregated viologens in solution.7,8
We also note that although at a first glance the spectra of 1˙+-H+ (Fig. 6c1) and 10-H+ (Fig. 6c4) look broadly similar, the fact that the spectrum of 10-H+ exhibits no transitions between 400 and 500 nm (Table S10†), provides a rapid fingerprint for the identity of the redox state.
Case of the metal complex [CdCl2(10)]: chelation and non-Kekulé structure aspect of 10
We provide a first example of a metal complex [CdCl2(10)] which illustrates the ability of such a ligand to chelate metal ions (Fig. 7). This proves that the neutral direduced species 10 can be obtained without being protonated unlike in the case of 10-H+, which reinforces the suggestion that the additional proton on N′2 is labile in solution. In addition, the crystals obtained are air stable, which is important for the further development of robust compounds.The fact that we were unable to isolate a pure phase of the cadmium complex precluded further characterization. Nevertheless, the reliable crystallographic dataset obtained for this compound has been used as a reference structure for further studies, especially regarding the investigation of the neutral direduced methylbiquinoxen 10 and its electronic structure.
The asymmetric unit is made of one entire complex [CdCl2(10)]. The cadmium adopts a distorted tetrahedral coordination environment of D2d symmetry.
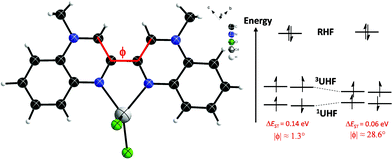
The 10 chelating ligand is cis-planar (ϕ = 1.3°) and very symmetric in terms of the relative bond distances (Table S15†). As for 10-H+, the multiple bond character of the central C3–C′3 bond (d = 1.449 Å) indicates a fully π-conjugated molecule, resulting in a homogenous distribution of the electron density.
Unrestricted DFT calculations for the singlet and triplet state of [CdCl2(10)] have been performed. The energy of the singlet state is slightly lower than the energy of the triplet state. The energy difference (ΔEST) amounts to 0.14 eV (Table S14†). However, the ground state shows a significant spin contamination. The analysis of the natural orbitals shows that there is a pair of partly occupied orbitals, one with bonding character between C3 and C′3, the other antibonding with occupation numbers of 1.5 and 0.5, respectively, showing partial occupation of the LUMO (Fig. S14†). In addition, the spin density (Fig. S15†) indicates a weakening of the bonding character similar to what is seen in a broken symmetry state. This behaviour is in line with the biradicaloids observed, for example, for under coordinated Sn atoms.52
Regarding 10 in terms of a symmetric and non-protonated structure, not to be confused with the dissymmetric closed-shell monoprotonated cation 10-H+, such an open-shell behaviour with a small ΔEST gap was predictable due to the non-classical (non-Kekulé)18,20,53,54 electronic structure of 10, as previously identified by Matheis, et al.18,20 in the prototype molecule, 1,1′-dimethyl-3,3′-bipyrazinium (Fig. S16†). By definition, non-Kekulé means a molecule which is “fully conjugated, but each of whose Kekulé structures contains at least two atoms that are not π-bonded”.55,56 Furthermore, the ΔEST gap can apparently be significantly reduced by simply twisting the molecule (ΔEST = f(ϕ) Fig. 7), thus giving a means to thermally access the triplet state.
Conclusions and Outlook
As is already known for viologens, the reversible and controllable structural, electronic, and electrostatic changes between the dication and radical species formed in solution invest the MBqn2+/˙+ redox couple with electron mediation properties. This should be useful for many radical-assisted reactions (photocatalytic H2 production, C–C bond cleavage, artificial photosynthesis, electrolyte, electrochromism etc.).7,57,58 Surprisingly, chelation to a cadmium centre allowed us to access the direduced solid state biradicaloid species MBqn0 as ligand. Additionally, as expected for such a biradicaloid species, the calculated small singlet to triplet energy gap opens perspectives for a range of exotic electronic, optical, magnetic, dielectric etc. properties within a single system composed of purely organic moieties or incorporating metalloid centres. Intriguingly, the superdiamagnetic behaviour observed for the doublet radical species in solution is such an unexpected property for an organic molecule that this demands further investigation.Acknowledgements
The authors thank the Alexander von Humboldt Foundation (fellowship to N. L) and Helmholtz POF “STN” for financial support. We also thank Christopher Anson and Magali Allain for helpful discussions regarding the crystal structure of the direduced monoprotonated species, Ingrid Freuze for the mass spectrometry measurements, Sven Stahl for the TGA measurements and Lutz Greb for the NMR measurement.Notes and references
- W. Kaim and B. Schwederski, Coord. Chem. Rev., 2010, 254, 1580–1588 CrossRef CAS.
- P. J. Chirik and K. Wieghardt, Science, 2010, 327, 794–795 CrossRef CAS PubMed.
