Open Access Article
Open Access ArticleChemistry and properties at a sub-nanometer scale
Bing
Ni
and
Xun
Wang
*
Key Lab of Organic Optoelectronics and Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing, 100084, China. E-mail: wangxun@mail.tsinghua.edu.cn
First published on 3rd March 2016
Abstract
Ultrathin materials at a sub-nanometer scale not only feature atomic scale size, but also possess unprecedented properties compared to conventional nanomaterials. The two aspects endow such materials with great potential. In sub-nanometric (SN) wires, the weak interactions may overwhelm the rigidity of inorganic compounds and dominate behaviours at this regime. Consequently intricate structures and polymer-like rheology can be obtained, shedding new light on chemistry as well as material design. As for 0D or 2D SN materials, clusters are analogous to molecules and SN sheets show unique electronic structures. Taking SN wire as an example, their growth mechanisms are discussed, as well as their applications and potentials. The chemistry at this regime can promote their application-oriented research, however, this is not yet well explored. In short, there is great potential at the sub-nanometer scale, although there are also many challenges ahead.
1. Introduction
As a subfield of chemistry, nanochemistry focuses on exploring and utilizing the chemical behaviours of materials at nanoscale1,2 to gain fundamental knowledge or build functional materials. In the world at the nanoscale, the size of materials greatly influences or even decides their properties. Nowadays inorganic “sub-nanometric (SN) material” is a burgeoning multi-disciplinary topic in nanoscience, after the huge progress in nanomaterials that has been made in the past several decades. Researchers have usually used the word “ultrathin” to describe a scale of several nanometers. However, the word “ultrathin” doesn't specify a certain size range,3 and thus it can also be used as a comparative word comparing with “thin”. By using “ultrathin” as a topic to search Web of Science, more than 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 results can be found, not to mention other relevant words like ultrafine and ultrasmall, however, only a small amount refers to sizes at the atomic level. Therefore we find it urgent to coin a new term, sub-nanometer, to delimit the scale at several nanometers or an even smaller size. The practical meaning of the term, sub-nanometric material (SNM), should include two aspects: one is that its size should be at the atomic level, and the other is that it shows distinct properties compared to its nano-counterparts with larger sizes. The former aspect refers to its chemical nature, and the latter one comes from the view of material science. Research on new materials should not be diverted from their chemical aspects,4 since it is chemistry that raises findings above the traditional “trial-and-error” paradigm in research. Vice versa, the materials viewpoint can move studies towards real applications.
000 results can be found, not to mention other relevant words like ultrafine and ultrasmall, however, only a small amount refers to sizes at the atomic level. Therefore we find it urgent to coin a new term, sub-nanometer, to delimit the scale at several nanometers or an even smaller size. The practical meaning of the term, sub-nanometric material (SNM), should include two aspects: one is that its size should be at the atomic level, and the other is that it shows distinct properties compared to its nano-counterparts with larger sizes. The former aspect refers to its chemical nature, and the latter one comes from the view of material science. Research on new materials should not be diverted from their chemical aspects,4 since it is chemistry that raises findings above the traditional “trial-and-error” paradigm in research. Vice versa, the materials viewpoint can move studies towards real applications.
Basically, inorganic SNMs can be divided into 3 categories: 0D clusters, 1D SN structures (especially SN wires, SNWs) and 2D sub-nanometric sheets (SNSs). Some complicated materials can be constituted of different structures. Clusters can be seen as a kind of special molecule containing a multi-metallic core, and research on this category could gain insight into chemical bonds. The most famous SNS must be graphene, which has provoked massive studies on its structure and properties, as have other star materials like transition metal dichalcogenides (TMDs), black phosphorus etc.5 As for SNWs, there are much fewer reports compared to 0D and 2D SNMs. This might be due primarily to the lack of synthesis methods. The growth mechanisms of SNWs have been progressively researched to date, and more syntheses can be foreseen. Our group focuses on both the synthesis and related properties of SNWs, and some fundamental knowledge about the relationship between their chemical nature and properties have been disclosed in past years.
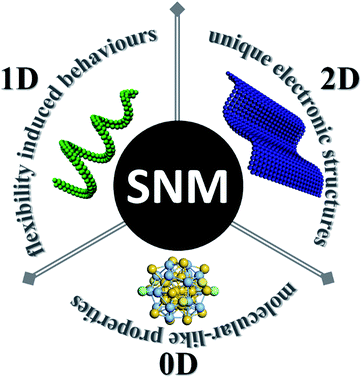
Besides the high content of surface atoms which may lead to better performances, SNMs possess some other unique features (Fig. 1). The most intriguing thing about SNMs is not their molecular scale size, but their unprecedented properties which are seldom seen in conventional nanomaterials, and we must bear this in mind when considering them. Here, we describe their most irreplaceable features organized by dimension. In terms of chemistry, intermolecular forces (or weak interactions hereafter) may play a vital role in this regime, which may overwhelm the inherent rigidity of inorganic materials and lead to unexpected flexibilities. This assumption has been perfectly practiced in SNWs. Accordingly, intricate morphologies or self-assembling structures can be achieved under the proper conditions. Based on the flexibility of SNWs, polymer-like rheological properties emerged. As for clusters, they can be considered as a new kind of molecule, and they can not only enrich the library of molecules, but also extend our knowledge about the foundation of chemistry. In the case of SNSs, they usually feature different electronic structures from thicker sheets, resulting in relevant outstanding properties.
This perspective is aimed at provoking new breakthrough points at this special scale, as well as applying more chemistry into material studies. Of the three kinds of SNMs, SNWs are a matchless example, which blur the boundary between inorganic materials and polymers, and the turning point is decided exactly by the size. To some extent, the intricate morphologies derived from SNWs conjure images of helical DNA strands or sophisticated biomolecular structures, showing greatly underutilized potential. Besides, there are lots of reviews discussing clusters and SNSs, and thus we would like to mainly focus on SNWs. In the following parts, we would like to start from the synthesis of SNWs, since this might be the largest contemporary obstacle toward the blossoming of relevant research. However, we had to relax the restriction on diameters to summarize their rules in this section since very few examples are elaborated due to various reasons. Then, structure and size related flexibility is discussed in the next section, followed by predictions to provide a comprehensive study on SNWs. In the case of clusters and SNSs, we prefer to talk about the similarities between the clusters and molecules, as well as the unique electronic structures in some single layered compounds. Then, complicated structures with multi-compositions are briefly discussed. A brief discussion on interfaces within atomic levels is also presented in this section. At last, we conclude the whole area and provide an outlook.
Although we have plenty of words for it, the discussion of chemistry should never stop. The concept of chemistry should be updated since enormous developments have been achieved.6 Contemporarily, there are two types of research: one is curiosity-oriented and another is application-oriented. One kind of research may revolutionize another kind at the proper time. For example, supramolecular chemistry helps to design functional molecular devices.7Vice versa, the creation of metal organic frameworks (MOFs) also led to the establishment of reticular chemistry.8 We believe there are still many opportunities in the gap between these two types of research, and the way to figure them out is by thinking about the constitution of materials and their interactions. In organic compounds, constitutions are relatively easy to determine and their types of interactions are well understood. However, things are very different in the case of inorganic compounds. Yet our group has found that weak interactions can decide the behaviours of SNWs, no matter what their constitutions are. But there is still a long way to go to establish new chemistry. Above all, the fabrication of SNWs is limited, which drastically retards the research. Secondly, another important hindrance is the lack of efficient in situ and non-destructive characterization tools, and thus we cannot clearly link their chemical structures with behaviours. However, chemical structures can dramatically benefit the development of research; for example, the design of organic molecular devices relies on the ingenious selection of functional groups and their arrangements.
The chemical formula has played a vital role in the spread of chemistry in history, however, it seems unable to represent the most important characteristics of materials in the view of applications nowadays, especially at this atomic nanoscale. For instance, allotropes of the same element, different crystal forms of the same compound, and different morphologies or sizes of the same crystal, may all show distinct properties despite the same chemical formula. Meanwhile in some cases, materials with different chemical formulas could also function similarly, like different SNWs can all show flexibilities. Maybe there should be another simple way to denote the material structure and briefly link with their properties. If there is, we believe that the size must be an important metric. Therefore we specify the diameters of the SNWs of interest in brackets in the following sections.
2. Growth mechanisms of SNWs
Up until now, the research focus has been largely on the synthesis of SNWs in this area, since it's the first step towards applications. The synthesis of conventional nanowires has shown much progress in the past decades, and several general methods have been established, such as the vapour-liquid-solid (VLS), solution-liquid-solid (SLS), selective-area epitaxy, template-directed, and oriented-attachment methods, etc.9,10 Although conventional nanowires and SNWs share something in common, such as the formation of both requiring the limitation of growth in two dimensions while another dimension is unconstrained, the SNWs are much more difficult to fabricate. In SNWs, the confinements in the two dimensions should be strong enough to only allow formation within several atoms while the growth in the other dimension is nearly unconfined. In polymers, the confinements are atomically-precise, satisfied by strong covalent bonds, such as C–H, C–X (X: F, Cl, Br), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, etc. However, this strategy is not transferable to inorganic compounds, since covalent bonds are not universal and typical there. In natural crystals, there are few kinds of compounds with one dimensional chains inside, such as M2Mo6X6 (M = Li, Na; X = Se, Te), poly(sulphur nitride), Se, Te,9 MCl2 (M = Pd, Cu, Be), etc. Thus it would be hard to utilize the inherent properties of materials to widely prepare SNWs, and so other bypasses must be found.
