Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polydopamine coated manganese oxide nanoparticles with ultrahigh relaxivity as nanotheranostic agents for magnetic resonance imaging guided synergetic chemo-/photothermal therapy†
Xing
Ding
ab,
Jianhua
Liu
bc,
Junqi
Li
a,
Fan
Wang
a,
Yinghui
Wang
*a,
Shuyan
Song
*a and
Hongjie
Zhang
*a
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: yhwang@ciac.ac.cn; songsy@ciac.ac.cn; hongjie@ciac.ac.cn
bState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
cDepartment of Radiology, The Second Hospital of Jilin University, Changchun, 130022, P. R. China
First published on 6th July 2016
Abstract
Mn-based nanoparticles have been regarded as a new class of probes for magnetic resonance imaging (MRI), but their low relaxivity is the major obstacle for applications in vivo. Herein, we designed and constructed a multifunctional nanotheranostic (FA-Mn3O4@PDA@PEG) for MRI guided combinatorial chemo-/photothermal therapy (PTT) for cancer. The ultrahigh relaxivity of 14.47 mM−1 s−1 makes the nanotheranostic an excellent contrast agent for MRI in vitro and in vivo, and provides comprehensive information for tumor diagnosis. When irradiated with an 808 nm NIR laser, FA-Mn3O4@PDA@PEG exhibits a remarkably improved and synergistic therapeutic effect compared to PTT or chemotherapy alone, providing high therapeutic efficiency and low side effects of drugs. These findings are of great interest and will inspire us to develop highly effective MRI guided synergetic chemo-/photothermal therapy for cancer treatment.
Introduction
Recent developments in the field of biomedicine have triggered significant research efforts to exploit multifunctional nanocomposites (so-called “theranostics”) that integrate imaging and therapy into a single system for imaging-guided cancer therapy since they play an important role in overcoming the limitations of traditional cancer therapy.1 Chemotherapy, as a general therapeutic approach for cancer, suffers greatly from limited therapeutic efficacy and severe side effects in patients. Designing multifunctional nanocomposites, which are responsive to external stimuli for on-demand chemotherapy, and are able to perform other therapeutic approaches for combined cancer therapy, is a promising strategy for addressing these problems.2 Lately, photothermal therapy (PTT) has received significant interest owing to its high efficiency and selectivity, minimal invasiveness, and favorable biosafety in normal tissues.3 Importantly, near-infrared (NIR) light could trigger the release of drugs loaded on nanocomposites, and improve the cellular uptake of the drugs. However, commonly used photothermal agents are currently inorganic nanomaterials, such as various gold nanostructures,4 copper sulfide nanoparticles,5 and carbon nanomaterials,6 which may cause long-term toxicity concerns in their further clinical implementation. Polydopamine (PDA), a type of melanin, has been proposed as a new generation of PTT agents with great potential for in vivo applications owing to its advantages of good biodegradability, no long-term toxicity, and high photothermal conversion efficiency (∼40%).7 Along this line, constructing theranostics based on PDA, which not only exhibits a strong photothermal effect, but also provides an active surface for loading aromatic chemotherapy drugs, could effectively improve therapeutic effectiveness and alleviate side effects.For