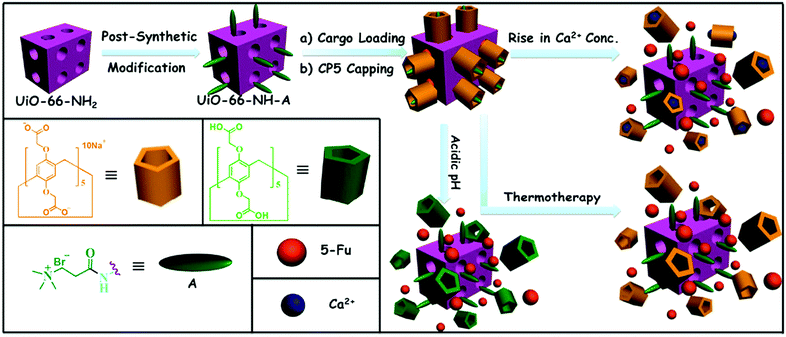
Ca2+, pH and thermo triple-responsive mechanized Zr-based MOFs for on-command drug release in bone diseases†
Li-Li
Tan
ab,
Nan
Song
a,
Sean Xiao-An
Zhang
b,
Haiwei
Li
c,
Bo
Wang
c and
Ying-Wei
Yang
 *a
*a
aState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, International Joint Research Laboratory of Nano-Micro Architecture Chemistry (NMAC), Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China. E-mail: ywyang@jlu.edu.cn
bState Key Laboratory of Supramolecular Structure and Materials, Jilin University, 2699 Qianjin Street, Changchun 130012, P. R. China
cKey Laboratory of Cluster Science, Ministry of Education of China, School of Chemistry, Beijing Institute of Technology, 5 South Zhongguancun Street, Beijing 100081, P. R. China
First published on 23rd November 2015
Abstract
We report a new approach towards the design of multi-stimuli responsive “gated scaffolds” based on the combination of capped metal–organic frameworks (MOFs) and supramolecular[2]pseudorotaxanes. These mechanized Zr-MOFs showed negligible premature release, high drug encapsulation, low cytotoxicity and good biocompatibility. Around or inside the bone tumour cells, the pH, lysosomal pH, and osteoclast pH are observed to be lowered (acidosis), and thus the resulting osteolysis increases the Ca2+ concentration (hypercalcemia). The drug release from the mechanized MOFs was triggered by the simultaneous variations of pH and Ca2+ concentration in bone tumour cells. Hyperthermia (also called thermal therapy or thermotherapy) as a popular type of cancer treatment technique can also control drug release in the above-mentioned system. This design opens up the possibility of developing smart biomaterials for bone regeneration and cancer therapy.
Introduction
Since bone is the third most common location for metastasis, bone tumours represent a significant clinical challenge that can cause severe pain, bone fractures, acidosis, spinal cord compression, hypercalcemia, anemia, spinal instability, decreased mobility, and rapid degradation in the quality of life for patients.1 The pH in bone tumour tissues is known to be more acidic than that in blood and normal tissues (pH 7.4), in view of the fact that their lysosomal pH levels are somewhat lower than those in healthy human cells.2 Bone tumour cells can directly break down bone and release minerals, and the concentration of Ca2+ will be extremely high at that instant, which is difficult to test at that moment (after diffusion, extracellular free Ca2+ will be increased to higher than 4 mM),4 resulting in a transfer of calcium from the bone fluid to the blood, causing hypercalcemia.3 In addition, osteoclasts and bone tumour cells lower the pH of the extracellular matrix (ECM) around the osteoclasts to approximately 4.5 or lower, which will lead to osteolysis, increasing bone resorption.4 These tumours also produce parathyroid hormone, prostaglandin E, vitamin D sterols, etc., which will trigger bone resorption. Bone resorption leads to the release of Ca2+ from bones, causing hypercalcemia. To address these important clinical issues, it is of great importance to design multifunctional bioactive scaffolds with the capability to stimulate osteogenesis and angiogenesis, anti-bacterial/cancer activity, etc.Porous biomaterials as scaffolds with a three-dimensional environment that preserves tissue volume, supports cell interactions, and delivers biological agents for repairing, maintaining, restoring or improving the function of organs and tissues have been widely studied and used for bone regeneration and bone cancer therapy in a multi-million market context.5 However, the most desirable course is to explore bioactive materials6 which can realize targeted, controlled release, and effectively solve the severe problem of premature drug leakage.