O, etc. However, this strategy is not transferable to inorganic compounds, since covalent bonds are not universal and typical there. In natural crystals, there are few kinds of compounds with one dimensional chains inside, such as M2Mo6X6 (M = Li, Na; X = Se, Te), poly(sulphur nitride), Se, Te,9 MCl2 (M = Pd, Cu, Be), etc. Thus it would be hard to utilize the inherent properties of materials to widely prepare SNWs, and so other bypasses must be found.
The details of SNW growth are not fully researched and understood yet. And the reports of strict SNWs which are thin enough to manifest organic-like behaviours are indeed scant, and thus summarizing their general rules would be difficult, and so we have tried to relax the restrictions on size in order to shed light on the possible rules for SNWs. Some reviews concerning the synthesis methods have been published by our group11 and other groups.3,12 To be concise, we ostensibly categorize the synthesis into oriented attached growth (OAG), template assisted growth (TAG), catalyst-guided growth (CGG) and ligand controlled growth (LCG) (Fig. 2, right). These four methods are not limited to SNWs, and some other SNMs can be prepared using them, such as nanotubes, SNSs, etc. Among these four methods, OAG is the most fascinating and promising tactic to prepare SNWs, and this process is an intuitive analogue to polymerizations (Fig. 2, left) which are the basis of polymer science, and thus we would like to introduce it first.
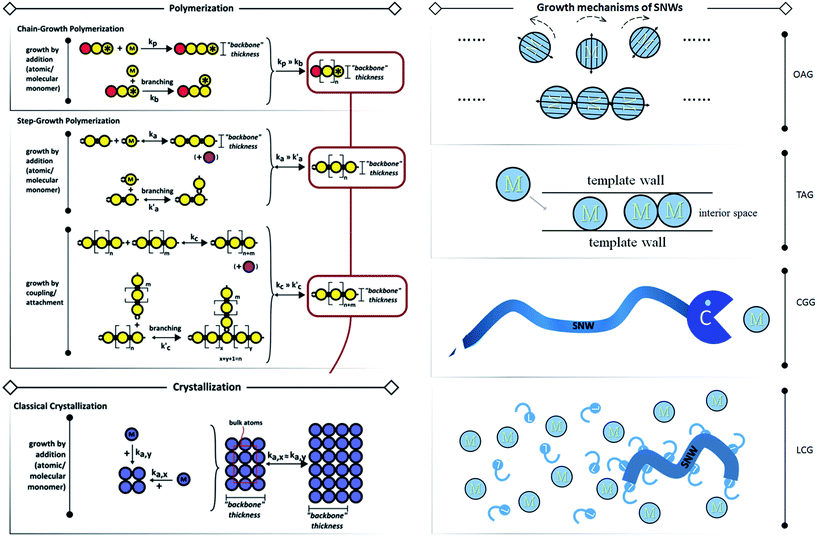 | ||
| Fig. 2 Growth mechanisms of polymerizations, traditional crystallization (left panel, reprinted from ref. 12 with permission of the 2013 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim) and SNWs (right panel). In the right panel, “M” indicates monomer, “C” stands for catalyst and “L” denotes ligand. In OAG, the monomers rotate to certain orientations for fusing driven by dipole or other forces described by arrows. In TAG, the monomer moves into the template and grows to form SNWs inside the interior space confined by template walls. In CGG, the catalyst “meets” the monomer and transfers it to the constructed SNW. In LCG, the SNW is formed with the help of ligands. We ostensibly categorise them into these four kinds, however, it's hard to describe the kinetics like that in the left panel due to their complexities. | ||
2.1 Oriented attachment and size effect
Considering the extreme confinements in two dimensions despite their non-1D crystal structures, it is conceivable that the formation of SNWs doesn't occur in the same way as traditional crystal growth mechanisms. Inspired by polymerization, oriented attachment (OA), which is a non-classical crystal growth mechanism, may be a solution to general SNW synthesis.OA, which was first described in 1998,13 demonstrates crystal growth by the cohesion of particles. This method is mainly found in one dimensional growth.14,15 So far, research of OA in nanosheets formation has been less reported,16 perhaps due to a lack of simple and convincing characterization tools, and small angle X-ray scattering analysis17 is helpful to illustrate the process in sheets. The small particles in OA can be considered as “monomers”, and the cohesion process may resemble the polymerization process.12 Unlike polymerization, the driving forces of cohesion are diverse and the structures of monomers are much more complicated which cannot be simply described by chemical formulas.
The structures of “monomers” are quite important in determining cohesion behaviours.16 The differences in atomic structures would result in different driving forces, and consequently the final morphologies are quite different. In the OA process of PdSe truncated cubes (∼5 nm),18 the different major elements (Pd or Se) on the terminated facets determine the direction of the dipole moments of the truncated cubes, leading to disparate cohesion manners. Sometimes the monomers need to adjust their relative orientations to find a perfect lattice match, followed by a sudden jump to join them, and finally their interface is eliminated to make them one particle.19
The more intriguing thing is that the diameter of the wires usually remains the same as the monomers,15 since the wires are formed by the one dimensional fusion of monomers, leaving the other dimensions alone. This is somehow confusing, as if the dot-like monomers are isotropic or highly symmetric, then why would they rotate and only elongate in one dimension? Thus we should believe that the monomers must possess certain asymmetric conformations besides steric crowding. This conjures an image of molecules with steric anisotropy, and then we can presume that the polymerization of such monomers leads to SNWs. Therefore the structures of monomers must be determined and the rules underlying cohesions must be studied to better guide the design of SNWs to fulfil their potentials.
Another interesting feature is that OA can show size effects.20 First, monodispersed Cu (2.2 nm), Cu (3.4 nm) and Cu (5.2 nm) were controllably synthesized, and then further solvothermal treatments proceeded to allow OA. It was found that Cu (<5 nm) seeds were able to produce Cu2S nanorods (lengths ∼30–100 nm, diameters ∼2–4 nm), or Cu2S nanodisks (diameters ∼6–13 nm, thickness ∼2–4 nm), while larger seeds could only result in irregular particles, or aggregations. This is easy to understand because as the size changes, their structures must be changed, followed by differences in the driving force, and consequently the final morphologies are different. Composition dependent OA was evidenced in PtAg alloy (2.8 ± 0.4 nm) nanowires.21 In this case, only Pt53Ag47 was able to achieve wire structures, while other compositions would remain as particles.
OA has been found in SNW or thin wire syntheses. CdOOH (<1 nm) SNWs have been synthesized in oleic acid and an oleylamine system, and their time profile experiments proposed a mechanism of OA.22 More reports on OA in nanowire formations can be found if we broaden the range of diameters of the materials of interest. The OA of Pt3Fe (average diameter ∼5.3 nm) dots was directly tracked during the formation of 1D nanorods using real time transmission electron microscopy (TEM).23 Pt–M alloy (M = Cu, Co, Ni, Fe, ∼4 nm) wormlike wires could be prepared by the OA of small alloy particles,24 as well as Pt (∼3 nm)25 or Pd (2.0 ± 0.6 nm)26 wires. As for non-metallic wires, GdF3 (2–3 nm) wires were fabricated by OA which was evidenced by time controlled experiments.27 ZnS (∼2 nm) wires were also prove to be grown with OA using time profiles.28
OA resembles polymerization, however, there is still a long way to realizing pragmatic and universal methods for SNW synthesis. Due to the diversities in substrates and driving forces, researchers are currently studying but not using OA. The intrinsic potential of OA will be fully realized when the monomers and cohesion driving forces are widely linked and concluded. To some extent, self-assembly is also similar to OA, except for material cohesion. Thus the insights of these two fields might apply to each other.
2.2 Other synthesis methods
Besides OA, there are 3 other kinds of growth methods, TAG, CGG, and LCG, as we suggested before. These 4 methods have also been long applied in the wet chemical syntheses of nanomaterials. TAG is the synthesis using preformed or in situ formed templates of thin 1D structures. CGG requires catalysts serving as growth active sites, and SNWs can elongate only at the positions with catalysts. LCG is another important method which involves ligands during synthesis. Actually, most of the syntheses require additive ligands, and some of them can be categorized into TAG, OAG or CGG after careful study, however, many of them have still not been researched in detail, and thus we could only put them into LCG.There are 2 types of templates, one is a constraining template and another is a sacrificing template. Constraining templates contain SN channels inside and their robust channels enable 1D growth only. Ag (0.4 nm) SNWs can be synthesized in the tubular channels of self-assembled calix[4]hydroquinone.29 This is an example of a hard template which is preformed and rigid. In addition, the templates can also be formed during synthesis. Amine is able to form tube-like micelles in the correct conditions, and accordingly Au (∼1.6 nm) wires can grow inside.30 Metallic thin wires can be formed in oleylamine,31 however, since the authors didn't focus on the formation of crude wires and details are missing, we will not abruptly conclude their methods to be TAG.
