 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Inexpensive polyphenylene network polymers with enhanced microporosity†
Kadhum J.
Msayib
and
Neil B.
McKeown
*
EastChem School of Chemistry, University of Edinburgh, David Brewster Road, Edinburgh, EH9 3JF, UK. E-mail: neil.mckeown@ed.ac.uk
First published on 17th June 2016
Abstract
Greatly enhanced microporosity is obtained for the amorphous porous polymers produced from the AlCl3-mediated coupling of aromatic hydrocarbons by using dichloromethane as the reaction solvent. A polymer of average BET surface area = 2435 m2 g−1 was obtained reproducibly from 1,3,5-triphenylbenzene with the additional porosity being provided as ultramicroporosity as demonstrated by very high CO2 adsorption at 273 K/1 bar.
The potential for applications in gas storage, carbon capture, adsorption, heterogeneous catalysis and molecular separations has inspired intensive research activity focused on the synthesis of microporous materials using molecular building units.1 From a structural perspective, there is an important distinction between crystalline porous materials derived from molecular precursors, such as the much-studied Metal Organic Frameworks (MOFs), and amorphous materials that possess a broader range of pore size. However, amorphous porous materials are attractive because they can be prepared using a greater diversity of chemistry and may offer enhanced stability as they do not require crystallisation via reversible bond formation.2 With a few notable exceptions,3 the generation of microporosity within amorphous polymers relies on the formation of a rigid and highly cross-linked network of covalent bonds. Among the rapidly growing number of reported porous network polymers, the Porous Aromatic Frameworks (e.g. PAF-1) and the structurally related Porous Polymer Networks (e.g. PPN-4) are particularly impressive with apparent Brunauer–Emmett–Teller (BET) surface areas rivalling those of the most porous MOFs (SABET > 5000 m2 g−1).4 PAFs and PPNs are prepared using the highly efficient Yamamoto aryl–aryl coupling reaction, which relies on the stoichiometric mediation of the expensive bis(1,5-cyclooctadiene)nickel(0) reagent. In addition, network polymer synthesis via Yamamoto coupling requires bromine-containing monomers which are either expensive to purchase or are prepared using a multi-step synthesis, such as tetra-(4-bromophenyl)methane.5 However, for many of the proposed applications of porous polymers, such as gas storage, water purification or carbon capture, low-cost and large-scale manufacture are necessary to provide competition with conventional inexpensive microporous materials such as activated carbons.1,6 Therefore, network forming reactions that involve cheap monomers and reagents such as the Friedel–Craft reaction7 or direct oxidative aryl–aryl coupling8 (i.e. the Scholl reaction9) are of interest. Unfortunately, to date, these reactions have failed to provide highly porous network polymers. Here we report a simple modification to an aromatic coupling polymerisation that provides highly microporous polymers from readily available and inexpensive starting materials and reagent.
As part of our on-going research programme on triptycene-based porous polymers,10 we performed an acylation of triptycene, following a literature procedure using acetyl chloride and AlCl3 in DCM.11 An insoluble material was isolated from the reaction that proved to be a network polymer with a surprisingly high surface area (SABET > 1000 m2 g−1). Optimisation studies, without acetyl chloride, gave polymer with SABET = 1750 m2 g−1, which is a significantly higher value than previously obtained from polymers derived from triptycene using the Friedel–Craft reaction (SABET = 1250–1430 m2 g−1)6c,7d and is comparable to results obtained using Yamamoto coupling of tribromo- or triiodo-triptycenes (SABET = 1300–1990 m2 g−1).12
This result encouraged the polymerisation of a range of readily available monomers, from which porous polymers had previously been prepared, using these reaction conditions (i.e. AlCl3, DCM at reflux). With the exception of tetraphenylmethane, highly porous polymers were obtained from each monomer (Table 1; Fig. 1). Indeed, for biphenyl, 1,3,5-triphenylbenzene (TPB), hexaphenylbenzene (HPB), spirobifluorene (SBF) and tetraphenylporphyrin (TPP) the SABET of the resulting polymer was greater than those reported for the polymers obtained previously from the same monomer using Friedel–Craft or Scholl reactions (ESI Table S1†). A particularly impressive result was obtained from the polymer derived from TPB that demonstrated reproducibly a SABET in the range 2328–2520 m2 g−1 greater than that of the equivalent porous polymer prepared from tri-brominated TPB using Yamamoto coupling (SABET = 1500–1870 m2 g−1; ESI Table S1†).13 This result allows access to an extremely porous polymer using cheap AlCl3 as reagent and from a monomer that is both inexpensive to buy and easily prepared on a large scale from acetophenone.14 Initial scale-up attempts showed that a 10 g batch of the TPB polymer was readily obtained and demonstrated the same SABET as polymers from smaller (1 g) batches. The lack of success from tetraphenylmethane is likely due its insolubility in DCM combined with low reactivity due to relatively poorly activated phenyl groups.
