Aldol condensation of biomass-derived platform molecules over amine-grafted hierarchical FAU-type zeolite nanosheets (Zeolean) featuring basic sites†
Thittaya
Yutthalekha
 a,
Duangkamon
Suttipat
a,
Duangkamon
Suttipat
 a,
Saros
Salakhum
a,
Saros
Salakhum
 a,
Anawat
Thivasasith
a,
Anawat
Thivasasith
 a,
Somkiat
Nokbin
a,
Somkiat
Nokbin
 b,
Jumras
Limtrakul
b,
Jumras
Limtrakul
 c and
Chularat
Wattanakit
c and
Chularat
Wattanakit
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, School of Energy Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong, 21210, Thailand. E-mail: chularat.w@vistec.ac.th
bDepartment of Chemistry, NANOTEC Center for Nanoscale Materials Design for Green Nanotechnology, and Center for Advanced Studies in Nanotechnology and Its Applications in Chemical, Food and Agricultural Industries, Faculty of Science, Kasetsart University, Bangkok 10900, Thailand
cDepartment of Materials Science and Engineering, School of Molecular Science and Engineering, Vidyasirimedhi Institute of Science and Technology, Rayong 21210, Thailand
First published on 20th October 2017
Abstract
The superior catalytic performance of amine-grafted hierarchical basic FAU-type zeolite nanosheets for the aldol condensation of 5-hydroxymethylfurfural (5-HMF) and acetone (Ac) has been achieved due to the synergistic effect of hierarchical structures, featuring basic active sites together with surface modification. This results in an unprecedented yield of 4-[5-(hydroxymethyl)furan-2-yl]but-3-en-2-one (HMB) close to 100%. The catalytic activity can be easily tuned by changing the degree of basicity corresponding to the nature of basic sites and surface modification.
The conversion of renewable feedstocks to chemicals is one of the most fascinating research topics, especially in catalysis.1 Aldol condensation is a well-known reaction forming a carbon–carbon bond between two aldehyde/ketone molecules. To combine this reaction together with biomass-derived platform molecules, the direct condensation of 5-hydroxymethylfurfural (5-HMF) with acetone (Ac), which is also available from renewable biomass via fermentation, is proposed to be a promising approach to increase the number of the carbon chain length (from C7 to C15) of biomass precursors in order to obtain biofuels.2,3
Base-catalysed aldol condensation is a typical reaction generating an enolate intermediate, which is subsequently transformed to a C–C condensation product.4 However, there are drawbacks with homogeneous mineral base catalysts as they cannot undergo the reaction because of the formation of an alkoxide ion and they are very difficult to recover from the reaction mixtures.5 Therefore, heterogeneous solid catalysts have significant advantages; however, they often suffer from rapid catalyst deactivation when using solid bases due to the presence of acids,6 which have a large portion of fermented biomass, for example, acetic acid.1 To overcome these limitations, the development of alternative highly efficient solid-base catalysts is still a great challenge.7
The application of zeolites as heterogeneous base catalysts has been attracting tremendous interest over the past few decades due to their outstanding selectivity in fine-chemistry related reactions,8 simplified process, and waste treatment. Moreover, their moderate basicity made them more attractive in aldol-type condensation, while stronger bases (e.g., NaOH, MgO) promote the formation of undesired side-products.9–11
Since this type of reaction always deals with bulky molecules, aluminum-rich FAU-type zeolites have been considered as one of the most suitable candidates. Although there are many advantages, such as shape selectivity to bulky reactants and high ion exchange capacity due to their large pores and low Si/Al ratio,12 conventional FAU zeolites often suffer from diffusion limitation of their sole microporous structures.10,13 Therefore, hierarchical zeolite nanosheets have become more interesting for the aldol condensation of bulky molecules.14–16 Recently, our group demonstrated that the hierarchical acid zeolite nanosheets exhibit superior performances in various catalytic reactions dealing with bulky molecules, such as alkylation and aromatization of hydrocarbons.17–19 However, a very limited number of studies regarding the catalytic behavior of hierarchical base zeolite nanosheets have been reported,17 and in particular, such catalysts have not yet been demonstrated for the aldol-type condensation involving bulky reactants.
