Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantifying ingested debris in marine megafauna: a review and recommendations for standardization†
Jennifer F.
Provencher
 *ab,
Alexander L.
Bond
*ab,
Alexander L.
Bond
 c,
Stephanie
Avery-Gomm
d,
Stephanie B.
Borrelle
c,
Stephanie
Avery-Gomm
d,
Stephanie B.
Borrelle
 e,
Elisa L.
Bravo Rebolledo
f,
Sjúrður
Hammer
e,
Elisa L.
Bravo Rebolledo
f,
Sjúrður
Hammer
 g,
Susanne
Kühn
f,
Jennifer L.
Lavers
h,
Mark L.
Mallory
b,
Alice
Trevail
i and
Jan A.
van Franeker
f
g,
Susanne
Kühn
f,
Jennifer L.
Lavers
h,
Mark L.
Mallory
b,
Alice
Trevail
i and
Jan A.
van Franeker
f
aDepartment of Biology, Carleton University, 1125 Colonel By Drive, Ottawa, Ontario K1A 0H3, Canada. E-mail: jennifpro@gmail.com
bDepartment of Biology, Acadia University, 33 Westwood Avenue, Wolfville, Nova Scotia, Canada B4P 2R6
cRSPB Centre for Conservation Science, Royal Society for the Protection of Birds, The Lodge, Sandy, Bedfordshire SG19 2DL, UK
dCentre for Biodiversity and Conservation Science, University of Queensland, St. Lucia, Queensland, 4067, Australia
eInstitute of Applied Ecology New Zealand, Auckland University of Technology, Private Bag 92006, Auckland 1142, New Zealand
fWageningen Marine Research, Ankerpark 27, 1781 AG Den Helder, The Netherlands
gInstitute of Biodiversity, Animal Health and Comparative Medicine, University of Glasgow, Graham Kerr Building, Glasgow, G12 8QQ, UK
hInstitute for Marine and Antarctic Studies, University of Tasmania, 20 Castray Esplanade, Battery Point, Tasmania 7004, Australia
iDepartment of Environmental Sciences, University of Liverpool, Nicholson Building, Liverpool, L69 3GP, UK
First published on 3rd October 2016
Abstract
Plastic pollution has become one of the largest environmental challenges we currently face. The United Nations Environment Program (UNEP) has listed it as a critical problem, comparable to climate change, demonstrating both the scale and degree of the environmental problem. Mortalities due to entanglement in plastic fishing nets and bags have been reported for marine mammals, turtles and seabirds, and to date over 690 marine species have been reported to ingest plastics. The body of literature documenting plastic ingestion by marine megafauna (i.e. seabirds, turtles, fish and marine mammals) has grown rapidly over the last decade, and it is expected to continue grow as researchers explore the ecological impacts of marine pollution. Unfortunately, a cohesive approach by the scientific community to quantify plastic ingestion by wildlife is lacking, which is now hindering spatial and temporal comparisons between and among species/organisms. Here, we discuss and propose standardized techniques, approaches and metrics for reporting debris ingestion that are applicable to most large marine vertebrates. As a case study, we examine how the use of standardized methods to report ingested debris in Northern Fulmars (Fulmarus glacialis) has enabled long term and spatial trends in plastic pollution to be studied. Lastly, we outline standardized metric recommendations for reporting ingested plastics in marine megafauna, with the aim to harmonize the data that are available to facilitate large-scale comparisons and meta-analyses of plastic accumulation in a variety of taxa. If standardized methods are adopted, future plastic ingestion research will be better able to inform questions related to the impacts of plastics across taxonomic, ecosystem and spatial scales.
1 Introduction
Since the invention of plastic in the early twentieth century, it has been polluting the marine environment. Plastic pollution has become one of the largest environmental challenges we currently face. The United Nations Environment Program (UNEP) has listed it as a critical problem, comparable to climate change, demonstrating both the scale and degree of the environmental problem.1,2 Marine plastic pollution occurs from the Arctic to the Antarctic, with several areas of significant concentrations in regions where ocean currents converge in gyres.3 Plastic pollution has also been documented in freshwater ecosystems,4,5 illustrating that few aquatic ecosystems are unaffected. Importantly, plastic pollution impacts wildlife through both entanglement and ingestion. Mortalities due to entanglement in plastic fishing nets and bags have been reported for marine mammals, turtles and seabirds (hereafter referred to as marine megafauna),6,7 and to date over 690 marine species have been reported to ingest plastics.8–10Over the last few decades, as interest in plastics in marine environments has increased,2,11 so too has the number of papers documenting plastic ingestion by marine animals. Since 2004, there have been some attempts to introduce standardized methods to plastic ingestion methods and protocols;12–17 however, a cohesive approach by the scientific community to quantify plastic ingestion by wildlife is lacking. Unfortunately, this has undermined attempts to detect spatial and temporal trends in plastic ingestion, or to perform meta-analyses. It may also obscure our ability to fully understand the impacts of plastic ingestion on wildlife. Although it has been shown that plastic ingestion may lead to deleterious effects through a number of physical and biochemical pathways,18,19 there is a paucity of research rigorously investigating population and ecosystem level effects of plastic ingestion.20 Improved standardization of sampling for plastic ingestion may help to facilitate an understanding of these higher order effects.
With this lack of framework in mind, the objective of this paper is to help advance the field of marine plastic ingestion. First, we provide a historical overview of the scientific reporting of plastic ingestion in marine megafauna. Second, we present a review of the plastics ingestion literature with a focus on methods of collection, characterization of ingested plastics, the reporting of metrics on ingested plastics and interpretation of results. Most plastic ingestion studies concern marine birds, therefore we use this group as a model to understand patterns in methods and draw lessons that are applicable to plastic ingestion studies in other megafauna groups. Third, we present an example of how employing standardized techniques across oceans enables spatial and temporal comparisons of plastic ingestion and informs science and policy; the seabird Northern Fulmar (Fulmarus glacialis). Finally, we offer recommendations for standardized metrics when reporting ingested plastics in marine megafauna, with the aim to harmonize the available data to facilitate large-scale comparisons and meta-analyses of plastic ingestion.
2 Methods and results
2.1 Review of reporting plastic ingestion in marine megafauna
We used the Web of Science search engine and citation index between November 2015 and July 2016 to search for publications using “seabird (or turtle or cetacean or pinniped or fish)*plastic”, “seabird (or turtle or cetacean or pinniped or fish)*debris”, and “seabird (or turtle or cetacean or pinniped or fish)*pollution”. To capture information on plastics from older publications that often reported plastic ingestion in diet studies, we also reviewed several summary papers on the topic including Laist,8 Kühn et al.,9 Provencher et al.,21 and Ryan.11 Our literature search spanned records from 1949 to 2015.We limited our literature search to seabirds as defined by Gaston,22 which includes penguins (Sphenisciformes), tubenoses (Procellariiformes), cormorants and gannets (Pelecaniformes), tropicbirds (Phaethontiformes), auks, terns, skuas, phalaropes and gulls (Charadriiformes). We included loons (Gaviiformes), and marine sea ducks and mergansers (Anseriformes; Merginae only) as most species spend almost the entire year in marine environments.22 We also included marine turtles (both Cheloniidae and Dermochelyidae), and mammals, namely cetaceans, sea cows, pinnipeds (sea lions, walruses and seals), otters (Mustelidae; sea otters and marine otters only) and bears (Ursidae; polar bears only) that reside in marine environments. Lastly, we included fish (Agnatha, Chondrichthyes, Acanthodii, and Osteichthyes) using http://fishbase.org to subset only marine species.9 While we aimed to cover all peer-reviewed literature on plastic ingestion in marine megafauna, the results presented here likely miss some peer-reviewed entries, and do not represent reports from the grey literature or popular media, we feel it is representative of the research field.
Marine megafauna are susceptible to ingesting a range of debris sizes. Although other papers in this special edition focus almost exclusively on microplastics, we include microplastics within a broader category of plastics. For the purpose of this paper, and in line with this special issue, we use the following categories of plastics as defined by Barnes;23 microplastics (1–5 mm), mesoplastics as (<5–20 mm), and macroplastics (>20–100 mm), while also including megaplastics (>100 mm). While the ingestion of pieces from micro- to macro-plastics has been recorded for many species of marine megafauna, most of these report the range and mean piece size, but do not typically quantify the number of pieces that fall into size categories. As such, we cover plastic ingestion in marine megafauna in general, while recognizing that the plastics reported often span the size categories described above. Additionally, marine megafauna ingest numerous other types of debris such as metal and paraffin wax.13,24 The majority of the debris found in seabirds is plastics, often >90%.13,25,26 Therefore, we shape our recommendations for standardization with this in mind. While most papers report plastic ingestion, what is actually measured in almost all papers is the accumulation of ingested plastics. Researchers rarely report birds in the act of ingestion (although see ref. 27 and 28), and more often report the accumulation of ingested plastics found in seabird gastrointestinal tracts but use the term ingestion widely. We recognize the difference between plastic ingestion and accumulation of plastics, but for the purposes of this review we use the term plastic ingestion to refer to the accumulated plastics that can be measured in birds through examination of gastrointestinal contents.