Taking Au wires as an example (Fig. 3), some other features of SNW formation can be disclosed. Halder and Ravishankar found that OA was the reason for Au (∼2 nm) wire formation in a synthesis using oleylamine, oleic acid and HAuCl4 in toluene as reactants, and the attachment manner was evidenced using transmission electron microscopy (TEM, Fig. 3A and B).32 On the other hand, Peidong Yang et al. have used similar conditions and claimed that the synthesis of Au (∼1.6 nm) wires was due to the formation of soft templates constituted by oleylamine tubular micelles (Fig. 3C), which was further proved using small angle X-ray diffraction (XRD).33 What's more, the syntheses in quite different conditions can all lead to Au wires of very similar diameters (actually the diameters are acquired using TEM, and the wires are rough, and thus the measuring errors might be large), and such a feature is evocative of the magic number in clusters. Xing et al. used a small amount of triisopropylsilane in the synthesis to facilitate the growth of Au (1.6 nm) wires.34 Zhiqun Lin et al. employed cetyltrimethylammonium bromide (CTAB) templates to prepare Au (∼2 nm) wires, which is a procedure extremely similar to the widely used method for Au nanorods, except for the adding of toluene to tune the diameters of the CTAB templates, and they suggested that it is the tubular micelles formed during synthesis that resulted in Au (∼2 nm) wires (Fig. 3D).35 Younan Xia et al. introduced NaOH solution into the CTAB template method, and they proved OA in the formation of Au (∼10 nm) wavy wires.36 Together with this literature, we may extrapolate that the formation of Au SNWs in soft templates might still proceed as OA in confined spaces, similar diameters in different conditions arise from similar or the same sized monomers. However, whether this is applicable to other systems is still unknown.
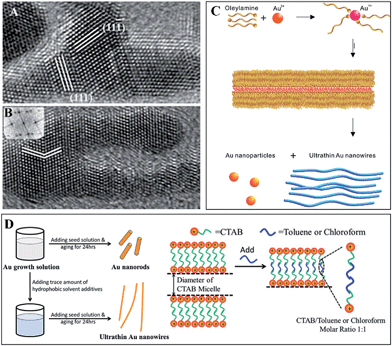 | ||
| Fig. 3 Formations of Au (∼1.6 nm) wires. (A) A particle attaches along a (111) facet that is different from the growing direction; (B) the branched region branches again to form a new segment. These two TEM images were taken at the early stage of formation. Reprinted from ref. 32 with permission of the 2007 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (C) TAG via the formation of an oleylamine soft template. Reprinted from ref. 33 with permission of the 2008 American Chemistry Society (ACS). (D) Using toluene or chloroform to tune the diameters of CTAB templates so that Au (∼2 nm) wires would be synthesized. Reprinted from ref. 35 with permission of the 2015 Royal Society of Chemistry (RSC). | ||
Sacrificing templates serve as reactants as well as morphology determining agents in syntheses. Firstly an easy-accessible 1D structure is prepared as a template, followed by adding other reactants to react with the template. During the reaction, reactants etch and deposit on the templates, and thus new products replicate the morphology of the templates. Te nanowires are relatively easy to prepare due to their 1D chain in crystal structures, and meanwhile they are chemically active, and thus they are usually employed as sacrificing templates. Te can reduce many metals and alloys with them, and thus PdTe, CdTe, Bi2Te3, Ag2Te, Cu1.75Te (>10 nm) wires,37 Pd, and Pd@Pt (∼5 nm)38 wires can be prepared using Te wire templates. Though this strategy cannot prepare SNWs yet, some interesting tricks can be introduced into the etching process, which endows the method with great potential. By using the Kirkendall effect in the etching process, nanotubes can be achieved.39
Thanks to the great advancement of the skills in synthesis, we have a large library of templates now, and the only requirement is time to make use of them. There are many kinds of metal organic frameworks or covalent organic frameworks consisting of SN channels40,41 which may be used as the confining templates. As the amount of SNWs increases, more selections of sacrificing templates are becoming available.
The VLS method is usually a kind of CGG method. Utilizing a sub-nanometric dot as a growing site can lead sub-nanometric materials to 1D growth. Si (∼3.5 nm) wires can be prepared using Au (∼2 nm) particles as catalysts.42 The sophisticated selection of catalysts enables controlled growth behaviour, and this is especially true in carbon nanotubes. (116) facet enclosed W6Co7 prefers the generation of single walled carbon nanotubes of (16, 0) chirality.43 Though CGG is much less researched in SNWs to date, we are confident that it will earn its status in the future, since this is a designed, guided method full of chemical knowledge.
As for LCG, here we briefly show some examples, for example, Bi2S3 (1.6 nm) wires can be prepared by injecting sulfur into bismuth citrate oleylamine solutions.44 Oleylamine is a good reducing agent as well as a good ligand which can protect nanowires. ZnSe (<2 nm) nanorods can be prepared by injecting zinc oleate into a mixture of selenium and oleylamine.45 Other organic ligands could also function well to synthesize thin wires. W18O49 (<1 nm) wires could be prepared by the hydrolysis of WCl6 in ethanol.46
The mechanisms in many LCG methods are not well explained. Ozin et al. successfully used static light scattering (SLS) analysis to debunk the myth underlying the synthesis of Bi2S3 (∼2 nm) SNWs.47 In this way, they quantitatively proved that the SNWs were analogous to polymers both in formation kinetics and their worm-like conformations. However, they were unable to clearly figure out the structure of the monomers. In short, LCG needs more attention from researchers on its mechanisms, and not only its procedures, to realize its potential.
2.3 Challenges in the synthesis
The synthesis of SNWs requires extremely anisotropic growth, causing lots of challenges. A big challenge is that the details of the reactions are little known in many cases. This is partly because of the lack of simple but convincing analysis tools, as the focus of researchers is on the applications but not the formation once they get SNWs, but mainly because of the obstacles in synthesizing new SNWs. The first two reasons can be bypassed by the motivations of researchers. As for the last reason, we may expect a general system. If there is a universal system in which various SNWs can be fabricated of high quality, it would greatly benefit both curiosity-oriented and application-oriented research. Our group was approaching this goal in previous years, and we experimentally found that a “good solvent and poor solvent system” is efficient to synthesise SNMs, in particular 1D SNMs in spite of their crystal structures.A good solvent is a solvent in which products can be well dispersed, while products show poor solubility in poor solvent. The rational selection and mixing of good and poor solvents can help us to produce 1D SNMs, such as wires,22 belts,48,49 and tubes.50 In our group, the good solvents are mainly long-chain organics (octylamines, oleylamines, oleic acid, etc.) and poor solvents are mostly alcohols (especially ethanol). Another detail is that the reactants are dispersed and usually not fully dissolved at the beginning of the reactions. Maybe the phase behaviours during synthesis are beneficial to SNMs. Learning from a well-known mechanism of nanomaterial synthesis,51 we may presume that adding poor solvent into the system increases the supersaturation of the products, leading to the precipitation and formation of ligand-protect SNMs. If we review other groups' work, we can also find that good solvents and poor solvents are unintentionally employed in their synthesis.
SNWs are indeed a nascent field; we are currently at the stage of discovery, and valuable chemical knowledge of SNWs is accumulating. After vast numbers of SNWs have been discovered, we can move to the stage of using them. In order to facilitate this stage, the mechanisms underlying synthesis should be revealed. Although we categorize the current syntheses into OAG, TAG, CGG and LCG, this is not mainly due to their mechanism differences, but some superficial aspects of the reactions. Accordingly, there is still a long way to go to see the big picture of the formation mechanisms. In terms of chemical reactions, the SNWs formation may share many things in common with polymerization, and thus we can concentrate on some potential aspects. First, as the monomers are essential, we'll try our best to track their formations, structures and behaviours. Second, the interplay between monomers resembles classic chemical reactions. Yet we only find two ostensible phenomena: reaction with or without additive catalysts, indicating the different activities of the growing sites. As for the reaction rules at the atomic scale, we are far behind this ultimate goal, though SNMs are substantially at the atomic scale. Hitherto, the total synthesis of natural compounds has largely progressed on the basis of achievements in organic reactions. So it's also possible that the controlled synthesis of SNMs can also progress in light of the reactions between monomers. We can concentrate on several functional materials yet to be developed in this area, such as Au or other noble metals, hydroxides, or some other inherent SNMs like polyoxometalates (POMs).
