New synthetic routes towards MOF production at scale
Marta
Rubio-Martinez
a,
Ceren
Avci-Camur
 b,
Aaron W.
Thornton
a,
Inhar
Imaz
b,
Daniel
Maspoch
bc and
Matthew R.
Hill
b,
Aaron W.
Thornton
a,
Inhar
Imaz
b,
Daniel
Maspoch
bc and
Matthew R.
Hill
 *ad
*ad
aCSIRO, Private Bag 10, Clayton South, VIC 3169, Australia
bInstitut Català de Nanociencia i Nanotecnologia, ICN2, Esfera UAB, Campus UAB 08193, Bellaterra, Spain
cInstitució Catalana de Recerca i Estudis Avançats (ICREA), 08100 Barcelona, Spain
dDepartment of Chemical Engineering, Monash University, Clayton, VIC 3800, Australia. E-mail: matthew.hill@csiro.au; matthew.hill@monash.edu
First published on 22nd May 2017
Abstract
The potential commercial applications for metal organic frameworks (MOFs) are tantalizing. To address the opportunity, many novel approaches for their synthesis have been developed recently. These strategies present a critical step towards harnessing the myriad of potential applications of MOFs by enabling larger scale production and hence real-world applications. This review provides an up-to-date survey (212 references) of the most promising novel synthetic routes, i.e., electrochemical, microwave, mechanochemical, spray drying and flow chemistry synthesis. Additionally, the essential topic of downstream processes, especially for large scale synthesis, is critically reviewed. Lastly we present the current state of MOF commercialization with direct feedback from commercial players.
1. Introduction
Metal organic frameworks (MOFs) have emerged as a focus of academic fascination and commercial opportunity due to their unprecedented structures that imply a plethora of potential applications. The initial report from Hoskins and Robson instigated a new field of coordination chemistry, combining the tenets of organometallic cluster chemistry with established coordination motifs to form coordination polymers, a.k.a. MOFs.1,2 Later reports from key instigators including Yaghi,3,4 Kitagawa,5,6 Ferey,7,8 and Long9,10 projected the potential applications of MOFs, utilising the combination of unprecedented porosity with periodicity and versatile chemistries. The applications envisaged were largely based on separation, storage, sensing or release characteristics.11–15 As a result, it is possible that MOFs could have revolutionary performance in areas that include natural gas storage,16–18 petrochemical separation,19,20 CO2 capture,21,22 or drug delivery.23,24However, central to the translation of these new materials into disruptive technologies is the ability to manufacture MOFs at the required scale, purity and price for implementation. For example, the potential application of the enormous natural gas reserves globally as a fuel for vehicles, adsorbed in MOFs within the tank would immediately require megaton scale production of MOFs.25 Or in CO2 capture, more than 8000 million tons of CO2 are produced from coal-fired power stations annually, again requiring many millions of tons of MOF to capture it.26,27 In the laboratory, MOFs are most commonly produced in milligram scales, with multi-day reaction times in expensive organic solvents. Their synthesis is often the balance of a number of competing forces, with a range of kinetic and thermodynamic products possible, meaning that a narrow set of reaction conditions are often possible for a successful synthesis. The large gap between laboratory production and that required for commercial application has created a strong imperative to develop efficient and versatile means of producing MOFs at scale.
As scale up production methods are developed, parameters for assessing their viability have become important. Of these, the key parameter is the space-time yield (STY), a measure of the amount of MOF able to be produced per unit volume of reactor in a 24 hour period. In concert with this, we recently proposed that the absolute value (in g h−1) is also important. Many new production techniques are still in the early stages, meaning that the calculated STY may be prone to over-extrapolation. Other important factors are measures of product quality (such as surface area and phase purity), particle size control, yield, and the versatility of the technique. This article will seek to use these criteria in describing the prospective production methods featured herein.
There are several challenges common to the bulk of the prospective scale up methods:
(a) Use of organic solvents. At scale, their cost, toxicity and in some cases flammability become significant issues.
(b) Anion build up. Typically, metal salts are employed as precursor molecules. At scale, nitrates present a safety hazard, and anions such as chlorides can prove corrosive. Oxide and hydroxide metal precursors are preferable.
(c) Ligand availability. Many MOFs require bespoke organic ligands. Production methods that could also produce these starting materials are in development.
(d) Particle size control. Applications such as membranes require nanometre sized particles whereas in storage applications. Larger particles are desired to stop unwanted movement of the MOF particles. Control of this is an important attribute.