Metal–organic frameworks (MOFs)7 have recently been recognized as promising porous materials for stimuli-responsive functional nanocarriers because of their enormous porosity, high surface area, good biocompatibility, ease of functionalization, and a wide array of potential applications for cell imaging.8–11 The promising mechanical, biocompatible characteristics and extremely low toxicity of zirconium (Zr) compounds boost the burgeoning biomedical applications of Zr-containing agents,12 such as dental implants, total knee and hip replacement, middle-ear ossicular chain reconstruction surgery, hemofiltration, hemodialysis, wearable kidneys, and anticancer therapy.13 Therefore, Zr-based MOFs (Zr-MOFs) possess a wide array of potential biomedical applications due to their excellent stable structure and successful post-synthetic modification (PSM).14–16
Given the need to develop scaffolds for advanced therapies that can improve the features of conventional systems, herein, we first provide a new combination therapy principle. Mechanized Zr-MOFs with multi-stimuli responsive supramolecular gatekeepers (Fig. 1) that combine thermotherapy with chemical (low pH in osteoclasts and tumour cells) and biochemical triggers (high Ca2+ concentration caused by osteolysis and bone resorption) were designed. Our motivation is to find a better bone cancer therapy and bone regeneration method and ameliorate the adverse side effects of traditional therapy. The new strategy offers important prospects for potential bone regeneration and bone cancer therapy:
(1) Because of the high concentration of Ca2+ caused by osteolysis and bone resorption, and the lower pH in osteoclasts and tumour cells, drugs can be released in a controlled manner from smart containers near the targeted lesions to kill the cancer cells and help to regenerate bone.
(2) These nanocontainers can not only transport the desired drugs but also decrease adverse side effects and tune the pH and Ca2+ concentration in the patient body.
(3) The concentration that induces cargo release should be fine-tuned according to the varied Ca2+concentration and pH in patients. Our mechanized MOFs with supramolecular gates can achieve a better therapeutic effect by accounting for the different characteristics of patients.
(4) Hyperthermia has been widely used for cancer treatment and palliation of the painful bone metastases.17 Meanwhile, with elevated temperature, supramolecular host–guest interactions within the gating entities are weakened followed by the disassociation of rings from MOF surfaces to unblock the pores and the release the stored cargo molecules.
Experimental
UiO-66-NH2, UiO-66-NH-A, 5-fluorouracil (5-Fu)-loaded, carboxylatopillar[5]arene (CP5)-capped UiO-66-NH-A, and 5-Fu-loaded UiO-66-NH-A without CP5 capping were prepared according to our previously reported procedures.11 Ultrasound was used to get smaller particles.Construction of supramolecular mechanized MOFs
