Enhanced reactivity of high-index surface platinum hollow nanocrystals†
Edgar
González
abc,
Florind
Merkoçi
a,
Raúl
Arenal
de,
Jordi
Arbiol
fg,
Joan
Esteve
h,
Neus G.
Bastús
a and
Víctor
Puntes
*ag
aInstitut Català de Nanotecnologia (ICN), Campus de la UAB, 08193 Bellaterra, Spain
bCentro de Ciencia y Tecnología Nanoescalar, 111311 Bogotá, Colombia
cFacultad de Ingeniería, Instituto Geofísico Pontificia Universidad Javeriana, 110231 Bogotá, Colombia
dInstituto de Nanociencia de Aragon, Universidad de Zaragoza, 50018 Zaragoza, Spain
eARAID Foundation, Paseo Maria Agustin, 36, 50004 Zaragoza, Spain
fInstitut de Ciència de Materials de Barcelona (ICMAB-CSIC), Campus de la UAB, 08193 Bellaterra, Spain
gInstitució Catalana de Recerca i Estudis Avançats (ICREA), 08010 Barcelona, Spain. E-mail: victor.puntes@icn.cat
hDepartament de Física Aplicada i Óptica, Universitat de Barcelona, 08028, Barcelona, Spain
First published on 17th November 2015
Abstract
The precise morphological control of the surface of inorganic nanocrystals (NCs) is critical for the understanding of the unique properties of the materials at the nanoscale and useful in a wide range of applications, such as catalysis, where the development of highly active and low-cost materials represents a landmark for the development of industrial technologies. Here we show how combining solid state chemistry and colloidal synthesis allows us to prepare exotic materials, in particular, PtAg@Pt single-crystal hollow NCs with high-index planes synthesized at room temperature by controlled corrosion of silver templates, which minimize Pt consumption and maximize surface reactivity.
Introduction
Noble metal nanocrystals (NCs) have been extensively studied recently because of their promising wide application range owing to their intriguing optical and electronic properties, which arise from their constitutional and precise morphological variations. This is dramatic in the case of heterogeneous catalysis, where low-coordination surface atomic sites are largely responsible for the observed behavior.1,2 Consequently, it is known that the catalytic properties of metal and alloy NCs strongly depend on atomic local features such as coordination and crystallographic index planes, which in turn are determined by the method of preparation.3–5 A very important case is that of the expensive rare metal Pt and its widespread use in low temperature polymer electrolyte (proton exchange) membrane fuel cells.6 Consequently, very small NCs (<5 nm) to maximize the surface to volume (mass) ratio have been synthesized to increase the (economic) competitiveness of these materials. However, such small NCs are unstable and tend to sinter or detach from the support during operation.7,8 This has fuelled up investigation on hollow Pt NCs, as an efficient alternative to increase the surface to mass ratio.7,9–11 Initially, hollow Pt NCs were prepared by galvanic replacement using Co12 and Ag templates.13 Due to the high immiscibility of Ag and Pt at low temperature, NCs with very rough surfaces where obtained when Pt had to grow on the top of Ag. Because of this, other groups prepared Pd templates to produce hollow Pt NCs with smoother surfaces.14,15 Recently, ultrathin (down to 3 atomic layers) hollow Pt NCs have been prepared reaching a new limit in the optimization of the surface to mass ratio of Pt NCs.16 In addition to reducing the effective mass, the increase of surface activity per unit area is also a good strategy to increase Pt NC performance. Indeed, it is well known that for most catalytic reactions, high-index planes (low coordinated atoms), associated with atomic steps, edges and kinks have been considered responsible for the enhancement of catalytic performance.2 Thus, high-index surface Pt-based NCs have been sought and prepared showing strong thermal and chemical stability and enhanced catalytic activity (up to 400%).2,17,18In order to produce stable high-index faceted NCs, we observed that the processes of dissolution and deposition during galvanic replacement reactions could be used to control the atomic roughness of the NCs and promote the alloying of Pt and Ag despite their immiscibility.11 It is important to note that solid state diffusion processes are dramatically enhanced at the nanoscale (up to 10 orders of magnitude19). Thus, corrosion processes at the nanoscale show concomitant diffusion and migration of atoms in the solid matrix, allowing the production of designed materials by unexpected ways. This is the case of transformations of NCs' composition while maintaining their morphological integrity20 or the carving of complex patterns in multimetallic hollow NCs,19 where the void is an added degree of freedom to control the morphology in a regime where the structure as much as the composition determines the activity. This plasticity is manifested in enhanced diffusion coefficients,19,21,22 and is also reflected in high tolerance to crystal strain,23 low tolerance to crystal defects,24 decrease of the melting temperature, and the ease of defect curing in NCs.25,26 Here we show how to prepare different hollow Pt NCs and especially PtAg@Pt single-crystals showing stable high-index planes (such as {950}, {910}, {540} and/or {4−10}) synthesized at room temperature by controlled corrosion of silver templates, which minimize Pt consumption and maximize surface reactivity.