(e) Activation. MOFs require removal of non-volatile solvents and unreacted starting materials from their pores. This is a major consideration at large scale.
(f) Shaping of MOF powders produced is also required for using them in real industrial applications.
All these challenges, which are specific for each MOF family due to their different composition and coordination nature, together with their extreme porosity, make the synthesis of these materials more complex than zeolites; each MOF requires bespoke conditions. In recent years, a number of approaches for addressing these challenges have been considered, including electrochemical, microwave and mechanochemical syntheses as well as spray drying and continuous flow production. Electrochemical synthesis of MOFs was developed by BASF and their initial purpose was to exclude anions by using metal electrodes as metal sources. Microwave-assisted synthesis, flow chemistry and spray-drying synthesis allow for a faster crystallization rate and production of smaller MOF crystals. In mechanochemical synthesis, no external heating or solvent is needed, reducing the washing and activation labour after the synthesis.
Given the rapid progress in the development of these techniques, there has been a recent rise in commercial entities that seek to utilise and/or produce MOFs. This review seeks to provide an update on progress of these companies, with direct input from them. BASF pioneered large-scale, bespoke solvothermal techniques primarily for use in vehicular natural gas storage. Following this, spin-out companies have been established, often based upon novel reaction techniques originally developed in a research setting. Some spin-out companies also seek to develop MOF-based products in addition to the broader supply of MOFs to the research community.
In the following, we review the novel synthesis routes developed to date. We also discuss important downstream processing considerations and provide a summary of recent commercial developments.
2. Production of MOFs: from laboratory to industrial scale
In 1995, Nalco Chemical Company and Omar Yaghi claimed the use of solvothermal synthesis to obtain MOFs.28 Up until now, this synthetic approach is the most common way to obtain grams of MOFs in the laboratory around the world. This method involves mixing solutions of the inorganic salt with the organic linker in a sealed reactor vessel and subsequent heating to promote the growth of insoluble frameworks that precipitate as fine crystals.29,30 This sealed approach allows the reaction mixture be heated up to temperatures and pressures above the solvent's boiling point to solubilize, partially or completely, otherwise insoluble reagents and form extended networks. It has become a benchmark in MOF chemistry, and a large variety of MOF families such as MIL series,8,31 MOF-74,32,33 UiOs34–36 and PCNs37,38 have been synthesized following this principle.However, despite the tremendous academic interest that MOFs have generated in the last two decades, with thousands of new structures and with very promising applications, only very few of them are produced at large scale and used in real world applications.18,22,23,39–41 The main reasons for this are the lack of stability of most of the structures towards temperature and humidity, the high cost of the raw materials and, above all, the difficulty of scaling up the synthesis and the post-synthetic stages in a cost-effective way whilst maintaining the product quality and reproducibility between batches. In addition, while the solvothermal approach is a well-known industrial method for chemical synthesis, its application for large scale MOF production is not feasible as MOF synthesis relies on the nucleation at a reactor vessel surface. Up-scaling the reactor vessel significantly decreases the surface to volume ratio and consequently, reduces the efficiency of the reaction. Additional problems include: long reaction times (hours or days), large amounts of solvents used, low quality of materials obtained, high complexity and cost in the up-scaling.42
In order for any production MOF process to be industrially viable a number of key aspects have to be considered: (i) a versatile method is crucial in order to accommodate the maximum number of MOF structures with the same piece of equipment; (ii) the possibility to avoid harsh processing conditions such as high temperature and pressure will reduce capital and operating costs and alleviate safety concerns; (iii) a switch from batch to continuous processing would be beneficial offering higher output per unit time and a continuous steady-state operation leading to significantly reduced downtimes, labour costs, reactor volumes, as well as constant and consistent production; and (iv) a high space-time-yield (STY) parameter which measures the amount of MOF produced per m3 of reaction mixture per day.