UiO-66-NH-A (3 mg) was dispersed in 5-Fu (1 mL, 3.3 mM) aqueous solution under ultrasound. Under stirring, an excess amount of carboxylatopillar[5]arene (CP5) (4 equiv. of A, 48 mg) was added after 12 h. 5-Fu-loaded, CP5-capped UiO-66-NH-A was separated by centrifugation and washed with deionized H2O twice. Finally, the supramolecular mechanized MOFs were dried in a vacuum oven overnight.Controlled release experiments
5-Fu-loaded, CP5-capped UiO-66-NH-A nanomaterial (1 mg) was placed in a dialysis bag, which was immersed in a cuvette filled with 3 mL of aqueous solution under stirring. Cargo release was controlled by tuning the pH, the Ca2+ concentration (the initial concentration of Ca2+ is constant) and/or the solution temperature. To monitor the release of 5-Fu, the UV absorption spectrum of the solution was recorded in situ with the change of time. Control experiments were carried out with 5-Fu-loaded UiO-66-NH-A without capping (1 mg). These 5-Fu-loaded UiO-66-NH-A Zr-MOFs were suspended in solutions and stirred for 3 days to result in complete drug release.Encapsulation capacity
For the calculation of encapsulation capacity, 5-Fu release was triggered by the addition of CaCl2. Zr-MOFs (1 mg, 5-Fu-loaded, CP5-capped UiO-66-NH-A or 5-Fu-loaded UiO-66-NH-A without CP5 capping) were placed into a dialysis bag, which was immersed into a cuvette containing CaCl2 aqueous solution (3 mL, 600 mM) under gentle stirring. During this period of time, the UV-vis absorption spectra of the solution were recorded at predetermined times. According to the Lambert–Beer law, the encapsulation capacity can be calculated. The maximum absorption wavelength of 5-Fu is 265 nm, A = 17 ± 1 C, b = 1 cm (Fig. S9–S11, ESI†).Results and discussion
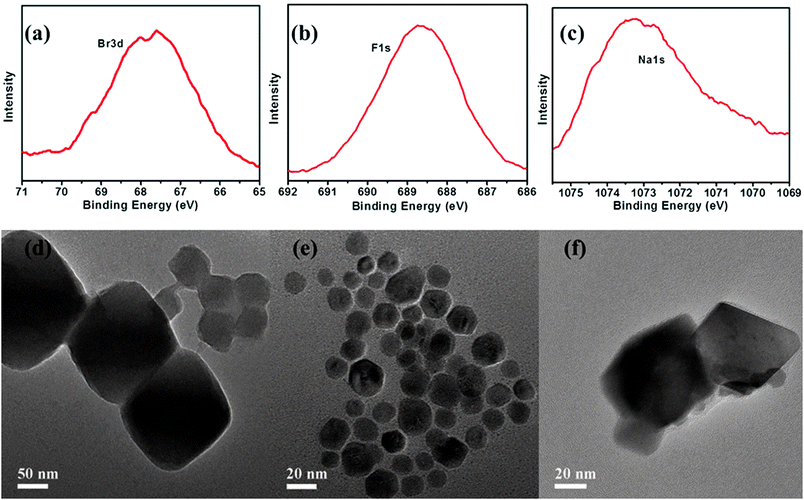
The fabrication of the CP5-based mechanized UiO-66-NH2 nanocarrier systems is depicted in Fig. 1. The synthesis and PSM of scaffold UiO-66-NH2 were carried out according to the reported procedure,11,18 followed by the loading of a drug, that is, 5-Fu (3.3 mM), in their nanopores at room temperature. Finally, CP5 rings19–32 were introduced to encircle the A stalks via host–guest complexation to form [2]pseudorotaxanes as the movable elements of the mechanized Zr-MOFs, thereby realizing the drug encapsulation. Upon adding excess CP5 to cap the pores followed by triggering with excess Ca2+ cations, the encapsulation capacity (247 μmol g−1) is more than 2 times larger than our previously reported Zn2+-triggered version,11 which also suggests the important role of CP5 supramolecular switches in our system.The appearance of Br− peaks of A stalks, F− peaks of 5-Fu and Na+ peaks of CP5 in the scanning electron microscope-energy dispersive spectrometer (SEM-EDS) (Fig. S3 and S4, ESI†) indicated that the A stalks were successfully anchored to UiO-66-NH2. As shown in Fig. 2, the appearance of Br3d, F1s and Na1s signals in the X-ray photoelectron spectrometer (XPS), the spectra of UiO-66-NH-A and 5-Fu-loaded, CP5-capped UiO-66-NH-A, verified the successful modification, loading and capping. To further test the microcrystallinity of our newly synthesized materials, powder X-ray diffraction (PXRD) patterns were obtained. As shown in Fig. S1 (ESI†), the original peaks still remain, which indicates that the porous scaffolds have not been damaged by PSM.