Surface modification is also an alternative way to fine-tune the surface properties of catalysts. To further increase the efficiency of basic zeolite, amine functionalized surfaces can enhance the basicity of catalysts. In this work, we present the benefits of hierarchical FAU-type zeolite nanosheets featuring basic sites, which are obtained by ion-exchange with various alkaline metals together with amine-grafted surface modification for potential application of the aldol condensation of 5-HMF and Ac. The synergistic effects of hierarchical porosity, the basicity of various alkaline metals and amine surface modification on catalytic performances in terms of the catalytic activity, desired product selectivity, and coke resistance of a nanosheet zeolite were also demonstrated.
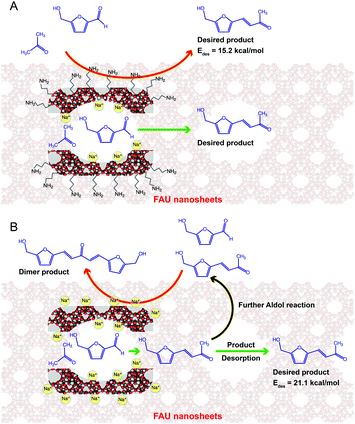
Fig. 1A presents the selective production of HMB as confirmed by the mass spectra (see Fig. S1, ESI†) via the aldol condensation of 5-HMF and Ac over amine-grafted surfaces of FAU nanosheets featuring ion-exchange basic sites. The amine modified surfaces not only promote the basic sites for the reaction but also weaken the adsorption of HMB, resulting in suppression of dimerization as a side reaction (Fig. 1). In strong contrast to this, unmodified zeolite surfaces facilitate the dimerization of products, eventually leading to a low selectivity of HMB (Fig. 1B). The production pathway of HMB is described by the base-catalysed mechanisms as illustrated in Fig. S2 (ESI†). The enolate adduct can be formed and then converted to α,β-unsaturated carbonyl compounds.
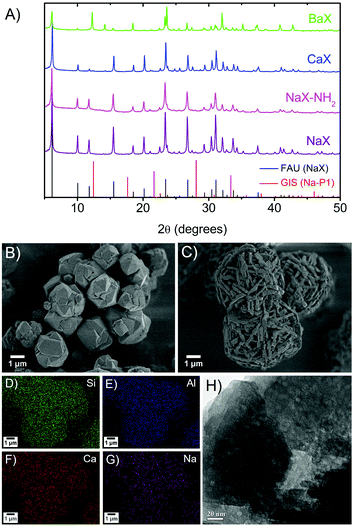
To provide high ion-exchange capacity of catalysts, hierarchical X-type zeolite nanosheets after complete removal of the organosilane template (see Fig. S3, ESI†) have been used because they have high aluminum content. In this case, various alkaline active sites (Na, Ca and Ba) have been successfully prepared with a high ion exchange degree (%IED) (∼95%, see Table S1, ESI†). In addition, the catalyst surfaces were modified by amine grafting of 3-aminopropyltriethoxysilane (APTES) in an ethanol solution at room temperature. All prepared zeolite X samples maintain their FAU structures (black pattern in Fig. 2A) without any competing crystalline impurity (e.g., GIS as shown in the red pattern) together with the same Si/Al ratio (1.3–1.4) even after several repeated ion exchange and amino grafting processes (see Table S1, ESI†). However, the ratio of the diffraction peak intensities was significantly changed in the case of the zeolite exchanged with larger cations. The reason for this phenomenon might be explained by the lower X-ray adsorption coefficient of large cations.20 For instance, the sample obtained by ion exchange with Ba exhibits weaker peak intensities and the disappearance of diffraction peaks at 2Θ of 10, 15, 20° was observed, while some intense peaks at 2Θ of 12, 14, 24° appeared instead. This relates to the fact that the structural distortion of the zeolite framework occurs due to the strong interaction of the larger cations and oxygen atoms in the framework.21
Compared with the conventional zeolite, the morphologies of the ion-exchanged hierarchical nanosheets were obviously different (Fig. 2B and C) and similar to those demonstrated in the previous report.17 The elemental mapping revealed a very uniform distribution of the alkaline cations in the zeolite framework (Fig. 2D–G). The existence of amine groups on the surface of catalysts was also confirmed by ATR-FTIR and 13C NMR spectroscopy (see details in the ESI† and Fig. S4). The intracrystalline mesopores were also found in the nanosheet structure as can be seen in Fig. 2H.