3. Flexibilities in SNWs
It is the unprecedented properties that render the unique structures (SNWs here) much more fantastic than conventional nanomaterials. Bearing this connotation in mind, we will move to the discussion of properties in this section. As we suggest in the first section, mechanical flexibility is the central part of SNWs' applications (in this perspective, flexibility only refers to mechanical flexibility, not versatility). This concept is a natural extension of polymers since both of them are SNWs. Polymers wield enormous influence on today's functional materials. Their versatile functions stem from their chain structures. Designing flexibilities, lengths or orientations contributes to the achievement of various products ranging from domestic plastic bags to military body armours. Frankly speaking, polymers not only endow our daily life with much convenience, but also support our entire society. However, the wide use of polymers also raises some debates mainly on their resistance to degradation, and thus society demands the optimizations of polymers or their substitutes. The structures of SNWs are intuitively like polymers as the diameter of polymer chains is approximately within the sub-nanometer scale, and it is almost at the same level as SNWs. Nonetheless, in order to comprehensively compare SNWs with polymers, more details such as atomic structures and functions must be illustrated.3.1 Structures of SNWs
Structures are usually the origin of functions. But unluckily, the atomic structures of SNWs are not well studied. Here we also relax the size limitation of SNWs for the sake of exploring more examples. The most common analysing tools for inorganic structures are mainly scanning electron microscopy (SEM), TEM, high resolution TEM (HRTEM, or high-angle annular dark field scanning TEM, HAADF-STEM), selected area electron diffraction (SAED), XRD, and X-ray photoelectron spectroscopy (XPS). However, due to the SN size, SEM can hardly reach that regime, and XRD patterns usually show weak signals because of the lack of a long-distance period. SNWs are usually not very stable since their surface areas are extremely high, and thus the energy in the high accelerating voltage of TEM is prone to damaging their original structures, while advanced HRTEMs capable of low voltage analysis are relatively rare in contemporary research institutes.The scant research on the structures of SNWs, mostly achieved by the lattice fingerprint acquired by HRTEM, shows that the behaviours or structures of ultrathin wires may not be just the same as their nano- or bulk counterparts, and some unique domains might exist. The formation of single- or multi-atom linear chains or reactive clusters on the side of Au (∼1.7 nm) wires was demonstrated using dynamic HAADF-STEM imaging in the deterioration process caused by electron beam in TEM.52 The packing manner of Au and Ag atoms in AuAg alloy (∼2 nm) wires is special as the packing of interpenetrated icosahedral and decahedral units forms 5-fold nanostructures, resembling quasicrystals (Fig. 4A–F).53 Taking advantage of this structure, a double helix conformation can be induced by additive metal depositions (Fig. 4G–I).54 This is an example to show that knowing detailed structures would be of benefit to applications. As for the non-metallic compounds, crystal lattices are only yet references to other characterizations (Fig. 4J–L)28,49,55 due to their varieties.
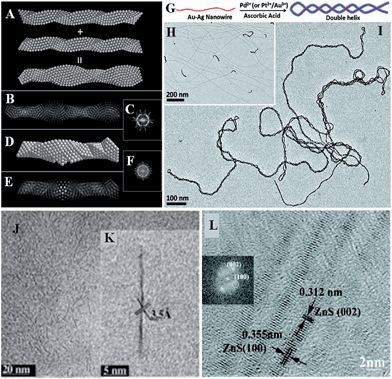 | ||
| Fig. 4 Structural characterizations using HRTEM. The interpenetration of two closely packed tetrahedral chains (A) results in the simulation of structures of (B), and (C) is the corresponding FFT pattern. (D–F) are the domain of a decahedral nanowire and corresponding FFT pattern. Reprinted with permission from ref. 53 of the 2013 ACS. (G) is the method to prepare a double helix (I) from such structures. (H) AuAg (∼2 nm) alloy wires. Reprinted with permission from ref. 54 of the 2011 ACS. (J and K) HRTEM images of TiO2 (<1 nm) wires. Reprinted with permission from ref. 55 of the 2010 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. (L) HRTEM image of ZnS (∼2 nm) wires. Reprinted with permission from ref. 28 of the 2011 ACS. | ||
The confusion on atomic structure would largely hinder the development of SNWs. To overcome this, simple and non-destructive benchmark methods must be created in the future. Along with the fast advancements in instruments, there might be two possible solutions. One is HRTEM capable of low voltage analysing, and another is X-ray adsorption spectra (XAS), such as extended X-ray adsorption fine structure. The former is able to directly show the atomic arrangement as well as the intact morphologies of SNWs at the same time. The latter can provide more details of bond structures. Both of them will greatly benefit the progress of SNWs.
After knowing the details of a single SNW, the focus may move forward to the ensemble structures of SNWs. In this regime, SEM and TEM are very powerful as ex situ tools to describe the aggregation and assembly of many SNWs. As for in situ detection, XAS may also offer some information. In light of polymers, there are some other useful methods. Dynamic light scattering and SLS can show the assembly behaviour without interfering with the pristine SNWs. The data are easy to achieve, however analysis requires some theoretical knowledge.
3.2 Flexibilities and related properties
Flexing morphologies have been observed in inorganic compounds,56 but only SNWs have been proven to show polymer-like flexible behaviours including tangling, coiling, and rheological behaviours, shedding new light on material design. In the case of GdOOH (<1 nm) SNWs, the polymer-like rheological behaviour emerges as a non-Newtonian fluid behaviour with apparent shear thinning at high shear rates (Fig. 5C). It is worth mentioning that their viscosities are size dependent, and that the viscosities would increase as the wires get longer or thinner (Fig. 5B).22 Taking advantage of this, the SNW solutions can form gels at the correct concentrations (Fig. 5A and D). Comparing with conventional nanowires, we can infer that the flexibility stems from the SN size, and that this kind of material is worthy of the name real SNWs.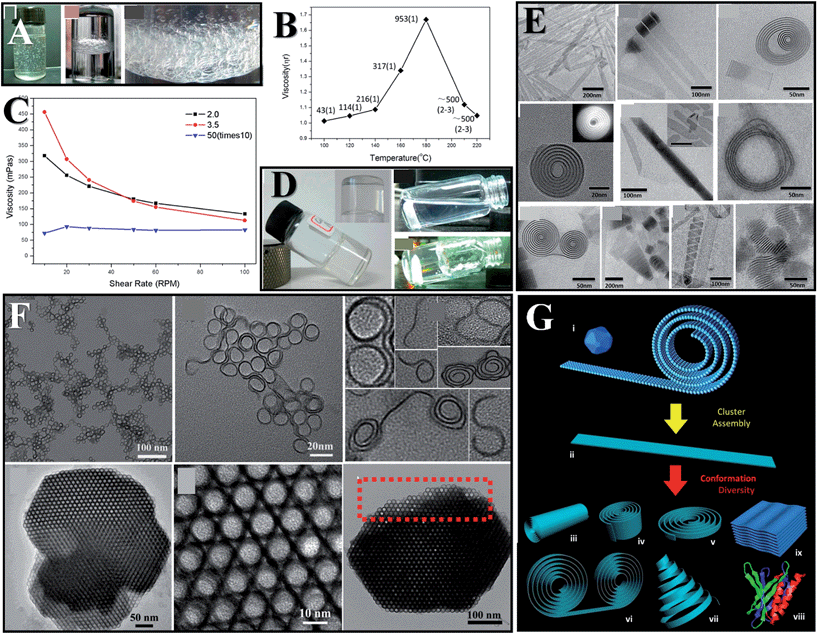 | ||
| Fig. 5 Flexibility induced properties. Air trapping (A) and gelation (D) induced by GdOOH (<1 nm) SNWs. (B) Viscosities of GdOOH wires synthesized at different temperatures. The numbers in the graph indicate the length of the wires and the bracket following denotes their diameters. (C) Rheology of GdOOH (<1 nm) SNWs. (E and G) Various flexible morphologies of Y(OH)3 (<1 nm) SN ribbons. Reprinted with permission from ref. 22 of the 2013 ACS. (F) Coiling and assembling structures of indium sulfide. Reprinted with permission from ref. 49 of the 2013 ACS. | ||
There is no experiment measuring the mechanical properties of single SNWs yet. And the mechanical properties of thin materials also show a discrepant tendency as the size changes in different materials. For example, theoretical calculations indicated that the Young's modulus of ZnO films would increase by 22% with decreasing thickness from 8 to 1 nm,57 while in Cu films, Young's modulus was expected to decrease with decreasing thickness from 12 to 1 nm.58 Mechanical tests on amorphous Al2O3 shells indicated that the elastic modulus would increase significantly when the thickness is smaller than 5 nm.59
However, things might be totally different in the sub-nanometer range, because the crystalline degree in SNWs might be extremely low, and defects which have huge impacts on mechanical properties are not easy to define in such structures. The mechanical flexibility in SNWs might be explained by conformational entropy.12 In that explanation, a larger length/diameter ratio contributes to a large conformational entropy, and thus wires can bend. Here we would like to provide another point of view from an energy perspective and specify the contributions of SN size (albeit in an oversimplified manner). The energy of a covalent bond is usually less than 400 kJ mol−1. The lattice energy of an ionic crystal is about several thousand kJ mol−1, however, in this regime their poor crystallinity must greatly compromise the value. Considering the relationship of ionic bonds and covalent bonds, let's say that the energies of ionic crystals at SN sizes are similar to that of covalent bonds. Bending the structures only requires a slight morphology change but not to an infinite distance, and thus the energy change might be of a smaller magnitude, and thus the energy required to change the morphology might be at the level of 40 kJ mol−1. As the energy of weak interactions could reach this scale (for example, hydrogen bonds: 10–102 kJ mol−1, and van der Waals interactions between hydrocarbon tails could reach about 6.7 kJ mol−1 per CH2 tail60), and also the number of weak interactions would be much more than chemical bonds at this SN scale due to their longer interplay distance, here the weak interactions can dominate the behaviours of SNWs, overwhelming the inherent rigidity in inorganic materials and leading to unexpected flexibilities. Hongyu Chen et al. once modified Au (∼1.7 nm) wires with polymers to enhance the weak interactions and indeed bending coils were realized61 which directly proved that weak interactions could take control at this scale. However, when the size increases, the degree of crystallinity would also increase, and consequently their energy increases. As the number and strength of weak interactions cannot increase significantly, they would lose control quickly as the size increases.
