Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective imaging and cancer cell death via pH switchable near-infrared fluorescence and photothermal effects†
Jingye
Zhang
a,
Zining
Liu
a,
Peng
Lian
a,
Jun
Qian
a,
Xinwei
Li
a,
Lu
Wang
c,
Wei
Fu
a,
Liang
Chen
d,
Xunbin
Wei
*b and
Cong
Li
*a
aKey Laboratory of Smart Drug Delivery, Ministry of Education, School of Pharmacy, Fudan University, Shanghai 201203, China. E-mail: congli@fudan.edu.cn
bState Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, School of Biomedical Engineering, Shanghai Jiao Tong University, 1954 Huashan Road, Shanghai, 200030, China. E-mail: xwei01@sjtu.edu.cn
cDepartment of Chemistry, National University of Singapore, Singapore 117543, Singapore
dDepartment of Neurosurgery, Huashan Hospital, Fudan University, Shanghai 200040, China
First published on 26th May 2016
Abstract
Accurately locating and eradicating sporadically distributed cancer cells whilst minimizing damage to adjacent normal tissues is vital in image-guided tumor ablation. In this work, we developed four heptamethine cyanine based theranostic probes, IR1–4, that demonstrated unique pH switchable near-infrared (NIR) fluorescence and photothermal efficiency. While their fluorescence quantum yields increased up to 1020-fold upon acidification from pH 7.4 to 2.4, their photothermal efficiencies decreased up to 7.1-fold concomitantly. Theoretical calculations showed that protonation of the probes in an acidic environment increased the orbital energy gaps and reduced the intramolecular charge transfer efficiency, resulting in the conversion of absorbed light energy to NIR fluorescence instead of hyperthermia. Substitutions at the terminal indole of the probes fine-tuned their pKafluo values to a narrow physiological pH range of 4.0–5.3. IR2, with a pKafluo of 4.6, not only specifically illuminated cancer cells by sensing their more acidic lysosomal lumen, but also selectively ablated cancer cells via its maximized photothermal effects in the alkaline mitochondrial matrix. As far as we are aware, these probes not only offer the highest physiological acidity triggered NIR fluorescence enhancement as small molecules, but are also the first to specifically visualize and eradicate cancer cells by sensing their altered pH values in cellular organelles. Considering that a disordered pH in organelle lumen is a common characteristic of cancer cells, these theranostic probes hold the promise to be applied in image-guided tumor ablation over a wide range of tumor subtypes.
1. Introduction
Cytoreductive surgery is the primary treatment for most solid tumors. Due to the invasive growth of cancer cells, however, clinicians encounter great challenges in identifying and completely removing the sporadically distributed tumor tissues. Residual neoplastic tissues post-surgical treatment usually lead to tumor recurrence and result in a poor prognosis. Intra-operative imaging shows great potential in guiding tumor resection by distinguishing neoplastic tissues with an improved target to background (T/B) ratio.1,2 Removing specific tumor foci amidst unresectable tissues such as major arteries, non-renewable nerves and intricate areas of the brain, however, is extremely difficult using conventional surgical instruments. The rapid development of image-guided tumor ablation3 has made it possible to precisely locate and eliminate tumor foci simultaneously with minimized damage to adjacent normal tissues. Theranostic probes for accurately locating and removing tumor foci intra-operatively are therefore invaluable for improving the surgical prognosis.Of the multiple imaging modalities used for image-guided therapy, optical imaging shows advantages including high sensitivity, rapid acquisition rate, convenience in manipulation and affordable running costs. Due to the minimized absorption and autofluorescence from endogenous molecules in the near-infrared (NIR) wavelength window (650–900 nm), NIR fluorescence can detect tumor foci at millimeter depth with low photocytotoxicity.4 Additionally, the absorbed NIR photons can lead to local hyperthermia and kill cancer cells with heat vulnerability.5 Visualizing and eliminating cancer cells simultaneously therefore depends on the availability of NIR theranostic probes. The FDA approved NIR fluorescent probe indocyanine green (ICG) not only successfully guided excisions of hepatocellular carcinomas and colorectal hepatic metastases in patients,6 but also efficiently photoablated tumors after NIR light irradiation.7 To accurately assess cytoreductive tumor surgery, Ntziachristos et al. showed the first proof-of-principle experiment in excising ovarian tumors in patients using the guidance of a folate receptor targeted fluorescence probe.8 The broad applicability of the receptor targeting strategy, however, is limited because these receptors are only expressed in a small percentage of patients. For example, less than 25% of breast cancer patients over-express Her2/neu,9 which is the most widely used biomarker for breast tumor targeted treatment. Furthermore, non-specific accumulation of the probes with an “always on” signal in normal tissues attenuates the T/B ratio. As a result, NIR theranostic probes that can localize tumors with wider applicability and improved T/B ratios are urgently needed.