| Monomera | SABET (m2 g−1) | V total (cm3 g−1) | V micro (cm3 g−1) | CO2 uptaked (mmol g−1) |
|---|---|---|---|---|
| a TPB = 1,3,5-triphenylbenzene; HPB = hexaphenylbenzene; TPP = tetraphenyl porphyrin; DPA = 9,10-diphenylanthracene; TPM = tetraphenylmethane. b Pore volume estimated from N2 uptake at 77 K and P/P0 = 0.98. c Micropore volume estimated from N2 uptake at 77 K and P/P0 = 0.01. d At 1 bar/273 K [1 bar/298 K]. | ||||
| Triptycene | 1750 | 1.0 | 0.6 | 5.8 [3.1] |
| TPB | 2435 | 1.6 | 1.0 | 5.9 [3.6] |
| TPB (CHCl3) | 1415 | 0.7 | 0.5 | 5.0 [3.2] |
| TPB (DCE) | 725 | 0.7 | 0.2 | 1.7 [1.1] |
| Spirobifluorene | 2035 | 1.0 | 0.7 | 5.8 [3.0] |
| HPB | 1790 | 0.9 | 0.6 | 4.5 [2.7] |
| Biphenyl | 1555 | 1.0 | 0.5 | 4.0 [2.7] |
| Biphenyl (CHCl3) | 800 | 0.5 | 0.2 | 3.3 [2.4] |
| Biphenyl (DCE) | 453 | 0.3 | 0.1 | 1.7 [1.1] |
| Triphenylene | 1180 | 0.7 | 0.4 | 4.0 [2.9] |
| DPA | 1020 | 0.6 | 0.3 | 3.2 [2.0] |
| TPP | 905 | 0.4 | 0.3 | 2.5 [2.0] |
| TPM | 125 | 0.2 | 0.0 | 2.0 [1.3] |
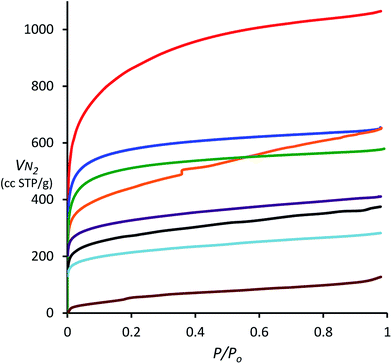 | ||
| Fig. 1 N2 adsorption isotherms obtained at 77 K for porous polymers derived from 1,3,5-triphenylbenzene (TPB, red), spirobifluorene (blue), triptycene (green), biphenyl (orange), triphenylene (purple), 9,10-diphenylanthracene (DPA, black), tetraphenylporphyrin (TPP, light blue) and tetraphenylmethane (TPM, brown) using DCM and AlCl3. Note that the isotherm of hexaphenylbenzene (HPB) is superimposable on that of triptycene and has been omitted for clarity (see ESI†). | ||
The greatly enhanced porosity of the TPB and biphenyl network polymers – twice the SABET of those reported in previous studies of AlCl3-mediated polymerisation – is surprising because the only difference is the use of DCM as solvent instead of CHCl3 (ESI Table S1†).8a,8c This prompted us to perform a direct comparison of the effect of DCM with commonly used CHCl3 or dichloroethane (DCE) solvent on the porosity of the resulting polymers from these two monomers (Table 1). It was found that the SABET values obtained from the TPB and biphenyl polymers prepared using CHCl3 as solvent were similar to those from previous studies.8a,8c Reactions using DCE as solvent produced polymer with only modest porosity. Therefore, it appears that the choice of DCM as solvent provides the dramatic enhancement of SABET. Consequently, it was important to determine whether DCM acts only as a solvent or also provides methylene cross-links via a Friedel–Craft reaction. The 13C solid-state NMR spectrum of all the polymers prepared using DCM shows a small peak between 30 and 40 ppm that may indicate the presence of methylene cross-links, originating from the DCM (ESI†). However, this peak is much smaller than that found previously from a network polymer prepared from a formal Friedel–Craft reaction between TPB and formyl dimethyl acetal as the methylene source.7a In addition, the non-aromatic peaks observed for the TPB polymer are of a similar size, relative to that of the much larger peak of the aromatic carbons, to those previously