All these factors make the scale-up of MOF production challenging and have motivated many researchers and engineers to explore and develop novel and commercially viable routes to produce MOFs in an efficient, reproducible and cost-effective way.43,44Fig. 1 shows the timeline of the most common synthetic processes developed in the last two decades. In this review, we will focus on energy-efficient processes with reduced reaction times that facilitate the up-scaling and the continuous operation. In the following sections, we will describe in detail the advances in electrochemical,45 microwave46 and mechanochemistry47 approaches and the more recent routes, the spray dryer48 and flow chemistry.49
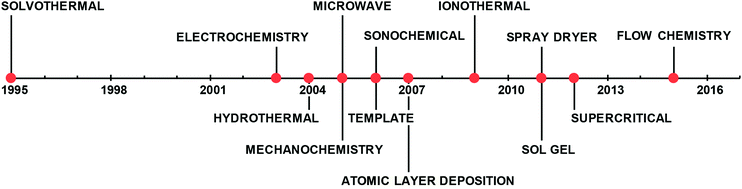 | ||
| Fig. 1 Timeline of the most common synthetic approaches patented for the synthesis of MOFs.28,45,46,48,49,205–210 | ||
2.1 Novel synthesis routes
Taking advantage of the potential of electrochemistry to synthesize materials and their large experience in the domain, the company BASF first patented the use of electrosynthesis to produce MOFs in 2005.50 The synthesis consisted on immersing a copper plate in a solution containing the organic linker, 1,3,5-benzenetricarboxylic acid (BTC), and an electrolyte. The copper plate, which acts as the electrode, was used as the source of Cu(II) ions. When a certain current or voltage was applied, the Cu(II) ions were released from the copper electrode to the solution and reacted with the dissolved linker. In this patent, a powder of electrochemically produced HKUST-1 that consisted of octahedral crystals (size: 0.5–5 μm) could be fabricated after applying a voltage of 12–19 V and a current of 1.3 A for 150 minutes. The surface area of this synthesized HKUST-1 was 1820 m2 g−1, which is higher than that reported for the solvothermally synthesized HKUST-1 (1550 m2 g−1).51
Since this first patent, electrochemical synthesis of MOFs has attracted great attention because it can offer many advantages. One of them is the possibility to run the synthesis of MOFs in a continuous way. It also allows their synthesis under milder conditions than typical solvothermal or microwave syntheses, reducing the reaction time. Indeed, while solvothermal synthesis might take hours or days, electrochemical methods can produce the MOF material within minutes or hours. In addition to these, the electrochemistry method provides the ability to control the MOF synthesis directly during the reaction by controlling the passed current or applied voltage. Furthermore, the electrochemistry method offers the possibility to synthesize homogeneous thin films or coatings.52
Electrosynthesis of MOFs can be classified in two main methods: (i) the anodic dissolution, which was the first route patented by BASF; and (ii) the cathodic deposition. In the anodic deposition, an applied electric potential induces the release of metal ions from the electrode, which then react with an organic linker present in the solution leading to the formation of a MOF film. In this case, the use of a metallic electrode (instead of metal salts) as the source of metal cations avoids the formation of any corrosive anions (mainly, nitrate and acetate anions) or any by-products. The anodic dissolution is typically carried out in a two-electrode set-up without a reference electrode, and the use of protic solvents is usually needed to ensure the evolution of hydrogen and avoid the reduction of metal ions at the counter electrode. In addition, the use of a sacrificial compound (e.g. acrylonitrile, acrylic or maleic esters) that are preferentially reduced or a counter electrode with a suitable overpotential for hydrogen evolution is recommended.53 In the cathodic deposition, a solution containing the organic linker, the metal ions, and a so-called probase is contacted with a cathodic surface. In this approach, the MOF film deposition results from increasing the pH near the cathodic surface, where the electrochemical reduction of the probase occurs. An example of a probase is the nitrite ions coming from the reduction of nitrates, which are able to deprotonate the organic linker and form the MOF.54
Since the electrochemical synthesis of HKUST-1 by BASF using the anodic dissolution was reported, there have been many efforts to understand and optimize this new route (Fig. 2a). Fransaer et al. recently proposed a mechanism for the anodic dissolution synthesis of HKUST-1 that consists in four phases: (i) initial nucleation; (ii) growth of HKUST-1 islands; (iii) intergrowth; and (iv) crystal detachment.55 When an electric potential is applied, the oxidation of the anode starts and the Cu(II) ions are released into the solution. The nucleation of the HKUST-1 phase starts once the critical ion concentration on the surface of the anode is reached. The nucleation is progressive and the dimensions of the crystals depend on the synthesis time and choice of the solvent. The HKUST-1 layers grow at the MOF–solution interface confirming that Cu(II) ions, which are dissolved at the interface, diffuse through the HKUST-1 crystals before they react with the organic linker. This migration of ions is accompanied by the creation of voids at the substrate–HKUST-1 interface, resulting in the formation of fragile layers of HKUST-1 crystals that are easily detached from the substrate. Simultaneously, van der Veen and Domke et al. described this anodic dissolution mechanism from a more chemical point of view.56 These authors identified that chemical species involved in the electrosynthesis of HKUST-1 are initially Cu(I)2O, which results from the oxidation of the copper plate in the presence of H2O or O2. Then, Cu(I)2O is further oxidized to Cu(II)O that can react with the organic linker and lead to the formation of HKUST-1 crystals.