The physicochemical properties of catalysts were measured using N2 sorption measurements. The isotherms of all conventional zeolites (Fig. S5A, ESI†) exhibit adsorption only at a relatively low pressure (P/P0 < 0.2) with a long horizontal plateau due to micropore filling, corresponding to the type-I isotherm, confirming the microporous characteristic. On the other hand, all hierarchical nanosheet catalysts (Fig. S5B, ESI†) exhibit adsorption at a relatively low pressure together with a hysteresis loop at P/P0 ∼ 0.45 due to capillary condensation in the mesopores, which is characteristic of the mesoporous structure. The combination of type-I and IV isotherms observed in the nanosheet catalysts confirms the hierarchical porous structure of catalysts. The hierarchical zeolite exchanged with Ca cations slightly alters the physicochemical properties compared with the NaX nanosheet (Table S2, ESI†). In contrast, the zeolite samples exchanged with the Ba cation and the amine-grafted zeolite could eventually lead to a significant reduction of the specific surface area and pore volume (about 30% and 25% decrease compared with the NaX nanosheet for BaX-NS and NaX-NS-NH2, respectively). The reason for this decrease in the surface area and pore volume of the Ba-exchanged zeolite related to the fact that Ba obstructs the accessible pore windows due to its large size and also occupies the zeolite pore. In the case of amine-grafted zeolite, the large number of amine-grafted moieties at the zeolite pore mouth also restricts the accessible site of the zeolite pores, resulting in the blockage of zeolite openings.
CO2-TPD was used to determine the basicity of catalysts. Theoretically, the basic strength of ion-exchanged zeolite could be related to the electrostatic potential of alkaline metal cations in the zeolite framework.22 From the CO2-TPD profiles (Fig. S6, ESI†), it was observed that all catalysts are composed of two distinct peaks centered at ∼170 °C and ∼320 °C. These results indicate that the basic strength of all exchanged zeolites is nearly similar. The number of accessible basic sites was increased noticeably from alkali cations (NaX) to alkaline earth cations (CaX) and decreased upon increasing the cation size (Ca2+ to Ba2+) (see Table S2, ESI†). This may be explained by the fact that large alkaline cations preferentially occupied the inaccessible sites or they formed reduced metal clustered species.23–25 Therefore, it becomes evident that the degree of basicity of the exchanged zeolite can be simply tuned depending on the type of alkaline cations.
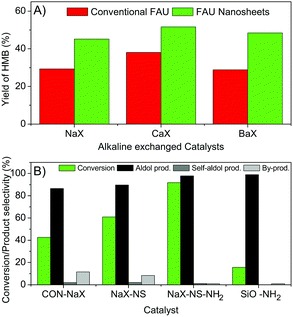
To demonstrate the catalytic performance, in this study the direct condensation of 5-HMF and Ac was carried out using a batch-type reactor at 130 °C. Typically, HMB and 4-hydroxy-4-methyl-2-pentanone (HMP) were formed as the desired and self-condensation products, respectively. For comparison with modified catalysts, the conventional NaX (CON-NaX) was used as the reference sample and the catalytic data are shown in Table S3 (ESI†). As expected, the desired product (HMB) was significantly reduced at a longer reaction time (from 100% at 6 h to 86.47% at 48 h, entries 1–4). Interestingly, the catalytic activity also changes depending on the type of alkaline cation. For example, in the case of a conventional alkaline exchange catalyst, the yield of HMB significantly increases from 29.32% to 38.05% when conventional CaX is used instead of NaX (Fig. 3A). However, the activity decreases when the alkaline earth metal size is increased. The yield of HMB was 28.84% in the case of the conventional BaX. This makes it clear that the catalytic activity towards HMB is directly related to the degree of basicity of the exchanged zeolite.
To further confirm the beneficial effect of hierarchical structures, the catalytic performances of conventional and hierarchical zeolite X nanosheets with various alkaline cations were compared (Fig. 3A). As expected, a significantly higher yield of HMB was observed in all cases of hierarchical zeolites. Although the yield of HMB as a function of the degree of basicity over nanosheet samples was also observed with a similar tendency, the effect of the type of alkaline cation is much less pronounced compared with that of the conventional one. The moderate yield of HMB was approximately 50% in all cases. It is reasonable to assume that the hierarchical structure benefits the increase of the accessible ability of large guest species.