Acidic tumor extracellular fluid (pH 6.2–6.9) is a hallmark of solid tumors, regardless of their genotypes or phenotypes.10 pH responsive NIR fluorescence probes have shown applicability in visualizing multiple types of tumors.11–13 Besides the pH discrepancy in extracellular fluids, the lysosomal pH (pHlys) in cancer cells (pHlys 3.8–4.7) also shows a higher acidity than that in normal cells (pHlys 4.5–6.0).14 This results in accelerated invasion and metastasis of cancer cells by maximizing the enzymatic activity of the exocytosed proteases that actively degrade the extracellular matrix.15 Compared to probes that are triggered in the extracellular fluid, probes activated inside lysosomes could offer higher T/B ratios because the background noise from fluorophores that have diffused into normal tissues can be minimized. Even though numerous lysosomal targeted theranostic probes have been developed,16 their therapeutic efficiency is less satisfactory due to the up-regulated self-restoration of the lysosomal membrane in cancer cells,17 which prevents lysosomal membrane permeability associated apoptosis.18 As another important organelle in eukaryotic cells, mitochondria play crucial roles in regulating energy production and triggering programmed cell death (PCD) by releasing pro-apoptotic proteins from mitochondria into the cytosol.19 Considering the high sensitivity of mitochondria to heat shock,20 disrupting the mitochondrial membrane via photothermal effects is a promising approach for eradicating cancer cells. For example, mitochondria targeted carbon nanotubes21 and gold nanoparticles22 were shown to kill cancer cells efficiently via NIR light induced hyperthermia. Additionally, due to the continuous proton pumping process across the mitochondrial inner membrane during oxidative phosphorylation (OXPHOS), the mitochondrial matrix demonstrates a unique alkaline environment (pHmito 7.5–8.2).23 There is promise therefore in selectively visualizing and eradicating cancer cells by sensing the altered pHlys and pHmito.
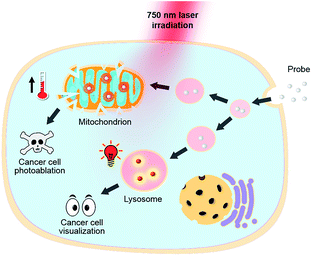
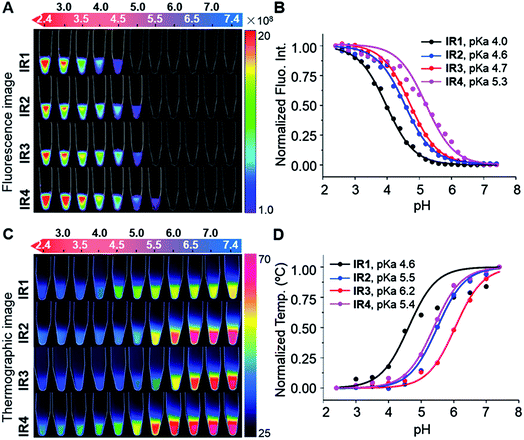
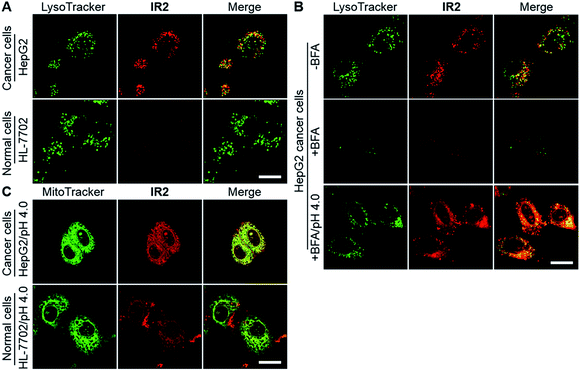
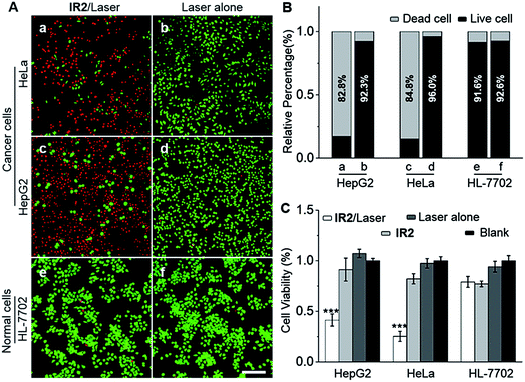
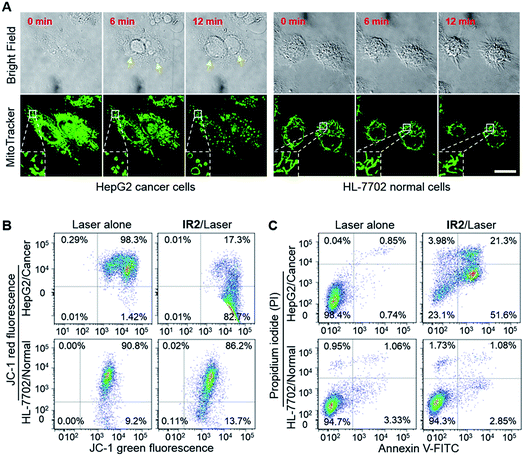