2.2 History of reporting plastic ingestion in marine megafauna
The first scientific publication of marine megafauna ingesting debris was in 1838 with Couch29 reporting part of a candle stick found in the gut of a Wilson's Storm–Petrel (Oceanites oceanicus) and Turner30 reporting a fish hook found inside a Sperm Whale (Physeter microcephalus) in 1895. These early reports illustrate that marine megafauna have always been susceptible to ingesting non-food items. The first reports we found identifying plastic debris specifically as an ingested item in marine megafauna groups was not until the 1960s (Fig. 1). By the 1970s, ingested plastic pollution had been reported in marine birds, mammals, turtles and fish.8,9,11 Since 1968, there has been an increase in publications related to ingested plastics by marine megafauna, with peaks in both the 1980s and 2000s. This pattern is largely driven by seabird publications (Fig. 1;11), likely due to several factors including: (1) numerous long term monitoring studies of seabirds; (2) the relative ease and accessibility of sampling seabirds when they breed on land in large colonies; and (3) their general use as biological indicators of the marine environment.31,32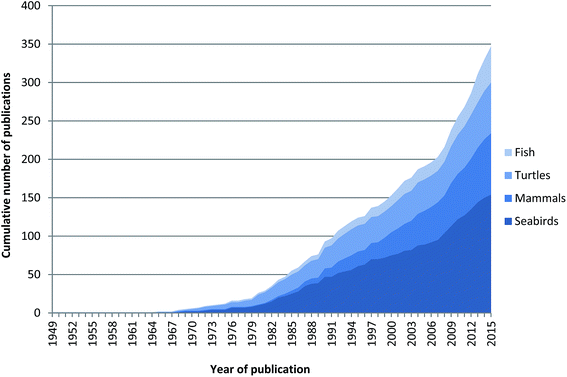 | ||
| Fig. 1 Cumulative number of publications reporting ingestion of plastic in marine megafauna, including seabirds, mammals, turtles and fish from 1949 to 2015. | ||
In general, while the number of studies varies among the megafauna groups (14–85; Table 1) the types of metrics found in the papers for each group is similar. By far the most often reported metric in literature reporting ingestion in marine megafauna is percentage frequency of occurrence (% FO), also described as incidence or prevalence (Table 1). In all groups, the number of pieces of plastics is the second most reported metric, with mass the third most commonly reported value. Interestingly, median values (of either number or mass) are the least reported measure of central tendency for any metric in all groups. The size and colour of ingested plastics are reported in roughly equal numbers in fish, turtles and mammals, whereas size is reported in marine birds almost 4× more frequently as colour. Importantly in relation to the call for standardized methods, <25% of the papers in all the megafauna groups examined noted the use of a standardized protocol in their methods for reporting plastics (Table 1). Overall this illustrates that studies reporting ingested plastic values for marine megafauna have variable reporting standards, and few use standardized protocols.
| Fish | Mammals | Birds | Turtles | |
|---|---|---|---|---|
| Number of studies | 43 | 14 | 85 | 34 |
| Frequency of occurrence | 72% | 64% | 89% | 100% |
| Number of pieces | 44% | 57% | 62% | 50% |
| Mass | 23% | 36% | 51% | 35% |
| Mean | 11% | 29% | 47% | 35% |
| Median | 2% | 0% | 4% | 9% |
| Range | 4% | 29% | 24% | 38% |
| Size | 25% | 57% | 36% | 35% |
| Colour | 30% | 14% | 32% | 29% |
| Reference to North Sea standardized protocol | 2% | 7% | 22% | 9% |
2.3 Plastic ingestion in seabirds as a model study group
To date, OSPAR, in particular the North Sea states, is the only jurisdiction that has implemented regulations aimed to track changes in plastic pollution through an environmental indicator, which is currently followed in all European Commission marine areas.15,16 The current definition of OSPAR's marine plastics Ecological Quality Objective (EcoQO) is: “there should be less than 10% of Northern Fulmars having 0.1 g or more plastic in the stomach in samples of 50–100 beached Fulmars from each of 5 different areas of the North Sea over a period of at least 5 years”.36 With a lack of policies in other regions, efforts to monitor marine plastic pollution or track ingestion by wildlife, researchers have been left to develop their own framework for studying and reporting plastic ingestion by marine megafauna, often as side projects. Although these various studies clearly add to our knowledge of plastic pollution in the marine environment, this growing field of metric papers without clear standardization of reporting metrics and techniques is not conducive to comparisons across space, time or taxa – or for use in larger meta-analyses and assessments.
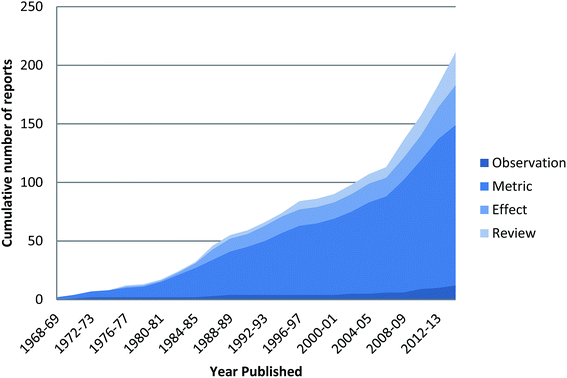 | ||
| Fig. 2 Cumulative number of published reports in the peer-reviewed literature on ingested plastics in seabirds from 1968 to 2015. | ||
2.3.2.1 Collection techniques. The objective of documenting plastic ingestion is to obtain a representative estimate of plastic ingestion for the wider population. Therefore, it is important to consider whether the collection method for specimens may influence the result. We reviewed 85 publications documenting plastic ingestion in seabirds and found a variety of specimen collection techniques were used. Most data were collected by necropsy of intact birds (70%) and examination of food remains (27%; Table 2), with only a handful (3%) not reporting the collection method. Among the 70% of studies that involved necropsies of intact birds, 5% of studies collected specimens intentionally (i.e., legal and confiscated from illegal hunting), 12% of studies already used carcasses that were in hand (i.e., from rehabilitation centers, fisheries bycatch), and the remaining 21% were collected from beaches following wrecking events (Table 2).
| Collection method | N | Percentage of total |
|---|---|---|
| Necropsy of stomach contents | 82 | 70% |
| Beached birds | 24 | 21% |
| Collected for other research | 21 | 18% |
| Found dead (i.e. predation on colony) | 16 | 14% |
| Fisheries bycatch | 12 | 10% |
| Legal hunting | 3 | 3% |
| Illegal poaching confiscation | 2 | 2% |
| Killed for plastics work | 2 | 2% |
| Rehab center | 2 | 2% |
| Food remains | 32 | 27% |
| Bolus | 12 | 10% |
| Regurgitation | 11 | 9% |
| Water-offloading | 8 | 7% |
| Emetics | 1 | 1% |
| Not specified | 3 | 3% |
| Present data on multiple collection methods | 22 | 26% |
| Compare between sampling methods | 8 | 9% |
Approximately a quarter (n = 22) of the plastic ingestion studies collected specimens using different methods, but only a small fraction (9%) compared ingestion results across collection techniques. Among these, we found contradictory results on whether collection methods influence accumulated plastics in seabirds. For example, Van Franeker & Meijboom38 reported no statistical difference in ingested plastic between fulmars that had died slowly and those that had died instantly (e.g., fisheries or collisions). Similarly, Colabuono et al.39 found no differences in the frequency of occurrence of plastic in eight Procellariiformes between beached birds and those recovered as bycatch by longline fisheries. Conversely, while Ryan40 found no difference in frequency of occurrence, mass or number of ingested plastics particles in Blue Petrels (Halobaena caerulea) either found beached or collected at sea, Ryan did find significantly higher numbers of plastics in beached birds as compared with collected birds in eight species examined. Auman et al.41 found that Laysan Albatross chicks that died naturally had greater masses of ingested plastics as compared with injured chicks. Importantly, these comparison studies represent only a small number of seabird species, suggesting that we know very little about how the collection methods affect ingested plastic detection in other groups. From a comparison of these studies we recommend that a researcher's ability to estimate true population level plastic loads may be influenced by the method of specimen collection, and therefore it is an important variable to consider when comparing results between studies and regions.
A major advantage of collecting data on plastic ingestion through necropsy of whole specimens is that one can examine the entire gastrointestinal tract for plastics, which provides a level of certainty in the findings. For example, in seabirds the proventriculus and gizzard are the main sections examined for accumulated debris. Conversely, in turtles, the entire length of the gastrointestinal tract is examined for plastics, as they frequently have plastics in the intestines as well as the stomach and esophagus.42 One can also examine different parts of the gastrointestinal tract for plastics, which may help elucidate ingestion and retention times.43 Importantly, regardless of the section examined, it should be reported to ensure comparability to other studies. A second advantage of examining plastic ingestion via necropsies is that the age, sex, possible cause of death and body condition of the birds can be determined. Because many seabirds are externally monomorphic, necropsies can allow an examination of differences between sexes. However, few studies have examined such differences. Only 11 of 85 (13%) studies reported testing for differences in plastic ingestion between sexes, with only one of those reporting a significant difference in just a few of the species examined.44 We discuss sex differences in plastic ingestion below, as they are related to species' ecology as well as sampling methods. Lastly, necropsies provide an opportunity to examine gastrointestinal contents using a common sized sieve allowing for multiple size classes of plastics to be examined. A 1 mm sieve is commonly used for species that can be examined with the laboratory (i.e., not the large whales), and the widespread uptake of this method will facilitate increased comparability between studies in the future.
For the remaining 27% of the 85 plastic ingestion papers, data on plastic load reviewed involved examination of food items from either live birds (17%) or found boluses (10%) to examine plastic ingestion (Table 2). Sampling of live birds is advantageous because it can be done systematically, although it is unclear whether 100% of the plastic loads can be collected via natural regurgitation, or induced regurgitations (i.e., stomach flushing or chemical emetics). Active sampling of live birds can be invasive, and researchers must give careful consideration of the ethical principle underlying the research before using such techniques. Sampling live birds, if complete stomach sampling can be achieved, is advantageous in that it does not rely on ad hoc sampling of birds (e.g., beached birds, although see below). Live birds can generally be sampled using three methods: natural regurgitation, induced regurgitation (via stomach pumping, also called lavage or water offloading), and chemical emetics.