In addition, the recyclability of the catalysts was examined by performing several cycles of the reaction. After the completion of each reaction cycle, the catalysts were regenerated by calcination to remove the deposited cokes before being reused in the next cycle. The O2-TPO profiles of such cokes indicated that the deactivation process of conventional zeolite was not the same as that of the nanosheet one (see details in the ESI† and Fig. S7). The catalytic activity of the regenerated nanosheet and conventional catalysts was unaffected after two or three reaction cycles exhibiting almost constant conversion of 5-HMF and desired product selectivity (Table S3, ESI†). Moreover, the reproducibility of these catalytic results was also demonstrated again. These results clearly confirm the comparable stability of the conventional and nanosheet structures of the catalysts.
Interestingly, the amine-grafted zeolites exhibited a dramatically high conversion of 5-HMF (91.74%) as shown in Fig. 3B. This makes it clear that such amine functionalized surfaces can be considered as additional basic sites. In addition, not only an improved activity but also a significantly higher selectivity of HMB was observed (89.67 and 97.81% for NaX-NS, NaX-NS-NH2, respectively). In strong contrast to this, amine-grafted silica (SiO2-NH2) showed a very low conversion of only 15.69%, and it is related to a very low external surface area of the support, eventually resulting in a low number of active basic sites (see Table S2, ESI†). Therefore, the hierarchical structure of the NaX zeolite can provide a large portion of additional basic sites compared to amorphous silica.
To verify the effect of amine-grafted surface modification on the catalytic selectivity towards HMB, DFT calculations were performed to investigate the interaction between the product (HMB) and catalytic sites as shown in Fig. S8 (ESI†) for optimized structures of HMB on unmodified surfaces (Fig. S8B, ESI†) and the amine-grafted zeolite X (Fig. S8C, ESI†) (see details in the ESI† and Fig. S8 and S9). It was found that in the case of unmodified NaX, the HMB molecule can be adsorbed on active sites via interaction between the oxygen of HMB (OHMB) and the alkaline sites (Na+) with a distance of 2.44 Å, providing a calculated adsorption energy of −21.1 kcal mol−1. In contrast, the adsorption energy of HMB on the amine-grafted surfaces was 6 kcal mol−1 lower than that of the unmodified NaX via interactions between the oxygen of HMB (OHMB) and the amine functional group. It is therefore reasonable to assume that the HMB product on amine grafted zeolite X can be somewhat easily desorbed compared with the unmodified system, resulting in suppression of the formation of dimeric by-products. This is consistent with the view that the HMB selectivity increases after the modification of surfaces with aminosilane molecules.
In summary, the superior catalytic performance in aldol condensation of 5-HMF and Ac has been reported using amine-grafted basic FAU-type zeolite nanosheets for the first time. The synergistic effect of hierarchical structures, featuring basic active sites together with surface modification results in an increase in the catalytic performances due to the following factors: (i) hierarchical structures can improve the accessibility of bulky molecules to catalytically basic sites, especially in the case of large cationic sizes; (ii) the catalytic activity can be easily tuned by changing the degree of basicity of accessible basic active sites; (iii) the introduction of basicity by amine-grafted surfaces provides exceptional catalytic activity and HMB selectivity due to the additional basic sites and the weakening of the interaction of HMB and the zeolite surface. This example opens up very interesting perspectives for the development of surface modified hierarchical zeolite nanosheets for applications regarding conversion of bulky biomass feedstocks to biofuels/chemicals.
This work was supported by the Research Fellowship from the Vidyasirimedhi Institute of Science and Technology and the Frontier Research Center. C.W would like to thank the Thailand Research Fund (TRF) (MRG5980114), the Office of Higher Education Commission (OHEC), and the Junior Research Fellowship Program of the French Embassy in Thailand.
Conflicts of interest
There are no conflicts to declare.Notes and references
- D. M. Alonso, J. Q. Bond and J. A. Dumesic, Green Chem., 2010, 12, 1493 RSC
.
- R. Xing, A. V. Subrahmanyam, H. Olcay, W. Qi, G. P. van Walsum, H. Pendse and G. W. Huber, Green Chem., 2010, 12, 1933 RSC
.