Heptamethine cyanines (Hcyanines) are widely used in developing NIR theranostic probes because of their high extinction coefficients (ε) and fluorescence quantum yields (Φ), good biocompatibility and photothermal/photodynamic effects.24 The first pH responsive Hcyanine probe to be reported sensed physiological acidity via a photo-induced electron transfer (PET) mechanism.25 Only a moderate fluorescence enhancement (2.5–3.5 times) was achieved, however, which was explained by the reduced energy gap between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) in the NIR region.26 We previously developed a Hcyanine probe based on self-aggregation induced quenching effects that offered a 15-fold fluorescence enhancement in physiological acidity.12,27 Recently, a hemicyanine derivative displayed a greater than 30-fold signal enhancement via an acidity triggered intramolecular spirocyclization mechanism.28 These probes, however, are not suitable for sensing pHlys due to the difficulty in fine tuning their pKa values. Intramolecular charge transfer (ICT) is a mechanism by which electrons are transferred from the electron donor (D) to acceptor (A) within the fluorophore.29 Displaying rapid and reversible responses to external stimuli,30 many ICT fluorescence probes have been developed to detect solvent polarity,31 metal ions32 and gasotransmitters.33 However, very few of them are reported to sense physiological acidity in the NIR region.34 In this work, we developed four Hcyanine based theranostic probes (IR1–4) that not only showed unprecedented pH switchable NIR fluorescence and photothermal effects (Scheme 1), but also possessed pKafluo values in the pHlys range by adjusting the ICT efficiency. While acidification from pH 7.4 to 4.0 led to a maximal 213-fold fluorescence quantum yield enhancement, the photothermal efficiencies decreased as low as 5.6-fold. As far as we are aware, IR1–4 demonstrate the highest physiological acidity triggered NIR fluorescence enhancement as small molecular probes. Of these probes, IR2, with satisfactory photostability, demonstrated intracellular uptake in both the lysosomes and mitochondria of multiple human cancer and normal cell lines (Fig. 1). Due to its optimized pKafluo (4.6), NIR fluorescence activation of IR2 in the lysosomes of cancer cells only, i.e. not in normal cells, was observed. Importantly, the combined use of IR2 and 750 nm laser irradiation led to cancer cell death with percentages of 58.7–74.8%. In contrast, only 19.8–32.9% viability loss was determined for the normal cells. Apart from instant cell death after the combined treatment, the remaining living cells continued to undergo apoptosis that was induced by mitochondrial membrane disruption. Overall, these pH responsive theranostic probes showed great potential in image-guided tumor ablation by precisely positioning and destroying tumor foci intra-operatively.