Whether it will be possible to sample plastic ingestion from natural or induced regurgitation in a seabird will depend on the species, age class, and time of the year. For most species, natural regurgitations represent chick meals that parents are returning to nest-bound offspring,45 or occasionally courtship feeding for a prospective mate. Regurgitations are less likely during the non-breeding season (or in non-breeding or pre-breeding individuals), and may also only represent the most recent meal because plastics accumulated previously may remain in the bird. Induced regurgitations via stomach pumping, also called lavage or water offloading, involves pumping the bird's stomach with water to induce regurgitation. Unlike natural regurgitations, any individual can be subject to stomach pumping, regardless of breeding status or age class. The technique has been used widely in penguins,46 fulmarine petrels,47 and shearwaters.48 Chemical emetics can also be used to induce regurgitation and obtain dietary samples, including ingested plastics. Emetics are pharmaceutical agents that induce vomiting.49 However, care must be taken to ensure the correct emetic and dose is chosen. Tartar emetic (antimony potassium tartarate) and apomorphine are more likely to cause adverse reactions than syrup of ipecachuana (ipecac).50,51 For larger species, the volumes required may be prohibitive,49 but for smaller species, where stomach flushing may not be recommended, using an emetic may be the best option. In a study of Leach's Storm–petrels (Oceanodroma leucorhoa), Bond and Lavers49 found that of 12 birds subjected to treatment with syrup of ipecac, all experienced complete emesis. Regardless of the technique, the underlying assumption to the approach is that all ingested plastic is available to be enumerated. However, this is likely not the case with most seabirds. For example, stomach pumping may not remove all plastics, with microplastics more likely to be left behind.52 In flesh-footed Shearwaters (Ardenna carneipes), 6% of plastic items in the proventriculus remained after stomach flushing.52 Plastics in the gizzard are also less likely to be retrieved from live birds given the constriction in the gastrointestinal tract between the proventriculus and gizzard.53–55 Therefore, stomach pumping and emetics should be used with caution, but particularly when reporting plastics in seabirds.
For species that regurgitate indigestible prey items regularly in the form of boluses, these can also be used to assess plastics (10% of the studies used this technique). Bolus examination is non-invasive, but opportunistic and can be repetitively collected from individuals. Unfortunately, boluses unlikely represent the full plastic load of an individual and caution must be used in assessing plastics when this technique is employed as small pieces of plastics are potentially lost to the environment before collection56 or vice versa, environmental particles sticking to the sample. It must also be recognized that to assign plastic ingestion to a species, year, or individual, particular care must be taken, and potentially the sampling region must be cleared of plastic at the end of each breeding season.
2.3.2.2 Sampling among age classes. We found that numbers of papers reporting plastic ingestion in seabirds equally reported on this phenomenon in adults and juveniles (59% and 53%; n = 78 studies reporting some age classification; total greater than 100% as some studies report on both groups). Ingested debris levels in adults may be indicative of individuals' larger range and distribution if ingested plastics accumulate in the gastrointestinal tract of individuals. Breeding stage can influence adult debris loads as adults can regurgitate plastics along with food items to young chicks (inter-generational transfer) resulting in a steady decrease in adult plastic levels over the breeding season.57 Therefore, when sampling adults it is important to consider annual cycles, migration paths and retention times when interpreting where ingested debris may have been acquired.
Few of the plastic ingestion studies (13%) we reviewed examined differences in plastic ingestion between adults and young birds collected at the same time and location. In general, young birds tended to have higher frequency of occurrence or mass of ingested plastics than adults.13,54,58–61 Some studies showed that adults had higher levels (mass, number of frequency of occurrence depending on species),55 while many studies showed no difference in plastic ingestion between age classes.62–64 Reporting age class and, if applicable, breeding stage are therefore essential to interpreting metrics of plastic ingestion.
Determining the source of plastics in juveniles' stomach contents can also be challenging. Since many species show long term accumulation of plastic debris in their stomachs during chick-rearing, chicks can be fed a mix of distant- and locally-foraged plastics. Adults may have accumulated marine pollution months before the breeding season, which is then fed to the chicks. For example, Wilson's Storm–petrel chicks in Antarctica had higher plastic frequency of occurrence than adults.60 In fact the plastic levels in chicks was so high that the authors attributed it to sources likely beyond the local foraging ranges.60 Depending on the species, this can include thousands of kilometers (e.g., albatrosses), or tens of kilometers around the colony (e.g., auklets).
2.3.2.3 Purposeful sampling and reporting. While 73% of the reports that we reviewed included the assessment of plastics as one of the primary objectives of the published work, only 1/85 studies indicated that the findings presented were part of a targeted long-term monitoring effort.13 All the other papers presented data on plastic ingestion that were collected through either one-time research efforts, or opportunistic sampling of birds collected for other purposes. While this ad hoc, opportunistic sampling may pose challenges for rigorously examining broad trends, early data on the presence, or absence, of plastic in seabird gastrointestinal tracts from diet studies are now informing changes in seabird plastic ingestion. For example, an analysis of prey items consumed by short-tailed Shearwaters (Ardenna tenuirostris) in Tasmania during 1978–1980 provided some of the first data on plastic ingestion by adults of this species.65 Recent work found the proportion of adult shearwaters consuming plastic has increased from around 37% of the population in 1978 (ref. 65) to 63% in 2010.58 Additionally, opportunistic sampling can take advantage of events that can yield large numbers of samples otherwise not available: wreck events where hundreds or thousands of seabirds wash up on beaches provide data on the type or quantity of plastic ingested by a range of species.58,66 Such surveys are useful as they provide a ‘snap shot’ view of the situation at the time.
Systematic sampling can offer advantages and avoid potential bias (e.g., unequal sampling intensity or preferential sampling of individuals or locations) introduced by the use of a single method, enabling inference of population trends over time as well as identification of variables affecting these parameters that could not be obtained with opportunistic designs alone. Studies that have surveyed individuals systematically over many years have yielded valuable insights into long-term trends in the abundance of plastic in regional waters, as assessed by regular sampling of the stomach contents of wildlife67,68 (Ryan 2008; Mrosovsky, Ryan & James 2009). Such sampling has contributed significantly to our understanding of patterns and processes over time, and also led to the development of marine pollution management targets, such as the EcoQO for North Sea Northern Fulmars.13 A combination of systematic and opportunistic sampling is recommended for studies that rely on beach-washed animals. For example, pairing data from beach-washed animals with sampling of live individuals (e.g., boluses or stomach pumping) can overcome any potential bias (due to unknown cause of death; though see above).
2.3.2.4 Types of data in published reports & terminology. There are a variety of metrics used when reporting ingested plastics in marine megafauna with little consistency in how these are interpreted and presented. The most common metric presented in the seabird literature reviewed was the percentage frequency of occurrence (% FO) of ingested plastic (89%; Table 2). This is the most basic information on plastic ingestion: what proportion of the sampled individuals contain plastic? In the ingested plastics literature, the terms prevalence and incidence are often used interchangeably for the % FO, though in other bodies of literature their meanings are quite different.69 Following diet studies of stomach contents,70 we therefore recommend to use the term ‘Percentage frequency of occurrence (% FO)’. The number of pieces of plastic is the second most often reported metric (62%). The number of plastics can be indicative of how much plastic an individual has consumed. However, it must be considered that as larger plastic items are likely broken down in the stomachs of seabirds the number of pieces accumulated in the stomach may not reflect the number of pieces ingested directly.
Data on the mass of plastics were reported in half of the papers reviewed (51%), though plastic mass is increasingly reported in the seabird literature. While only 21% of the papers published from 1968–1999 reported mass of plastic loads, 64% of those published from 2000–2015 included plastic mass in their results. The mass of accumulated plastics in seabirds is arguably the most important metric from a biological perspective. The mass of plastics relays information on the volume of plastics in an individual, which is important as plastics compete with food for space in the stomach. Many seabirds also rely on reducing wing-loading (body mass to wing size) for flight and diving, therefore adding mass to a seabird gives a plastic-loaded bird a disadvantage. While it is challenging to test for how the mass of plastics may affect seabirds, new research documenting effects of tracking devices attached to birds may provide some insights. Typically, most tracking devices deployed on birds are limited to be <5% of the birds body mass,71 but research suggests that when devices exceed 2.5% of the bird's body mass, year-to-year survival declines significantly.72 Therefore, mass of plastics carried by marine birds must be considered on a species-specific basis, and will benefit from applying information gained from the field of seabird science using tracking devices to examining the potential impacts from plastics.
2.3.2.5 Metrics presented in published reports. While there are some standard metrics reported in plastic ingestion studies, the terminology used to quantify the quantity and characteristics of ingested is inconsistent across studies. Though “intensity” is defined as a value derived from only affected individuals (i.e. the average mass or number of pieces across only those birds containing plastics), “abundance” is used in the parasitology literature to describe values from all individuals examined (i.e., an average value using all individuals examined73). While both intensity and abundance describe plastic burdens for a sample of individuals, their meanings and interpretations differ greatly.73 Most papers (95% of 85 papers) reported the mean or median abundance (either mass or number of pieces) from all individuals, but a subset actually reported the mean or median intensity (includes only counts from individuals containing plastics). While it can be argued that abundance values contain redundant information partly found in the frequency of occurrence data, it should be noted that abundance values are the most common throughout the literature, and therefore the most comparable among studies. At times, data on plastic debris ingestion can be highly skewed statistically, so reporting intensities can provide key information independent of frequency of occurrence, but this should be in addition to abundance values.