effective guidance for cancer therapy, an appropriate medical imaging modality is significant for identifying the tumor location and size, monitoring the biodistribution of nanocomposites and assessing the therapeutic efficacy. Magnetic resonance imaging (MRI), as a routine diagnostic tool in clinical medicine, is particularly attractive owing to its advantages of noninvasiveness, high spatial resolution and soft tissue contrast, and three-dimensional imaging.8 To compensate for the innate low sensitivity, a variety of contrast agents, especially positive or T1 contrast agents, are explored to increase contrast for obtaining an accurate diagnosis.9 Most T1 MRI contrast agents adopted currently are based on gadolinium (Gd3+) in the form of paramagnetic chelates or colloidal nanoparticles.10 Unfortunately, their potential renal toxicity, which is occasionally associated with nephrogenic system fibrosis, has aroused widespread concerns and promoted the search to find alternatives. Manganese (Mn)-based nanoparticles, especially Mn3O4 nanoparticles, have been regarded as promising alternatives owing to their lower intrinsic toxicity than Gd3+ ions, relatively high electronic spin, and fast water exchange rates. Nevertheless, although different size and shape Mn3O4 nanoparticles have been reported by several groups as potential T1 contrast agents,11 their relaxivities are usually lower than those of commercial Gd-based agents. Therefore, it is highly desirable to design and construct a theranostics nanoplatform based on Mn3O4 nanoparticles with high relaxivity for MRI guided synergetic chemo-/photothermal therapy, but it remains a great challenge.12
Herein, we designed and synthesized novel multifunctional Mn3O4@PDA core/shell nanocomposites using a water-in-oil microemulsion method, then applied them as MR imaging contrast agents, photothermal agents and drug carriers for combined anticancer therapy (PTT and chemotherapy), which has not been reported until now to our best knowledge (Fig. 1). Biamino polyethylene glycol (NH2–PEG–NH2) was modified on the surface of Mn3O4@PDA for further conjugation with folic acid (FA), improving the ability to target tumors and the stability in physiological conditions. The obtained FA-Mn3O4@PDA@PEG exhibited ultrahigh longitudinal relaxivity (14.47 mM−1 s−1), which is nearly three times higher than that of the commercial Magnevist (4.96 mM−1 s−1) and the highest value reported to date over all Mn-based nanoparticles. MRI experiments were employed and the results demonstrated that FA-Mn3O4@PDA@PEG can serve well as a contrast agent in vitro and in vivo. Doxorubicin (DOX) as a model anticancer drug was loaded on the surface of PDA via π–π stacking and hydrogen bonding interactions, and the release could be triggered by NIR light, reducing side effects owing to the on-demand drug release. Moreover, a synergistic effect of combined photothermal therapy and chemotherapy is expected to improve the therapeutic efficiency in both in vitro and in vivo experiments.
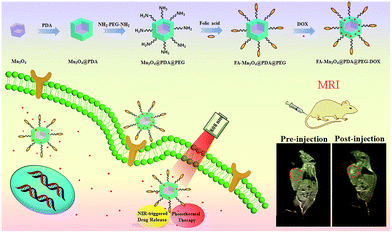 | ||
| Fig. 1 Schematic illustration of the design and synthesis of core/shell nanotheranostic Mn3O4@PDA for MRI guided synergetic chemo-/photothermal therapy. | ||
Results and discussion