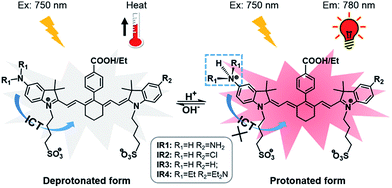 | ||
| Scheme 1 Structures of theranostic probes IR1–4 and the proposed mechanism of pH switchable NIR fluorescence and photothermal effects. | ||
2. Results and discussion
2.1. Design, synthesis and characterization of NIR theranostic probes
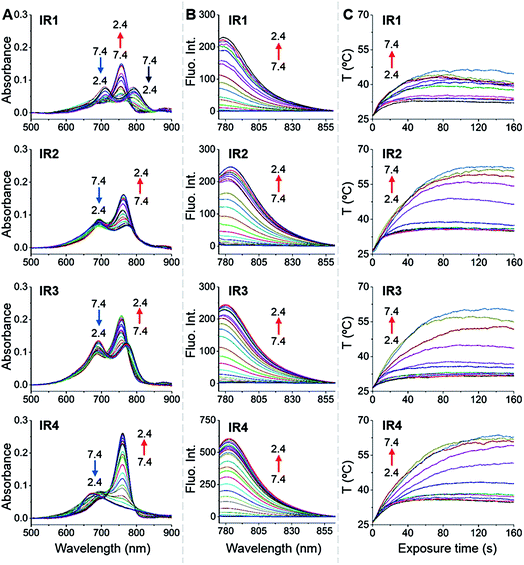
Cyanine dyes are composed of two terminal heterocyclic units linked by a polymethine bridge. Hcyanine derivative IR783 was chosen as a NIR fluorophore prototype to develop the theranostic probes because of its good biocompatibility, optimized emission wavelength and intracellular delivery into lysosomes and mitochondria via organic anion transporting peptide (OATP).35 A p-benzoic acid group was substituted on the meso position of IR783 via a C–C bond to increase its photostability and quantum yield.36 To achieve a pH response, an amine moiety with electron donating capability was incorporated at the 5-position on one terminal indole ring. At neutral pH, electron transfer from the amine to the Hcyanine will fully quench the fluorescence through non-radiative decay. In acidic environments, protonation of the amine will block the ICT process and the fluorescence will be regenerated. In order to trigger NIR fluorescence, specifically in the lysosomal lumen of cancer cells, selected functionalities with differing electron withdrawing capabilities were substituted on the other terminal indole. In this way, the pKa of the probes could be fine-tuned by adjusting the electron density on the Hcyanine scaffold. The structures of Hcyanine based theranostic probes IR1–4 are illustrated in Scheme S1.† All the probes were fully characterized by 1H NMR spectroscopy, 13C NMR spectroscopy, high resolution mass spectrometry (HRMS), and high performance liquid chromatography (HPLC) (spectra in ESI†).2.2. pH dependent optical and photothermal properties
| Probe | λ mono (nm) | λ agg (nm) | λ em (nm) | pKafluo | Φ (%) | pKaΔT |
|---|---|---|---|---|---|---|
| a Maximal absorption wavelength of monomers. b Maximal absorption wavelength of aggregates. c Maximal emission wavelength. d Quantum yield of the probes correlated to ICG (Q.Y.ICG = 0.12 in DMSO). e Parameters of deprotonated form measured at pH 7.4. f Parameters of protonated form measured at pH 2.4. g Not detected. | ||||||
| IR1 | 792e | 712e | 778 | 3.99 | 0.03e | 4.58 |
| 755f | 689f | 9.19f | ||||
| IR2 | 774e | 691e | 784 | 4.56 | 0.05e | 5.51 |
| 761f | 694f | 4.63f | ||||
| IR3 | 771e | 691e | 780 | 4.71 | 0.05e | 6.16 |
| 756f | 688f | 6.07f | ||||
| IR4 | N.D.g | 677e | 783 | 5.28 | 0.01e | 5.39 |
| 760f | 696f | 10.20f | ||||
2.3. Theoretical calculations of the acidity triggered photophysical properties