The morphology, size and monodispersity of UiO-66-NH2, UiO-66-NH-A and 5-Fu-loaded, CP5-capped UiO-66-NH-A were investigated using high-resolution transmission electron microscopy (HR-TEM). Interestingly, after modification and sonication, comparing with the work we published before,11 the nanoparticles become monodisperse with smaller and more homogeneous particle sizes (around ca. 20 nm, Fig. 2e), due to the biocompatibility of the A stalks and sonication. After loading and capping, the particle sizes became larger (around ca. 40 nm), which indicated the successful construction of the mechanical nanocarriers. As shown in Fig. 2d and e, these MOFs are mainly in a cubic shape, with an average size of ca. <100 nm in diameter, which was within the size range of nanoparticles that can be easily taken up by cells,33 making the mechanical nanocarriers constructed based on UiO-66-NH-A and CP5 promising candidates for drug storage and drug delivery.
In an acidic environment, neutralization of the CP5 sodium salts can result in the weakening of the host–guest interactions between the rings and the stalk components of the CP5-based supramolecular [2]pseudorotaxanes, which will lead to the unblocking of the nanopores.33 So, 5-Fu-loaded, CP5-capped UiO-66-NH-A MOF nanoparticles are able to contain 5-Fu drug molecules at neutral pH but release them under acidic pH, and the release rate of 5-Fu depends on the pH level. In order to demonstrate this pH-dependent feature, the release of 5-Fu entrapped in UiO-66-NH-A was evaluated at different pH values. The resulting release profiles are shown in Fig. 3. A flat baseline showed that 5-Fu was held firmly within the nanopores at neutral pH: there is no premature release, which is rare for MOF-based drug delivery systems. When the pH of the solution was lowered to 5, the supramolecular gates were opened and 5-Fu molecules were released. As expected, the release rate of 5-Fu correlated with the final solution pH. In the case of pH 5.0, 18% of 5-Fu was released in about 1 h, while at pH 4.0, about 54% of 5-Fu was released in the same period of time. As the pH in the areas of bone tumour tissues, their lysosomal pH levels, and pH of the extracellular matrix (ECM) around the osteoclast (approximately 4.5 or lower) are known to be more acidic than in blood and normal tissues (pH 7.4), the responsive drug delivery system can reduce undesired drug release during drug transportation in blood circulation and improve the effective release of anti-tumour drugs in tumour tissues. Moreover, since the drug is expected to be released much faster at the tumour site than in the surrounding normal tissues maintaining a physiological pH of 7.4, it is expected that the delivery of chemotherapeutic drugs via these systems may also reduce their adverse side effects, which in some cases can be severely debilitating. So, with no doubt, this pH-sensitive drug nanocarrier opened new perspectives in bone cancer therapy.
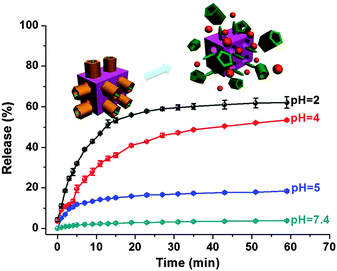 | ||
| Fig. 3 Controlled release profiles of the 5-Fu-loaded, CP5-capped UiO-66-NH-A operated by pH changes. | ||
Besides the pH-responsiveness, more importantly, these 5-Fu-loaded, CP5-capped UiO-66-NH-A nanoparticles have the capability of responding to Ca2+ due to the stronger binding between CP5 and Ca2+ (163.3 M−1) confirmed by NMR titration experiments (Fig. S8, ESI†). Ca2+ plays essential roles in a number of biochemical processes especially cancer, osteolysis and bone resorption will lead to the rapid increase of the Ca2+ concentration.34 To further investigate the effect of Ca2+ concentration and kinetics on controlled release, a different amount of Ca2+, e.g., 1 mM, 10 mM, 30 mM, 300 mM and 600 mM (these high concentrations may exist at the instant of bone resorption) was added and then 5-Fu-release was caused by dethreading of the CP5 rings from the A stalks (Ka = 118.2 M−1).11 When the concentration of Ca2+ (in vitro) is comparable to the concentration in the extracellular fluids (1 mM), only 5% of 5-Fu was released, indicating that Ca2+ could trigger the systems with extremely low premature release (Fig. 4a). It was found that with the increase of Ca2+ concentration, the release percentage and the release rate of 5-Fu gradually increased (Fig. 4b), which means that the drug release will be enhanced and made faster when the amount of Ca2+ in the treated region is greater and indicates the important roles of the CP5-based supramolecular switches on controlled cargo release. If the Ca2+ concentration was extremely high (up to 300 mM), all the drugs in the containers will be released after two days. What's more, this system not only acts as a calcitonin that is frequently used to reduce blood calcium, but also releases drugs to the targeted site in a controlled fashion, especially for bone cancer sites. Therefore, based on the above meaningful investigation, the Ca2+-triggered drug delivery of supramolecular switch-gated Zr-MOFs was demonstrated for the first time, which opens up new alternatives for bone cancer treatment.