Variable terminology also creates a challenge with the statistical descriptions of metrics of plastic ingestion across studies. Mean values are the most frequently reported, but have the disadvantage of misrepresenting the sample if there are a few individuals with extremely high values, or large numbers of individuals without plastics. Median values are useful for describing ingested plastics data as they are less sensitive to the effects of outliers within the sample, and hence give a more typical value in a skewed dataset. Consequently, mean and median values can differ substantially for the same sample: Provencher et al.74 found a mean mass of 9.5 g plastic ingestion per bird compared to a median of 2 g. Only 40% of the 85 studies reviewed here reported a mean value (for either mass or number of pieces), and only 3% report a median value.
The geometric mean mass is another way of reducing the influence of extreme values on the mean, yet with the advantage of using all of the data points. It calculates the mean of the data following logarithmic transformation.13 It loses mathematical value when there are many zero values (which some plastics data sets are prone to have), however the geometric mean can provide a good measure for comparing plastic ingestion over time. The disadvantage is that the geometric mean, if read as the ‘average’, can be misinterpreted as it underestimates the most commonly occurring plastic ingestion metrics. Such an issue is particularly relevant when using seabird ingestion studies to inform policy as it could undervalue the magnitude of marine litter pollution – sometimes we need to know the extreme values. The range and maximum values of plastic ingestion complement presentation of the average by providing context, particularly given the effect of data skew on averages. Only 24% of the published literature presented range values for plastic.
Both standard error and standard deviation are used in the ingested plastics literature. The standard error should be used to indicate the precision of the estimate of the mean, whereas the standard deviation should be used to indicate the dispersion of the sampled data. While plastic ingestion data are unlikely to be normally distributed, few studies treat the data accordingly. Commonly, the number of pieces of plastic is often a Poisson distribution,75 so the median, inter-quartile range, or 95% confidence intervals are more statistically appropriate. While confidence interval and standard error of the mean both indicate the reliability of the mean, standard error values include only about two-thirds of the values measures, whereas the 95% confidence intervals, by definition, include 95% of the values sampled, giving the reader a better sense of the range of the data.
An important metric that is only reported within the literature for Northern Fulmars is the percentage of birds above or below a certain level (see also Section 3.1).13 This species-specific approach allows for standard reporting metrics that is straightforward and easy to follow while circumventing some of the more complex idiosyncrasies of data presentation and interpretation as described above. More work is needed to develop such metrics for a broad set of indicator species beyond the Northern Fulmar. Based on our review of the seabird literature we conclude that within the large body of published work there is insufficient information to reconstruct the descriptive statistics and compare findings in meta-analyses.
2.3.2.6 Statistical power. Researchers are often interested in changes in the frequency of occurrence or abundance of ingested plastic over time. Monitoring that change is often challenging given the low sample sizes and low frequency of occurrence or abundance of plastics in most populations. The annual sample sizes required to detect a 20% change in the frequency of occurrence of plastic ingestion in Canadian seabirds ranged from 61 (Thick-billed Murres, Uria lomvia) to 193 (Northern Fulmars), depending on interannual variability and frequency of occurrence.21 Similarly, >600 Laysan Albatrosses (Phoebastria immutabilis) would need to be sampled annually to detect a 5% change in the frequency of ingested plastics.76 In the North Sea, Northern Fulmars have a high frequency of occurrence of plastic, so fewer individuals are required to detect changes over time: evaluation of annual sample variances in Dutch birds38 indicated that a sample of roughly 30 to 40 birds per year produced reasonably robust figures for frequency of occurrence and average number or mass of plastics. Power analyses of these data produced strongly variable results not only for the different metrics, but also when looking at industrial plastics, user plastics or their combination. Overall, van Franeker & Meijboom38 concluded that in the order of 4 to 8 years of samples of each around 40 fulmars would be needed to reliably detect changes in ingested plastic mass in the order of 25%. Given that most studies sample few individuals (usually <100), and species often have low frequency of occurrence of plastics, current sampling strategies are often only sufficient to monitor very coarse changes over time.
2.3.2.7 Importance of reporting plastic and non-plastic debris in a common framework for comparisons. The recognition of plastic debris as a threat to marine wildlife has grown over the past five decades,77 but what has received less attention is a similar treatment for other anthropogenic debris. While foams and rubber materials are often correctly lumped with “plastic”, other debris is clearly distinct and often mentioned but not given separate treatment. For example, paper and wood products, and pieces of cardboard are consumed by marine birds, but because they are composed of natural materials and break down (presumably more rapidly than plastic), they are not often reported with the same level of detail as plastics. Non-reporting of non-plastic items is a concern because some recent studies are finding surprisingly high frequency of occurrence of metal debris in some species.24 We would predict that metals, which presumably sink in water, would not be distributed as broadly as plastics across the ocean, or certainly not be as available to surface feeders. Holland et al.5 have found wax or plastic-coated wrapping papers in coastal marine birds in eastern Canada, and such materials are often grouped with other film-like plastics. Depending on digestion and breakdown rates, this type of product could have similar possibilities of blockage in avian digestive tracts, and would certainly be expected to be more common in scavenging species like gulls. However, in order for researchers to draw sound, statistical comparisons of the frequency of occurrence, abundance, and trends in ingestion of these other types of materials, they require the same rigorous reporting (size, mass, frequency, colour) as for plastic materials.38
3 Case study: what can be learned from taking a global standardized approach
3.1 Brief history of Ecological Quality Objective in Northern Fulmars
To meet the emerging need to track and monitor marine litter in the North Sea, the EcoQO was established based on plastic debris found in Northern Fulmar stomachs (both the proventriculus and gizzard), a species known to ingest plastics throughout its range at the time, and often found in beached bird surveys in the North Sea region.13 Although the initial EcoQO was based on plastic loads found in Northern Fulmars, during the early phases of policy development, working groups of the International Council for the Exploration of the Sea (ICES) and OSPAR worded a preliminary target definition of the proposed EcoQO as plastics in stomachs of ‘seabirds’ as ‘the proportion of birds having 10 or more pieces of plastic in the stomach should be below 2%’.13,78 This target had no substantiated background of ecological or individual or population health. It represented an arbitrary target considered to reflect ‘acceptable ecological quality’ as used in policy documents.After the original level of 2% was determined, the Northern Fulmar became the chosen species for EcoQO monitoring in the policy discussions. At that stage, it was recommended to OSPAR and ICES that the target definition would be more ecologically meaningful in terms of plastic if mass was used instead of number of particles. Early Dutch studies indicated that in terms of ‘mass’ of plastics in Northern Fulmar stomachs, the critical level of 10 particles equaled to about 0.1 g of plastic.38 Dutch studies also showed that nearly every Northern Fulmar in the southern North Sea had plastic in the proventriculus, with an average mass of 0.6 g per bird (about 0.1% of the species average body mass) between 1996–2000.38 Consequently, the policy aim of <2% of Northern Fulmars exceeding 0.1 g of plastic became unrealistic for the foreseeable future. OSPAR and ICES then followed the advice to redefine the less strict target to <10% of beached Northern Fulmars exceeding 0.1 g of plastic in the stomach.
This new target still lacked an ecological background. The arbitrary proportion of 10% of birds was chosen from the definition for the EcoQO on oil pollution, which used Common Murre (Uria aalge) as an indicator species with an EcoQO target of <10% of beached guillemots having oil in their feathers.36 The OSPAR target level is an arbitrary political choice, but was aimed to match pollution levels in environments where anthropogenic influence is expected to be low. Only later, the Northern Fulmar <10% with <0.1 g EcoQO target proved to be somewhat reasonable, when reports for Northern Fulmars in the Canadian high Arctic showed to be close to such a target level.13,79 The Canadian high Arctic can be viewed as a relatively clean environment, with few local sources of marine pollution, and limited shipping in the region.40 While it would be unrealistic to have a target below such a level in regions more heavily affected by plastic pollution, aiming at these remote level targets seems reasonable if the goal is to reduce plastics in the environment. Importantly, although the EcoQO target provides no evidence for an acceptable threshold of harm to individuals or negative impacts on populations, it has created a standardized protocol that researchers throughout the northern hemisphere have been able to employ leading to a cohesive approach to monitoring marine plastic pollution, and a framework for similar approaches elsewhere.
