Open Access Article
Open Access ArticleSymmetry breaking above room temperature in an Fe(II) spin crossover complex with an N4O2 donor set†
Wasinee
Phonsri
 a,
Casey G.
Davies
b,
Guy N. L.
Jameson
b,
Boujemaa
Moubaraki
a,
Jas S.
Ward
c,
Paul E.
Kruger
c,
Guillaume
Chastanet
d and
Keith S.
Murray
a,
Casey G.
Davies
b,
Guy N. L.
Jameson
b,
Boujemaa
Moubaraki
a,
Jas S.
Ward
c,
Paul E.
Kruger
c,
Guillaume
Chastanet
d and
Keith S.
Murray
 *a
*a
aSchool of Chemistry, Monash University, Clayton, Victoria 3800, Australia. E-mail: keith.murray@monash.edu
bDepartment of Chemistry & MacDiarmid Institute for Advanced Materials and Nanotechnology, University of Otago, PO Box 56, Dunedin, 9054, New Zealand
cDepartment of Chemistry & MacDiarmid Institute for Advanced Materials and Nanotechnology, University of Canterbury, Private Bag 4800, Christchurch 8041, New Zealand
dCNRS, Université de Bordeaux, ICMCB, 87 avenue du Dr A. Schweitzer, Pessac 33608, France
First published on 6th January 2017
Abstract
[Fe(qsal-Cl)2] is one of the few known Fe(II) spin crossover compounds with an N4O2 donor atom environment. It shows an abrupt two-step spin transition at 308 and 316 K and importantly, symmetry breaking at the highest temperature reported, to date, in spin crossover compounds.
The study of spin crossover (SCO) materials continues to generate a great deal of interest from both fundamental and applied perspectives.1–6 Fe(II) d6 and Fe(III) d5 compounds remain the metal systems of choice in these studies. Within these systems, mixed N/O-ligand donor sets are well known for Fe(III) but rare for Fe(II) SCO complexes.7–16 Specifically, the qsal ligands are known to produce SCO with Fe(III).12,17–20 However, only one qsal derivative of Fe(II), [FeII(qsal-NO2)2] (qsal-NO2 = 5-nitro-N-(8-quinolyl)-salicylaldimine), has been reported to show SCO (incomplete) up to 400 K.21 Generally, the space group symmetry of SCO compounds is unchanged following transition between different spin states. There are a limited number of compounds for which symmetry breaking takes place and accompanies complete SCO.22–25 Notably, there are only two compounds that have shown symmetry breaking in an [FeIIN4O2] system i.e. Fe(II) with a hexadentate N4O2 ligand, [Fe(5-NO2-sal-N(1,4,7,10))].26 This compound shows two-step SCO with T1/2 (heating) = 136 and 171 K, and three different space groups at 103, 153 and 292 K. Another example is a 1D chain of [FeL1(azpy)] where L1 is the tetradentate N2O2 ligand (L1 = diethyl(E,E)-2,2′-[1,2-phenyl-bis(iminomethylidyne)]bis[3-oxobutanoate]-(2-)-N,N′,O3,O3′).27 Herein, we report the first example of symmetry breaking with re-entrant behaviour in a complex containing a tridentate N2O ligand, viz. [Fe(qsal-Cl)2] 1. Interestingly, 1 shows complete, abrupt two-step SCO above room temperature, the highest temperature yet reported so far for a mononuclear SCO compound.
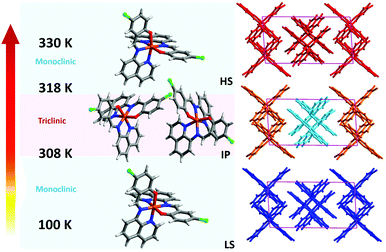
Complex 1 was synthesized by a multi-layering method: a dichloromethane solution of Hqsal-Cl and Et3N formed the bottom layer; neat MeOH formed the middle ‘buffering’ layer; whilst FeCl2·4H2O in MeOH formed the top layer. Black crystals formed in good yield within one week. Variable-temperature single crystal X-ray diffraction experiments on 1 were obtained at six temperatures, viz. 100, 298, 308, 312, 318 and 330 K that encompass all the different spin states observed (vide infra), Fig. 1.‡ Intriguingly, crystals of 1 show symmetry breaking. At 100 and 298 K, 1 exists in the monoclinic space group P21/n and a single molecule of 1 is observed in the asymmetric unit. Between 308 and 312 K, the cell parameters α and γ deviate significantly from 90° and the symmetry changes to triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . At this intermediate stage (IP), there are two molecules of 1 in the asymmetric unit (labelled Fe1 and Fe51) with different spin states (1LS–1HS), Fig. 1c. At temperatures above 318 K, the crystal system reverts to P21/n, thus showing re-entrant behaviour.