In order to study the mechanism of photophysical changes upon acidification, quantum chemistry calculations were carried out using Gaussian 09.33 The conformations of IR1–4 in their deprotonated and protonated states were optimized by means of density functional theory (DFT) methods with the B3LYP/6-311G basis set levels prior to the population analysis of the frontier molecular orbitals (FMOs) of these compounds. In the deprotonated form, the HOMO localized both on the Hcyanine scaffold and bridgehead amine, whereas the LUMO only spread over the polymethine chain, indicating a significant charge transfer from the terminal amine to the polymethine upon excitation44 (Fig. 4A and S4†). In contrast, in the protonated form, electrons in the HOMO of the conjugated polymethine chain exhibited a large overlap with those in the LUMO. A more detailed conformational change of the bridgehead amine is highlighted in the optimized three-dimensional molecular structures in Fig. 4B. While it was demonstrated that a planar conformation (ICT state) existed in neutral environments, transformation into a pyramidal geometry (LE state) was observed after its quaternarization in acidic conditions.50 The transfer from the ICT to the LE state is often accompanied with an increased energy gap.43 The calculated energy gaps between the HOMO and LUMO increase by 0.109, 0.085, 0.108 and 0.211 eV (ΔEgap = ΔE1 − ΔE2) for the protonation of IR1–4 respectively. The increase in the energy gaps further explains the hypsochromic shift of absorption in acidic environments. Overall, the FMO analysis confirmed that IR1–4 underwent ICT at neutral pH whereas this process was inhibited upon protonation of the amine moiety in acidic environments.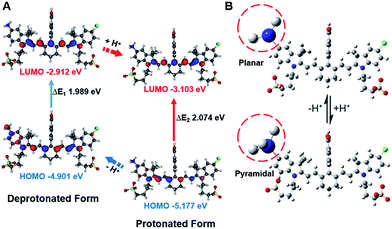 | ||
| Fig. 4 Frontier molecular orbital plots (A) and geometries (B) of IR2 in deprotonated and protonated forms. The corresponding HOMO and LUMO energy levels and HOMO–LUMO energy gaps are indicated. | ||
2.4. Theranostic probe for specifically imaging and killing cancer cells
3. Conclusion
Overall, we developed four Hcyanine based theranostic probes showing pH switchable NIR fluorescence and photothermal efficiency under physiological environments. By fine-tuning the pKa values of these probes, IR2, with a pKafluo value of 4.6, specifically visualized and eradicated multiple types of cancer cells by maximizing its NIR fluorescence intensity in acidic lysosomal lumen and its photothermal effects in the alkaline mitochondrial matrix. Considering that a disordered organellar pH is a common characteristic of cancer cells regardless of their genotypes or phenotypes, these theranostic probes hold promise in assisting image-guided tumor photoablation by completely eradicating tumor foci and improving prognosis.Acknowledgements
This work was financially supported by the National Basic Research Program of China (973 Program, 2013CB932500, 2015CB755500), the National Natural Science Foundation of China (No. 81171384, 81371624), the Nanotechnology Program of Shanghai Science and Technology Committee (13NM1400400, 15140901300), and the National Outstanding Young Scientist Award (61425006). We appreciate the kind help of Mr Ji Tang for fluorescence microscopic studies.Notes and references
- C. Chi, Y. Du, J. Ye, D. Kou, J. Qiu, J. Wang, J. Tian and X. Chen, Theranostics, 2014, 4, 1072–1084 CrossRef PubMed.
- T. F. Brewer and C. J. Chang, J. Am. Chem. Soc., 2015, 137, 10886–10889 CrossRef CAS PubMed.