Results and discussion
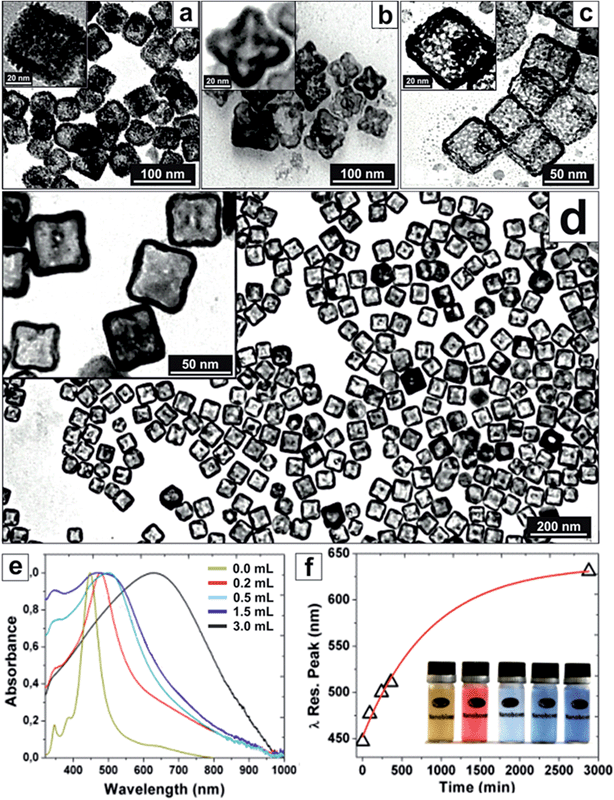
Platinum NCs grown on silver templates tend to grow in a Volmer–Weber (island by island) mode due to their lattice mismatch and high immiscibility at low T. Therefore, normally, few nm (2–3 nm) rough Pt NC surfaces are obtained when Ag templates are used,9,13 as those shown in Fig. 1a. Thus, to promote PtAg alloying and a more epitaxial growth, several strategies were employed allowing metal inter-diffusion to play a significant role in NC synthesis.27,28 Thus, by working at low T, using insoluble platinum salts (where Pt2+ is slowly solubilized and consumed maintaining a low but constant concentration of reactive Pt in solution), and using mild reducing agents, allows to decrease Pt2+ avidity for the Ag 5s electrons. It is also worth noting that in the mixture, as a by-product, there is generation of highly insoluble AgCl and AgBr compounds, which here are solubilized by the presence of surfactants.19 As a consequence of these reaction conditions, the island growth mode (rough surfaces) is avoided and interdiffusion is promoted allowing the formation of NC smooth surfaces exposing high index planes and PtAg alloys (Fig. 1d).Thus, by using silver NCs as sacrificial templates, polyvinylpyrrolidone (PVP), benzylalkyldimethylammonium chloride (BDAC) and cetyltrimethylammonium bromide (CTAB) as surfactants and complexing agents, platinum salts (K2PtCl4, H2PtCl6, PtCl2) as precursors and oxidizing agents, and ascorbic acid as the reducing agent, distinct hollow PtAg and Pt NCs were obtained (Fig. 1). We observed that Pt dosing was critical to control the reaction towards the desired products. Initially, when using K2PtCl4 as a precursor, controlled corrosion was not achieved (Fig. S1c†). However, when a more oxidizing form of Pt (H2PtCl6) was used, hollow PtAg nanoflowers with symmetrically distributed pores were systematically obtained (Fig. 1b). By modifying the reaction conditions, using CTAB as the surfactant and PtCl2 as the precursor, which is poorly soluble in water, hollow NCs were formed displaying the formation of Pt islands at the NC rough surface (Fig. 1a), as a witness of a Volmer–Weber type growth due to the low affinity of the substrate for the deposited material.29 This mode of growth can be seen when using a number of surfactants and precursors (Fig. S2†). In those cases, as expected, no degree of PtAg alloying was observed. However, using BDAC as the surfactant and PtCl2 as the precursor resulted in the formation of single-crystal nanoboxes with smooth surfaces exhibiting high-index atomic planes, forming a stable and homogeneous alloy between Ag and Pt, coated by a thin overlayer of pure platinum (PtAg@Pt hollow NCs) (Fig. 1d and 2). Here, it seems that the Volmer–Weber growth was suppressed allowing an atom-by-atom Pt growth to take place.