- A. Heckmann and C. Lambert, Angew. Chem., Int. Ed., 2012, 51, 326–392 CrossRef CAS PubMed.
- A. C. Fahrenbach, C. J. Bruns, H. Li, A. Trabolsi, A. Coskun and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 482–493 CrossRef CAS PubMed.
- O. R. Luca and R. H. Crabtree, Chem. Soc. Rev., 2013, 42, 1440–1459 RSC.
- N. Martin, Chem. Commun., 2013, 49, 7025–7027 RSC.
- P. M. S. Monk, The Viologens: Physicochemical Properties, Synthesis, and Application of the Salts of 4,4′-Bipyridine, Wiley, 1998 Search PubMed.
- T. M. Bockman and J. K. Kochi, J. Org. Chem., 1990, 55, 4127–4135 CrossRef CAS.
- N. Mercier, Eur. J. Inorg. Chem., 2013, 19–31 CrossRef.
- R. J. Mortimer, Electrochim. Acta, 1999, 44, 2971–2981 CrossRef CAS.
- M.-S. Wang, G. Xu, Z.-J. Zhang and G.-C. Guo, Chem. Commun., 2010, 46, 361–376 RSC.
- N. Leblanc, M. Allain, N. Mercier and L. Sanguinet, Cryst. Growth Des., 2011, 11, 2064–2069 CAS.
- A. S. Abouelwafa, V. Mereacre, T. S. Balaban, C. E. Anson and A. K. Powell, CrystEngComm, 2010, 12, 94–99 RSC.
- A. S. Abouelwafa, C. E. Anson, A. Hauser, H. H. Patterson, F. Baril-Robert, X. Li and A. K. Powell, Inorg. Chem., 2012, 51, 1294–1301 CrossRef CAS PubMed.
- M. Freitag, L. Gundlach, P. Piotrowiak and E. Galoppini, J. Am. Chem. Soc., 2012, 134, 3358–3366 CrossRef CAS PubMed.
- A. Trabolsi, N. Khashab, A. C. Fahrenbach, D. C. Friedman, M. T. Colvin, K. K. Cotí, D. Benítez, E. Tkatchouk, J.-C. Olsen, M. E. Belowich, R. Carmielli, H. A. Khatib, W. A. Goddard, M. R. Wasielewski and J. F. Stoddart, Nat. Chem., 2010, 2, 42–49 CrossRef CAS PubMed.
- N. Leblanc, N. Mercier, L. Zorina, S. Simonov, P. Auban-Senzier and C. Pasquier, J. Am. Chem. Soc., 2011, 133, 14924–14927 CrossRef CAS PubMed.
- W. Kaim and W. Matheis, Chem. Ber., 1990, 123, 1323–1325 CrossRef CAS.
- W. Matheis and W. Kaim, J. Chem. Soc., Faraday Trans., 1990, 86, 3337–3339 RSC.
- W. Matheis, J. Poppe, W. Kaim and S. Zalis, J. Chem. Soc., Perkin Trans. 2, 1994, 1923–1928 RSC.
- O. N. Chupakhin, E. O. Sidorov, S. M. Shein and I. I. Bil'kis, Zh. Org. Khim., 1976, 12, 2464–2468 CAS.
- E. G. Cox, D. W. J. Cruickshank and J. a. S. Smith, Nature, 1955, 175, 766 CrossRef CAS.
- F. H. Allen, O. Kennard, D. G. Watson, L. Brammer, A. G. Orpen and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- TURBOMOLE V6.6 2014, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, since 2007; available from http://www.turbomole.com.
- A. Di Matteo, Chem. Phys. Lett., 2007, 439, 190–198 CrossRef CAS.
- D. Jacquemin, E. A. Perpète, G. E. Scuseria, I. Ciofini and C. Adamo, J. Chem. Theory Comput., 2008, 4, 123–135 CrossRef CAS PubMed.
- D. Chasseau, J. Gaultier, C. Hauw, R. Fugnitto, V. Gianis and H. Strzelecka, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 1629–1631 CrossRef.
- H. R. Luss and D. L. Smith, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 1580–1588 CrossRef.
- A. Schulz, W. Kaim and H.-D. Hausen, J. Chem. Soc., Faraday Trans. 1, 1988, 84, 3207–3214 RSC.
- O. Back, B. Donnadieu, P. Parameswaran, G. Frenking and G. Bertrand, Nat. Chem., 2010, 2, 369–373 CrossRef CAS PubMed.
- X. Pan, X. Chen, T. Li, Y. Li and X. Wang, J. Am. Chem. Soc., 2013, 135, 3414–3417 CrossRef CAS PubMed.
- X. Pan, Y. Su, X. Chen, Y. Zhao, Y. Li, J. Zuo and X. Wang, J. Am. Chem. Soc., 2013, 135, 5561–5564 CrossRef CAS PubMed.