In polymers, flexibilities originating from chains can result in versatile conformations and complicated structures, while in inorganic compounds, flexibilities are also able to generate complicated structures (Fig. 5E–G). Intricate assembling structures can be obtained in SNWs due to the effect of weak interactions. In Y(OH)3 (<1 nm) SN ribbons, coiling and rolling structures are commonly seen (Fig. 5E). In the case of indium sulfide (∼0.9 nm) SN coils, “S” and “J” shaped or coiling structures would be produced after reactions.49 Further optimizing the conditions helps to generate uniform self-assembling structures driven by weak interactions (Fig. 5F). Incubating the SN coils for several days would lead to the formation of crystal-like macroscopic solids constituted of coils. In nature, weak interactions enable the multilevel structures of biomolecules, bringing about versatile functions. However, the library of organics is smaller than that of inorganics. If we could arbitrarily design complicated structures of inorganics in a similar way to organics, the material world would be very different.
There is plenty of room for flexibilities, and several kinds of inorganic single-walled nanotubes (SWNTs) can be fabricated originating from the flexible nature of SN structures. Elongating the reaction time of the aforementioned indium sulfide system or elevating the reaction temperature would result in the formation of indium sulfide SNWTs. Further careful studies proved that the self-adjusting and OA of indium sulfide coils brings about the formation of SWNTs (Fig. 6A, C and D).
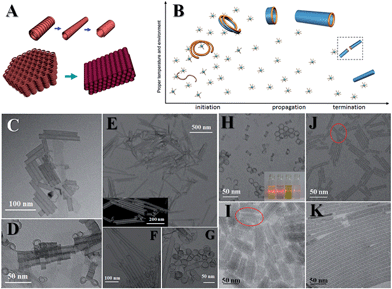 | ||
| Fig. 6 Inorganic SWNTs. (A and B) Illustrations of mechanisms. (C, E, J, K) TEM images of indium sulfide, Co(OH)2, nickel phosphate and POM SWNTs; and (D, F, G, H, I) are their corresponding coiling structures. Reprinted with permission from ref. 50 of the 2015 Nature Publishing Group (NPG). | ||
In searching for general methods of producing inorganic SWNTs, we have found that it is the flexibilities which lead to the formation of the coiling or spiral structures that initiate the formation of SWNTs apart from crystal structures.50 Co(OH)2, nickel phosphate and POM SWNTs are therefore synthesised (Fig. 6B, E–K). Here we use Co(OH)2 to detail the formation process. First, SN strands coil to form initiators, followed by the fusion of strands and formation of short nanotubes: this is the initiation stage of the formation mechanism. Since the coiled structures are asymmetrical, the tubes elongate in a unidimensional direction: this is the propagation stage. Finally, two elongating tubes fuse together to terminate the propagation, resulting in the termination stage. If the precursors formed small dots (1.2–5 nm) at the beginning, they would reside on the walls and couldn't reconstruct onto the walls, suggesting that the monomers are smaller materials, i.e. SNMs. During the synthesis, we can introduce ethanol into the system to weaken the strength of interaction, and thus double-walled or multi-walled nanotubes can be made. What's more, replacing the solvent with some other solvents of a similar dielectric constant will not influence the final products. Taking into account that the strength of interaction is mainly determined by the dielectric constant, we are sure that weak interactions play a vital role in this regime.
Though reports are scarce in this subfield, achievements are progressively advancing. Now we have paved a way from size, passing flexibility, and finally to multilevel and complicated structures such as rolls, coils and SWNTs. In this road, the brick is 1D SNMs in spite of crystal structures, and the concrete to adhere the bricks is weak interactions. Using these bricks and concrete we can build sophisticated buildings. More complicated structures or even biomolecular structures might just stand in the way not very far from now. A foreseeable interesting aspect of inorganic SNWs is their chirality. Bending, coiling or flexing strands have been achieved, then if they can be sterically hindered or guided, non-racemic products should be achievable, opening new gates for material design and even the origin of chirality. The symmetry breaking in the self-assembly of achiral molecules can be achieved by the design of structures. An achiral C3-symmetric benzene-1,3,5-tricarboxylate substituted with methyl cinnamate through ester bonds was found to form optic active assemblage in cyclohexane, driven by π–π stacking. As well as solvents, the chiral structures might also be tuned by other sophisticatedly designed small molecules,62 thermal treatment63 and light.64 In the conversion of CdTe to CdS with the help of light, right-(left-) handed circular light would result in the imbalanced right- or left-handedness of the final twisted nanoribbons.65 Even stirring66 or magnetic fields67 can induce the formation of helical structures. However, it would be hard to design chirality in SNWs without the knowledge of structures, and inorganic chirality is rare still.
Reflecting on our achievements and also learning from polymer science, we can find that our results are mainly qualitatively showing the potential but not quantitatively utilizing their flexibilities yet, while polymer physics has been already established to make full use of their latent properties. We have emphasized that SN sizes induce and influence flexibilities no matter what their constitutions are. However, it is also conceivable that the constitution would also have an impact on the quantitative results or even determine them, because in polymers the different chain structures show greatly different flexible behaviours, not to mention that we only know a little about the chain structures of SNWs. So there are lots of opportunities in SNWs.
3.3 Beyond mechanical flexibility
SNWs can do more beyond flexibility due to the virtue of their constitutions. Their elevated amount of surface atoms augments the utilization of functional atoms and reduces the amount of noble metals used. This straightforward idea has already come true in Au, Pt, Pd and their alloy SNWs, which have shown superior electrocatalytic activities.24–26,31,38 Highly active catalysts usually prefer a high amount of active sites, while it is reasonable to deem that not all surface atoms are active in nanomaterials, and thus revealing the active sites and enlarging their ratio would be a clever strategy to take full advantage of catalysts. Au (∼2 nm) wires with pentagonal cross sections feature an enormous amount of edge atoms which have been identified as active sites for the conversion of CO2 to CO, and thus they showed a superior performance compared to Au particles. And the longer the Au (∼2 nm) wires were, the better performances they showed.68 The atomic-thin SNWs might be intuitively considered as a chain of clusters. While clusters can serve as promising catalysts in various reactions,69 SNWs might be also promising. And the recycling of SNWs would be much easier than clusters due to their long chain structures.The creation of catalysts primarily relies on the inherent activities of constitutions, and thus limits the universality of SNW utilizations. However, size-derived properties would be more promising in the general realization of SNWs’ potentials. In light of polymer science and technologies, there are lots of unrealized possibilities for SNWs still, considering the merging of the merits of inorganic and organic compounds. Here we try to make some forecasts.
In the synthesis of various nanomaterials, polymers (such as polyvinyl pyrrolidone) are extensively involved as capping agents to protect from aggregations, however this lowers their performance sometimes because entire encapsulation would impede the sufficient contact of substrates with nanomaterials, or hinder charge transfer between nanomaterials. Using inorganic compounds as capping agents will alleviate such issues, because properly designed inorganic compounds with suitable energy bands will prompt the charge or mass transfer between substrates and nanomaterials as well as stabilizing them. Chalcogenides70 and POMs71 have been tested as inorganic capping agents in some cases, however, the realizations are scarce yet. Then would it be possible to use inorganic SNWs as capping ligands? The flexible long chains might be able to well enclose the nanoparticles. Together with the affinity of inorganic compounds, it would be reasonable that SNWs can serve well as capping agents without compromising products' uniformities. However, the main hindrance ahead is the stability of SNWs.