The overall synthetic procedure for FA-Mn3O4@PDA@PEG is illustrated in Fig. 1. Briefly, Mn3O4 nanocrystals were firstly synthesized using a low-temperature method, and the average diameter was determined to be 16.8 nm using transmission electron microscopy (TEM), as presented in Fig. 2a. The crystalline nature of the as-prepared nanocrystals is further confirmed by the corresponding X-ray diffraction (XRD) pattern (Fig. S1, see ESI†), which can be indexed well to the hausmannite Mn3O4 (JCPDS 24-0734) phase.13 X-ray photoelectron spectroscopy (XPS) was further employed to analyze the Mn oxidation states. As shown in Fig. S2a,† in the survey spectrum, the detected peaks of Mn2p and O1s confirm the presence of Mn and O elements in the sample. The high-resolution Mn2p3/2 spectrum (Fig. S2b, see ESI†) can be resolved into two peaks at 641.2 and 642.9 eV, corresponding to the data reported for Mn2+ and Mn4+, respectively.14 Afterwards, Mn3O4 nanocrystals were coated with uniform PDA by a water-in-oil microemulsion method. The TEM image in Fig. 2b reveals that the PDA wrapping process on Mn3O4 was successful and the thickness of the PDA shell is approximately 4 nm. The XPS spectrum clearly shows the peaks of N1s, confirming the presence of PDA on the Mn3O4 (Fig. S3, see ESI†). With the aim of improving the accumulation of nanoparticles in tumors, NH2–PEG–NH2 was modified on the surface of the Mn3O4@PDA through a reaction between the amine group of PEG and PDA, and then the retained amine groups were available for reacting with FA via a carbodiimide conjugation reaction and the ether bond of the PEG chains. The amount of PEG conjugated on the surface of the Mn3O4@PDA was approximately 4%, which was assessed using thermogravimetric analysis (Fig. S4, see ESI†). The successful PEG and FA targeted functionalization is evidenced by Fourier-transform infrared spectroscopy (FT-IR) and zeta potential (Fig. S5 and S6, see ESI†). The as-obtained FA-Mn3O4@PDA@PEG is still uniform in size, and HAADF-STEM and elemental mapping analysis further confirm its core–shell structure (Fig. 2c–e).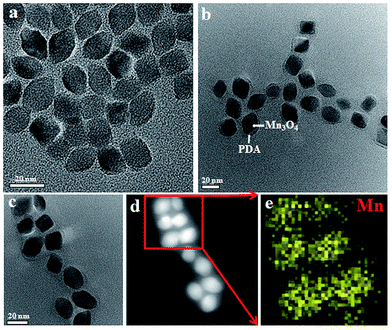 | ||
| Fig. 2 (a–c) TEM images of Mn3O4, Mn3O4@PDA, and FA-Mn3O4@PDA@PEG, (d) HAADF-STEM image of FA-Mn3O4@PDA@PEG, and (e) EDS mapping of Mn according to the image in (d). | ||
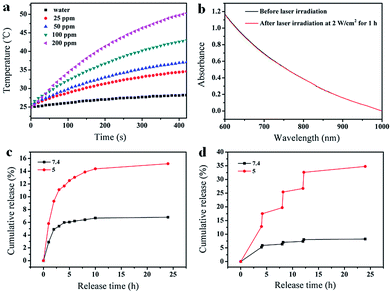
The FA-Mn3O4@PDA@PEG exhibits excellent colloidal stability in different dispersants including H2O, PBS, and 0.9% NaCl solution (Fig. S7, see ESI†). Importantly, such FA-Mn3O4@PDA@PEG shows strong NIR absorption owing to the oxidation of dopamine and the following self-polymerization process (Fig. S8, see ESI†).7a Then, to investigate the photothermal performance, FA-Mn3O4@PDA@PEG aqueous solutions with different concentrations were exposed to an 808 nm NIR laser at 2 W cm−2 for 420 s. In marked contrast to pure water, FA-Mn3O4@PDA@PEG displays effective photothermal heating of the solutions (Fig. 3a). The temperatures of the solutions increase with increasing FA-Mn3O4@PDA@PEG concentration or irradiation time. After irradiation for 420 s, a rapid temperature increase of the FA-Mn3O4@PDA@PEG solution (Mn content: 200 ppm) is noted from 25 °C to 50 °C (the temperature of efficient killing of cancerous cells). Furthermore, there is no colour change or absorption decrease of the FA-Mn3O4@PDA@PEG after irradiation by an 808 nm NIR laser for 1 h (Fig. 3b and S9, see ESI†), indicating that FA-Mn3O4@PDA@PEG has high photostability. Such high photothermal efficiency and photostability makes FA-Mn3O4@PDA@PEG superior as a promising PTT agent.