- N. Leblanc, N. Mercier, O. Toma, A. H. Kassiba, L. Zorina, P. Auban-Senzier and C. Pasquier, Chem. Commun., 2013, 49, 10272–10274 RSC.
- A. G. Evans, J. C. Evans and M. W. Baker, J. Am. Chem. Soc., 1977, 99, 5882–5884 CrossRef CAS.
- D. W. Clack, J. C. Evans, A. Y. Obaid and C. C. Rowlands, Tetrahedron, 1983, 39, 3615–3620 CrossRef CAS.
- T. Fujihara, T. Wada and K. Tanaka, Inorg. Chim. Acta, 2004, 357, 1205–1212 CrossRef CAS.
- N. Terkia-Derdra, R. Andreu, M. Sallé, E. Levillain, J. Orduna, J. Garín, E. Ortí, R. Viruela, R. Pou-Amérigo, B. Sahraoui, A. Gorgues, J.-F. Favard and A. Riou, Chem.–Eur. J., 2000, 6, 1199–1213 CAS.
- V. L. Ginzburg, Solid State Commun., 1981, 39, 991–992 CrossRef CAS.
- V. L. Ginzburg, A. A. Gorbatsevich, Y. V. Kopayev and B. A. Volkov, Solid State Commun., 1984, 50, 339–343 CrossRef CAS.
- M. Haruki and P. Wachter, J. Magn. Magn. Mater., 1992, 104–107, 475–476 CrossRef CAS.
- D. F. Evans, J. Chem. Soc., 1959, 2003–2005 RSC.
- W. W. Porter and T. P. Vaid, J. Org. Chem., 2005, 70, 5028–5035 CrossRef CAS PubMed.
- J. M. Spruell, A. Coskun, D. C. Friedman, R. S. Forgan, A. A. Sarjeant, A. Trabolsi, A. C. Fahrenbach, G. Barin, W. F. Paxton, S. K. Dey, M. A. Olson, D. Benítez, E. Tkatchouk, M. T. Colvin, R. Carmielli, S. T. Caldwell, G. M. Rosair, S. G. Hewage, F. Duclairoir, J. L. Seymour, A. M. Z. Slawin, W. A. Goddard, M. R. Wasielewski, G. Cooke and J. F. Stoddart, Nat. Chem., 2010, 2, 870–879 CrossRef CAS PubMed.
- G.-P. Yong, C.-F. Li, Y.-Z. Li and S.-W. Luo, Chem. Commun., 2010, 46, 3194–3196 RSC.
- L. Roullier and E. Laviron, Electrochim. Acta, 1980, 25, 795–804 CrossRef CAS.
- D. R. Eaton, J. M. Watkins and R. J. Buist, J. Am. Chem. Soc., 1985, 107, 5604–5609 CrossRef CAS.
- W. Kaim, Rev. Chem. Intermed., 1987, 8, 247–286 CrossRef CAS.
- T. Yutaka, A. Kazuyuki, I. Toshiaki, K. Hitomi and I. Kazuhiko, Chem. Lett., 1972, 1, 847–848 Search PubMed.
- F. Gaillard and E. Levillain, J. Electroanal. Chem., 1995, 398, 77–87 CrossRef.
- M. Dias, P. Hudhomme, E. Levillain, L. Perrin, Y. Sahin, F.-X. Sauvage and C. Wartelle, Electrochem. Commun., 2004, 6, 325–330 CrossRef CAS.
- O. Alévêque, E. Levillain and L. Sanguinet, Electrochem. Commun., 2015, 51, 108–112 CrossRef.
- C. Schrenk, A. Kubas, K. Fink and A. Schnepf, Angew. Chem., Int. Ed., 2011, 50, 7273–7277 CrossRef CAS PubMed.
- S. Inagaki, Orbitals in Chemistry, Springer, 2009 Search PubMed.
- W. T. Borden, H. Iwamura and J. A. Berson, Acc. Chem. Res., 1994, 27, 109–116 CrossRef CAS.
- H. C. Longuet-Higgins, J. Chem. Phys., 1950, 18, 265–274 CrossRef CAS.
- V. I. Minkin, Pure Appl. Chem., 1999, 71, 1919–1981 CrossRef CAS.
- M. D. Forbes, Carbon-Centered Free Radicals and Radical Cations: Structure, Reactivity, and Dynamics, Wiley, 2010 Search PubMed.
- P. J. Cappillino, H. D. Pratt, N. S. Hudak, N. C. Tomson, T. M. Anderson and M. R. Anstey, Adv. Energy Mater., 2014, 4, 1300566 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1043174, 1043175 and 1437399. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5sc04904k |
| This journal is © The Royal Society of Chemistry 2016 |