The ability to entangle enables polymers to form glue or film. From this point, SNWs may also be able to create useful glues. Nontoxic inorganic glues could be used in medical cures to heal wounds. Conducting or semiconducting inorganic glues might be a good choice for joining circuits. We are not sure whether SNWs can convolute to double helices, let alone duplicities, however, we are also not able to discount these possibilities. The ability to form films may enable the realization of portable electric devices. Since weak interactions dominate this scale, such portable electric devices might be able to self-heal. Considering the vast library of compounds, inorganic films might show ion conductivities, which are essential in fuel cells. The formation of films might also enable the invention of inorganic plastic bags. Some progress has been made in the past several years, although the compounds are not strict SNWs. SiO2 dots have been applied in tissue adhesion,72 chalcogenides have been used as solders,73 Pt2+ cations have been introduced to link ligands around nanoparticles to form inorganic gels,74 and some metal complexes enable the welding of crystals into single-crystalline entities.75
4. Other SNMs
The attentions on SNWs cannot be on par with other SNMs, such as 0D clusters and 2D SNSs. There exist plenty of reviews discussing their properties, especially SNSs such as graphene.5,69,76,77 Thus we will only talk briefly about them here from the views of chemistry. Then the combination of different nanomaterials in which at least one of them is an SNM is briefly presented to fulfil this versatile area.4.1 Clusters and SNSs
The term “cluster” doesn't explicitly specify a certain object actually, and thus the term is also widely used referring to a small dot or domain in chemistry. Here we use this term to refer to a bunch of atoms with a certain chemical formula containing metal cores. Non-metallic clusters, such as C60, POMs, and boron clusters, are excluded here. The interest in clusters preferably focuses on noble metals due to their stabilities and functions. In the past few decades, Au, Ag, Pt, Pd, and Cu clusters69,76,78,79 have been carefully explored. Among them, Au and Ag clusters might be the most well studied subjects. They showed many properties like small molecules.Their finite atom structures endow them with molecule-like energy levels, resulting in distinct light absorption properties compared to nano- or bulk metals.76 Intuitively speaking, clusters should be a natural concept expansion of small molecules, and there is no reason why clusters won't show molecular properties, i.e., chirality, isomerism, reactivity etc. The chirality of clusters usually arises from the chirality of chiral ligands, and chiral metal cores are rare.76 Isomerism is an important concept in small organics, and this concept has been found in Au clusters (Fig. 7A),80,81 suggesting that clusters might be as versatile as small organics. Clusters also provided insights into chemical bonds.
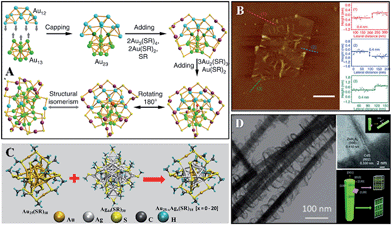 | ||
| Fig. 7 Other SNMs. (A) Anatomy of an Au38 cluster and its isomer. Reprinted with permission from ref. 80 of the 2015 NPG. (C) The core exchange reaction between Au25(SR)18 and Ag44(SR)30 (SR, alkyl/aryl thiolate). Reprinted with permission from ref. 83 of the 2016 ACS. (B) AFM images of Rh single-layered sheets. Reprinted with permission from ref. 89 of the 2014 NPG. (D) Spiral growth of ZnIn2S4 on CdS. Reprinted with permission from ref. 90 of the 2014 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
Research on reactions between clusters has started. Though much more complicated than small molecules due to the fact that all of the sites can be active, several different reaction types have been categorized: ligand exchange reactions, and core exchange reactions. The former one enabled the understanding of the localization of electronic transitions to the cluster moiety,82 and the latter one gained insights towards cluster formation. Researchers found that the mechanism of core exchange reactions might be caused by the degradation of pristine clusters to atom domains, followed by reassembling (Fig. 7C).83
The SNSs are another important regime at the SN scale. Quantum confinement effects contribute to the change in materials' electronic structures, leading to many unprecedented properties.84 Not only is this the case for the star material “graphene”, this is also true in other SNSs. Reducing the layers of MoS2 would change the indirect-band gap (few-layer and bulk) to a direct-band gap (monolayer), and the stacking manner could influence the band gap, too.85 The changing of electronic structures is able to modify the electrical chemistry activities. Co(OH)2 single-layered sheets feature a higher density of state at the Fermi level, leading to superior energy storage properties.86 Thin bimetallic hydroxides (Fe, Co, Ni) showed excellent catalytic activities toward the oxygen evolution reaction.87
In 0D and 1D SNMs, metals are significant subjects, however, metallic SNSs are relativity rare, especially single layer metallic sheets. Reducing the layers of metal sheets would greatly risk their stability, and thus challenge our skill of synthesis. The primary intention to prepare metallic SNSs is to fabricate active metal sheets with only surface atoms and no interior atoms, which might dramatically reduce the use of precious metals while maintaining their activities, since all other morphologies, even SNWs, have interior atoms. Pd (less than 10 layers) SNSs showed promising photothermal activities and 2.5 times higher activities than commercial Pd black catalysts toward the oxidation of formic acid.88 On the other hand, single-layered metal may feature unique bonds. Rh (single layer, Fig. 7B) SNSs include a δ-bonding framework, which helps to stabilize the single-layered structure.89
4.2 Complicated structures
A judicious combination of different functional materials will facilitate the design of better materials. The interplay between various parts complements each other. The characteristic advantages of SNWs are flexibility and of clusters are molecule-like properties, and of SNSs are unique electronic structures and energy bands. The addition of other materials may jeopardize the size-derived flexibilities of SNWs, and thus it would not be a clever choice to combine the flexibility of SNWs with other materials. Therefore, the candidates remain clusters and SNSs.What's more, the selections are usually not arbitrary at this SN scale. In order to take complete advantage of each material, the structures should not interfere a lot, requiring less but efficient contact. Since the engaging atoms are limited, the interactions should be as strong as possible, and thus their crystal lattices should be well matched.
Here we introduce a case utilizing the lattice matches to combine materials of different dimensions. In this case,90 ZnIn2S4 (less than 3 layers) SNSs were successfully deposited on the lateral surface of CdS (∼20 nm) wires with a tilted manner due to the matching of the lattice mismatch and dangling bonds (Fig. 7D). To be precise, the CdS wire stretched along the [001] axis, parallel to the [001] axis of ZnIn2S4 that exposed its (006) planes in its two unconfined dimensions. This judicious design promotes charge separation and transport, leading to enhanced photoelectrochemical properties. A similar design was achieved in the growth of CdS dots onto the edges of Bi2Se3 sheets.91
The support can modify the metal's properties, resulting in detrimental or beneficial effects. Graphene can modify the performances of materials simply due to its high conductivities of electrons and heat. If we only need to use the inherent properties of each constitution to play a part in catalytic reactions without changing them, then simply reducing their distance can reach such goal. Pt nanowires deposited on Ni(OH)2 SNSs can make use of the dissociation of H2O by Ni(OH)2 sheets and formation of H2 by Pt to prepare excellent electrochemical catalysts towards H2 evolution from water.92
A catalytic reaction usually evolves several elementary reactions, in which the stabilization of some intermediates and destabilization of some other intermediates can facilitate the whole reaction. Thus the interfaces between different components can play a vital role in catalyst design, meanwhile such interfaces are usually a 2D plane area and the utilization of such an area usually relies on their edges.93 The combination of different components can also help to tune the binding energies of the substance and intermediates and the energy band of the catalysts, and consequently the catalytic reaction is influenced. We have discussed this area in our previous review.94 There we discussed the controlled synthesis of interfaces and the challenging issues, as well as the link between interfaces and catalysis towards renewable energy.
5. Outlook
There is ample room at the SN scale, especially for SNWs. Weak interactions enable unprecedented flexibility in inorganic materials, obscuring the gap between SNWs and polymers. Accordingly complicated or multilevel structures can be obtained from inorganics which were previously thought to be rigid materials. In this regime, the assembling behaviours are much more interesting. The interplay between SNWs and solvents endows solutions with polymer-like rheological behaviours. The self-assembling structures of SNWs can be achieved by the self-adjustment of building blocks first, followed by the ordering of them, to construct crystal-like solids. All of these properties are exactly based on the SN size and are barely seen in conventional nanomaterials. Other SNMs, such as clusters and SNSs, all feature some astonishing properties. Clusters are the natural extension of molecules and they share many things in common, while SNSs possess unique electronic structures, resulting in relevant superior properties.However, there are many obstacles to making full use of SNMs. First, the synthesis or discovery of different SNMs is still the main hindrance now. Second, their stabilities usually suffer when they are subjected to advanced characterization tools with high energy. These two problems are common in nanoscience, however, they are much more severe in the study of SNMs. Besides, the attention on the 3 kinds of SNMs is unbalanced, SNSs attracting by far the most, and this may impede full understanding at this scale. Compared with clusters and SNSs, SNWs are much less studied and have fewer applications as yet. The old cliche that size determines many things is absolutely right, however, we are not confident whether there is a certain threshold of size at which rigid rods turn into flexible wires. What's more, the skills to strongly control the diameters of nanowires are not well-known in current “nano” communities, limiting the wide realizations of polymer-like properties. In this perspective, we are very careful in using the word “SNW”, since the diameters determined by TEM could be not accurate, especially in such a small regime. We only use the word “SNW” when predicting or with materials proved to show polymer like behaviours in this manuscript.
Opportunities accompany the challenges. As synthesis skill advances and the discovery of SNMs accumulates, the underlying potential should be progressively realized. Not to say more about SNSs and clusters, SNWs promise a radically different future for material design. Soft material is already an important aspect in technology, however, this is usually realized by organics or polymers. If one can substitute SNWs for conventional organics to design soft materials, new functions must appear since the merits of both inorganics and organics would be merged. Yet this area is largely unexplored.