. At this intermediate stage (IP), there are two molecules of 1 in the asymmetric unit (labelled Fe1 and Fe51) with different spin states (1LS–1HS), Fig. 1c. At temperatures above 318 K, the crystal system reverts to P21/n, thus showing re-entrant behaviour.
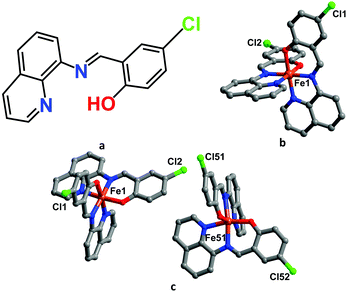 | ||
| Fig. 1 Molecular structures of (a) Hqsal-Cl ligand and the asymmetric unit of 1 at (b) LS and HS state (c) intermediate states. | ||
The Fe(II) centres coordinate to N4O2 donors from two tridentate anionic qsal-Cl ligands chelating in meridional fashion to yield neutral 1. Fe–L bond lengths for the compound are shown in Table S2 (ESI†). At 100 and 298 K, Fe–L distances (Fe–O ≈ 1.96 and Fe–N ≈ 1.94–1.99 Å) and the octahedral distortion parameters28,29 (Σ ≈ 33° and Θ = 65–74°) indicate that the Fe(II) centre is in the LS state.30,31 Upon warming the crystal of 1 to between 308 and 312 K, there are two molecules in the asymmetric unit. The Fe–L bond lengths and octahedral distortion parameters suggest an intermediate state consisting of 1LS and 1HS Fe(II) centre in the asymmetric unit. At temperatures above 318 K only one molecule of 1 is again observed in the asymmetric unit, suggesting the fully HS structure exists at 330 K (Fe–O ≈ 2.01 and Fe–N ≈ 2.16 Å).
Although symmetry breaking is observed in 1, the packing of the Fe(II) moieties within the crystal remains unchanged, Fig. 2. The supramolecular arrangements that remain similar during the symmetry breaking have also been reported in previous systems.32–34 The deficiency of diffuse scattering and the extra Bragg reflections in the IP of compound 1 suggest that the spin transition relates to the long-range order characteristic of the IP.24
The packing in 1 is reminiscent of those seen in Fe(III)-qsal systems (albeit without the presence of an anion).12,35–38 Thus, a chain of Fe(II) moieties interact through two sets of π–π interactions via sal⋯quin rings of the qsal-Cl ligands and C–H⋯O interactions along the b axis (Fig S1, ESI†). Moreover, along the a axis, C–H⋯Cl interactions and two sets of parallel fourfold aryl embraces (P4AE)39 are observed to link the Fe moieties into higher dimension. P4AE is believed to be the reason for the observation of complete abrupt spin crossover in 112,40 (see Magnetic section). Furthermore, in the ac plane, there are two extra sets of π–π interactions holding the Fe molecules in the plane. It is important to note that two types of P4AE interactions and the extra two sets of π–π interactions described here are not seen in Fe(III)-(qsal-X) complexes. That is because anions and/or solvents occupy spaces between the [Fe(III)(qsal-Cl)2] molecules and prevent those interactions taking place in those complexes. Such interactions are believed to be responsible for the greater cooperativity as they enhance the surface contacts between Fe(II) moieties.41 Therefore, they result in the exceptionally high spin transition temperatures observed in 1 (vide infra).