3.2 Results from a standardized approach
At a regional level, the standardized approach from the North Sea has allowed temporal trends to be assessed over the last three decades (Fig. 3). Importantly, the framework has allowed researchers to examine patterns in pollution type which has helped to inform policy and mitigation practices as demonstrated by the reduction of industrial plastics in beached birds in the recent decades after industry standards were changed.13,67 The standardized approaches to assessing the accumulation of plastics in Northern Fulmars has also allowed policies related to target pollution levels to be re-evaluated over time, and re-considered (Fig. 4; EcoQO change from 2% to 10% and from number to mass metric in 2006;80).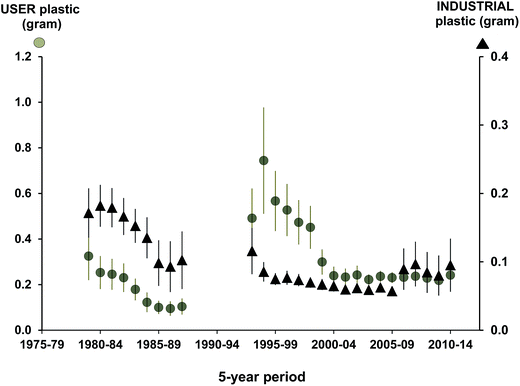 | ||
| Fig. 3 Five year running arithmetic averages (with standard error bars) for user and industrial plastics (both in grams) in Northern Fulmars (Fulmarus glacialis) in the Netherlands collected as part of the standardized ‘Save the North Sea’ project.13 | ||
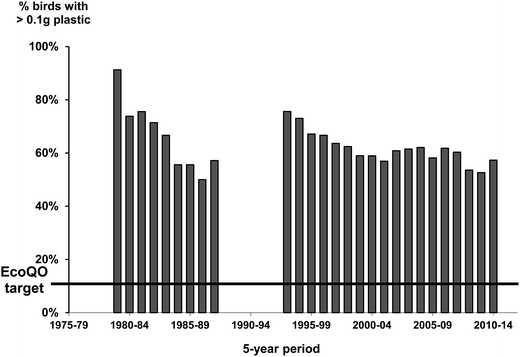 | ||
| Fig. 4 Percentage of Northern Fulmars with >0.1 g plastic using a running 5 year mean to examine changes in plastic ingestion in the Netherlands collected as part of the standardized ‘Save the North Sea’ project.13 The black line represents the Ecological Quality Objective from the North Sea of birds to have 10% or less of >0.1 g of plastic in their stomachs. | ||
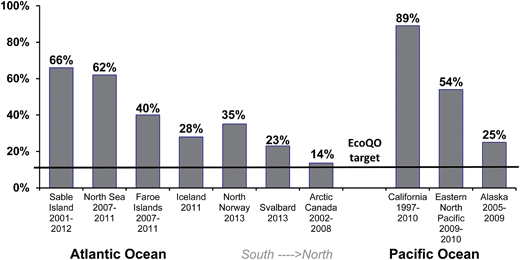
While the North Sea Northern Fulmar program has shown great utility in using standardized approaches, the real benefit of the methods were demonstrated when the wider scientific community studying Northern Fulmars adopted the protocol allowing for comparisons among regions at the ocean basin scale. First, as discussed above, the arbitrary target of 10% has since been shown to be realistic in less polluted environments as demonstrated by studies of Northern Fulmars in the Canadian Arctic (Fig. 5). Second, global patterns can also be assessed from the compiled data, allowing us to examine hypotheses developed in earlier studies, for example that the ingestion of plastics decreased with increasing latitude.81–83 It is only with recent studies of Northern Fulmars throughout their range that this can be tested across multiple sites in two ocean basins (Fig. 6).
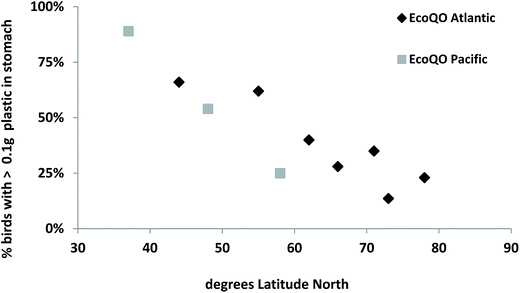 | ||
| Fig. 6 Ecological Quality Objective performance in Northern Fulmars (Fulmarus glacialis) along a gradient of latitude (south to north) in the Atlantic and Pacific Oceans. | ||
While applying the EcoQO to other species is problematic due to its political origins (i.e., lacking known biological level of impact), applying a similar approach to other species could be beneficial. Target levels could, as in the Northern Fulmar, be set at ingestion levels observed in low pollution areas. A wider use of EcoQO may also encourage local governments to adopt similar approaches within their own regions using the species that are present and available for study.
4 Recommendations for plastic ingestion studies in marine megafauna
One of our objectives was to outline a standardized set of methods for measuring and reporting accumulated plastics in marine megafauna to help shape future studies of ingested plastics, contribute to our global understanding of marine pollution, and facilitate easier comparisons among studies, regions, and species.84 With this is mind, we make several recommendations here that are specific to seabirds, but useful for all taxonomic groups where ingested pollution is studied.To this end, standardized methods and metrics should be used across all studies. For seabirds, the methods used by the North Sea Fulmar Study13,14 have been adopted widely, but not universally. We recommend that all publications reporting ingested plastics in seabirds use this protocol as it offers a comprehensive and flexible framework for the quantification and classification of marine debris. In addition to this method and classification framework, we highly recommend that reporting of data on variables that have been shown to influence plastic accumulation in marine megafauna, and particularly information on collection method, date and location of collection, age, and sex. For other groups of marine megafauna we recommend adopting the North Sea Fulmar Study plastic classification system, which separates debris into user and industrial categories, and then further subtypes (see Section 4.1 below), as well as the same standard minimum metrics outlined above. In a European context such standardization has started with recommended procedures for marine turtle and fish15 and results for plastic ingestion by seals.17 Below we present some guidelines for each classification for ingested plastics that will help standardize data reporting and cross-study comparisons.
4.1 Plastic type
Distinction of ingested litter into categories provides an arguably fundamental insight into the source of debris and thus required mitigation actions, and is key when using seabirds as indicators of environmental pollution. All plastic items should be broadly categorized as either industrial plastic pellets (or ‘nurdles’) or user plastic (all non-industrial remains of plastic objects;85). Industrial plastic pellets are small, often cylindrical-shaped microplastic granules approximately 4 mm in diameter, but oval, disk-like and rectangular shapes also occur. User plastic includes the following commonly reported categories: sheet plastics (e.g., plastic bags), threadlike plastic (e.g., rope or netting), foamed synthetics (e.g., polystyrene) and hard fragments (from mostly unidentifiable larger objects). A “miscellaneous” category can be used for uncommon items such as cigarette filters, pieces of balloon rubber, etc., however, reports should preferably include specific details of the items included in this category. Online supplemental material or depositing raw data in an online repository is a useful way to include these details if they are not directly relevant to the original study. Such classification has been extensively employed by studies of seabirds, turtles and marine mammals and has enabled valuable studies of long term changes in marine debris composition.13,67,86 A comprehensive guide to the above categories can be found in the online supplement of Van Franeker et al.13Increasingly, there is interest in knowing more about the plastic types in the marine environment.87,88 If the focus of the study is to provide a baseline for the type of plastics consumed by marine megafauna, or present in the environment, plastic items should be further categorized according to polymer type (e.g., polyethylene) using Fourier Transform Infrared spectroscopy (FT-IR;89,90) or Raman Spectroscopy.91 From a biological perspective, this level of classification of plastics is of particular interest in relation to how different chemical contaminants are associated with marine plastics.
4.2 Plastic size
The most commonly used plastic size classes include mega- (>100 mm), macro- (>20–100 mm), meso- (5–20 mm) and microplastics (<5 mm),92–94 but no globally accepted definitions exist. As a result, the term “microplastic” has been used to describe plastic particles ranging in size from <1 mm to 20 mm.95,96 The lack of standardized sizes classifications, and their relevancy to marine biota has been recognized as a research priority.97,98 While marine megafauna ingest several sizes of plastics, we advocate the use of the categories suggested by Barnes23 as the most relevant and applicable. This includes megaplastic (>100 mm), macroplastics (>20–100 mm), mesoplastics (>5–20 mm), and microplastics (1–5 mm) as these are the most likely detected in marine megafauna and easily summarized from plastic accumulation studies. We recognize that studies focused on small biota (e.g., plankton, bivalves) may also report smaller plastics (<1 mm), often also referred to as micro-plastic which may be referred to ultrafine-plastics (1 μm to 1 mm), and nanoplastics (1 nm to 1 μm) following the terminology used by the field of nano-ecotoxicology.99 Specifically, “nano-” refers to particles that have potential to interact with biota at the cellular level, and should be used accordingly. In order to better contribute to the overall assessment of plastics in the environment and biota, we recommend that authors report the percentage of plastics in each of the four size categories.4.3 Plastic colour
Plastic colour is important to report, but often not reported (only reported in 27% of 85 papers reviewed). First, assessing colour in marine megafauna can give insights into how organisms may select plastics from the environment. Plastic size and colour can influence the chance of being ingested by different animals with different foraging strategies.100,101 However, to assess selectivity, organismal data needs to be paired with environmental assessments on the availability of different coloured plastics in the environment, which are currently lacking in many regions. Future studies may allow us to hindcast environmental data onto megafauna reports and examine selection of plastics from the environment. Second, plastic colour may also be associated with higher exposure to some chemicals.102,103The specific method of colour determination of plastics particles found ingested by marine megafauna is often not mentioned,48,58,104 or done by two different observers to confirm the colour;101 in all these cases it is impossible to compare studies on plastic colours. Ideally, plastic items should be assigned to a colour by comparing individual pieces to a colour wheel or standardized chart. To accurately and consistently report colour across plastic ingestion studies we recommend a two-step colour sorting process. First, a colour wheel (that includes secondary and tertiary colours) should be used to assign plastic pieces to specific colour categories. A Munsell chart or 72-colour wheel can be used for this step (widely available for order online). Second, the specific colour assignments should be grouped into eight broad colour designations; off/white-clear, grey-silver, black, blue-purple, green, orange-brown, red-pink, and yellow.61 This use of a large colour wheel to assign pieces to a board range of colours, and then collapsing these categories into only broad groupings allows for both observer accuracy (via the large initial categories), and systematic presentation of a few comparable groups (the eight broad colour groupings). Distinguishing colour down beyond these categories is likely to lead spurious results due to differences in categorization. Additional scales (e.g., black to grey to white) may also be used, but the source and type of scale should be made clear to the reader to enable comparison. Importantly, plastics, especially white pieces, are frequently discoloured following digestion, so researchers should endeavor to report the likely original colour of each piece.