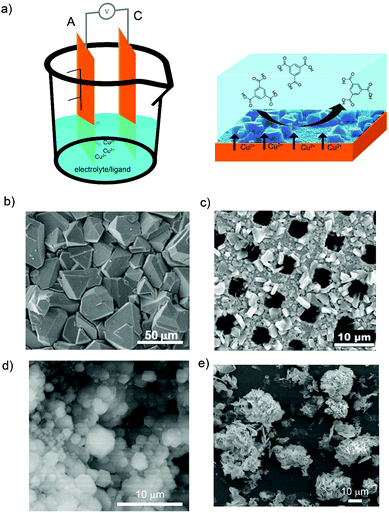 | ||
| Fig. 2 Electrosynthesis of MOFs by anodic dissolution. (a) Schematic illustration showing the anodic dissolution cell (left) and the formation of HKUST-1 on the anode electrode (right). (b) SEM image of HKUST-1 on a copper electrode. (c) SEM image of HKUST-1 on a copper mesh. (d) SEM image of ZIF-8 particles on a zinc electrode. (e) SEM images of flower-shaped MOF-5 on a zinc electrode. (© Elsevier, American Chemical Society and Royal Society of Chemistry, reprinted with permission from ref. 57, 59, 65 and 68). | ||
To date, it is known that small variations of the applied electric potential and passed current, the nature of the solvent and its conductivity, and nature of the electrolyte have a strong influence on the anodic dissolution synthesis of HKUST-1. For example, the applied electric potential is important due to the direct influence on the generation of Cu(II) ions from the copper electrode. As observed by De Vos et al., higher voltages applied by square wave functions provided higher concentration of Cu(II) cations because of the higher dissolution rate of the copper metal (Fig. 2b). These conditions led to the formation of coatings with smaller crystals of HKUST-1 in agreement with the nucleation theory.57 In the same line, Gascón et al. observed better results in terms of HKUST-1 coverage of the electrode when square wave functions were used instead of a continuous mode.58 Denayer et al. found that the frequency of these square wave functions does not influence the HKUST-1 coating of the copper electrode.59
Solvent media is also influencing the electrochemical crystallization of HKUST-1. De Vos et al.57 and Denayer et al.59 observed the formation of larger crystals when the amount of water (from 10 to 50% in volume) was increased in the electrolyte water/ethanol solution because it slowed down the reaction by the hydration of the Cu(II) cations. In addition, detachment of HKUST-1 crystals from the electrode was observed for water contents higher than 50%. Under these conditions, Gascón et al. detected the formation of a secondary phase consisting of a catena-triaqua-μ-(1,3,5-benzene-tricarboxylate)-copper(II) compound.60 More recently, Deyaner et al. investigated the effect of other organic solvents (e.g. methanol, ethanol, 2-propanol, acetonitrile, N,N-dimethylformamide (DMF), and dimethylsulfoxide (DMSO)) in the electrochemical formation of HKUST-1 (Fig. 2c).61 They observed that the crystal size increases when increasing the water content in methanol and ethanol; less dense and uniform HKUST-1 layers are obtained in 2-propanol; HKUST-1 crystal morphology is different when using acetonitrile instead of methanol or ethanol; octahedral crystals are generated in DMF; and the amount of water does not influence the synthesis of HKUST-1 in DMSO, as it does in methanol or ethanol.