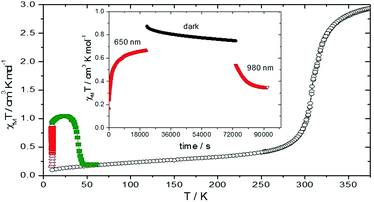
The variable-temperature magnetic susceptibility data for 1 were obtained between 100–360 K using various scan rates i.e. 2, 5 and 10 K min−1. The magnetic results for the compound are independent of the scan rate and are illustrated in Fig. 3. Upon closer inspection, together with differential scanning calorimetry results, two steps have been detected in 1 with a pseudo-plateau of width of about 8 K. Upon heating from 100 up to 250 K, the χMT values of 1 is invariant with temperature, with χMT values of about 0.3 cm3 K mol−1, indicative of the LS Fe(II) forms. Upon further warming, spin crossover takes place and 1 shows a two-step transition with T1/2 = 308 and 316 K for the 1st and 2nd steps, respectively, before reaching the fully HS form at high temperatures. There is a small thermal hysteresis of about 1–2 K width present in this compound. The magnetic profiles are reproducible in subsequent cycles.
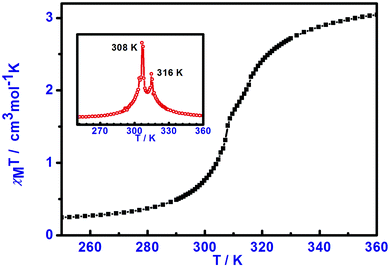 | ||
| Fig. 3 Variable-temperature magnetic susceptibility (χMT) measurements for compound 1, an inset shows the first order differentiation of the magnetic plot. | ||
Variable temperature single crystal X-ray diffraction indicates concerted SCO and symmetry breaking in 1via LS(P21/n) ↔ 1LS–1HS(P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) ↔ HS(P21/n) steps. This also agrees with the discontinuous changes of the unit cell parameters around 300 K (Fig S2, ESI†). Thus, there is a strong correlation between SCO and structural changes in this compound.
) ↔ HS(P21/n) steps. This also agrees with the discontinuous changes of the unit cell parameters around 300 K (Fig S2, ESI†). Thus, there is a strong correlation between SCO and structural changes in this compound.
According to the reviewed information in Table S4 (ESI†), there is only a small number of examples of symmetry breaking with complete, two steps SCO that have been characterized. To the best of our knowledge, [Fe(qsal-Cl)2] is the first Fe(II) complex with a N2O ligand donor set that exhibits symmetry breaking involving a two-step SCO, above RT, the highest temperatures reported for symmetry breaking in mononuclear Fe–SCO compounds. The highest temperature noted previously was ∼250 K.
For a deeper comparison of a related symmetry breaking Fe(III) compound, [FeIII(qsal-Br)2]NO3·2MeOH,352 shows a two-step SCO with a large plateau of about 96 K while 1 has a small pseudo-plateau with width about 8 K. Consideration of intermediate state structures shows that compound 1 and 2 both have two Fe centres, 1LS–1HS. However, these Fe centres interact in different fashion. In the case of 1, a plane of the same spin state of [Fe(II)(qsal-Cl)2] is formed via two sets of π–π and P4AE interactions. In contrast, a chain of the same Fe(III) molecules connect, solely, through two sets of π–π interactions (Fig S3, ESI†). As both of the compounds show re-entrant behaviour, chains of 1LS and 1HS Fe(III) moieties in the intermediate state have to propagate through the whole compound in 2D and 3D packing to revert to 1HS upon heating. Whereas the 2D-plane of Fe(II) molecules in 1 is more facile to induce two unique Fe(II) sheets reaching one Fe(II) centre with fully HS behaviour. Such differences possibly result in a significantly narrower intermediate phase temperature gap in 1.
The effect of light irradiation on compound 1 was also studied. Irradiations at 405, 510, 650, 830 and 980 nm were tested with 650 nm irradiation inducing the most efficient photoconversion. The T(LIESST) curve was recorded. A common feature to 1 is the shape of the T(LIESST) curve. Increasing the temperature from 10 K in the dark, after photo-saturation was reached, induces an increase of the χMT value. This usually follows from the Fe(II) zero-field splitting.42 After a maximum in the T(LIESST) curve, the χMT value drops down to the base line. Regarding the photoconversion efficiency, it is 30% with T(LIESST) = 40 K. An interesting point is that upon irradiation at 980 nm, a partial depopulation of the photo-induced HS state is observed, due to the reverse-LIESST process. Compared to the relaxation in the dark measured at 10 K (inset in Fig. 4), the reverse LIESST is clearly operative, even if it is not complete.