Under our conditions, the formation of these hollow NCs took up to 50 hours. Aliquots taken at intermediate times of the reaction showed intermediate corrosion states, in which we could appreciate also the formation of Kirkendall voids that disappeared in the final product (Fig. S1†). Finally, further oxidation of these structures with Pt2+ led to PtAg dealloying and the formation of highly porous Pt nanocages (Fig. 1c). During this time the colour of the solution changed with a strong redshift and a dramatic broadening of the absorbance spectrum, indicative of the formation of plasmonic cavities13,30–32 (Fig. 1e and f). Note that these observed absorption bands are different from those obtained for other reported Pt–Ag NCs, which indeed consisted of layers of metals rather than a mixture (alloy) at the atomic level.33
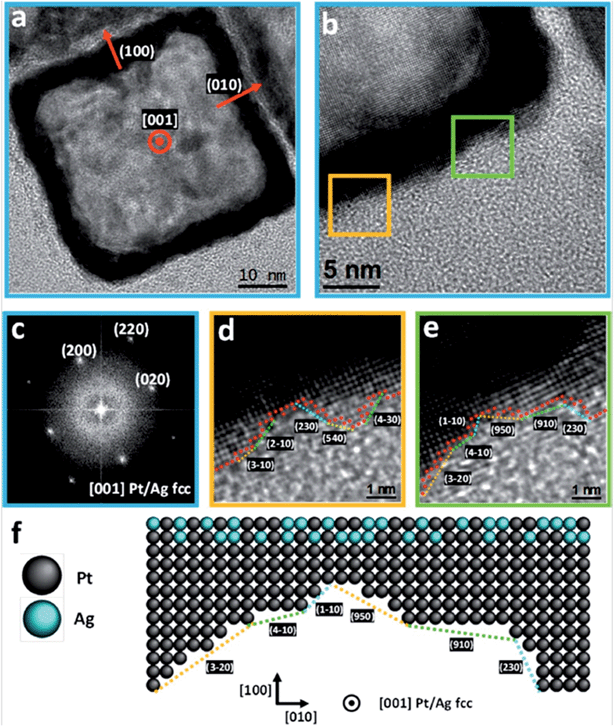
Fig. 2 shows high-resolution TEM images of the obtained PtAg@Pt smooth surface hollow NCs. The outer surface roughness is composed of monoatomic steps leading to highly reactive high-index surfaces (Fig. 2d–f, and Fig. S3 and S4†) associated with a known higher catalytic activity.2,17,34 The atomic representation extracted from the atomic-resolved image corresponds to the 2D projection of the 3D structure. Therefore, the real roughness is smoothed by this integration and the real atom de-coordination (or very low coordination) is even higher. Note that for standard high performance Pt NCs their surfaces are much smoother.16,35 For comparison, an example of a perfect {100} solid Pt nanocube of similar dimensions showing monoatomic flat surfaces36,37 is presented in Fig. S5.† All these studied nanoboxes are monocrystalline (Fig. 2c) and narrowly dispersed with no other by-product present in the synthesis solution (Fig. 1d).
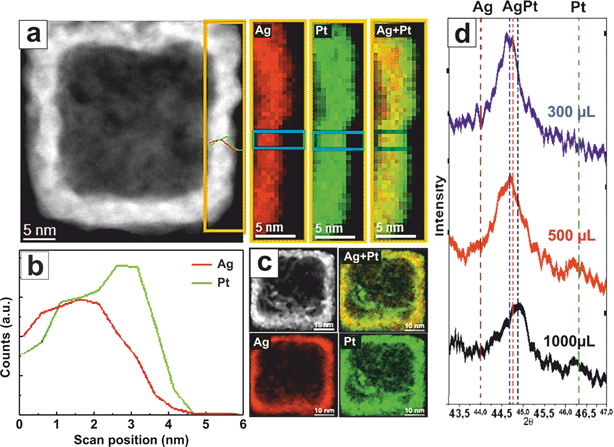
In Fig. 3 we present a detailed high-resolution Energy-Dispersive X-ray Spectroscopy (EDS) analysis of one of the lateral facets of a representative PtAg@Pt nanobox. The wall has a thickness of around 5 nm, the first – inner – 2.5 nm surface is composed of a PtAg alloy while the external – outer – 2.5 nm surface is mainly composed of Pt, as observed in the maps and the EDS profile (Fig. 3a–c). This anisotropy together with the formation of a stable PtAg solid solution are indicative footprints of the synthetic process. It is known that Pt in the bulk form does not readily undergo solid–solid diffusion with Ag at temperatures below 900 K.13
However, theoretical studies, and some limited experimental data have shown that miscibility between metal elements can be significantly altered at the nanoscale, including the formation of PtAg alloys in the immiscible area,38–40 despite their different reduction kinetics and thermodynamic immiscibility. In fact, due to the higher number of vacancies and enhanced mobility at the nanoscale,24 the formation of large PtAg-alloy single crystals at RT is enabled. Of course, when forced to mix at RT, Pt and Ag should relax the consequent crystal strains in highly atomic-uneven surfaces. These PtAg alloy NCs are then passivated and stabilized with a final Pt deposition (∼2–3 nm) which seals all the open galvanic gaps, as in the case of the originally reported Au nanoboxes.41 The PtAg alloy at RT is forcedly strained and therefore serves as a substrate for the deposition of the high-index Pt facets. The formation of the PtAg alloy is confirmed by the EDS spectra and the XRD patterns (Fig. 3d and S6†).