- M. Ahmed, L. Solbiati, C. L. Brace, D. J. Breen, M. R. Callstrom, J. W. Charboneau, M. H. Chen, B. I. Choi, T. de Baere, G. D. Dodd 3rd, D. E. Dupuy, D. A. Gervais, D. Gianfelice, A. R. Gillams, F. T. Lee Jr, E. Leen, R. Lencioni, P. J. Littrup, T. Livraghi, D. S. Lu, J. P. McGahan, M. F. Meloni, B. Nikolic, P. L. Pereira, P. Liang, H. Rhim, S. C. Rose, R. Salem, C. T. Sofocleous, S. B. Solomon, M. C. Soulen, M. Tanaka, T. J. Vogl, B. J. Wood and S. N. Goldberg, A. International Working Group on Image-guided Tumor, P. Interventional Oncology Sans Frontieres Expert, R. Technology Assessment Committee of the Society of Interventional, C. Standard of Practice Committee of the and E. Interventional Radiological Society of, Radiology, 2014, 273, 241–260 CrossRef PubMed.
- E. M. Sevick-Muraca, Annu. Rev. Med., 2012, 63, 217–231 CrossRef CAS PubMed.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, J. Am. Chem. Soc., 2006, 128, 2115–2120 CrossRef CAS PubMed.
- F. P. Verbeek, J. R. van der Vorst, B. E. Schaafsma, M. Hutteman, B. A. Bonsing, F. W. van Leeuwen, J. V. Frangioni, C. J. van de Velde, R. J. Swijnenburg and A. L. Vahrmeijer, Journal of Hepato-Biliary-Pancreatic Sciences, 2012, 19, 626–637 CrossRef PubMed.
- Z. Sheng, D. Hu, M. Zheng, P. Zhao, H. Liu, D. Gao, P. Gong, G. Gao, P. Zhang, Y. Ma and L. Cai, ACS Nano, 2014, 8, 12310–12322 CrossRef CAS PubMed.
- G. M. van Dam, G. Themelis, L. M. Crane, N. J. Harlaar, R. G. Pleijhuis, W. Kelder, A. Sarantopoulos, J. S. de Jong, H. J. Arts, A. G. van der Zee, J. Bart, P. S. Low and V. Ntziachristos, Nat. Med., 2011, 17, 1315–1319 CrossRef CAS PubMed.
- H. J. Burstein, N. Engl. J. Med., 2005, 353, 1652–1654 CrossRef CAS PubMed.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed.
- Y. Wang, K. Zhou, G. Huang, C. Hensley, X. Huang, X. Ma, T. Zhao, B. D. Sumer, R. J. De Berardinis and J. Gao, Nat. Mater., 2014, 13, 204–212 CrossRef CAS PubMed.
- L. Wang, X. Zhu, C. Xie, N. Ding, X. Weng, W. Lu, X. Wei and C. Li, Chem. Commun., 2012, 48, 11677–11679 RSC.
- C. Zhang, T. Liu, Y. Su, S. Luo, Y. Zhu, X. Tan, S. Fan, L. Zhang, Y. Zhou, T. Cheng and C. Shi, Biomaterials, 2010, 31, 6612–6617 CrossRef CAS PubMed.
- G. Kroemer and M. Jaattela, Nat. Rev. Cancer, 2005, 5, 886–897 CrossRef CAS PubMed.
- J. P. Luzio, P. R. Pryor and N. A. Bright, Nat. Rev. Mol. Cell Biol., 2007, 8, 622–632 CrossRef CAS PubMed.
- X. Wu, M. Yu, B. Lin, H. Xing, J. Han and S. Han, Chem. Sci., 2015, 6, 798–803 RSC.
- T. Kirkegaard, A. G. Roth, N. H. Petersen, A. K. Mahalka, O. D. Olsen, I. Moilanen, A. Zylicz, J. Knudsen, K. Sandhoff, C. Arenz, P. K. Kinnunen, J. Nylandsted and M. Jaattela, Nature, 2010, 463, 549–553 CrossRef CAS PubMed.