Because of the low conductivity of the reaction media, electrolytes that enhance charge transport in solution are generally used. Tributylmethylammonium methyl sulfate (MTBS) is usually recommended for syntheses carried out in organic media and, indeed, it showed a positive role on the HKUST-1 synthesis. For example, increase of conductivity by increasing the concentration of MTBS in the electrolyte solution reduced the ohmic drop of the solution and increased the production yield of HKUST-1. However, Deyaner et al. found some disadvantages related to the use of MTBS in the electrochemical formation of HKUST-1 thin films. They observed structural damages of the copper mesh and the generation of non-adhesive HKUST-1 crystals at the surface of the anode. On the contrary, they could get more control over the synthesis in the absence of MTBS because of lower current density in the system.62 Another disadvantage of using MTBS was reported by Hartmann et al., who observed a decrease in the surface area of HKUST-1 that was attributed to the presence of the electrolyte salt in its pores.63
Beyond the archetypical HKUST-1, the syntheses of other MOFs have been envisaged using anodic dissolution. Remarkably, Gascón et al. demonstrated the possibility to electrochemically synthesize ZIF-8, MIL-53 and MIL-100(Al).58 Since then, De Vos and Fransaer et al. optimized the quality of the synthesized MIL-100(Fe) by performing the electrochemical synthesis under high pressure and high temperature.64 Also, Attfield and Dryfe et al. improved the synthesis of zeolitic imidazolate frameworks (ZIF) (e.g. ZIF-4, ZIF-7, ZIF-8, ZIF-14, and ZIF-67) coatings (Fig. 2d) by increasing the reaction times (a proxy for higher metal ion concentration), the organic linker concentration and the reaction temperature.65 With this anodic dissolution, luminescent rare earth based MOFs were also prepared by Fransaer et al. on electrically conductive solid substrates.66 Here, Tb-BTC and Gd-BTC layers were electrochemically synthesized on terbium and gadolinium metal foils by immersing the foil in a water–ethanol solution containing the organic linker, the electrolyte (MTBS) and applying a constant current of 1 mA cm−2.
Within this variety of MOFs, the electrochemical synthesis of MOF-5 has also been largely investigated. Cao et al. reported the anodic dissolution electrosynthesis of thin films of rod-like MOF-5 crystals.67 They could generate dense and thick MOF-5 films by using zinc electrodes in an aqueous solution containing H2BDC and ammonium fluoride as the electrolyte salt and applying voltage (2 V) at 65 °C. Liang et al. synthesized MOF-5 in the form of flower shape by using molten salt in the electrolyte solution and 1-butyl-3-methylimidazole (BMiM) bromine as a template (Fig. 2e).68,69 This MOF-5 was synthesized using a zinc plate as the anode, a titanium plate as the cathode, and a DMF and BMIM bromide mixture containing H2BDC and zinc nitrate hexahydrate as the electrolyte solution. The reaction was done in atmospheric conditions and applying a current density of 0.025 A cm−2 for 2 hours.
As state above, the second main route for the electrochemical synthesis of MOFs is the cathodic deposition. In 2011, Dinca et al. first investigated the cathodic deposition of MOFs70 to resolve two major limitations of the anodic dissolution (Fig. 3a): (i) the deposition surface (anode surface) is used to produce the metal cations and thus, it is eroded in a continuous manner throughout the synthesis; and (ii) the selection of the anode metal is limited since the anode is also used as the metal resource. In this cathodic deposition, the metal salt, which is dissolved in the electrolyte solution together with the organic linker and the probase, is used as the metal precursor. To show the potentiality of this approach, Dinca et al. showed the synthesis of HKUST-1 and MOF-5 in only 15 min at room temperature (Fig. 3b). For it, they used fluorine doped tin oxide (FTO) as the working electrodes, Ag/Ag(cryptand) as the reference electrode, and a DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (100
water (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (v
1) (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) mixture containing the organic linkers and the metal salts as the electrolyte solvent. In these syntheses, it was found that the nature of the metal salt plays a crucial role. This importance is due to the nature of the counteranions, which act as a probase and can inhibit or favour the formation of the desired MOF.71 For example, as the synthesis of MOF-5 starts with the formation of Zn5(OH)8(H2O)2(NO3)2, the use of chlorine anions can inhibit its formation due to the formation of Zn5(OH)8(Cl)2(H2O)2. On the contrary, the use of nitrate anions can help on its formation since they can act as the probase and participate in the formation of the intermediate specie.
v) mixture containing the organic linkers and the metal salts as the electrolyte solvent. In these syntheses, it was found that the nature of the metal salt plays a crucial role. This importance is due to the nature of the counteranions, which act as a probase and can inhibit or favour the formation of the desired MOF.71 For example, as the synthesis of MOF-5 starts with the formation of Zn5(OH)8(H2O)2(NO3)2, the use of chlorine anions can inhibit its formation due to the formation of Zn5(OH)8(Cl)2(H2O)2. On the contrary, the use of nitrate anions can help on its formation since they can act as the probase and participate in the formation of the intermediate specie.