Along with the capability of acting as a photothermal agent for PTT of cancer, FA-Mn3O4@PDA@PEG can load therapeutic molecules for chemotherapy. We chose an extensively used clinical chemotherapy drug DOX as a model to evaluate the carrying and releasing properties of FA-Mn3O4@PDA@PEG. After incubating with FA-Mn3O4@PDA@PEG, DOX was loaded on the surface of FA-Mn3O4@PDA@PEG at a content of 0.8014 mg mg−1. Such high loading capacity could be attributed to the strong π–π stacking and hydrogen bond interactions of the PDA shell with DOX since PDA has abundant phenyl, amino, and hydroxyl groups on its surface.15 The subsequent test of sustained release of the drug under different pH values in Fig. 3c shows that a slow drug release from the obtained FA-Mn3O4@PDA@PEG-DOX is detected at pH 7.4. By comparison, the carrier shows a relatively faster release process at pH 5, which contributes to the protonation of the amino group in the DOX molecule that offers DOX a positive charge, thus facilitating drug release under acidic conditions. Importantly, after irradiation under an 808 nm NIR laser (2 W cm−2, 10 min for each pulse), the release of DOX sharply increases from the FA-Mn3O4@PDA@PEG-DOX at pH 5.0 (as shown in Fig. 3d). However, there is only limited DOX release from FA-Mn3O4@PDA@PEG-DOX at pH 7.4 under the same conditions. Such pH-dependent NIR-responsive release properties show that FA-Mn3O4@PDA@PEG-DOX could be applied as a better on-demand drug delivery system which can enhance antitumor efficacy and minimize side effects of the drug.
Prior to the theranostic application of FA-Mn3O4@PDA@PEG, investigation of the cytotoxicity is an important prerequisite, so the cytotoxicity of FA-Mn3O4@PDA@PEG was assessed using a traditional MTT assay. Fig. S10† shows the in vitro cell viability of human breast cancer cells (MCF-7) incubated with FA-Mn3O4@PDA@PEG with different concentrations ranging from 25 to 800 μg mL−1 for 24 h. It can be seen that the cell viabilities are still higher than 88%, even at the highest concentration of 800 μg mL−1. Such findings demonstrate the near non-cytotoxicity in vitro for all dosages of FA-Mn3O4@PDA@PEG, which implies that FA-Mn3O4@PDA@PEG can potentially serve as a theranostic probe for simultaneous MRI and combinatorial PTT and chemotherapy of cancer.
The cellular uptake process and targeting recognition capability of FA-Mn3O4@PDA@PEG-DOX were investigated using a confocal laser scanning microscope (CLSM). Fig. 4 shows CLSM images of MCF-7 cells incubated with Mn3O4@PDA@PEG-DOX and FA-Mn3O4@PDA@PEG-DOX for 1 h and 3 h at 37 °C. Obviously, the stronger red fluorescence from the cells incubated with FA-Mn3O4@PDA@PEG-DOX than that from Mn3O4@PDA@PEG-DOX can be seen, which indicates that more folate-modified nanocomposites were uptaken specifically by MCF-7 cells. These results demonstrate that the as-prepared FA-Mn3O4@PDA@PEG-DOX is a promising candidate for targeted imaging and combinatorial PTT and chemotherapy of cancer.
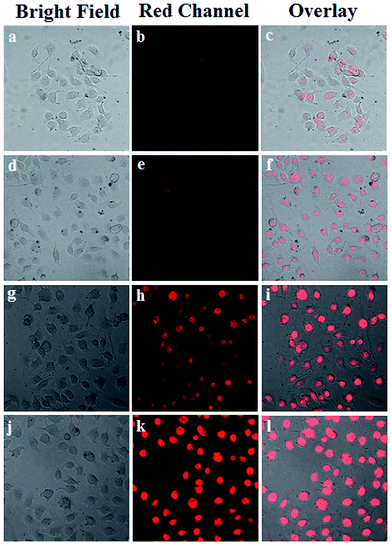 | ||
| Fig. 4 (a) CLSM images of MCF-7 cells incubated with Mn3O4@PDA@PEG-DOX for 1 h (a–c), and 3 h (d–f), and FA-Mn3O4@PDA@PEG-DOX for 1 h (g–i), and 3 h (j–l) at 37 °C. | ||
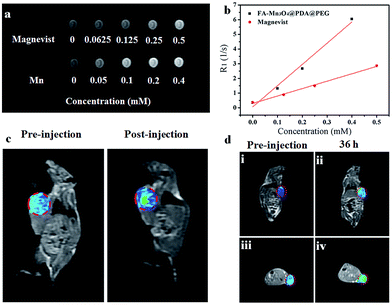