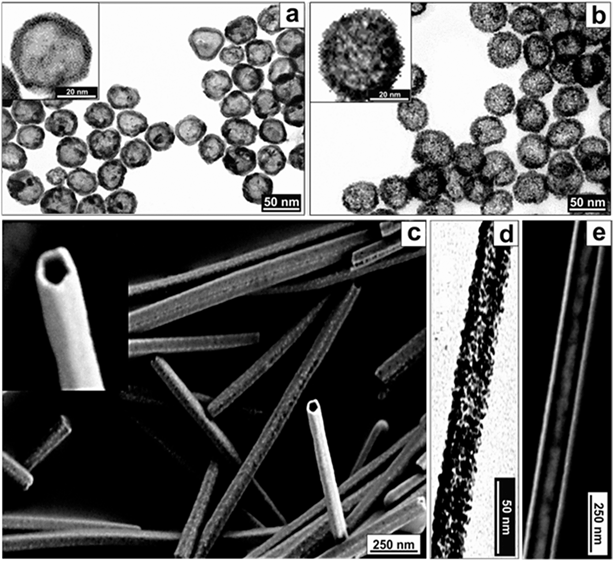
This synthetic strategy can be applied to different Pt/Ag compositions (Fig. 3d and S6 and S7†) and sacrificial templates (Fig. 4). Thus, using Ag spheres or wires as templates, both the immiscible Volmer–Weber (rough) growth and the generation of (smooth) PtAg@Pt single crystal hollow spheres and tubes could be achieved (Fig. 4). It is important to note that Pt nanotubes, because of their shape and aspect ratio, are very appealing since they could act, for example, as both catalysts and electrodes in fuel cells, where one main drawback is the degradation of the C electrodes that support the Pt catalytic centers.42
As Pt surfaces are known to be excellent catalysts, we wanted to test the surface reactivity of different types of engineered Pt NC surfaces. We compared three different types of particles with different types of Pt NCs: (i) rough surfaces produced by a Volmer–Weber growth, similar to those reported by Zhang et al.,40 (ii) smooth ones (PtAg@Pt nanoboxes) produced by strained epitaxial growth, and (iii) treated commercial Pt black. Note that here rough surfaces display high atomic coordination and low crystallographic index planes while smooth surfaces show low atomic coordination and very high crystallographic index planes. We use processed Pt black as the control material (see methods in the ESI†). The process consists of suspending in a PVP solution the Pt black as received, leaving it for a while and then discarding the sediment to select the fraction of the smaller NPs present in Pt black. Otherwise, large crystal and aggregates will further decrease the Pt black performance when compared to the hollow NCs. The overall reduction of p-nitrophenol (p-NP) to p-aminophenol (p-AP) by sodium borohydride (NaBH4) has been chosen as a model reaction, as established in the literature,43–45 to evaluate the surface reactivity, related to the catalytic capability of our different Pt NCs. This reaction has been proved effective to test the efficacy and selectivity of several noble metal catalysts.28,46,47 The reduction cycles were carried out under the same conditions for all samples and then normalized to the NC concentration, total mass and total surface area (Table S1†).
The conversion rate efficiency during 10 consecutive cycles of 25 minutes reduction (with no catalyst purification between each cycle) is shown in Fig. 5 (see experimental details). Fig. 5a plots the UV-vis spectra showing the decay of the p-NP signal of normalized absorption in the 300–500 nm visible range, where it can be clearly seen how the presence of Pt NCs in the solution leads to the rapid vanishment of the p-NP absorption peak, being faster when the smooth surface (high index planes) PtAg@Pt NCs are used. In contrast, the signal coming from the p-NP is much more stable in the presence of treated Pt black. An intermediate signal decay behavior is observed when Pt NCs with rough surfaces are used. These different behaviors are very clear when plotting the maximum absorption (at 400 nm) versus time (Fig. 5b) for each individual cycle. Here the behavior associated with the different surface reactivity of the NCs is clearly seen, and translates into faster reaction rates as we engineer the NC surface. This is properly assessed when calculating the apparent kinetic rate constants (kapp), normalized to the material mass (cost) and surface area (intrinsic activity per unit area), as plotted in Fig. 5c–e. The obtained data were analyzed according to the first-order rate law. The negative natural logarithm of the absorbance at 400 nm was plotted against time and the steepest part of the curve was fitted, the slope of which was considered the apparent rate constant kapp. It is clearly shown here the superior surface reactivity (up to two orders of magnitude) and higher stability of smooth (high index) surfaces vs. the rough (low index) or controls (Pt black). Regarding kapp, it is important to note its stability and repeatability, indicating the robustness of the PtAg@Pt NCs during operation, which allows us to conclude the absence of sample poisoning under the present conditions. The observed progressive decrease in reaction yield with time is attributed to the increased presence of products in the reaction vial as the number of cycles goes by. Importantly, the kapp remains constant throughout all the cycles. Note that despite possessing a well defined Pt surface composition, the presence of Ag in the inner layer as the remnant of the original Ag template may also have some contribution to the observed catalytic behavior. According to the literature, the presence of Ag in the Pt catalyst may improve, mainly, its structural robustness after catalytic cycling.38 Other effects related to modifications of the Fermi levels due to alloying and proximity effects should not be discarded and will be the subject of future studies.