The 57Fe Mössbauer spectra of 1 were measured at low (∼5 K) and room temperatures to confirm the presence of LS and HS forms. The spectral parameters of the compounds are shown in Fig. 5 and Table S5 (ESI†). The results are typical for Fe(II) complexes with quadrupole splitting, ΔEQ, and isomer shift, δ of ca. 1 and 0.5 mm s−1 for LS forms while they are about 2 and 1 mm s−1 for HS forms, respectively.43,44 For compound 1, the fully Fe(II) LS forms exist at low temperatures. At room temperature, some fractions of Fe(II) HS are also present. These Mössbauer data correspond well with the magnetic results confirming exceptionally high T1/2 in the compounds. Unfortunately, we cannot collect the fully HS Fe(II) spectrum as this occurs at too high a temperature for our Mössbauer facility.
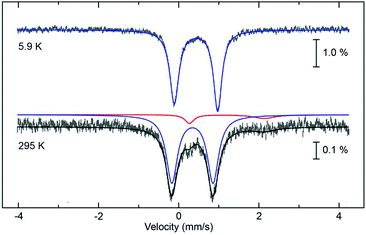 | ||
| Fig. 5 Variable temperature 57Fe Mössbauer spectral plots for 1. At low temperature the sample is fully low spin (blue fit). At 295 K the sample is a mixture of low spin (blue) and high spin (red). Fitted parameters are given in Table S5 (ESI†). | ||
Differential scanning calorimetry (DSC) data were collected on compound 1 using a 10 K min−1 scan rate. From the DSC plots in Fig. S4 (ESI†), there are two peaks for endo- and exothermic measurements. All the phase transition temperatures from the DSC data are in the same range as the spin transition results from magnetic measurements. As mentioned above, the initial magnetic plot for 1 roughly suggested a one-step SCO with an abrupt spin transition. The DSC result confirms the two step SCO present in this compound. Moreover, the small ΔS value of 19.8 J mol−1 K−1 in 1, agrees with data by Wang and Gao et al. who reported that symmetry breaking was also associated with a two-step SCO and led to a significant lowering in ΔH and ΔS values.40
In summary, [Fe(II)(qsal-Cl)2] 1 has provided an unprecedented array of magnetic and spin crossover properties. It is the first Fe(II) compound with a N2O donor ligand to exhibit symmetry breaking accompanied by a two-step SCO above room temperature, the highest temperatures reported thus far for symmetry breaking SCO compounds (viz. T1/2 = 308 and 316 K). In general, compared to iron(III) analogues [Fe(III)(qsal-X)2]Y,12,45 we propose that the abrupt SCO at such a high temperature in 1 is due to an absence of anions/solvents in the lattice that allow the Fe(II) molecules to have larger surface contact (via two extra sets of π–π and P4AE interactions) than for the Fe(III) complexes which enhances the strong cooperativity. Subsequently, to design a potential spin crossover material, the fundamental requirements for an abrupt spin crossover, particularly in Fe-qsal complexes, is the combination of the symmetrical two sets of π–π and P4AE interactions. To show spin transition at exceptionally high temperatures, the extra two sets of π–π and P4AE interactions are further recommended.
This work was supported by an Australian Research Council Discovery grant (to K. S. M.). P. E. K. gratefully acknowledges the Royal Society of New Zealand Marsden Fund for financial support. We thank Professor David Harding (of Walailak University, Thailand) for valuable discussions. This paper is dedicated to Professor Leone Spiccia (1957–2016), a fine person, friend and excellent scientist.
Notes and references
- R. J. Deeth, C. M. Handley and B. J. Houghton, in Spin-Crossover Materials, ed. M. A. Halcrow, John Wiley & Sons Ltd, 2013, pp. 443–454 Search PubMed.
- P. N. Martinho, C. Rajnak and M. Ruben, in Spin-Crossover Materials, ed. M. A. Halcrow, John Wiley & Sons Ltd, 2013, pp. 375–404 Search PubMed.