Owing to their intrinsic paramagnetic properties, Mn3O4 nanoparticles are potential T1-MRI contrast agents by accelerating the longitudinal relaxation of water protons. To evaluate the capacity of FA-Mn3O4@PDA@PEG as a T1-MRI contrast agent for diagnostics, we investigated the magnetic resonance signals of FA-Mn3O4@PDA@PEG and Magnevist (a traditional MRI contrast agent currently used in clinic) using a 1.2 T MRI system. Fig. 5a displays the T1-weighted relaxivity of FA-Mn3O4@PDA@PEG and shows that the MRI contrast is enhanced by increasing the concentration of Mn. The longitudinal relaxivity value (r1) is calculated to be 14.47 mM−1 s−1 from the slope of the concentration-dependent relaxation 1/T1 (Fig. 5b). This value is nearly three times higher than that of Magnevist (4.96 mM−1 s−1) under the same testing conditions, which may be ascribed to the greater number of free sites of FA-Mn3O4@PDA@PEG for water ligation. Most importantly, it is the highest value reported to date for Mn-based NPs (Table S1, see ESI†), which reduces the concentration required for contrast agent detection by MRI. This result further confirms that FA-Mn3O4@PDA@PEG could operate as an excellent positive MRI contrast agent.
To assess the in vivo MR imaging, we intratumorally injected FA-Mn3O4@PDA@PEG into a tumor-bearing mouse, and used a 3.0 T human MRI scanner. As shown in Fig. 5c, the significantly enhanced MRI signal can be clearly observed in the injection area. Then, FA-Mn3O4@PDA@PEG was intravenously administered into a tumor-bearing mouse to further evaluate its targeting MRI capacity in vivo. As shown in Fig. 5d, different from the homogeneous and slightly dark image before injection, the whole tumor area becomes brighter after 36 h injection. Such results demonstrate that a large amount of FA-Mn3O4@PDA@PEG accumulates in the tumor owing to the active targeting ability of the FA and the enhanced permeability and retention (EPR) effect occurring in the vessels of the cancer tissues. Importantly, the enhancement of the MRI signals in the tumor is capable of continuing for 36 hours, which provides adequate time for guiding the following treatment. The active targeting and high MRI contrast ability make FA-Mn3O4@PDA@PEG a promising candidate for accurate cancer diagnosis and locating the tumor site to guide the external NIR laser irradiation for photothermal therapy and controlling the drug release on-demand.
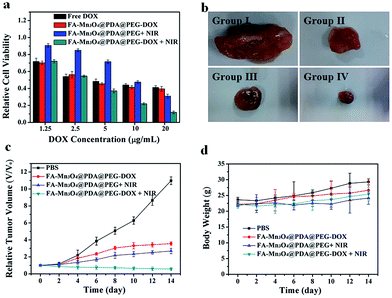
Inspired by the properties of high photothermal efficiency and NIR triggered controllable drug release, we further investigated the antitumor effects of FA-Mn3O4@PDA@PEG-DOX in vitro and in vivo. MCF-7 cells were treated with free DOX, FA-Mn3O4@PDA@PEG, and FA-Mn3O4@PDA@PEG-DOX for 10 min with or without NIR laser irradiation. As shown in Fig. 6a, after exposure to the NIR laser for 10 min, FA-Mn3O4@PDA@PEG-DOX exhibits the highest statistically cytotoxic effect at all the tested concentrations compared to the chemotherapy and photothermal therapy alone. For example, when DOX concentration is 20 μg mL−1, the cell viability for FA-Mn3O4@PDA@PEG-DOX + NIR is remarkably reduced to 11%, which is evidently lower than that of free DOX, FA-Mn3O4@PDA@PEG-DOX alone, and FA-Mn3O4@PDA@PEG + NIR. The remarkably improved therapeutic effect may be attributed to the photothermal effect, which can not only kill the cancer cells but also effectively enhance the sensitivity of the delivery and release of DOX into cells for improved chemotherapy for cancer.