observed from a TPB network obtained using CHCl3.8a,8c Therefore, it appears that network formation is predominately based on direct aryl–aryl coupling via the Scholl reaction, however, it seems likely that the DCM may contribute some cross-links to the network but only to a similar extent as CHCl3 or DCE. Therefore, the effect of DCM in enhancing SABET is predominately related to its role as a solvent ensuring a more efficient network formation and/or facilitating the generation of a more microporous structure by behaving as a better porogen.15 Similarly large solvent effects on SABET have been reported for other types of reactions to make porous network polymers.7e,16
The N2 adsorption isotherms also provide information about the pore size distribution within the porous polymers. For the polymers derived from TPB, spirobifluorene and triptycene, the relatively large uptake of N2 at 77 K at low relative pressure (P/P0 < 0.01), demonstrates that micropores (<2 nm) contribute a large portion (>60%) of the total pore volume (Table 1). The absence of significant hysteresis between the adsorption and desorption isotherms (ESI†) for all polymers, with the exception of that from biphenyl, indicates both low degree of mesoporosity and that the pore structure appears fixed on adsorption of N2 with no significant swelling.
CO2 adsorption isotherms obtained at 273 K (ESI and Table S1†) also provide information on pore size distribution. Such data are also useful as an indicator of the suitability of a polymer for use in carbon capture using pressure-swing adsorption.6a,b CO2 uptake for the triptycene, SBF and TPB network polymers are all approximately 5.8 mmol g−1 at 1 bar/273 K (3.0–3.6 mmol g−1 at 1 bar/295 K), which is impressive in comparison with other polymers derived from these building units (ESI Table S1†) and are similar to the best performing network polymers (∼6.0 mmol g−1 at 1 bar/273 K and 3.6 mmol g−1 at 1 bar/295 K).17 The high uptake of CO2 suggests that the use of DCM provides additional porosity in the form of ultramicropores (i.e. pores of diameter less that 0.7 nm), which are the physisorption sites for CO2 at 273 K/1 bar. The porosity and gas adsorption of the polymer derived from TPB is strikingly similar to that of one of the best performing activated carbons for CO2 capture.18
Despite the large number of reported porous polymers, prepared using a wide variety of monomers and network-forming reactions,2 only a few demonstrate SABET in excess of 2000 m2 g−1 and all of these require preparation from monomers that need a multistep synthesis or expensive precursors.4a,b,5,13b,17a,19 This work now allows access to highly microporous network polymers, of surface areas in excess of those of commercial activated carbons, from readily obtained starting materials.
Acknowledgements
We thank the EPSRC for funding (EP/K008102/2 & EP/M01486X/1) and the EPSRC UK Solid State NMR Service at Durham University.Notes and references
- A. G. Slater and A. I. Cooper, Science, 2015, 348, 988 CrossRef CAS PubMed
.