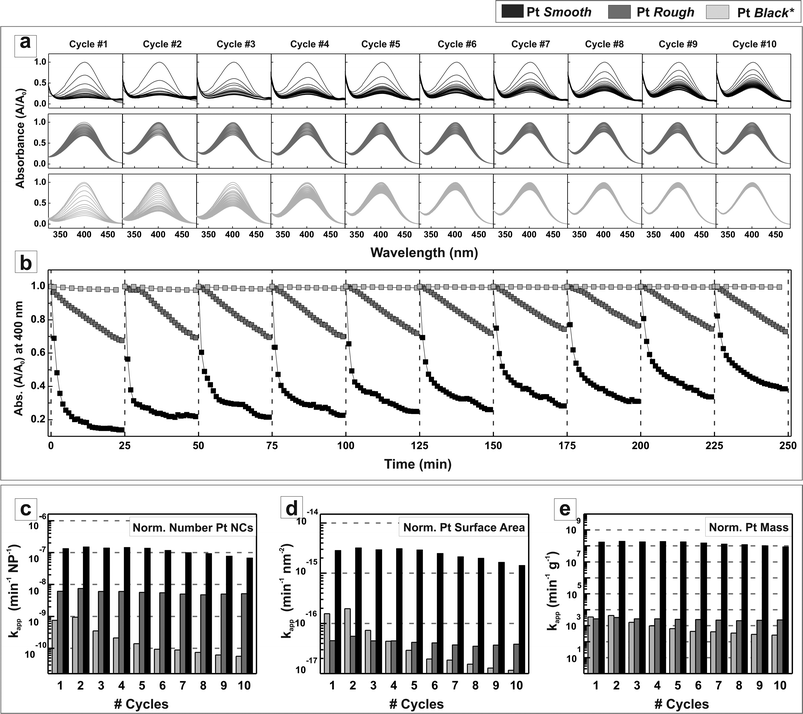 | ||
| Fig. 5 Conversion rate efficiency in the overall degradation of p-nitrophenol into p-aminophenol in the presence of Pt NCs in 10 consecutive 25 minutes reduction cycles. (a) Decay of the normalized absorbance in the 300–500 nm visible range, indicating the reduction of nitrophenolate ions when using the three different surface shaped PtNCs: Pt ‘Smooth’, Pt ‘Rough’ and Pt ‘Black*’ (*treated commercial Pt black) (b) decay of the normalized absorbance at 400 nm. Since the Pt ‘Rough’ and Pt ‘Smooth’ catalysed reactions involved the same amount of total nanoparticles while the Pt ‘Black’ one had 32 times more (see Table S1†), the absorbance of nitrophenolate when using Pt black was re-normalized (in terms of NCs per mL) for its comparison. The kapp values obtained for each PtNC in each reduction cycle were plotted against its respective cycle number after normalizing them as a function of (c) total number of NCs, (d) total amount of Pt surface and (e) total amount of Pt mass needed to carry out the reaction. | ||
Pt NC's stability was further studied by analyzing the morphology and performance of the NCs before and after the reduction cycles and thermal stress. For the later, the NCs, prepared at RT, were boiled at 100 °C for 24 hours, exposed to e-beam showers (field emission 300 keV) for 30 minutes, and kept under intense artificial sun exposure for 7 consecutive days. In any case, no morphological alteration was detected (see TEM images in Fig. S9†). Additionally, after purification at the end of the 10 reduction cycles or the thermal processes, recovered NCs just performed as initially. This observed stability could be related to the pinning of the high-index planes by the underneath strained PtAg alloy and the hollow nature of these NCs (since heat is preferably generated in the bulk and dissipated at the surface). Also, it has been observed how the presence of Ag in Pt stabilizes the Pt nanostructure, what has been attributed to the shift of the d-band center of Pt in PtAg alloys.38 This is important since nanobox collapse and dealloying during use would be otherwise expected.