To shed more light on the combined therapeutic effects of FA-Mn3O4@PDA@PEG-DOX, we further investigated the effectiveness of tumor inhibition in vivo using H22 tumor-bearing mice. The H22 (murine hepatocarcinoma) xenograft model was established by injecting H22 cancer cells into the right axilla of female Balb/c mice. The mice bearing tumors were randomly divided into four groups (group I to IV) and treated with PBS (as control), FA-Mn3O4@PDA@PEG-DOX, FA-Mn3O4@PDA@PEG + NIR irradiation, and FA-Mn3O4@PDA@PEG-DOX + NIR irradiation, respectively. The tumor dimensions were tracked every 2 days with a caliper for 14 days. After treatment, the tumors were isolated from the different groups of mice and weighed. As shown in Fig. 6b and c, the tumor growth of group II and group III was only slightly delayed compared to group I, indicating that either chemotherapy or photothermal therapy by itself exhibited only partial inhibitory effects. In contrast, when the mice were injected with FA-Mn3O4@PDA@PEG-DOX and then exposed to 808 nm NIR laser triggered photothermal therapy and drug release, the tumor growth was remarkably inhibited with tumor growth inhibition (TGI) of 94.81%, demonstrating that combination chemotherapy and PTT exhibited the optimal therapeutic efficacy. Moreover, the body weight of all groups did not decrease with the time prolonged, indicating no significant acute toxicity of our theranostic agent. Such results clearly confirm that FA-Mn3O4@PDA@PEG-DOX has potential applications for in vivo combinatorial chemotherapy and PTT for cancer.
Conclusions
In summary, a multifunctional core/shell FA-Mn3O4@PDA@PEG nanotheranostic has been successfully constructed for MRI guided combinatorial chemo-/photothermal therapy for cancer. The ultrahigh relaxivity of 14.47 mM−1 s−1 makes the nanotheranostic an excellent contrast agent for MRI in vitro and in vivo, and provides comprehensive information for tumor diagnosis. The PDA shell not only is employed as a high photothermal conversion agent for PTT and aromatic anticancer drug carrier, but also endows the nanotheranostic with robust biocompatibility owing to its natural characteristics. Moreover, the PTT and on-demand drug release are simultaneously triggered by 808 nm NIR laser irradiation, which effectually enhances the anticancer effect and reduces adverse side effects of drugs. Compared with chemotherapy or photothermal treatment alone, the combined treatment shows a synergistic effect, resulting in higher therapeutic efficacy for in vitro and in vivo cancer therapy. Therefore, this multifunctional nanocomposite is expected to be employed as a powerful theranostics platform for MRI guided therapeutics allied with significant therapeutic effectiveness but low side effects in future oncotherapy.Acknowledgements
This work was supported by financial aid from the National Natural Science Foundation of China (Grant No. 51502284, 21521092, 21590794, 51372242, and 21210001), the Hong Kong, Macao and Taiwan Science and Technology Cooperation Special Project of Ministry of Science and Technology of China (No. 2014DFT10310), the Program of Science and Technology Development Plan of Jilin Province of China (No. 20140201007GX), the National Key Basic Research Program of China (No. 2014CB643802), the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB20030300), and the Jilin Province Youth Foundation of China (20150520007JH).Notes and references
- (a) F. Greco and M. Vicent, Adv. Drug Delivery Rev., 2009, 61, 1203 CrossRef CAS PubMed; (b) D. Lane, Nat. Biotechnol., 2006, 24, 163 CrossRef CAS PubMed.
- (a) J. Liu, C. Wang, X. Wang, X. Wang, L. Cheng, Y. Li and Z. Liu, Adv. Funct. Mater., 2015, 25, 384 CrossRef CAS; (b) H. Gong, L. Cheng, J. Xiang, H. Xu, L. Feng, X. Shi and Z. Liu, Adv. Funct. Mater., 2013, 23, 6059 CrossRef CAS; (c) H. Y. Liu, D. Chen, L. L. Li, T. L. Liu, L. F. Tan, X. L. Wu and F. Q. Tang, Angew. Chem., Int. Ed., 2011, 50, 891 CrossRef CAS PubMed.