4.4 Metrics reported
All publications reporting plastic ingestion should report the frequency of occurrence of plastics (with a 95% confidence interval using the Jeffreys interval105 [also see http://epitools.ausvet.com.au/content.php?page=CIProportion]), sample size and the methods for the collection of the samples. These are the basic components of any study on plastic ingestion in marine megafauna. At a minimum, we also recommend that all authors include data on the mean (with standard deviation), median and range of mass of the ingested plastics per individual, including individuals that contain no plastics (Box 1). Standard Error, as used in Fulmar protocols, can be calculated from standard deviation and sample size, which is critical to ensure future comparisons for this species. Studies should also report the mean, standard deviation, median, and range of all plastic debris metrics (number of pieces, total mass of debris pieces by debris category; see ESI†). All summary statistics should give abundance values (which include all individuals examined not just those containing plastics). Reporting absence data are crucial, as the amount of plastic in the oceans is expected to increase over time.23,106 For studies examining how the amount and distribution of plastics change over time, authors should include information on temporal trends in the size classes, colour and type of plastics as well. This will be facilitated if data are collected using comparable methods (see above). Data on ingestion of plastics should be reported in tabular form, not graphical form for papers presenting novel plastic ingestion data for marine megafauna. This practice, along with raw data archiving in open access forums, will facilitate spatial and temporal comparisons, without having to guess where bars or points line up with a scantily labeled axis. Luckily the use of online supplemental material makes this practice increasingly easier.
Box 1 – Recommended reporting guidelines for all marine megafauna plastic ingestion studiesAs a minimum all studies should report:• Location and timing of sampling • Method of sampling • Sample size • Frequency of occurrence of ingested plastics (with a 95% confidence interval; Jeffreys interval) • Mean (with standard deviation and error), median and range of mass of ingested plastics/individual (including all individuals sampled) • Mean (with standard deviation and error), median and range of all plastics reported by debris category (user/industrial; fragment/foam/sheet/thread/other) Additional information to be provided: • Size of plastics reported by size classes (mega/macro/meso/micro/ultra-fine/nano) • Colour reported in 8 broad colour groups (see text for more details) |
4.5 Future areas of study
Votier et al.114 fed six Great Skua (Stercorarius skua) breeding pairs fish that contained small numbered plastic markers that were date-stamped inserted within fish heads. From 76 fish fed to the six pairs of skuas, eight (12.3%) of the plastic markers were recovered in pellets. No markers were discovered in faeces, suggesting the birds are unable to pass the plastic items. The bulk of the cast pellets were produced between 6 and 24 hours (53%). Also some species cast pellets, regurgitating indigestible materials such as bones, feathers, or shells. This is particularly the case with gulls and skuas, but occurs in a range of species including albatrosses and shearwaters. Despite this, pellets are unlikely to eliminate plastics completely, though the reasons why only some pieces are regurgitated remain unknown.
Retention times can also be influenced by life history. Adults can have very low frequency of occurrence of debris when feeding chicks, as they offload plastics to their nest-bound young.57,58,115 These chicks have no mechanism for eliminating plastics fed to them by their parents (though some species can cast pellets to eliminate some items; see above), and consequently fledge carrying the plastics accumulated during the often lengthy chick-rearing period.
Plastic debris has been found in a variety of marine prey items, either in the wild or with ingestion shown to occur in experimental studies, including zooplankton,116 sessile molluscs,117 cephalopods,118 and large crustaceans like crabs and lobsters.119 Even among marine megafauna there may be transfer of accumulated plastics. Trophic transfer has been reported between seabirds,56,120 and an extreme example of this was reported by Perry et al.121 who found marine debris within Dovekie (Alle alle), that had been consumed by a Goosefish (Lophius americanus). Consequently, researchers have proposed that marine megafauna may in fact acquire plastic debris by consuming prey which themselves have ingested particles.122,123
Confirming this hypothesis is challenging. There is no obvious means of distinguishing between directly consumed plastic particles from pieces that were first consumed by fish, which were then eaten and digested by a bird. One way to solve this problem would be to capture and analyze the diet of seabirds that have recently been feeding (e.g., following direct observation). However, this presents another challenge, in that digestion times can be very rapid in seabirds.124 For example, even when collecting auks which had just been diving for fish and pouring alcohol into their digestive tracts to preserve contents, Provencher et al.125 found that fish which had just been consumed were already partially digested, minutes after capture. Therefore, at present researchers must (safely) assume that plastic in seabird prey becomes plastic in seabirds,126,127 but distinguishing plastics in species that are directly consumed versus consumed through trophic transfer is very difficult to quantify. Given that seabirds accumulate plastics, the source of the plastics (either direct or indirect) is perhaps irrelevant from a toxicological perspective. Therefore, studies interested in the trophic transfer of plastics within the food web should approach this from both a bioaccumulation and a biomagnification standpoint, similar to other ecotoxicology studies interested in biological effects induced from environmental pollution.
5 Conclusions
Over the last 60 years, reporting of plastics ingested by marine megafauna has gone from semi-noteworthy in a report, to a growing body of literature with a rapidly increasing number of publications specifically reporting plastics in marine biota and their potential impacts on the marine environment. While simply reporting plastics within a diet study was once sufficient, the growing interest in marine plastics and their impacts, and purpose-driven publications on plastic accumulation in marine biota now demand a higher standard. Standards for reporting data are required to make studies comparable84 and to provide data suitable for statistically rigorous meta-analyses. These standards should include consistent reporting of the collection and sampling method, type of debris, the mass, the number, the colour, and the characteristics of the material, as well as the method of sorting and identifying materials. While other metrics and measures should continue to be explored by researchers to ensure creative and novel approaches which will drive researchers to explore new questions, the inclusion of basic metrics as discussed above is critical. The large quantity of data collected in recent years and increased awareness of the problems around marine plastic pollution can enable scientists to answer questions on a larger ecological scale when data are collected and reported using a standardized approach.Acknowledgements
We are indebted to the many colleagues with whom we have had numerous fruitful discussions, especially H. G. Gilchrist, I. Hutton, and G. J. Robertson. In general research on plastics received funding from Environment and Climate Change Canada, Detached Foundation (P. Clive & B. Neill), the Natural Sciences and Engineering Research Council of Canada, the Northern Contaminants Program (Indigenous and Northern Affairs Canada), and the W. Garfield Weston Foundation. The long term fulmar monitoring program in the Netherlands is funded by RWS Water, Traffic and Living Environment (RWS-WVL) of the Ministry of Infrastructure and the Environment (I&M). We thank C. Rochman and R. Thompson for inviting us to contribute to this special issue. Lastly, we thank the two reviewers of this manuscript that helped to improve this work.References
- UNEP, 2011.
- UNEP, UNEP Year Book 2014 emerging issues update, United Nations Environment Programme, Nairobi, Kenya, 2014 Search PubMed.
- E. van Sebille, C. Wilcox, L. Lebreton, N. Maximenko, B. D. Hardesty, J. A. van Franeker, M. Eriksen, D. Siegel, F. Galgani and K. L. Law, Environ. Res. Lett., 2015, 10, 124006 CrossRef.
- R. Dris, H. Imhof, W. Sanchez, J. Gasperi, F. Galgani, B. Tassin and C. Laforsch, Environ. Chem., 2015, 12, 539 CrossRef CAS.
- E. R. Holland, M. L. Mallory and D. Shutler, Sci. Total Environ., 2016, 571, 251–258 CrossRef CAS PubMed.
- T. P. Good, J. A. June, M. A. Etnier and G. Broadhurst, Mar. Pollut. Bull., 2010, 60, 39–50 CrossRef CAS PubMed.
- S. C. Votier, K. Archibald, G. Morgan and L. Morgan, Mar. Pollut. Bull., 2011, 62, 168–172 CrossRef CAS PubMed.
- D. W. Laist, in Marine Debris: Sources, Impacts, and Solutions, ed. J. M. Coe and D. B. Rogers, Springer-Verlag, New York, NY, 1997, pp. 99–140 Search PubMed.
- S. Kühn, E. L. B. Rebolledo and J. A. Van Franeker, in Marine Anthropogenic Litter, ed. M. A. Bergman, L. Gutow and M. Klages, Springer Open, Bremerhaven, Germany, 2015 Search PubMed.
- S. C. Gall and R. Thompson, Mar. Pollut. Bull., 2015, 92(1–2), 170–179, DOI:10.1016/j.marpolbul.2014.12.041.
- P. Ryan, in Marine Anthropogenic Litter, ed. M. Bergmann, L. Gutow and M. Klages, Springer International, New York, 2015 Search PubMed.
- J. A. van Franeker, M. Heubeck, K. Fairclough, D. M. Turner, M. Grantham, E. W. M. Stienen, N. Guse, J. Pedersen, K. O. Olsen, P. J. Andersson and B. Olsen, Alterra-Rapport, 2005, 1162, 1–70.
- J. A. van Franeker, C. Blaize, J. Danielsen, K. Fairclough, J. Gollan, N. Guse, P. L. Hansen, M. Heubeck, J. K. Jensen, G. Le Guillou, B. Olsen, K. O. Olsen, J. Pedersen, E. W. M. Stienen and D. M. Turner, Environ. Pollut., 2011, 159, 2609–2615 CrossRef CAS PubMed.
- OSPAR, Guidelines for Monitoring of plastic particles in stomachs of fulmars in the North Sea area, OSPAR, Texel, the Netherlands, 2015 Search PubMed.
- MSFD-TSML, Guidance on Monitoring of Marine Litter in European Seas, Publications Office of the European Union, Luxembourg, 2013 Search PubMed.
- European Commission, Reporting on monitoring programmes for Marine Strategy Framework Directive Article 11, DG Environment, Brussels, 2014 Search PubMed.
- E. L. B. Rebolledo, J. A. Van Franekar, O. E. Jansen and S. M. J. M. Brasseur, Mar. Pollut. Bull., 2013, 67, 200–202 CrossRef PubMed.
- R. G. Santos, R. Andrades, M. A. Boldrini and A. S. Martins, Mar. Pollut. Bull., 2015, 93, 37–43 CrossRef CAS PubMed.