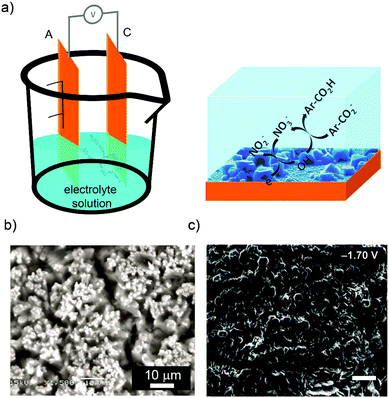 | ||
| Fig. 3 MOF synthesis by cathodic deposition. (a) Schematic illustration showing the cathodic dissolution cell (left) and the reaction that takes place on the cathode electrode (right). (b) SEM images of MOF-5 deposited on the cathode surface. (c) SEM images of bilayer structures of MOF-5 and (Et3NH)2Zn3(BDC)4. (© Royal Society of Chemistry and American Chemical Society, reprinted with permission from ref. 71 and 72). | ||
The aptitude of the cathodic deposition to favour the formation films was also exploited by the same authors to form more complex biphasic MOF thin films at room temperature from single deposition baths using potential bias as the main user input.72 In this case, bilayer structures of MOF-5 and (Et3NH)2Zn3(BDC)4 (applied potential: −1.7 V) (Fig. 3c), mixed structures of MOF-5 and (Et3NH)2Zn3(BDC)4 (applied potential: −1.1 V), and layers of only (Et3NH)2Zn3(BDC)4 (applied potential: −1.5 V) were fabricated tuning the applied potential.
Later innovations on the electrochemical synthesis of MOFs have been centered on the development of new methodologies such as electrophoretic deposition,73,74 galvanic displacement,75 anodic–cathodic deposition76 or bipolar electrochemistry.77 For example, Ameloot et al. combined both anodic and cathodic deposition to perform the modulated synthesis of UiO-66 simultaneously on both anode and cathode surfaces.76 For this synthesis, zirconium films were used as electrodes and H2BDC was dissolved in a mixture of DMF, nitric acid (electrolyte), water and acetic acid (AA). AA was used as a modulator to increase the amount of linker defects and therefore, the BET surface area. However, increase of AA also decreased the crystallinity because of the increase of the competition between BDC and AA. It was found that denser packed films with smoother surfaces were formed on the anode when an AA concentration of 0.5 M or 1 M was used, and that larger octahedral UiO-66 crystals were obtained for AA concentrations higher than 5 M. In this process, when the AA concentration increased, the complexation of released Zr(IV) ions also increased leading to a decrease of the anodic deposition. On the contrary, when the concentration of AA decreased, the concentration of released Zr(IV) ions increased, thereby increasing the deposition on the cathode.
Another interesting example was reported by Bradshaw and Kuhn et al. who used bipolar electrochemistry (BE) to produce Janus-type MOF composites inducing the site selective ZIF-8 or HKUST-1 crystallization on a polarized metallic wire under an electric filed. In BE, a conducting object is exposed to an electric field established between two electrodes in a solution this induces a positive and negative polarization between the two opposite sides of the object and a redox reactions can occur.77
The pioneering work on MW synthesis of MOFs by Jhung et al. reported the water-based synthesis of the chromium trimesate MIL-100 MOF in the presence of hydrofluoric acid.82 The synthesis was performed in a microwave oven at 220 °C for 1, 2 or 4 hours with the reaction mixture in a sealed Teflon autoclave. The results showed the presence of unreacted metallic chromium species for reaction times less than 2 hours. The crystal yield obtained after 4 h was 44%, which is comparable to the 45% achieved in the conventional synthesis in 4 days. Two years later, the same group reported the synthesis of spherical nanocrystals of chromium terephthalate MIL-101 MOF.83 In this work, they showed that crystal size increases with increasing irradiation time, ultimately allowing the isolation of particles with a high surface area.
The MW synthesis of IRMOF-1, IRMOF-2, and IRMOF-3 was reported by Ni et al. who obtained microcrystals with a relatively uniform size and identical cubic morphology in less than 2 minutes.84 They showed that the crystal size can be varied from micrometer to submicrometer by manipulating the concentration of the starting material. The same synthesis was conducted by Choi et al. who investigated how the power level, irradiation time, temperature, solvent concentration and substrate composition affected the crystallinity and morphology of MOF-5.85 The microwave irradiation lead to crystals after only 30 min of reaction time while 24 h were necessary with the conventional method. The optimum microwave conditions lead to uniform cubic crystals with average size of 20–25 μm and with a BET surface area of 3008 m2 g−1.