In summary, by slowing down the reaction and controlling Pt dosing, we can obtain a set of Pt NCs with increasing reactivity per unit mass and area. This strategy enables the control of the ratio between Pt and Ag in the alloy with high morphological and structural quality, and the process provides novel insight into the advanced control of matter at the nanoscale. Finally, this process is also advantageous because it is carried out at room temperature, which results in a more cost-effective and scalable process.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge funding from grants 2009 SGR 770, 2009 SGR 776, 2014 SGR 612, 2014 SGR 1638, XaRMAE, CSD2009-00013, MAT2006-13572-C02-02, MAT2009-14734-C02-01, MAT2010-15138, MAT2012-33330, MAT2014-51480-ERC, VALTEC09-2-0085 and VALTEC09-2-0089. J. A. acknowledges NanoAraCat funding for the use of advanced (S)TEM facilities at Instituto de Nanociencia de Aragón–Laboratorio de Microscopia Avanzada. N. G. B. acknowledges financial support through the Juan de la Cierva program (JCI-2011-09678) and European Commission for the Career Integration Grant (322153-MINE) Marie Curie Action. A patent application related to this work was filed on 14 March 2011 (European Union application number EP11158024.7).References
- G. A. Somorjai, A. M. Contreras, M. Montano and R. M. Rioux, Clusters, surfaces, and catalysis, Proc. Natl. Acad. Sci. U. S. A., Early Ed., 2006, 103(28), 10577–10583 CrossRef CAS PubMed.
- T. Yu, D. Y. Kim, H. Zhang and Y. Xia, Platinum Concave Nanocubes with High-Index Facets and Their Enhanced Activity for Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2011, 50(12), 2773–2777 CrossRef CAS PubMed.
- N. S. Porter, H. Wu, Z. Quan and J. Fang, Shape-Control and Electrocatalytic Activity-Enhancement of Pt-Based Bimetallic Nanocrystals, Acc. Chem. Res., 2013, 46(8), 1867–1877 CrossRef CAS PubMed.
- J. Gu, Y.-W. Zhang and F. Tao, Shape control of bimetallic nanocatalysts through well-designed colloidal chemistry approaches, Chem. Soc. Rev., 2012, 41(24), 8050–8065 RSC.
- J. Chen, B. Lim, E. P. Lee and Y. Xia, Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications, Nano Today, 2009, 4(1), 81–95 CrossRef CAS.
- J. Wu and H. Yang, Platinum-Based Oxygen Reduction Electrocatalysts, Acc. Chem. Res., 2013, 46(8), 1848–1857 CrossRef CAS PubMed.
- H. Zhang, M. Jin and Y. Xia, Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd, Chem. Soc. Rev., 2012, 41(24), 8035–8049 RSC.
- F. De Bruijn, V. Dam and G. Janssen, Review: durability and degradation issues of PEM fuel cell components, Fuel Cells, 2008, 8(1), 3 CrossRef CAS.
- X. Xia, Y. Wang, A. Ruditskiy and Y. Xia, 25th Anniversary Article: Galvanic Replacement: A Simple and Versatile Route to Hollow Nanostructures with Tunable and Well-Controlled Properties, Adv. Mater., 2013, 25(44), 6313–6333 CrossRef CAS PubMed.
- Z. Chen, M. Waje, W. Li and Y. Yan, Supportless Pt and PtPd Nanotubes as Electrocatalysts for Oxygen-Reduction Reactions, Angew. Chem., Int. Ed., 2007, 46(22), 4060–4063 CrossRef CAS PubMed.
- H. M. Chen, R.-S. Liu, M.-Y. Lo, S.-C. Chang, L.-D. Tsai, Y.-M. Peng and J.-F. Lee, Hollow Platinum Spheres with Nano-Channels: Synthesis and Enhanced Catalysis for Oxygen Reduction, J. Phys. Chem. C, 2008, 112(20), 7522–7526 CAS.
- H.-P. Liang, H.-M. Zhang, J.-S. Hu, Y.-G. Guo, L.-J. Wan and C.-L. Bai, Pt Hollow Nanospheres: Facile Synthesis and Enhanced Electrocatalysts, Angew. Chem., 2004, 116(12), 1566–1569 CrossRef.
- J. Chen, B. Wiley, J. McLellan, Y. Xiong, Z.-Y. Li and Y. Xia, Optical Properties of Pd–Ag and Pt–Ag Nanoboxes Synthesized via Galvanic Replacement Reactions, Nano Lett., 2005, 5(10), 2058–2062 CrossRef CAS PubMed.
- B. Lim, M. Jiang, P. H. C. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Pd–Pt Bimetallic Nanodendrites with High Activity for Oxygen Reduction, Science, 2009, 324(5932), 1302–1305 CrossRef CAS PubMed.
- L. Wang and Y. Yamauchi, Metallic Nanocages: Synthesis of Bimetallic Pt–Pd Hollow Nanoparticles with Dendritic Shells by Selective Chemical Etching, J. Am. Chem. Soc., 2013, 135(45), 16762–16765 CrossRef CAS PubMed.