- (a) S. Lal, S. E. Clare and N. J. Halas, Acc. Chem. Res., 2008, 41, 1842 CrossRef CAS PubMed; (b) J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, D. Vinh and H. Dai, J. Am. Chem. Soc., 2011, 133, 6825 CrossRef CAS PubMed.
- (a) Y. Xia, W. Li, C. M. Cobley, J. Chen, X. Xia, Q. Zhang, M. Yang, E. C. Cho and P. K. Brown, Acc. Chem. Res., 2011, 44, 914 CrossRef CAS PubMed; (b) L. Dykman and N. Khlebtsov, Chem. Soc. Rev., 2012, 41, 2256 RSC; (c) E. Boisselier and D. Astruc, Chem. Soc. Rev., 2009, 38, 1759 RSC.
- (a) Q. Tian, M. Tang, Y. Sun, R. Zou, Z. Chen, M. Zhu, S. Yang, J. Wang and J. Hu, Adv. Mater., 2011, 23, 3542 CrossRef CAS PubMed; (b) Q. Tian, F. Jiang, R. Zou, Q. Liu, Z. Chen, M. Zhu, S. Yang, J. Wang, J. Wang and J. Hu, ACS Nano, 2011, 5, 9761 CrossRef CAS PubMed.
- (a) K. Yang, S. Zhang, G. Zhang, X. Sun, S.-T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318 CrossRef CAS PubMed; (b) K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530 RSC; (c) C. Li, S. Bolisetty, K. Chaitanya, J. Adamcik and R. Mezzenga, Adv. Mater., 2013, 25, 1010 CrossRef CAS PubMed.
- (a) Y. Liu, K. Ai, J. Liu, M. Deng, Y. He and L. Lu, Adv. Mater., 2013, 25, 1353 CrossRef CAS PubMed; (b) J. Stritzker, L. Kirscher, M. Scadeng, N. C. Deliolanis, S. Morscher, P. Symvoulidis, K. Schaefer, Q. Zhang, L. Buckel, M. Hess, U. Donat, W. G. Bradley, V. Ntziachristos and A. A. Szalay, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 3316 CrossRef CAS PubMed; (c) Y. Gao, X. Wu, L. Zhou, Y. Su and C. M. Dong, Macromol. Rapid Commun., 2015, 36, 916 CrossRef CAS PubMed.
- (a) J. A. Feshitan, F. Vlachos, S. R. Sirsi, E. E. Konofagou and M. A. Borden, Biomaterials, 2012, 33, 247 CrossRef CAS PubMed; (b) X. Q. Yang, J. J. Grailer, I. J. Rowland, A. Javadi, S. A. Hurley, V. Z. Matson, D. A. Steeber and S. Q. Gong, ACS Nano, 2010, 4, 6805 CrossRef CAS PubMed; (c) C. Sanson, O. Diou, J. Thevenot, E. Ibarboure, A. Soum, A. Brulet, S. Miraux, E. Thiaudiere, S. Tan, A. Brisson, V. Dupuis, O. Sandre and S. Lecommandoux, ACS Nano, 2011, 5, 1122 CrossRef CAS PubMed; (d) J. S. Basuki, L. Esser, H. T. T. Duong, Q. Zhang, P. Wilson, M. R. Whittaker, D. M. Haddleton, C. Boyer and T. P. Davis, Chem. Sci., 2014, 5, 715 RSC.
- (a) S. H. Wang, X. Shi, M. Van Antwerp, Z. Cao, S. D. Swanson, X. Bi and J. R. Baker, Adv. Funct. Mater., 2007, 17, 3043 CrossRef CAS; (b) X. Shi, S. H. Wang, S. D. Swanson, S. Ge, Z. Cao, M. E. Van Antwerp, K. J. Landmark and J. R. Baker, Adv. Mater., 2008, 20, 1671 CrossRef CAS; (c) H. Cai, K. Li, M. Shen, S. Wen, Y. Luo, C. Peng, G. Zhang and X. Shi, J. Mater. Chem., 2012, 22, 15110 RSC.