- G. W. Huber, J. N. Chheda, C. J. Barrett and J. A. Dumesic, Science, 2005, 308, 1446 CrossRef CAS PubMed
.
- S. Van de Vyver, C. Odermatt, K. Romero, T. Prasomsri and Y. Román-Leshkov, ACS Catal., 2015, 5, 972 CrossRef CAS
.
- A. V. Subrahmanyam, S. Thayumanavan and G. W. Huber, ChemSusChem, 2010, 3, 1158 CrossRef CAS PubMed
.
-
K. Tanabe, New solid acids and bases: their catalytic properties, Kodansha, 1989 Search PubMed
.
- J. Li, A. Corma and J. Yu, Chem. Soc. Rev., 2015, 44, 7112 RSC
.
- J. Weitkamp, M. Hunger and U. Rymsa, Microporous Mesoporous Mater., 2001, 48, 255 CrossRef CAS
.
- L. Faba, E. Díaz and S. Ordóñez, Appl. Catal., B, 2012, 113–114, 201 CrossRef CAS
.
- T. C. Keller, S. Isabettini, D. Verboekend, E. G. Rodrigues and J. Perez-Ramirez, Chem. Sci., 2014, 5, 677 RSC
.
- A. Corma, V. Fornés, R. M. Martín-Aranda, H. García and J. Primo, Appl. Catal., 1990, 59, 237 CrossRef CAS
.
- Y. Watanabe, H. Yamada, J. Tanaka, Y. Komatsu and Y. Moriyoshi, Sep. Sci. Technol., 2005, 39, 2091 CrossRef
.
- O. Kikhtyanin, V. Kelbichová, D. Vitvarová, M. Kubů and D. Kubička, Catal. Today, 2014, 227, 154 CrossRef CAS
.
- A. Inayat, I. Knoke, E. Spiecker and W. Schwieger, Angew. Chem., Int. Ed., 2012, 51, 1962 CrossRef CAS PubMed
.
- M. Choi, K. Na, J. Kim, Y. Sakamoto, O. Terasaki and R. Ryoo, Nature, 2009, 461, 246 CrossRef CAS PubMed
.
- X. Zhang, D. Liu, D. Xu, S. Asahina, K. A. Cychosz, K. V. Agrawal, Y. Al Wahedi, A. Bhan, S. Al Hashimi, O. Terasaki, M. Thommes and M. Tsapatsis, Science, 2012, 336, 1684 CrossRef CAS PubMed
.
- T. Yutthalekha, C. Wattanakit, C. Warakulwit, W. Wannapakdee, K. Rodponthukwaji, T. Witoon and J. Limtrakul, J. Cleaner Prod., 2017, 142, 1244 CrossRef CAS
.
- P. Wuamprakhon, C. Wattanakit, C. Warakulwit, T. Yutthalekha, W. Wannapakdee, S. Ittisanronnachai and J. Limtrakul, Microporous Mesoporous Mater., 2016, 219, 1 CrossRef CAS
.
- W. Wannapakdee, C. Wattanakit, V. Paluka, T. Yutthalekha and J. Limtrakul, RSC Adv., 2016, 6, 2875 RSC
.
- A. Borgna, S. Magni, J. Sepúlveda, C. L. Padró and C. R. Apesteguía, Catal. Lett., 2005, 102, 15 CrossRef CAS
.
- Y. H. Yeom, S. B. Jang, Y. Kim, S. H. Song and K. Seff, J. Phys. Chem. B, 1997, 101, 6914 CrossRef CAS
.
- S.-T. Yang, J. Kim and W.-S. Ahn, Microporous Mesoporous Mater., 2010, 135, 90 CrossRef CAS
.
- G. M. Lari, K. Desai, C. Mondelli and J. Perez-Ramirez, Catal. Sci. Technol., 2016, 6, 2706 CAS
.
- T. C. Keller, E. G. Rodrigues and J. Pérez-Ramírez, ChemSusChem, 2014, 7, 1729 CrossRef CAS PubMed
.
- T. C. Keller, J. Arras, S. Wershofen and J. Pérez-Ramírez, ACS Catal., 2015, 5, 734 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7cc06375j |
| This journal is © The Royal Society of Chemistry 2017 |