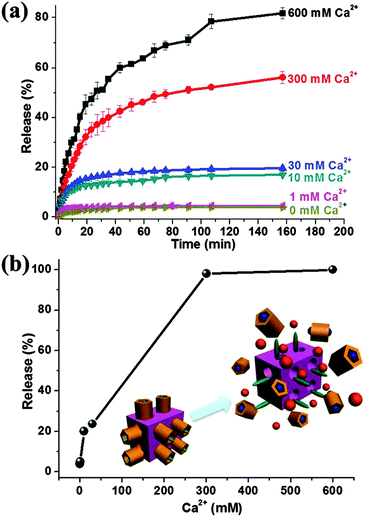 | ||
| Fig. 4 Controlled release profiles of the 5-Fu-loaded, CP5-capped UiO-66-NH-A. (a) Operation by Ca2+ addition. (b) The release percent of 5-Fu for 2 days as a function of Ca2+ concentration. | ||
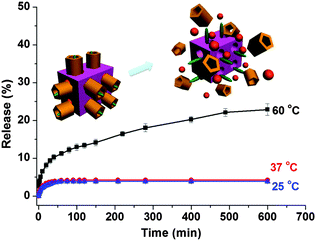
Hyperthermia (also called thermal therapy or thermotherapy) is a type of cancer treatment in which body tissue is exposed to higher temperatures.35 Research has shown that high temperatures can damage and kill cancer cells, usually with minimal injury to normal tissues.36 By killing cancer cells and damaging proteins and structures within cells, hyperthermia may shrink tumours. The host–guest interactions between the CP5 rings and the A stalks on Zr-MOFs can be weakened by elevating the temperature to 60 °C, and 5-Fu released gradually (Fig. 5).35 The premature release at 25 °C and 37 °C is not significantly obvious. Therefore, the multi-stimuli responsive supramolecular switch-gated MOFs combine traditional therapy with additional controlled release at the same time, which provides a new design principle for bone cancer treatment.
A series of control experiments have also been conducted to certify the functionalization of the CP5 supramolecular switches in the UiO-66-NH-A drug delivery system by comparing the difference of the release performance of 5-Fu-loaded, CP5-capped UiO-66-NH-A and 5-Fu-loaded UiO-66-NH2 without CP5 capping by Ca2+ activation. From Fig. S12 (ESI†), we could reach the following conclusions: (1) premature leakage without attaching the CP5 rings was more obvious than with attaching the CP5 rings. It indicated the important role of the CP5 supramolecular switches in our system that effectively prevented premature leakage of cargo. (2) The encapsulation efficiency of MOFs without attaching the CP5 rings (42 μmol g−1) was significantly lower than that with the attachment of CP5 rings (247 μmol g−1). This is because, without CP5 supramolecular switches on the surfaces of the UiO-66-NH-A Zr-MOFs, not only 5-Fu from physisorption but also 5-Fu partially from the pore interiors of materials can be washed away in the process of cleaning and centrifugation. These reveal the important role of the CP5-based supramolecular switches in our system for tuning the loading capacity of drugs.
As shown in Fig. 6, with the increase of concentration of UiO-66-NH-A and CP5-capped UiO-66-NH-A, as deduced from the fact that the cell viabilities were higher than 96% even though their concentration was as high as 50 μg mL−1, indicated that our new “gated scaffolds” had only negligible cytotoxicity to normal human cells. After capping, this system shows lower cytotoxicity due to the biological friendly properties of the CP5 rings.33 Overall, the nanomaterials, before and after CP5 capping, possess negligible cell cytotoxicity at low concentrations, allowing them to be used as nanocontainers for controlled drug delivery.