The connotations of chemistry are updating as time flies. And chemistry at this level has just started to be established. This scale merges supramolecular chemistry, physical inorganic chemistry and molecular chemistry. That's to say, weak interactions are the vital external parameters which need to be finely controlled, in order to construct sophisticated structures. The intrinsic properties of each building blocks still play a substantial part in the performances of such structures, and this aspect should be ascribed to physical inorganic chemistry or molecular chemistry. Since these materials are located exactly at the scale of molecules, new notations for such materials should be developed. Here we suggest that the size of materials should be included in the expression of compounds. However, this is only a small aspect for the optimization of their names. Surface ligands are essential for the performances of nanomaterials, especially at SN scale, because the amount of them is not negligible. Researchers may use methods like calcinations or irradiations to lower the effect of surface ligands, but cannot eliminate their existence. The amounts and structures of them are very hard to quantify. Surface chemistry at this scale is highly demanding. In short, there are many things that need to be done to end such helplessness.
Acknowledgements
This work was supported by NSFC (21431003, 21521091).Notes and references
- G. A. Ozin, Adv. Mater., 1992, 4, 612–649 CrossRef CAS.
- G. M. Whitesides, J. P. Mathias and C. T. Seto, Science, 1991, 254, 1312–1319 CAS.
- L. Cademartiri and G. A. Ozin, Adv. Mater., 2009, 21, 1013–1020 CrossRef CAS.
- K. Muellen, Angew. Chem., Int. Ed., 2015, 54, 10040–10042 CrossRef CAS PubMed.
- H. Zhang, ACS Nano, 2015, 9, 9451–9469 CrossRef CAS PubMed.
- G. M. Whitesides, Angew. Chem., Int. Ed., 2015, 54, 3196–3209 CrossRef CAS PubMed.
- J.-M. Lehn, J. Inclusion Phenom., 1988, 6, 351–396 CrossRef CAS.
- N. W. Ockwig, O. Delgado-Friedrichs, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2005, 38, 176–182 CrossRef CAS PubMed.
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353–389 CrossRef CAS.
- N. P. Dasgupta, J. Sun, C. Liu, S. Brittman, S. C. Andrews, J. Lim, H. Gao, R. Yan and P. Yang, Adv. Mater., 2014, 26, 2137–2184 CrossRef CAS PubMed.
- S. Hu and X. Wang, Chem. Soc. Rev., 2013, 42, 5577–5594 RSC.
- S. Shaw and L. Cademartiri, Adv. Mater., 2013, 25, 4829–4844 CrossRef CAS PubMed.
- R. L. Penn and J. F. Banfield, Science, 1998, 281, 969–971 CrossRef CAS PubMed.
- J. Zhang, F. Huang and Z. Lin, Nanoscale, 2010, 2, 18–34 RSC.
- W. Kechun and H. Weidong, Nanotechnology, 2015, 26, 382001 CrossRef PubMed.
- C. Schliehe, B. H. Juarez, M. Pelletier, S. Jander, D. Greshnykh, M. Nagel, A. Meyer, S. Foerster, A. Kornowski, C. Klinke and H. Weller, Science, 2010, 329, 550–553 CrossRef CAS PubMed.
- Y. Bekenstein, B. A. Koscher, S. W. Eaton, P. Yang and A. P. Alivisatos, J. Am. Chem. Soc., 2015, 137, 16008–16011 CrossRef CAS PubMed.
- K.-S. Cho, D. V. Talapin, W. Gaschler and C. B. Murray, J. Am. Chem. Soc., 2005, 127, 7140–7147 CrossRef CAS PubMed.
- D. Li, M. H. Nielsen, J. R. I. Lee, C. Frandsen, J. F. Banfield and J. J. De Yoreo, Science, 2012, 336, 1014–1018 CrossRef CAS PubMed.
- S. Shen, J. Zhuang, X. Xu, A. Nisar, S. Hu and X. Wang, Inorg. Chem., 2009, 48, 5117–5128 CrossRef CAS PubMed.
- Z. Peng, H. You and H. Yang, ACS Nano, 2010, 4, 1501–1510 CrossRef CAS PubMed.
- S. Hu, H. Liu, P. Wang and X. Wang, J. Am. Chem. Soc., 2013, 135, 11115–11124 CrossRef CAS PubMed.
- H.-G. Liao, L. Cui, S. Whitelam and H. Zheng, Science, 2012, 336, 1011–1014 CrossRef CAS PubMed.
- X. Yu, D. Wang, Q. Peng and Y. Li, Chem.–Eur. J., 2013, 19, 233–239 CrossRef CAS PubMed.
- B. Y. Xia, H. B. Wu, Y. Yan, X. W. Lou and X. Wang, J. Am. Chem. Soc., 2013, 135, 9480–9485 CrossRef CAS PubMed.
- Y. Wang, S.-I. Choi, X. Zhao, S. Xie, H.-C. Peng, M. Chi, C. Z. Huang and Y. Xia, Adv. Funct. Mater., 2014, 24, 131–139 CrossRef CAS.
- Y. Tian, J. Tian, X. Li, B. Yu and T. Shi, Chem. Commun., 2011, 47, 2847–2849 RSC.
- G. Zhu, S. Zhang, Z. Xu, J. Ma and X. Shen, J. Am. Chem. Soc., 2011, 133, 15605–15612 CrossRef CAS PubMed.
- B. H. Hong, S. C. Bae, C.-W. Lee, S. Jeong and K. S. Kim, Science, 2001, 294, 348–351 CrossRef CAS PubMed.
- C. Morita, H. Tanuma, C. Kawai, Y. Ito, Y. Imura and T. Kawai, Langmuir, 2013, 29, 1669–1675 CrossRef CAS PubMed.
- L. Bu, J. Ding, S. Guo, X. Zhang, D. Su, X. Zhu, J. Yao, J. Guo, G. Lu and X. Huang, Adv. Mater., 2015, 27, 7204–7212 CrossRef CAS PubMed.
- A. Halder and N. Ravishankar, Adv. Mater., 2007, 19, 1854–1858 CrossRef CAS.
- Z. Huo, C.-K. Tsung, W. Huang, X. Zhang and P. Yang, Nano Lett., 2008, 8, 2041–2044 CrossRef CAS PubMed.
- H. Feng, Y. Yang, Y. You, G. Li, J. Guo, T. Yu, Z. Shen, T. Wu and B. Xing, Chem. Commun., 2009, 1984–1986 RSC.
- B. Li, B. Jiang, H. Tang and Z. Lin, Chem. Sci., 2015, 6, 6349–6354 RSC.
- C. Zhu, H.-C. Peng, J. Zeng, J. Liu, Z. Gu and Y. Xia, J. Am. Chem. Soc., 2012, 134, 20234–20237 CrossRef CAS PubMed.
- H. Yang, S. W. Finefrock, J. D. Albarracin Caballero and Y. Wu, J. Am. Chem. Soc., 2014, 136, 10242–10245 CrossRef CAS PubMed.
- H.-H. Li, S.-Y. Ma, Q.-Q. Fu, X.-J. Liu, L. Wu and S.-H. Yu, J. Am. Chem. Soc., 2015, 137, 7862–7868 CrossRef CAS PubMed.
- W.-B. Luo, X.-W. Gao, S.-L. Chou, J.-Z. Wang and H.-K. Liu, Adv. Mater., 2015, 27, 6862–6869 CrossRef CAS PubMed.
- H. Deng, S. Grunder, K. E. Cordova, C. Valente, H. Furukawa, M. Hmadeh, F. Gandara, A. C. Whalley, Z. Liu, S. Asahina, H. Kazumori, M. O'Keeffe, O. Terasaki, J. F. Stoddart and O. M. Yaghi, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- P. J. Waller, F. Gándara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- Y. Wu, Y. Cui, L. Huynh, C. J. Barrelet, D. C. Bell and C. M. Lieber, Nano Lett., 2004, 4, 433–436 CrossRef CAS.
- F. Yang, X. Wang, D. Zhang, K. Qi, J. Yang, Z. Xu, M. Li, X. Zhao, X. Bai and Y. Li, J. Am. Chem. Soc., 2015, 137, 8688–8691 CrossRef CAS PubMed.
- J. W. Thomson, L. Cademartiri, M. MacDonald, S. Petrov, G. Calestani, P. Zhang and G. A. Ozin, J. Am. Chem. Soc., 2010, 132, 9058–9068 CrossRef CAS PubMed.
- T. Yao, Q. Zhao, Z. Qiao, F. Peng, H. Wang, H. Yu, C. Chi and J. Yang, Chem.–Eur. J., 2011, 17, 8663–8670 CrossRef CAS PubMed.
- G. Xi, S. Ouyang, P. Li, J. Ye, Q. Ma, N. Su, H. Bai and C. Wang, Angew. Chem., Int. Ed., 2012, 51, 2395–2399 CrossRef CAS PubMed.
- L. Cademartiri, G. Guerin, K. J. M. Bishop, M. A. Winnik and G. A. Ozin, J. Am. Chem. Soc., 2012, 134, 9327–9334 CrossRef CAS PubMed.
- J. He, H. Liu, B. Xu and X. Wang, Small, 2015, 11, 1144–1149 CrossRef CAS PubMed.
- P.-P. Wang, Y. Yang, J. Zhuang and X. Wang, J. Am. Chem. Soc., 2013, 135, 6834–6837 CrossRef CAS PubMed.