- L. Zhang, L. T. Roling, X. Wang, M. Vara, M. Chi, J. Liu, S.-I. Choi, J. Park, J. A. Herron, Z. Xie, M. Mavrikakis and Y. Xia, Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets, Science, 2015, 349(6246), 412–416 CrossRef CAS PubMed.
- N. Tian, Z.-Y. Zhou, S.-G. Sun, Y. Ding and Z. L. Wang, Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity, Science, 2007, 316(5825), 732–735 CrossRef CAS PubMed.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, Highly Concave Platinum Nanoframes with High-Index Facets and Enhanced Electrocatalytic Properties, Angew. Chem., Int. Ed., 2013, 52(47), 12337–12340 CrossRef CAS PubMed.
- E. González, J. Arbiol and V. F. Puntes, Carving at the Nanoscale: Sequential Galvanic Exchange and Kirkendall Growth at Room Temperature, Science, 2011, 334(6061), 1377–1380 CrossRef PubMed.
- Y. Yin, R. M. Rioux, C. K. Erdonmez, S. Hughes, G. A. Somorjai and A. P. Alivisatos, Formation of Hollow Nanocrystals Through the Nanoscale Kirkendall Effect, Science, 2004, 304(5671), 711–714 CrossRef CAS PubMed.
- Y. Yin, C. K. Erdonmez, A. Cabot, S. Hughes and A. P. Alivisatos, Colloidal Synthesis of Hollow Cobalt Sulfide Nanocrystals, Adv. Funct. Mater., 2006, 16(11), 1389–1399 CrossRef CAS.
- B. Wiley, T. Herricks, Y. Sun and Y. Xia, Polyol Synthesis of Silver Nanoparticles:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Use of Chloride and Oxygen to Promote the Formation of Single-Crystal, Truncated Cubes and Tetrahedrons, Nano Lett., 2004, 4(9), 1733–1739 CrossRef CAS.
Use of Chloride and Oxygen to Promote the Formation of Single-Crystal, Truncated Cubes and Tetrahedrons, Nano Lett., 2004, 4(9), 1733–1739 CrossRef CAS. - G. Catalan, A. Lubk, A. H. G. Vlooswijk, E. Snoeck, C. Magen, A. Janssens, G. Rispens, G. Rijnders, D. H. A. Blank and B. Noheda, Flexoelectric rotation of polarization in ferroelectric thin films, Nat. Mater., 2011, 10(12), 963–967 CrossRef CAS PubMed.
- G. Guisbiers, Schottky Defects in Nanoparticles, J. Phys. Chem. C, 2011, 115(6), 2616–2621 CAS.
- Y. Yin and A. P. Alivisatos, Colloidal nanocrystal synthesis and the organic–inorganic interface, Nature, 2005, 437(7059), 664–670 CrossRef CAS PubMed.
- R. L. Penn and J. F. Banfield, Imperfect Oriented Attachment: Dislocation Generation in Defect-Free Nanocrystals, Science, 1998, 281(5379), 969–971 CrossRef CAS PubMed.
- Y. Yu, Q. Zhang, Q. Yao, J. Xie and J. Y. Lee, Architectural Design of Heterogeneous Metallic Nanocrystals—Principles and Processes, Acc. Chem. Res., 2014, 47(12), 3530–3540 CrossRef CAS PubMed.
- N. G. Bastús, F. Merkoçi, J. Piella and V. Puntes, Synthesis of Highly Monodisperse Citrate-Stabilized Silver Nanoparticles of up to 200 nm: Kinetic Control and Catalytic Properties, Chem. Mater., 2014, 26(9), 2836–2846 CrossRef.
- X. W. Lou, L. A. Archer and Z. Yang, Hollow Micro-/Nanostructures: Synthesis and Applications, Adv. Mater., 2008, 20(21), 3987–4019 CrossRef CAS.
- J. Chen, B. Wiley, Z. Y. Li, D. Campbell, F. Saeki, H. Cang, L. Au, J. Lee, X. Li and Y. Xia, Gold Nanocages: Engineering Their Structure for Biomedical Applications, Adv. Mater., 2005, 17(18), 2255–2261 CrossRef CAS.
- J. Gao, X. Ren, D. Chen, F. Tang and J. Ren, Bimetallic Ag–Pt hollow nanoparticles: Synthesis and tunable surface plasmon resonance, Scr. Mater., 2007, 57(8), 687–690 CrossRef CAS.
- Q. Zhang, J. Xie, J. Y. Lee, J. Zhang and C. Boothroyd, Synthesis of Ag@AgAu Metal Core/Alloy Shell Bimetallic Nanoparticles with Tunable Shell Compositions by a Galvanic Replacement Reaction, Small, 2008, 4(8), 1067–1071 CrossRef CAS PubMed.