- R. Yamashita, H. Takada, M. A. Fukuwaka and Y. Watanuki, Mar. Pollut. Bull., 2011, 62, 2845–2849 CrossRef CAS PubMed.
- C. M. Rochman, M. A. Browne, A. J. Underwood, J. A. van Franeker, R. C. Thompson and L. A. Amaral-Zettler, Ecology, 2016, 97, 302–312 Search PubMed.
- J. F. Provencher, A. L. Bond and M. L. Mallory, Environ. Rev., 2015, 23, 1–13 CrossRef.
- A. J. Gaston, Seabirds: A natural history, Yale University Press, New Haven, 2004 Search PubMed.
- D. K. A. Barnes, F. Galgani, R. C. Thompson and M. Barlaz, Philos. Trans. R. Soc., B, 2009, 364, 1985–1998 CrossRef CAS PubMed.
- M. D. English, G. J. Robertson, S. Avery-Gomm, D. Pirie-Hay, S. Roul, P. C. Ryan, S. I. Wilhelm and M. L. Mallory, Mar. Pollut. Bull., 2015, 98, 349–353 CrossRef CAS PubMed.
- M. L. Brandão, K. M. Braga and J. L. Luque, Mar. Pollut. Bull., 2011, 62, 2246–2249 CrossRef PubMed.
- J. L. Lavers, J. C. Hodgson and R. H. Clarke, Mar. Pollut. Bull., 2013, 77, 320–324 CrossRef CAS PubMed.
- N. Janinhoff, H. Verdaat and J. A. van Franeker, Sula, 2010, 23(1), 40–45 Search PubMed.
- G. C. Cadee, Mar. Pollut. Bull., 2002, 44, 1294–1295 CrossRef CAS PubMed.
- J. Couch, Proc. Linn. Soc. London, 1838, 1, 2–3 Search PubMed.
- W. Turner, Proc. - R. Soc. Edinburgh, Sect. A: Math., 1904, 24, 423–436 CrossRef.
- J. F. Piatt, W. J. Sydeman and F. Wiese, Mar. Ecol.: Prog. Ser., 2007, 352, 199–204 CrossRef.
- D. K. Cairns, Journal of Marine Biology & Oceanography, 1987, 5, 261–271 Search PubMed.
- J. R. Henderson, Mar. Pollut. Bull., 2001, 42, 584–589 CrossRef CAS PubMed.
- European Commission, Official Journal of the European Union, 2010, 53, C232, DOI:10.3000/17252423.C_2010.232.eng.
- J. Mouat, R. L. Lozano and H. Bateson, Economic impacts of marine litter, KIMO, Shetland, 2010 Search PubMed.
- OSPAR, The OSPAR System of Ecological Quality Objectives for the North Sea: a Contribution to OSPAR's Quality Status Report 2010, OSPAR Publication 404/2009, London, en Rijkswaterstaat VenW, Rijswijk, 2010 Search PubMed.
- W. Threlfall, Can. Field-Nat., 1968, 82, 176–180 Search PubMed.
- J. A. van Franeker and A. Meijboom, Litter NSV - Marine litter monitoring by northern fulmars: a pilot study, Alterra, Wageningen, the Netherlands, 2002, vol. ALTERRA-Ra Search PubMed.
- F. I. Colabuono, S. Taniguchi and R. C. Montone, Mar. Pollut. Bull., 2010, 60, 630–634 CrossRef CAS PubMed.
- P. G. Ryan, Mar. Environ. Res., 1987, 23, 175–206 CrossRef.
- H. J. Auman, J. P. Ludwig, J. P. Giesy and T. Colborn, in Albatross: biology and conservation, Surrey Beatty & Sons Pty Limited, 1998 Search PubMed.
- C. Poli, D. O. Mesquita, C. Saska and R. Mascarenhas, Iheringia. Série Zoologia, 2015, 105, 265–270 CrossRef.
- M. L. Mallory, Mar. Pollut. Bull., 2008, 56, 1501–1504 CrossRef CAS PubMed.
- S. Jimenez, A. Domingo, A. Brazeiro, O. Defeo and R. A. Phillips, Mar. Pollut. Bull., 2015, 96, 149–154 CrossRef CAS PubMed.
- A. L. Bond, I. L. Jones, J. C. Williams and G. V Byrd, Mar. Pollut. Bull., 2010, 60, 1346–1349 CrossRef CAS PubMed.
- R. Wilson, J. Ornithol., 1984, 55, 109–112 Search PubMed.
- J. A. Van Franeker, R. Williams, M. J. Imber and W. J. Wolff, PhD thesis, University of Groningen, 2001.
- J. L. Lavers, A. L. Bond and I. Hutton, Environ. Pollut., 2014, 187, 124–129 CrossRef CAS PubMed.
- A. L. Bond and J. L. Lavers, Mar. Pollut. Bull., 2013, 70, 171–175 CrossRef CAS PubMed.
- J. D. Carlisle and R. L. Holberton, J. Ornithol., 2006, 77, 126–135 CrossRef.
- R. P. Prŷs-Jones, L. Schifferli and D. W. Macdonald, Ibis, 2008, 116, 90–94 CrossRef.
- I. N. Hutton, N. Carlile and D. Priddel, Pap. Proc. R. Soc. Tasmania, 2008, 142, 67–72 Search PubMed.
- P. Ryan and S. Jackson, Auk, 1986, 103, 427–428 Search PubMed.
- P. G. Ryan, Condor, 1988, 90, 446–452 CrossRef.
- L. B. Spear, D. G. Ainley and C. A. Ribic, Mar. Environ. Res., 1995, 40, 123–146 CrossRef CAS.
- S. Hammer, R. G. Nager, P. C. D. Johnson, R. W. Furness and J. F. Provencher, Mar. Pollut. Bull., 2016, 103, 206–210 CrossRef CAS PubMed.
- M. J. Carey, Emu, 2011, 111, 229–234 CrossRef.
- H. Acampora, Q. A. Schuyler, K. A. Townsend and B. D. Hardesty, Mar. Pollut. Bull., 2014, 78, 63–68 CrossRef CAS PubMed.
- M. P. Harris and S. Wanless, Mar. Pollut. Bull., 1994, 28, 54–55 CrossRef.
- J. A. van Franeker and P. J. Bell, Mar. Pollut. Bull., 1988, 19, 672–674 CrossRef CAS.
- K. M. Verlis, M. L. Campbell and S. P. Wilson, Mar. Pollut. Bull., 2013, 72, 244–249 CrossRef CAS PubMed.
- D. T. Fife, G. J. Robertson, D. Shutler, B. M. Braune and M. L. Mallory, Mar. Pollut. Bull., 2015, 91, 368–371 CrossRef CAS PubMed.
- P. C. Harper and J. A. Fowler, Notornis, 1987, 34, 65–70 Search PubMed.
- S. Avery-Gomm, P. D. O'Hara, L. Kleine, V. Bowes, L. K. Wilson and K. L. Barry, Mar. Pollut. Bull., 2012, 64, 1776–1781 CrossRef CAS PubMed.
- I. J. Skira, Aust. Wildl. Res., 1986, 13, 481–488 CrossRef.
- S. M. Reid, Notornis, 1981, 4, 139–240 Search PubMed.
- P. G. Ryan, Mar. Pollut. Bull., 2008, 56, 1406–1409 CrossRef CAS PubMed.
- N. Mrosovsky, G. D. Ryan and M. C. James, Mar. Pollut. Bull., 2009, 58, 287–289 CrossRef CAS PubMed.
- R. G. Botzler and R. N. Brown, Foundations of wildlife diseases, UC Press, 2014 Search PubMed.
- D. C. Duffy and S. Jackson, Colon. Waterbirds, 1986, 9, 1–17 CrossRef.
- R. Kenward, A manual for wildlife radio tagging, Academic Press, 2001 Search PubMed.
- J. Rodríguez-Ruiz, D. Parejo, J. de la Puente, F. Valera, M. A. Calero-Torralbo, A. Bermejo, I. Catry and J. M. Avilés, Ibis, 2016, 158, 179–183 CrossRef.
- L. Rozsa, J. Reiczigel and G. Majoros, J. Parasitol., 2000, 86, 228–232 CrossRef CAS PubMed.
- J. F. Provencher, A. L. Bond, A. Hedd, W. A. Montevecchi, S. Bin Muzaffar, S. J. Courchesne, G. Gilchrist, S. Jamieson, F. Merkel, J. Durnick and M. L. Mallory, Mar. Pollut. Bull., 2014, 84, 411–417 CrossRef CAS PubMed.
- A. L. Bond, J. F. Provencher, R. D. Elliot, P. C. Ryan, S. Rowe, I. L. Jones, G. J. Robertson and S. I. Wilhelm, Mar. Pollut. Bull., 2013, 77, 192–195 CrossRef CAS PubMed.
- J. Lavers and A. L. Bond, Mar. Pollut. Bull., 2016, 110(1), 493–500 CrossRef CAS PubMed.
- J. G. B. Derraik, Mar. Pollut. Bull., 2002, 44, 842–852 CrossRef CAS PubMed.
- ICES-WGSE, Report of the working group on seabird ecology, Copenhagen, 2001 Search PubMed.
- M. L. Mallory, G. J. Robertson and A. Moenting, Mar. Pollut. Bull., 2006, 52, 813–815 CrossRef CAS PubMed.
- OSPAR MASH, Further development of the EcoQ on plastic particles in the stomachs of seabirds, (incl. Annex I ICES-ACE Report 2006 Section1.5.5.5.), Horta, Azores, 2006 Search PubMed.
- R. Day, MSc thesis, University of Alaska, 1980.
- J. A. van Franeker, Mar. Pollut. Bull., 1985, 16, 367–369 CrossRef.