- P. Gütlich, A. Hauser and H. Spiering, Angew. Chem., Int. Ed. Engl., 1994, 33, 2024–2054 CrossRef.
- A. Bousseksou, G. Molnar, L. Salmon and W. Nicolazzi, Chem. Soc. Rev., 2011, 40, 3313–3335 RSC.
- K. S. Murray, in Spin-Crossover Materials, ed. M. A. Halcrow, John Wiley & Sons Ltd, 2013, pp. 1–54 Search PubMed.
- M. A. Halcrow, Chem. Commun., 2013, 49, 10890–10892 RSC.
- M. Nihei, T. Shiga, Y. Maeda and H. Oshio, Coord. Chem. Rev., 2007, 251, 2606 CrossRef CAS.
- P. Gütlich and H. A. Goodwin, in Spin Crossover in Transition Metal Compounds I, ed. P. Gütlich and H. A. Goodwin, Springer Berlin Heidelberg, Berlin, Heidelberg, 2004, pp. 1–47 Search PubMed.
- B. Weber, E. Kaps, J. Weigand, C. Carbonera, J.-F. Létard, K. Achterhold and F. G. Parak, Inorg. Chem., 2008, 47, 487–496 CrossRef CAS PubMed.
- B. Weber, W. Bauer and J. Obel, Angew. Chem., Int. Ed., 2008, 47, 10098–10101 CrossRef CAS PubMed.
- S. Dorbes, L. Valade, J. A. Real and C. Faulmann, Chem. Commun., 2005, 69–71 RSC.
- D. J. Harding, P. Harding and W. Phonsri, Coord. Chem. Rev., 2016, 313, 38–61 CrossRef CAS.
- B. Weber, Coord. Chem. Rev., 2009, 253, 2432–2449 CrossRef CAS.
- L. Zhang, G.-C. Xu, H.-B. Xu, V. Mereacre, Z.-M. Wang, A. K. Powell and S. Gao, Dalton Trans., 2010, 39, 4856–4868 RSC.
- L. Zhang, G.-C. Xu, Z.-M. Wang and S. Gao, Eur. J. Inorg. Chem., 2013, 1043–1048 CrossRef.
- T. Romero-Morcillo, M. Seredyuk, M. C. Muñoz and J. A. Real, Angew. Chem., Int. Ed., 2015, 54, 14777–14781 CrossRef CAS PubMed.
- S. Hayami, Z.-z. Gu, H. Yoshiki, A. Fujishima and O. Sato, J. Am. Chem. Soc., 2001, 123, 11644–11650 CrossRef CAS PubMed.
- K. Takahashi, H. Mori, H. Kobayashi and O. Sato, Polyhedron, 2009, 28, 1776–1781 CrossRef CAS.
- D. J. Harding, D. Sertphon, P. Harding, K. S. Murray, B. Moubaraki, J. D. Cashion and H. Adams, Chem. – Eur. J., 2013, 19, 1082–1090 CrossRef CAS PubMed.
- K. Fukuroi, K. Takahashi, T. Mochida, T. Sakurai, H. Ohta, T. Yamamoto, Y. Einaga and H. Mori, Angew. Chem., Int. Ed., 2014, 53, 1983–1986 CrossRef CAS PubMed.
- O. Iasco, E. Rivière, R. Guillot, M. Buron-Le Cointe, J.-F. Meunier, A. Bousseksou and M.-L. Boillot, Inorg. Chem., 2015, 54, 1791–1799 CrossRef CAS PubMed.
- K. Nakano, S. Kawata, K. Yoneda, A. Fuyuhiro, T. Yagi, S. Nasu, S. Morimoto and S. Kaizaki, Chem. Commun., 2004, 2892–2893 RSC.
- N. Bréfuel, H. Watanabe, L. Toupet, J. Come, N. Matsumoto, E. Collet, K. Tanaka and J.-P. Tuchagues, Angew. Chem., Int. Ed., 2009, 48, 9304–9307 CrossRef PubMed.
- D. Chernyshov, M. Hostettler, K. W. Törnroos and H.-B. Bürgi, Angew. Chem., Int. Ed., 2003, 42, 3825–3830 CrossRef CAS PubMed.
- N. Ortega-Villar, M. Muñoz and J. Real, Magnetochemistry, 2016, 2, 16 CrossRef.