MW irradiation is an attractive method to synthesize MOFs with biomedical applications, such as iron-carboxylate MOFs, because uniform nanocrystals are easily achievable. For instance, in 2009, Lin and co-workers described the MW synthesis of 200 nm nanoparticles of iron-MIL-101 MOF and its amino functionalized version.86 The starting materials were dissolved in DMF and then rapidly heated to 150 °C and held at this temperature for 10 minutes.
Several studies have been performed comparing conventional electric (CE) heating, MW and ultrasound (US) methods in order to understand the accelerated US and MW syntheses.87–90 For example, in 2009, Haque et al. performed a kinetic study on the synthesis of MIL-53-Fe.91 They found that the crystallisation rate (both nucleation and crystal growth) decreased in the order: US > MW ≫ CE. These results suggested that physical effects, such as hot spots, are more important than chemical effects in the accelerated syntheses performed under US and MW conditions (see Fig. 4). A similar study was performed by Chalati et al. where the synthesis of iron fumarate MIL-88A nanoparticles was compared with the classical solvothermal, MW and US methods.92 With the CE heating a polydesperse sample of 200 nm nanoparticles was obtained, whereas 100 nm monodisperse nanoparticles but with very low yields were obtained with the US method and <100 nm monodisperse nanoparticle with high yields were obtained with the MW method.
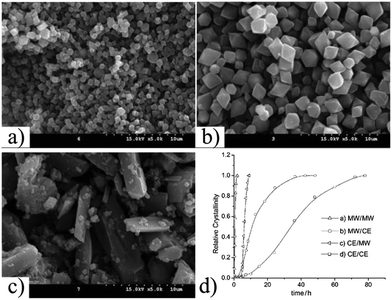 | ||
| Fig. 4 SEM images of synthesis of MIL-53(Fe). Synthesis at 70 °C with (a) ultrasounds for 35 min, (b) microwave for 2 h, and (c) conventional electric heating for 3 days (d) comparison crystallization curves for the synthesis of MIL-53(Fe) in two steps by (a) microwave, (b) microwave and conventional electric heating, (c) conventional electric heating and microwave and (d) conventional electric heating.91 (© Elsevier, reprinted with permission from ref. 80). | ||
The zeolitic imidazolate framework, ZIF-8, has been synthesized with MW irradiation and CE heating at 140 °C in 4 hours and 20 hours respectively.93 In addition to the reduced reaction time, the ZIF-8 obtained by microwave heating had a larger surface area and micropore volume compared with the ZIF-8 synthesized with CE heating.
There are a few reports available showing the effectiveness of MW irradiation for the synthesis of lanthanide–organic frameworks. For example, Silva et al. obtained quality single-crystals of the microporous cationic [Ce2(pydc)2(Hpydc)(H2O)2]Cl (where pydc corresponds to the 2,5-pyridinedicarboxylic acid) by applying MW heating for 20 min at 200 °C.94 In 2014, Vileda et al., synthesized a series of lanthanide (Eu, Gd and Tb) bisphosphonates using conventional hydrothermal synthesis (180 °C, 3 days), MW-assisted heating (40 °C, 5 seconds) and US-assisted synthesis (room temperature, 5 minutes).95 Under CE heating, microcrystalline materials were obtained which did not possess any significant catalytic activity, whereas the application of MW and US resulted in nanocrystals that exhibited relatively high catalytic activity and excellent selectivity to 2-methoxy-2-phenylethanol (100% yield within 48 hours of reaction time). A recent work by Cao and co-workers showed the gram scale production of nine isostructural microporous lanthanide MOFs via a microwave over 5 min.96 The same synthesis but under conventional solvothermal reaction required seven days to produce the same materials with a similar yield. Moreover, with the solvothermal method only 10 milligrams of quality material could be obtained while MW synthesis yielded up to 2 grams.
In recent years, zirconium-based MOFs have attracted great attention due to their exceptionally high thermal and chemical stability. In 2013, D'Alessandro and co-workers reported the efficient synthesis of MIL-140A, MIL-140B and MIL-140A-NH2 frameworks using MW irradiation.97 They obtained products with purer phase and higher quality in significantly less time than the CE heating method. Recently, a process optimisation for the UiO-66 MW assisted synthesis was presented by Taddei and co-workers.98 The optimized synthesis required 15 minutes of pre-mixture of the initial solutions and 15 minutes at 120 °C. The reaction yield was 83% and no significant negative effects on morphology, crystal size, or defects were found from the use of MW assisted heating in comparison with those synthesized by CE heating. One exciting area that it has been explored with MW in the last 2 years is the defect engineering of UiO-66. Babarao and co-workers presented an experimental and theoretical study showing the correlation between the defect concentration composition in UiO-66 and their carbon dioxide adsorption properties.99 They presented a detailed MW-assisted solvothermal synthesis protocol to prepare pure phases of high-quality crystalline UiO-66 frameworks with different defect concentrations. Highly crystalline UiO-66 octahedral shaped crystals were obtained in a short reaction time of 5 minutes using hydrochloric acid and formic acid as modulators.