- M. D. Robards, J. F. Piatt and K. D. Wohl, Mar. Pollut. Bull., 1995, 30, 151–157 CrossRef CAS.
- S. Avery-Gomm, M. Valliant, C. R. Schacter, K. R. Robbins, M. Liboiron, P. Y. Daoust, L. M. Rios and I. L. Jones, Mar. Pollut. Bull., 2016 DOI:10.1016/j.marpolbul.2016.08.062.
- J. A. Van Franeker and A. Meijboom, Fulmar litter EcoQO monitoring in the Netherlands 1982-2004 in relation to the EU Directive 2000/59/EC on port reception facilities, Alterra, Wageningen UR, Texel, the Netherlands, 2006 Search PubMed.
- L. S. Vlietstra and J. A. Parga, Mar. Pollut. Bull., 2002, 44, 945–955 CrossRef CAS PubMed.
- K. Enders, R. Lenz, C. A. Stedmon and T. G. Nielsen, Mar. Pollut. Bull., 2015, 100, 70–81 CrossRef CAS PubMed.
- L. M. R. Mendoza and P. R. Jones, Environ. Chem., 2015, 12, 611–617 CrossRef.
- M. Mecozzi, M. Pietroletti and Y. B. Monakhova, Mar. Pollut. Bull., 2016, 106, 155–161 CrossRef CAS PubMed.
- Q. Qiu, Z. Tan, J. Wang, J. Peng, M. Li and Z. Zhang, Estuarine, Coastal Shelf Sci., 2016, 176, 102–109 CrossRef CAS.
- J. Lenzi, M. F. Burgues, D. Carrizo, E. Machín and F. Teixeira-de Mello, Mar. Pollut. Bull., 2016, 107, 71–76 CrossRef CAS PubMed.
- T. Romeo, B. Pietro, C. Pedà, P. Consoli, F. Andaloro and M. C. Fossi, Mar. Pollut. Bull., 2015, 95, 358–361 CrossRef CAS PubMed.
- P. G. Ryan, C. J. Moore, J. A. van Franeker and C. L. Moloney, Philos. Trans. R. Soc., B, 2009, 364, 1999–2012 CrossRef CAS PubMed.
- W. Sanchez, C. Bender and J.-M. Porcher, Environ. Res., 2014, 128, 98–100 CrossRef CAS PubMed.
- D. V. Dantas, M. Barletta and M. F. da Costa, Environ. Sci. Pollut. Res. Int., 2012, 19, 600–606 CrossRef PubMed.
- V. Hidalgo-Ruz, L. Gutow, R. C. Thompson and M. Thiel, Environ. Sci. Technol., 2012, 46, 3060–3075 CrossRef CAS PubMed.
- A. C. Vegter, M. Barletta, C. Beck, J. Borrero, H. Burton, M. L. Campbell, M. Eriksen, C. Eriksson, A. Estrades, K. Gilardi, B. D. Hardesty, J. A. Ivar do Sul, J. L. Lavers, B. Lazar, L. Lebreton, W. J. Nichols, C. A. Ribic, P. G. Ryan, Q. A. Schuyler, S. D. A. Smith, H. Takada, K. A. Townsend, C. C. C. Wabnitz, C. Wilcox, L. Young and M. Hamann, Endangered Species Research, 2014, 25, 225–247 CrossRef.
- S. Moret-Ferguson, K. L. Law, G. Proskurowski, E. K. Murphy, E. E. Peacock and C. M. Reddy, Mar. Pollut. Bull., 2010, 60, 1873–1878 CrossRef CAS PubMed.
- D. M. Brown, M. R. Wilson, W. MacNee, V. Stone and K. Donaldson, Toxicol. Appl. Pharmacol., 2001, 175, 191–199 CrossRef CAS PubMed.
- M. L. Moser and D. S. Lee, Colon. Waterbirds, 1992, 15, 83–94 CrossRef.
- R. G. Santos, R. Andrades, L. M. Fardim and A. S. Martins, Environ. Pollut., 2016, 214, 585–588 CrossRef CAS PubMed.
- R. M. Christie, Polym. Int., 1994, 34, 351–361 CrossRef CAS.
- S. Endo, R. Takizawa, K. Okuda, H. Takada, K. Chiba, H. Kanehiro, H. Ogi, R. Yamashita and T. Date, Mar. Pollut. Bull., 2005, 50, 1103–1114 CrossRef CAS PubMed.
- E. Barbieri, Braz. Arch. Biol. Technol., 2009, 52, 341–348 CrossRef.
- L. Brown, T. Cat and A. DasGupta, Stat. Sci., 2001, 16, 101–133 Search PubMed.
- S. L. Wright, R. C. Thompson and T. S. Galloway, Environ. Pollut., 2013, 178, 483–492 CrossRef CAS PubMed.
- J. A. Van Franeker and K. L. Law, Environ. Pollut., Ser. A, 2015, 203, 89–96 CrossRef CAS PubMed.
- J. F. Provencher, A. J. Gaston, M. L. Mallory, P. D. O'Hara and H. G. Gilchrist, Mar. Pollut. Bull., 2010, 60, 1406–1411 CrossRef CAS PubMed.
- P. Ryan, Environ. Pollut., 2015, 207, 438–440 CrossRef CAS PubMed.
- T. N. Pettit, G. S. Grant and G. C. Whittow, Auk, 1981, 98, 839–841 Search PubMed.
- P. G. Ryan and S. Jackson, Mar. Pollut. Bull., 1987, 18, 217–219 CrossRef CAS.
- P. Lutz, in Proceedings of the Second International Conference on Marine Debris, ed. R. Shomura and M. Godfrey, U.S. Dept. of Commerce report no. NOM-TM-NMFS-SUFSC-15, 1990, pp. 719–735 Search PubMed.
- R. H. Day and D. G. Shaw, Mar. Pollut. Bull., 1987, 18, 311–316 CrossRef CAS.
- S. C. Votier, S. Bearhop, N. Ratcliffe and R. W. Furness, Bird Study, 2001, 48, 373–376 CrossRef.
- J. L. Lavers and A. L. Bond, Mar. Environ. Res., 2016, 113, 1–6 CrossRef CAS PubMed.
- E. Besseling, B. Wang, M. Lürling and A. A. Koelmans, Environ. Sci. Technol., 2014, 48, 12336–12343 CrossRef CAS PubMed.
- J. Li, D. Yang, L. Li, K. Jabeen and H. Shi, Environ. Pollut., 2015, 207, 190–195 CrossRef CAS PubMed.
- H. E. Braid, J. Deeds, S. L. DeGrasse, J. J. Wilson, J. Osborne and R. H. Hanner, Mar. Biol., 2012, 159, 25–31 CrossRef CAS.
- F. Murray and P. R. Cowie, Mar. Pollut. Bull., 2011, 62, 1207–1217 CrossRef CAS PubMed.
- P. G. Ryan and M. W. Fraser, Emu, 1988, 88, 16–19 CrossRef.
- M. C. Perry, G. H. Olsen, R. A. Richards and P. C. Osenton, Northeastern Naturalist, 2013, 20, 148–154 CrossRef.
- J. Ramos, M. Barletta and M. Costa, Aquat. Biol., 2012, 17, 29–34 CrossRef.
- C. Eriksson and H. Burton, AMBIO: A Journal of the Human Environment, 2003, 32, 380 CrossRef.
- G. M. Hilton, R. W. Furness and D. C. Houston, J. Avian Biol., 2000, 31, 36–46 CrossRef.
- J. F. Provencher, A. J. Gaston, P. D. O'Hara and H. G. Gilchrist, Mar. Ecol.: Prog. Ser., 2012, 454, 171–182 CrossRef.
- E. M. Foekema, C. De Gruijter, M. T. Mergia, J. A. van Franeker, A. J. Murk and A. A. Koelmans, Environ. Sci. Technol., 2013, 130711150255009 Search PubMed.
- A. Cozar, F. Echevarria, J. I. Gonzalez-Gordillo, X. Irigoien, B. Ubeda, S. Hernandez-Leon, A. T. Palma, S. Navarro, J. Garcia-de-Lomas, A. Ruiz, M. L. Fernandez-de-Puelles and C. M. Duarte, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10239–10244 CrossRef CAS PubMed.
- C. M. Rochman, in Marine Anthropogenic Litter, ed. M. Bergmann, L. Gutow and M. Klages, Springer International Publishing, 2015, pp. 117–140 Search PubMed.
- E. Nakashima, A. Isobe, S. Kako, T. Itai, S. Takahashi and X. Guo, Mar. Pollut. Bull., 2016, 107, 333–339 CrossRef CAS PubMed.
- Y. Mato, T. Isobe, H. Takada, H. Kanehiro, C. Ohtake and T. Kaminuma, Environ. Sci. Technol., 2001, 35, 318–324 CrossRef CAS PubMed.
- K. Tanaka, H. Takada, R. Yamashita, K. Mizukawa, M. Fukuwaka and Y. Watanuki, Mar. Pollut. Bull., 2013, 69, 219–222 CrossRef CAS PubMed.
- E. L. Teuten, J. M. Saquing, D. R. U. Knappe, M. A. Barlaz, S. Jonsson, A. Bjorn, S. J. Rowland, R. C. Thompson, T. S. Galloway, R. Yamashita, D. Ochi, Y. Watanuki, C. Moore, H. V Pham, T. S. Tana, M. Prudente, R. Boonyatumanond, M. P. Zakaria, K. Akkhavong, Y. Ogata, H. Hirai, S. Iwasa, K. Mizukawa, Y. Hagino, A. Imamura, M. Saha and H. Takada, Philos. Trans. R. Soc., B, 2009, 364, 2027–2045 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ay02419j |
| This journal is © The Royal Society of Chemistry 2017 |