- L. M. Liz-Marzan and A. P. Philipse, Stable hydrosols of metallic and bimetallic nanoparticles immobilized on imogolite fibers, J. Phys. Chem., 1995, 99(41), 15120–15128 CrossRef CAS.
- G. A. Somorjai and D. W. Blakely, Mechanism of catalysis of hydrocarbon reactions by platinum surfaces, Nature, 1975, 258(5536), 580–583 CrossRef CAS.
- L. Y. Chang, A. S. Barnard, L. C. Gontard and R. E. Dunin-Borkowski, Resolving the Structure of Active Sites on Platinum Catalytic Nanoparticles, Nano Lett., 2010, 10(8), 3073–3076 CrossRef CAS PubMed.
- S. I. Lim, I. Ojea-Jimenez, M. Varon, E. Casals, J. Arbiol and V. Puntes, Synthesis of Platinum Cubes, Polypods, Cuboctahedrons, and Raspberries Assisted by Cobalt Nanocrystals, Nano Lett., 2010, 10(3), 964–973 CrossRef CAS PubMed.
- B. N. Wanjala, J. Luo, R. Loukrakpam, B. Fang, D. Mott, P. N. Njoki, M. Engelhard, H. R. Naslund, J. K. Wu, L. Wang, O. Malis and C.-J. Zhong, Nanoscale Alloying, Phase-Segregation, and Core−Shell Evolution of Gold−Platinum Nanoparticles and Their Electrocatalytic Effect on Oxygen Reduction Reaction, Chem. Mater., 2010, 22(14), 4282–4294 CrossRef CAS.
- Z. Peng, H. You and H. Yang, An Electrochemical Approach to PtAg Alloy Nanostructures Rich in Pt at the Surface, Adv. Funct. Mater., 2010, 20(21), 3734–3741 CrossRef CAS.
- Z. Peng and H. Yang, Ag–Pt alloy nanoparticles with the compositions in the miscibility gap, J. Solid State Chem., 2008, 181(7), 1546–1551 CrossRef CAS.
- W. Zhang, J. Yang and X. Lu, Tailoring Galvanic Replacement Reaction for the Preparation of Pt/Ag Bimetallic Hollow Nanostructures with Controlled Number of Voids, ACS Nano, 2012, 6(8), 7397–7405 CrossRef CAS PubMed.
- Y. Sun and Y. Xia, Shape-Controlled Synthesis of Gold and Silver Nanoparticles, Science, 2002, 298(5601), 2176–2179 CrossRef CAS PubMed.
- P. J. Ferreira, G. J. la O', Y. Shao-Horn, D. Morgan, R. Makharia, S. Kocha and H. A. Gasteiger, Instability of Pt
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) /C Electrocatalysts in Proton Exchange Membrane Fuel Cells: A Mechanistic Investigation, J. Electrochem. Soc., 2005, 152(11), A2256–A2271 CrossRef.
/C Electrocatalysts in Proton Exchange Membrane Fuel Cells: A Mechanistic Investigation, J. Electrochem. Soc., 2005, 152(11), A2256–A2271 CrossRef. - Y.-Y. Shen, Y. Sun, L.-N. Zhou, Y.-J. Li and E. S. Yeung, Synthesis of ultrathin PtPdBi nanowire and its enhanced catalytic activity towards p-nitrophenol reduction, J. Mater. Chem. A, 2014, 2(9), 2977–2984 CAS.
- J. Zeng, Q. Zhang, J. Chen and Y. Xia, A Comparison Study of the Catalytic Properties of Au-Based Nanocages, Nanoboxes, and Nanoparticles, Nano Lett., 2009, 10(1), 30–35 CrossRef PubMed.
- G. W. Qin, W. Pei, X. Ma, X. Xu, Y. Ren, W. Sun and L. Zuo, Enhanced Catalytic Activity of Pt Nanomaterials: From Monodisperse Nanoparticles to Self-Organized Nanoparticle-Linked Nanowires, J. Phys. Chem. C, 2010, 114(15), 6909–6913 CAS.
- T. Yu, J. Zeng, B. Lim and Y. Xia, Aqueous-Phase Synthesis of Pt/CeO2 Hybrid Nanostructures and Their Catalytic Properties, Adv. Mater., 2010, 22(45), 5188–5192 CrossRef CAS PubMed.
- X. Yu, A. Shavel, X. An, Z. Luo, M. Ibañez and A. Cabot, Cu2ZnSnS4–Pt and Cu2ZnSnS4–Au Heterostructured Nanoparticles for Photocatalytic Water Splitting and Pollutant Degradation, J. Am. Chem. Soc., 2014, 136(26), 9236–9239 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details about the preparation, characterization and analysis of the surface of hollow nanocrystals together with the evaluation of the surface reactivity. See DOI: 10.1039/c5ta07504a |
| This journal is © The Royal Society of Chemistry 2016 |