- A. C. Johansson, H. Appelqvist, C. Nilsson, K. Kagedal, K. Roberg and K. Ollinger, Apoptosis, 2010, 15, 527–540 CrossRef CAS PubMed.
- D. R. Green and J. C. Reed, Science, 1998, 281, 1309–1312 CrossRef CAS PubMed.
- H. S. Jung, J. Han, J.-H. Lee, J. H. Lee, J.-M. Choi, H.-S. Kweon, J. H. Han, J.-H. Kim, K. M. Byun, J. H. Jung, C. Kang and J. S. Kim, J. Am. Chem. Soc., 2015, 137, 3017–3023 CrossRef CAS PubMed.
- F. Zhou, S. Wu, B. Wu, W. R. Chen and D. Xing, Small, 2011, 7, 2727–2735 CrossRef CAS PubMed.
- L. Wang, Y. Liu, W. Li, X. Jiang, Y. Ji, X. Wu, L. Xu, Y. Qiu, K. Zhao, T. Wei, Y. Li, Y. Zhao and C. Chen, Nano Lett., 2011, 11, 772–780 CrossRef CAS PubMed.
- J. Santo-Domingo and N. Demaurex, J. Gen. Physiol., 2012, 139, 415–423 CrossRef CAS PubMed.
- A. Yuan, J. Wu, X. Tang, L. Zhao, F. Xu and Y. Hu, J. Pharm. Sci., 2013, 102, 6–28 CrossRef CAS PubMed.
- B. Tang, F. Yu, P. Li, L. Tong, X. Duan, T. Xie and X. Wang, J. Am. Chem. Soc., 2009, 131, 3016–3023 CrossRef CAS PubMed.
- Y. Urano, M. Kamiya, K. Kanda, T. Ueno, K. Hirose and T. Nagano, J. Am. Chem. Soc., 2005, 127, 4888–4894 CrossRef CAS PubMed.
- C. Li, J. Xia, X. Wei, H. Yan, Z. Si and S. Ju, Adv. Funct. Mater., 2010, 20, 2222–2230 CrossRef CAS.
- X. J. Wu, B. J. Lin, M. Z. Yu, L. Yang, J. H. Han and S. F. Han, Chem. Sci., 2015, 6, 2002–2009 RSC.
- P. Fromherz and A. Heilemann, J. Phys. Chem. C, 1992, 96, 6864–6866 CrossRef CAS.
- Z. R. Grabowski, K. Rotkiewicz and W. Rettig, Chem. Rev., 2003, 103, 3899–4032 CrossRef PubMed.
- S. S. Palayangoda, X. Cai, R. M. Adhikari and D. C. Neckers, Org. Lett., 2008, 10, 281–284 CrossRef CAS PubMed.
- Q. Li, M. Peng, H. Li, C. Zhong, L. Zhang, X. Cheng, X. Peng, Q. Wang, J. Qin and Z. Li, Org. Lett., 2012, 14, 2094–2097 CrossRef CAS PubMed.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 2852–2854 RSC.
- Y. Li, Y. Wang, S. Yang, Y. Zhao, L. Yuan, J. Zheng and R. Yang, Anal. Chem., 2015, 87, 2495–2503 CrossRef CAS PubMed.
- X. Yang, C. Shi, R. Tong, W. Qian, H. E. Zhau, R. Wang, G. Zhu, J. Cheng, V. W. Yang, T. Cheng, M. Henary, L. Strekowski and L. W. Chung, Clin. Cancer Res., 2010, 16, 2833–2844 CrossRef CAS PubMed.
- H. Lee, J. C. Mason and S. Achilefu, J. Org. Chem., 2008, 73, 723–725 CrossRef CAS PubMed.
- A. Mishra, R. K. Behera, P. K. Behera, B. K. Mishra and G. B. Behera, Chem. Rev., 2000, 100, 1973–2012 CrossRef CAS PubMed.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- B. Wang and C. Yu, Angew. Chem., Int. Ed., 2010, 49, 1485–1488 CrossRef CAS PubMed.
- N. C. Maiti, S. Mazumdar and N. Periasamy, J. Porphyrins Phthalocyanines, 1998, 2, 369–376 CrossRef CAS.