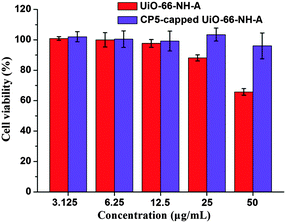 | ||
| Fig. 6 MTT cytotoxicity assay of 293 cells treated with UiO-66-NH-A and CP5-capped UiO-66-NH-A at various concentrations. | ||
Conclusions
In summary, we report herein a new approach for the design of multi-stimuli responsive “gated scaffolds” which combine the thermotherapy and pathology of bone cancer to control drug release and decrease the adverse side effects of anticancer drugs. XPS was first used to confirm the successful modification of the novel multi-stimuli responsive “gated scaffolds”. The obtained mechanized Zr-MOFs capped with CP5-based pseudoro[2]taxanes remain tightly in place at the normal Ca2+ concentration, pH, and temperature of a healthy human body yet are able to release drugs in a controlled manner around or in bone tumour cells with increasing Ca2+ concentration, decreasing pH, and/or by using thermal therapy. What's more, the particle size is smaller, and the encapsulation capacity is 2 times higher than a similar system we found earlier. This unique MOF-based multi-stimuli responsive nanovalve system showed negligible premature release, large pore sizes for drug encapsulation, noncytotoxicity, good biocompatibility, and potential application in cell imaging, which, we envision, will find applications in regenerative medicine and bone cancer therapy. We also expect that the preparation approach of this type of multi-stimuli responsive supramolecular host–guest “gated scaffold” will find broader applications in the future.Acknowledgements
Y. W. Y. is thankful for financial support from the National Natural Science Foundation of China (21272093 and 51473061), the JLU Cultivation Fund for the National Science Fund for Distinguished Young Scholars, and the Fundamental Research Funds for the Central Universities (No. JCKY-QKJC05).References
- M. Vallet-Regí and E. Ruiz-Hernández, Adv. Mater., 2011, 23, 5177–5218 CrossRef PubMed.
- S. Angelos, N. M. Khashab, Y.-W. Yang, A. Trabolsi, H. A. Khatib, J. F. Stoddart and J. I. Zink, J. Am. Chem. Soc., 2009, 131, 12912–12914 CrossRef CAS PubMed.
- G. R. Monteith, D. McAndrew, H. M. Faddy and S. J. Roberts-Thomson, Nat. Rev. Cancer, 2007, 7, 519–530 CrossRef CAS PubMed.
- S. L. Teitelbaum, Am. J. Pathol., 2007, 170, 427–435 CrossRef CAS PubMed.
- N. Mas, D. Arcos, L. Polo, E. Aznar, S. Sánchez-Salcedo, F. Sancenón, A. García, M. D. Marcos, A. Baeza, M. Vallet-Regí and R. Martínez-Máñez, Small, 2014, 10, 4859–4864 CrossRef CAS PubMed.
- Y.-W. Yang, Y.-L. Sun and N. Song, Acc. Chem. Res., 2014, 47, 1950–1960 CrossRef CAS PubMed.
- J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed.
- L.-L. Tan, H. Li, Y.-C. Qiu, D.-X. Chen, X. Wang, R.-Y. Pan, Y. Wang, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Chem. Sci., 2015, 6, 1640–1644 RSC.
- T. Faust, Nat. Chem., 2015, 7, 270–271 CrossRef CAS.
- L.-L. Tan, H. Li, Y. Zhou, Y. Zhang, X. Feng, B. Wang and Y.-W. Yang, Small, 2015, 11, 3807–3813 CrossRef CAS PubMed.
- D. B. N. Lee, M. Robert, C. G. Bluchel and R. A. Odell, ASAIO J., 2010, 56, 550–556 CrossRef CAS PubMed.
- C. Wu and J. Chang, J. Controlled Release, 2014, 193, 282–295 CrossRef CAS PubMed.