-
(a) N. B. McKeown and P. M. Budd, Macromolecules, 2010, 43, 5163–5176 CrossRef CAS
; (b) R. Dawson, A. I. Cooper and D. J. Adams, Prog. Polym. Sci., 2012, 37, 530–563 CrossRef CAS
; (c) D. Wu, F. Xu, B. Sun, R. Fu, H. He and K. Matyjaszewski, Chem. Rev., 2012, 112, 3959–4015 CrossRef CAS PubMed
; (d) Y. Zhang and S. N. Riduan, Chem. Soc. Rev., 2012, 41, 2083–2094 RSC
; (e) Z. Chang, D.-S. Zhang, Q. Chen and X.-H. Bu, Phys. Chem. Chem. Phys., 2013, 15, 5430–5442 RSC
; (f) Z. Xiang and D. Cao, J. Mater. Chem. A, 2013, 1, 2691–2718 RSC
; (g) M. Rose, ChemCatChem, 2014, 6, 1166–1182 CAS
.
- Polymers of intrinsic microporosity and some other high free volume polymers exhibit microporosity without a network structure – see N. B. McKeown and P. M. Budd, Chem. Soc. Rev., 2006, 35, 675–683 RSC
.
-
(a) T. Ben, H. Ren, S. Q. Ma, D. P. Cao, J. H. Lan, X. F. Jing, W. C. Wang, J. Xu, F. Deng, J. M. Simmons, S. L. Qiu and G. S. Zhu, Angew. Chem., Int. Ed., 2009, 48, 9457–9460 CrossRef CAS PubMed
; (b) D. Yuan, W. Lu, D. Zhao and H.-C. Zhou, Adv. Mater., 2011, 23, 3723–3725 CrossRef CAS PubMed
; (c) C. Pei, T. Ben and S. Qiu, Mater. Horiz., 2015, 2, 11–21 RSC
.
- W. Lu, D. Yuan, D. Zhao, C. I. Schilling, O. Plietzsch, T. Muller, S. Braese, J. Guenther, J. Bluemel, R. Krishna, Z. Li and H.-C. Zhou, Chem. Mater., 2010, 22, 5964–5972 CrossRef CAS
.
-
(a) R. Dawson, E. Stoeckel, J. R. Holst, D. J. Adams and A. I. Cooper, Energy Environ. Sci., 2011, 4, 4239–4245 RSC
; (b) C. F. Martin, E. Stockel, R. Clowes, D. J. Adams, A. I. Cooper, J. J. Pis, F. Rubiera and C. Pevida, J. Mater. Chem., 2011, 21, 5475–5483 RSC
; (c) R. T. Woodward, L. A. Stevens, R. Dawson, M. Vijayaraghavan, T. Hasell, I. P. Silverwood, A. V. Ewing, T. Ratvijitvech, J. D. Exley, S. Y. Chong, F. Blanc, D. J. Adams, S. G. Kazarian, C. E. Snape, T. C. Drage and A. I. Cooper, J. Am. Chem. Soc., 2014, 136, 9028–9035 CrossRef CAS PubMed
; (d) H. A. Patel, F. Karadas, A. Canlier, J. Park, E. Deniz, Y. Jung, M. Atilhan and C. T. Yavuz, J. Mater. Chem., 2012, 22, 8431–8437 RSC
.
-
(a) B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44, 2410–2414 CrossRef CAS
; (b) G. Liu, Y. Wang, C. Shen, Z. Ju and D. Yuan, J. Mater. Chem. A, 2015, 3, 3051–3058 RSC
; (c) K. Schute and M. Rose, ChemSusChem, 2015, 8, 3419–3423 CrossRef CAS PubMed
; (d) C. Zhang, P.-C. Zhu, L. Tan, J.-M. Liu, B. Tan, X.-L. Yang and H.-B. Xu, Macromolecules, 2015, 48, 8509–8514 CrossRef CAS
; (e) N. Fontanals, R. M. Marce, F. Borrull and P. A. G. Cormack, Polym. Chem., 2015, 6, 7231–7244 RSC
.
-
(a) B. Li, Z. Guan, X. Yang, W. D. Wang, W. Wang, I. Hussain, K. Song, B. Tan and T. Li, J. Mater. Chem. A, 2014, 2, 11930–11939 RSC
; (b) L. Li, H. Ren, Y. Yuan, G. Yu and G. Zhu, J. Mater. Chem. A, 2014, 2, 11091–11098 RSC
; (c) L. Li, K. Cai, P. Wang, H. Ren and G. Zhu, ACS Appl. Mater. Interfaces, 2015, 7, 201–208 CrossRef CAS PubMed
.