- B. Ni, H. Liu, P.-P. Wang, J. He and X. Wang, Nat. Commun., 2015, 6, 8756 CrossRef CAS PubMed.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- L.-M. Lacroix, R. Arenal and G. Viau, J. Am. Chem. Soc., 2014, 136, 13075–13077 CrossRef CAS PubMed.
- J. J. Velázquez-Salazar, R. Esparza, S. J. Mejía-Rosales, R. Estrada-Salas, A. Ponce, F. L. Deepak, C. Castro-Guerrero and M. José-Yacamán, ACS Nano, 2011, 5, 6272–6278 CrossRef PubMed.
- Y. Wang, Q. Wang, H. Sun, W. Zhang, G. Chen, Y. Wang, X. Shen, Y. Han, X. Lu and H. Chen, J. Am. Chem. Soc., 2011, 133, 20060–20063 CrossRef CAS PubMed.
- C. Liu, H. Sun and S. Yang, Chem.–Eur. J., 2010, 16, 4381–4393 CrossRef CAS PubMed.
- B. Yuan and L. Cademartiri, J. Mater. Sci. Technol., 2015, 31, 607–615 Search PubMed.
- G. Cao and X. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 165407 CrossRef.
- A. J. Kulkarni, M. Zhou and F. J. Ke, Nanotechnology, 2005, 16, 2749 CrossRef CAS.
- Y. Chen, Q. Gao, Y. Wang, X. An, X. Liao, Y.-W. Mai, H. H. Tan, J. Zou, S. P. Ringer and C. Jagadish, Nano Lett., 2015, 15, 5279–5283 CrossRef CAS PubMed.
- M. A. Boles, D. Ling, T. Hyeon and D. V. Talapin, Nat. Mater., 2016, 15, 141–153 CrossRef CAS PubMed.
- J. Xu, H. Wang, C. Liu, Y. Yang, T. Chen, Y. Wang, F. Wang, X. Liu, B. Xing and H. Chen, J. Am. Chem. Soc., 2010, 132, 11920–11922 CrossRef CAS PubMed.
- G.-F. Liu, L.-Y. Zhu, W. Ji, C.-L. Feng and Z.-X. Wei, Angew. Chem., 2016, 128, 2457–2461 CrossRef.
- M. Yamauchi, T. Ohba, T. Karatsu and S. Yagai, Nat. Commun., 2015, 6, 8936 CrossRef CAS PubMed.
- Y. Cai, Z. Guo, J. Chen, W. Li, L. Zhong, Y. Gao, L. Jiang, L. Chi, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2016, 138, 2219–2224 CrossRef CAS PubMed.
- J. Yeom, B. Yeom, H. Chan, K. W. Smith, S. Dominguez-Medina, J. H. Bahng, G. Zhao, W.-S. Chang, S.-J. Chang, A. Chuvilin, D. Melnikau, A. L. Rogach, P. Zhang, S. Link, P. Král and N. A. Kotov, Nat. Mater., 2015, 14, 66–72 CrossRef CAS PubMed.
- C. Tan, X. Qi, Z. Liu, F. Zhao, H. Li, X. Huang, L. Shi, B. Zheng, X. Zhang, L. Xie, Z. Tang, W. Huang and H. Zhang, J. Am. Chem. Soc., 2015, 137, 1565–1571 CrossRef CAS PubMed.
- G. Singh, H. Chan, A. Baskin, E. Gelman, N. Repnin, P. Král and R. Klajn, Science, 2014, 345, 1149–1153 CrossRef CAS PubMed.
- W. Zhu, Y.-J. Zhang, H. Zhang, H. Lv, Q. Li, R. Michalsky, A. A. Peterson and S. Sun, J. Am. Chem. Soc., 2014, 136, 16132–16135 CrossRef CAS PubMed.
- G. Li and R. Jin, Acc. Chem. Res., 2013, 46, 1749–1758 CrossRef CAS PubMed.
- M. V. Kovalenko, M. Scheele and D. V. Talapin, Science, 2009, 324, 1417–1420 CrossRef CAS PubMed.
- M. Raula, G. Gan Or, M. Saganovich, O. Zeiri, Y. Wang, M. R. Chierotti, R. Gobetto and I. A. Weinstock, Angew. Chem., Int. Ed., 2015, 54, 12416–12421 CrossRef CAS PubMed.
- S. Rose, A. Prevoteau, P. Elziere, D. Hourdet, A. Marcellan and L. Leibler, Nature, 2014, 505, 382–385 CrossRef CAS PubMed.
- D. S. Dolzhnikov, H. Zhang, J. Jang, J. S. Son, M. G. Panthani, T. Shibata, S. Chattopadhyay and D. V. Talapin, Science, 2015, 347, 425–428 CrossRef CAS PubMed.
- A. Singh, B. A. Lindquist, G. K. Ong, R. B. Jadrich, A. Singh, H. Ha, C. J. Ellison, T. M. Truskett and D. J. Milliron, Angew. Chem., Int. Ed., 2015, 54, 14840–14844 CrossRef CAS PubMed.
- C. R. R. Adolf, S. Ferlay, N. Kyritsakas and M. W. Hosseini, J. Am. Chem. Soc., 2015, 137, 15390–15393 CrossRef CAS PubMed.
- H. Qian, M. Zhu, Z. Wu and R. Jin, Acc. Chem. Res., 2012, 45, 1470–1479 CrossRef CAS PubMed.
- Y. Zhu, S. Murali, W. Cai, X. Li, J. W. Suk, J. R. Potts and R. S. Ruoff, Adv. Mater., 2010, 22, 3906–3924 CrossRef CAS PubMed.
- J. Yan, H. Su, H. Yang, S. Malola, S. Lin, H. Häkkinen and N. Zheng, J. Am. Chem. Soc., 2015, 137, 11880–11883 CrossRef CAS PubMed.
- T.-A. D. Nguyen, Z. R. Jones, B. R. Goldsmith, W. R. Buratto, G. Wu, S. L. Scott and T. W. Hayton, J. Am. Chem. Soc., 2015, 137, 13319–13324 CrossRef CAS PubMed.
- S. Tian, Y.-Z. Li, M.-B. Li, J. Yuan, J. Yang, Z. Wu and R. Jin, Nat. Commun., 2015, 6, 8667 CrossRef CAS PubMed.
- W.-L. Li, H.-T. Liu, T. Jian, G. V. Lopez, Z. A. Piazza, D.-L. Huang, T.-T. Chen, J. Su, P. Yang, X. Chen, L.-S. Wang and J. Li, Chem. Sci., 2016, 7, 475–481 RSC.
- Y. Song and R. W. Murray, J. Am. Chem. Soc., 2002, 124, 7096–7102 CrossRef CAS PubMed.
- K. R. Krishnadas, A. Ghosh, A. Baksi, I. Chakraborty, G. Natarajan and T. Pradeep, J. Am. Chem. Soc., 2016, 138, 140–148 CrossRef CAS PubMed.
- Y. Sun, S. Gao, F. Lei, C. Xiao and Y. Xie, Acc. Chem. Res., 2015, 48, 3–12 CrossRef CAS PubMed.
- J. Yan, J. Xia, X. Wang, L. Liu, J.-L. Kuo, B. K. Tay, S. Chen, W. Zhou, Z. Liu and Z. X. Shen, Nano Lett., 2015, 15, 8155–8161 CrossRef CAS PubMed.
- S. Gao, Y. Sun, F. Lei, L. Liang, J. Liu, W. Bi, B. Pan and Y. Xie, Angew. Chem., Int. Ed., 2014, 53, 12789–12793 CrossRef CAS PubMed.
- B. Ni and X. Wang, Chem. Sci., 2015, 6, 3572–3576 RSC.
- X. Huang, S. Tang, X. Mu, Y. Dai, G. Chen, Z. Zhou, F. Ruan, Z. Yang and N. Zheng, Nat. Nanotechnol., 2011, 6, 28–32 CrossRef CAS PubMed.
- H. Duan, N. Yan, R. Yu, C.-R. Chang, G. Zhou, H.-S. Hu, H. Rong, Z. Niu, J. Mao, H. Asakura, T. Tanaka, P. J. Dyson, J. Li and Y. Li, Nat. Commun., 2014, 5, 3093 Search PubMed.
- B. Xu, P. He, H. Liu, P. Wang, G. Zhou and X. Wang, Angew. Chem., Int. Ed., 2014, 53, 2339–2343 CrossRef CAS PubMed.
- B. Xu, H. Li, H. Yang, W. Xiang, G. Zhou, Y. Wu and X. Wang, Nano Lett., 2015, 15, 4200–4205 CrossRef CAS PubMed.
- H. Yin, S. Zhao, K. Zhao, A. Muqsit, H. Tang, L. Chang, H. Zhao, Y. Gao and Z. Tang, Nat. Commun., 2015, 6, 6430 CrossRef CAS PubMed.
- B. Ni and X. Wang, Adv. Sci., 2015, 2, 1500085 Search PubMed.
- Z.-c. Zhang, B. Xu and X. Wang, Chem. Soc. Rev., 2014, 43, 7870–7886 RSC.
| This journal is © The Royal Society of Chemistry 2016 |