- D. Boinnard, A. Bousseksou, A. Dworkin, J. M. Savariault, F. Varret and J. P. Tuchagues, Inorg. Chem., 1994, 33, 271–281 CrossRef CAS.
- W. Bauer, T. Pfaffeneder, K. Achterhold and B. Weber, Eur. J. Inorg. Chem., 2011, 3183–3192 CrossRef CAS.
- J. K. McCusker, A. L. Rheingold and D. N. Hendrickson, Inorg. Chem., 1996, 35, 2100–2112 CrossRef CAS.
- M. Marchivie, P. Guionneau, J.-F. Letard and D. Chasseau, Acta Crystallogr., Sect. B: Struct. Sci., 2005, 61, 25–28 CrossRef PubMed.
- L. Zhang, G.-C. Xu, H.-B. Xu, T. Zhang, Z.-M. Wang, M. Yuan and S. Gao, Chem. Commun., 2010, 46, 2554–2556 RSC.
- T. Kuroda-Sowa, Z. Yu, Y. Senzaki, K. Sugimoto, M. Maekawa, M. Munakata, S. Hayami and Y. Maeda, Chem. Lett., 2008, 37, 1216–1217 CrossRef CAS.
- T. M. Ross, B. Moubaraki, K. S. Wallwork, S. R. Batten and K. S. Murray, Dalton Trans., 2011, 40, 10147–10155 RSC.
- H. Watanabe, N. Bréfuel, E. Collet, L. Toupet, K. Tanaka and J.-P. Tuchagues, Eur. J. Inorg. Chem., 2013, 710–715 CrossRef CAS.
- S. Bonnet, M. A. Siegler, J. S. Costa, G. Molnar, A. Bousseksou, A. L. Spek, P. Gamez and J. Reedijk, Chem. Commun., 2008, 5619–5621 RSC.
- D. J. Harding, W. Phonsri, P. Harding, K. S. Murray, B. Moubaraki and G. N. L. Jameson, Dalton Trans., 2015, 44, 15079–15082 RSC.
- D. J. Harding, W. Phonsri, P. Harding, I. A. Gass, K. S. Murray, B. Moubaraki, J. D. Cashion, L. Liu and S. G. Telfer, Chem. Commun., 2013, 49, 6340–6342 RSC.
- K. Takahashi, T. Sato, H. Mori, H. Tajima and O. Sato, Phys. B, 2010, 405, S65–S68 CrossRef CAS.
- D. Sertphon, D. J. Harding, P. Harding, K. S. Murray, B. Moubaraki, J. D. Cashion and H. Adams, Eur. J. Inorg. Chem., 2013, 788–795 CrossRef CAS.
- V. Russell, M. Scudder and I. Dance, J. Chem. Soc., Dalton Trans., 2001, 789–799 RSC.
- W. Zhang, F. Zhao, T. Liu, M. Yuan, Z.-M. Wang and S. Gao, Inorg. Chem., 2007, 46, 2541–2555 CrossRef CAS PubMed.
- M. A. Halcrow, in Spin-Crossover Materials, ed. M. A. Halcrow, John Wiley & Sons Ltd, 2013, pp. 147–169 Search PubMed.
- J.-F. Letard, J. Mater. Chem., 2006, 16, 2550–2559 RSC.
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419–427 RSC.
- O. Hietsoi, P. W. Dunk, H. D. Stout, A. Arroyave, K. Kovnir, R. E. Irons, N. Kassenova, R. Erkasov, C. Achim and M. Shatruk, Inorg. Chem., 2014, 53, 13070–13077 CrossRef CAS PubMed.
- W. Phonsri, PhD thesis, Walailak University, Thailand, 2014.
Footnotes |
| † Electronic supplementary information (ESI) available: Tables S1–S3 structural data; Table S4 review of two step symmetry breaking compounds; Tables S5 and S6 Mössbauer and DSC results; Fig. S1–S3 structures; Fig. S4–S6 photomagnetism, Mössbauer and Fig. S4 DSC results. CCDC 1495808–1495813. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc10040f |
| ‡ Synthesis details and crystallographic data at variables temperatures are presented in the ESI.† |
| This journal is © The Royal Society of Chemistry 2017 |