- V. Bon, V. Senkovskyy, I. Senkovska and S. Kaskel, Chem. Commun., 2012, 48, 8407–8409 RSC.
- D. Cunha, M. B. Yahia, S. Hall, S. R. Miller, H. Chevreau, E. Elkaïm, G. Maurin, P. Horcajada and C. Serre, Chem. Mater., 2013, 25, 2767–2776 CrossRef CAS.
- X. Zhu, J. Gu, Y. Wang, B. Li, Y. Li, W. Zhao and J. Shi, Chem. Commun., 2014, 50, 8779–8782 RSC.
- A. Matsumine, K. Takegami, K. Asanuma, T. Matsubara, T. Nakamura, A. Uchida and A. Sudo, Int. J. Clin. Oncol., 2011, 16, 101–108 CrossRef PubMed.
- V. Guillerm, S. Gross, C. Serre, T. Devic, M. Bauer and G. Férey, Chem. Commun., 2010, 46, 767–769 RSC.
- H. Li, D.-X. Chen, Y.-L. Sun, Y. B. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS PubMed.
- D.-X. Chen, Y.-L. Sun, Y. Zhang, J.-Y. Cui, F.-Z. Shen and Y.-W. Yang, RSC Adv., 2013, 3, 5765–5768 RSC.
- D.-D. Zheng, D.-Y. Fu, Y.-Q. Wu, Y.-L. Sun, L.-L. Tan, T. Zhou, S.-Q. Ma, X. Zha and Y.-W. Yang, Chem. Commun., 2014, 50, 3201–3203 RSC.
- Y. Zhou, L.-L. Tan, Q.-L. Li, X.-L. Qiu, A.-D. Qi, Y. Tao and Y.-W. Yang, Chem. – Eur. J., 2014, 20, 2998–3004 CrossRef CAS PubMed.
- L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Adv. Mater., 2014, 26, 7027–7031 CrossRef CAS PubMed.
- Q. Zhao, J. W. C. Dunlop, X. Qiu, F. Huang, Z. Zhang, J. Heyda, J. Dzubiella, M. Antonietti and J. Yuan, Nat. Commun., 2014, 5 DOI:10.1038/ncomms5293.
- C. Li, Chem. Commun., 2014, 50, 12420–12433 RSC.
- Y. Chang, K. Yang, P. Wei, S. Huang, Y. Pei, W. Zhao and Z. Pei, Angew. Chem., Int. Ed., 2014, 53, 13126–13130 CrossRef CAS PubMed.
- N. L. Strutt, H. Zhang, S. T. Schneebeli and J. F. Stoddart, Acc. Chem. Res., 2014, 47, 2631–2642 CrossRef CAS PubMed.
- T. Ogoshi and T. Yamagishi, Chem. Commun., 2014, 50, 4776–4787 RSC.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed.
- W. Si, P. Xin, Z.-T. Li and J.-L. Hou, Acc. Chem. Res., 2015, 48, 1612–1619 CrossRef CAS PubMed.
- X. Ma and Y. Zhao, Chem. Rev., 2015, 115, 7794–7839 CrossRef CAS PubMed.
- Y.-W. Yang and D. Cao, Chin. J. Chem., 2015, 33, 303 CrossRef CAS.
- Y.-L. Sun, Y.-W. Yang, D.-X. Chen, G. Wang, Y. Zhou, C.-Y. Wang and J. F. Stoddart, Small, 2013, 9, 3224–3229 CAS.
- Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762–10769 CrossRef CAS PubMed.
- H. Li, L.-L. Tan, P. Jia, Q.-L. Li, Y.-L. Sun, J. Zhang, Y.-Q. Ning, J. Yu and Y.-W. Yang, Chem. Sci., 2014, 5, 2804–2808 RSC.
- J. V. Zee, Ann. Oncol., 2002, 13, 1173–1184 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic schemes, electron microscopy images and nitrogen adsorption/desorption isotherms of the nanoparticles, FT-IR spectra, NMR spectra and DLS results for nanoparticle stability. See DOI: 10.1039/c5tb01789k |
| This journal is © The Royal Society of Chemistry 2016 |