- M. Grzybowski, K. Skonieczny, H. Butenschoen and D. T. Gryko, Angew. Chem., Int. Ed., 2013, 52, 9900–9930 CrossRef CAS PubMed
.
-
(a) B. S. Ghanem, M. Hashem, K. D. M. Harris, K. J. Msayib, M. Xu, P. M. Budd, N. Chaukura, D. Book, S. Tedds, A. Walton and N. B. McKeown, Macromolecules, 2010, 43, 5287–5294 CrossRef CAS
; (b) M. Carta, M. Croad, R. Malpass-Evans, J. C. Jansen, P. Bernardo, G. Clarizia, K. Friess, M. Lanc and N. B. McKeown, Adv. Mater., 2014, 26, 3526–3531 CrossRef CAS PubMed
; (c) R. G. D. Taylor, C. G. Bezzu, M. Carta, K. J. Msayib, J. Walker, R. Short, B. M. Kariuki and N. B. McKeown, Chem.–Eur. J., 2016, 22, 2466–2472 CrossRef CAS PubMed
.
- P.-F. Li and C.-F. Chen, J. Org. Chem., 2012, 77, 9250–9259 CrossRef CAS PubMed
.
- C. Zhang, Y. Liu, B. Li, B. Tan, C.-F. Chen, H.-B. Xu and X.-L. Yang, ACS Macro Lett., 2012, 1, 190–193 CrossRef CAS
.
-
(a) H. Ren, T. Ben, F. Sun, M. Guo, X. Jing, H. Ma, K. Cai, S. Qiu and G. Zhu, J. Mater. Chem., 2011, 21, 10348–10353 RSC
; (b) Z. Xiang, X. Zhou, C. Zhou, S. Zhong, X. He, C. Qin and D. Cao, J. Mater. Chem., 2012, 22, 22663–22669 RSC
.
-
(a) Y. Zhao, J. Li, C. Li, K. Yin, D. Ye and X. Jia, Green Chem., 2010, 12, 1370–1372 RSC
; (b) S. Zhao, L. Kang, H. Ge, F. Yang, C. Wang, C. Li, Q. Wang and M. Zhao, Synth. Commun., 2012, 42, 3569–3578 CrossRef CAS
; (c) D. Prasad, A. Preetam and M. Nath, C. R. Chim., 2013, 16, 252–256 CrossRef CAS
; (d) H. R. Safaei, M. Davoodi and M. Shekouhy, Synth. Commun., 2013, 43, 2178–2190 CrossRef CAS
.
- D. C. Sherrington, Chem. Commun., 1998, 2275–2286 RSC
.
- R. Dawson, A. Laybourn, Y. Z. Khimyak, D. J. Adams and A. I. Cooper, Macromolecules, 2010, 43, 8524–8530 CrossRef CAS
.
-
(a) S. Hug, L. Stegbauer, H. Oh, M. Hirscher and B. V. Lotsch, Chem. Mater., 2015, 27, 8001–8010 CrossRef CAS
; (b) Y. Zhu, H. Long and W. Zhang, Chem. Mater., 2013, 25, 1630–1635 CrossRef CAS
.
- M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 1765–1771 Search PubMed
.
-
(a) J. H. Ahn, J. E. Jang, C. G. Oh, S. K. Ihm, J. Cortez and D. C. Sherrington, Macromolecules, 2006, 39, 627–632 CrossRef CAS
; (b) P. Kuhn, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2008, 47, 3450–3453 CrossRef CAS PubMed
; (c) E. Preis, C. Widling, G. Brunklaus, J. Schmidt, A. Thomas and U. Scherf, ACS Macro Lett., 2013, 2, 380–383 CrossRef CAS
; (d) Z. Xiang, R. Mercado, J. M. Huck, H. Wang, Z. Guo, W. Wang, D. Cao, M. Haranczyk and B. Smit, J. Am. Chem. Soc., 2015, 137, 13301–13307 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Information of synthesis and characterisation of porous polymers. See DOI: 10.1039/c6ta03257e |
| This journal is © The Royal Society of Chemistry 2016 |