- (a) P. Caravan, Chem. Soc. Rev., 2006, 35, 512 RSC; (b) P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Chem. Rev., 1999, 99, 2293 CrossRef CAS PubMed; (c) R. E. Lauffer, Chem. Rev., 1987, 87, 901 CrossRef CAS; (d) L. M. Randolph, C. L. M. LeGuyader, M. E. Hahn, C. M. Andolina, J. P. Patterson, R. F. Mattrey, J. E. Millstone, M. Botta, M. Scadeng and N. C. Gianneschi, Chem. Sci., 2016, 7, 4230 RSC.
- (a) C. C. Huang, N. H. Khu and C. S. Yeh, Biomaterials, 2010, 31, 4073 CrossRef CAS PubMed; (b) J. Xiao, X. M. Tian, C. Yang, P. Liu, N. Q. Luo, Y. Liang, H. B. Li, D. H. Chen, C. X. Wang, L. Li and G. W. Yang, Sci. Rep., 2013, 3, 3424 CAS; (c) K. An, M. Park, J. H. Yu, H. B. Na, N. Lee, J. Park, S. H. Choi, I. C. Song, W. K. Moon and T. Hyeon, Eur. J. Inorg. Chem., 2012, 2148 CrossRef CAS; (d) T. Yu, J. Moon, J. Park, Y. Park II, H. B. Na, B. H. Kim, I. C. Song, W. K. Moon and T. Hyeon, Chem. Mater., 2009, 21, 2272 CrossRef CAS; (e) J. Shin, R. M. Anisur, M. K. Ko, G. H. Im, J. H. Lee and I. S. Lee, Angew. Chem., Int. Ed., 2009, 48, 321 CrossRef CAS PubMed; (f) T. L. Ha, H. J. Kim, J. Shin, G. H. Im, J. W. Lee, H. Heo, J. Yang, C. M. Kang, Y. S. Choe, J. H. Lee and I. S. Lee, Chem. Commun., 2011, 47, 9176 RSC.
- (a) M. Nafiujjaman, M. Nurunnabi, S. Kang, G. R. Reeck, H. A. Khand and Y. Lee, J. Mater. Chem. B, 2015, 3, 5815 RSC; (b) H. Hu, A. Dai, J. Sun, X. Li, F. Gao, L. Wu, Y. Fang, H. Yang, L. An, H. Wu and S. Yang, Nanoscale, 2013, 5, 10447 RSC; (c) R. Savla, O. B. Garbuzenko, S. Chen, L. Rodriguez-Rodriguez and T. Minko, Pharm. Res., 2014, 31, 3487 CrossRef CAS PubMed.
- Y. Tan, C. Xu, G. Chen, X. Fang, N. Zheng and Q. Xie, Adv. Funct. Mater., 2012, 22, 4584 CrossRef CAS.
- (a) V. Di Castro and G. Polzonetti, J. Electron Spectrosc. Relat. Phenom., 1989, 48, 117 CrossRef CAS; (b) S. Ardizzone, C. L. Bianchi and D. Tirelli, Colloids Surf., A, 1998, 134, 305 CrossRef CAS; (c) K. J. Kim, M. S. Park, J. H. Kim, U. Hwang, N. J. Lee, G. Jeong and Y. J. Kim, Chem. Commun., 2012, 48, 5455 RSC.
- F. Liu, X. He, Z. Lei, L. Liu, J. Zhang, H. You, H. Zhang and Z. Wang, Adv. Healthcare Mater., 2015, 4, 559 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, supplementary figures and table of relaxivity of the present work and reported Mn-based nanoparticles. See DOI: 10.1039/c6sc01320a |
| This journal is © The Royal Society of Chemistry 2016 |