- K. Zhou, H. Liu, S. Zhang, X. Huang, Y. Wang, G. Huang, B. D. Sumer and J. Gao, J. Am. Chem. Soc., 2012, 134, 7803–7811 CrossRef CAS PubMed.
- T. Myochin, K. Kiyose, K. Hanaoka, H. Kojima, T. Terai and T. Nagano, J. Am. Chem. Soc., 2011, 133, 3401–3409 CrossRef CAS PubMed.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3–40 CrossRef CAS.
- M. Sowmiya, A. K. Tiwari, Sonu and S. K. Saha, J. Photochem. Photobiol., A, 2011, 218, 76–86 CrossRef CAS.
- Z. Wan, H. Mao, M. Guo, Y. Li, A. Zhu, H. Yang, H. He, J. Shen, L. Zhou, Z. Jiang, C. Ge, X. Chen, X. Yang, G. Liu and H. Chen, Theranostics, 2014, 4, 399–411 CrossRef PubMed.
- U. Rosch, S. Yao, R. Wortmann and F. Wurthner, Angew. Chem., Int. Ed. Engl., 2006, 45, 7026–7030 CrossRef PubMed.
- M. Levitus and S. Ranjit, Q. Rev. Biophys., 2011, 44, 123–151 CrossRef CAS PubMed.
- G. K. Vegesna, J. Janjanam, J. H. Bi, F. T. Luo, J. T. Zhang, C. Olds, A. Tiwari and H. Y. Liu, J. Mater. Chem. B, 2014, 2, 4500–4508 RSC.
- Y. Ma, V. Abbate and R. C. Hider, Metallomics, 2015, 7, 212–222 RSC.
- X. Peng, F. Song, E. Lu, Y. Wang, W. Zhou, J. Fan and Y. Gao, J. Am. Chem. Soc., 2005, 127, 4170–4171 CrossRef CAS PubMed.
- J. A. Dickson and S. K. Calderwood, Ann. N. Y. Acad. Sci., 1980, 335, 180–205 CrossRef CAS PubMed.
- C. Abels, S. Fickweiler, P. Weiderer, W. Bäumler, F. Hofstädter, M. Landthaler and R.-M. Szeimies, Arch. Dermatol. Res., 2000, 292, 404–411 CrossRef CAS PubMed.
- T. Yoshimori, A. Yamamoto, Y. Moriyama, M. Futai and Y. Tashiro, J. Biol. Chem., 1991, 266, 17707–17712 CAS.
- X. Tan, S. Luo, D. Wang, Y. Su, T. Cheng and C. Shi, Biomaterials, 2012, 33, 2230–2239 CrossRef CAS PubMed.
- M. Sebbagh, C. Renvoize, J. Hamelin, N. Riche, J. Bertoglio and J. Breard, Nat. Cell Biol., 2001, 3, 346–352 CrossRef CAS PubMed.
- D. N. Wheatley, C. Kerr and D. W. Gregory, Int. J. Hyperthermia, 1989, 5, 145–162 CrossRef CAS PubMed.
- M. G. Sun, J. Williams, C. Munoz-Pinedo, G. A. Perkins, J. M. Brown, M. H. Ellisman, D. R. Green and T. G. Frey, Nat. Cell Biol., 2007, 9, 1057–1065 CrossRef CAS PubMed.
- S. K. Ko, S. K. Kim, A. Share, V. M. Lynch, J. Park, W. Namkung, W. Van Rossom, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Nat. Chem., 2014, 6, 885–892 CrossRef CAS PubMed.
- I. R. Indran, G. Tufo, S. Pervaiz and C. Brenner, Biochim. Biophys. Acta, 2011, 1807, 735–745 CrossRef CAS PubMed.
- Y. Tian, J. Sun, H. Yan, Z. Teng, L. Zeng, Y. Liu, Y. Li, J. Wang, S. Wang and G. Lu, Analyst, 2015, 140, 750–755 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, experimental details and other figures and spectra. See DOI: 10.1039/c6sc00221h |
| This journal is © The Royal Society of Chemistry 2016 |