The three different mechanochemical approaches used for MOF production are Solvent-Free Grinding (SFG) which is the simplest method and avoids the use of solvent; Liquid-Assisted Grinding (LAG) which is more versatile, and quicker, as it uses catalytic amounts of liquid phases which increase the mobility of the reagents; and finally, Ion-and-Liquid Assisted Grinding (ILAG) which uses a catalytic liquid with traces of salt additives to accelerate the MOF formation. Using these techniques, the synthesis for almost all families of MOFs has been demonstrated, and selected studies will be explained in this section.109
A first work by James and co-workers employed the SFG method, milling a dry mixture of copper acetate and isonicotinic acid (Hina) powder for 10 minutes resulting in the formation of copper(II) isonicotinate MOF with acetic acid and water molecules occluded in the pores (see Fig. 5a).110 Using the same approach, the same group performed a screening study, grinding sixty different combinations of twelve different divalent metal salts, composed of copper, nickel and zinc together with five different carboxylate organic linkers for 15 minutes.111 As a result several crystalline structures, including two microporous metal–organic frameworks HKUST-1 and Cu(INA)2 were obtained.
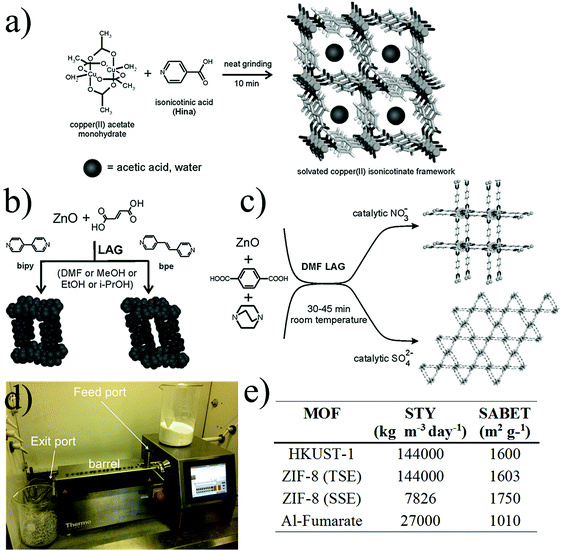 | ||
| Fig. 5 Mechanochemical synthesis of MOFs. (a) Neat grinding, (b) liquid-assisted grinding (c) ion- and liquid-assisted grinding, exploiting the catalytic effect of nitrates and sulphates (d) twin screw extruder with key parts highlighted. (e) Table of space time yields (STYs) and BET surface area for the synthesis of MOFs synthesized by extrusion methods (© The Royal Society of Chemistry, reprinted with permission from ref. 106 and 107). | ||
One important advantage of this approach is the possibility to synthesize MOFs with only water as a by-product allowing the complete elimination of the purification stage. This is achieved by using hydroxides or oxides as the metal source which then in combination with the protons generated from the organic ligand forms H2O.
Following this strategy, Tanaka and co-workers presented the mechanical dry conversion of zinc oxide and an imidazole ligand into ZIF-8.112 The process was investigated for reaction times of 3 to 240 hours, yielding the best BET surface area (1480 m2 g−1) at 96 hours. The decrease of the BET surface area after 96 hours was due to the formation of amorphous domains during the mechanochemical reaction. The same year, Balema and co-workers reported the preparation of the yttrium based MIL-78 MOF under completely liquid-free conditions and using a metal hydride for the first time as a starting material and forming hydrogen as a by-product.113
Very recently, Xu and co-workers reported the synthesis of MIL-101(Cr) without the addition of solvent and hydrofluoric acid.114 The chromium salt and the terephthalic acid were ground for 30 minutes at room temperature and then transferred into an autoclave at 220 °C for 4 hours, yielding a material with a BET surface area of 3517 m2 g−1 and with a reduced particle size compared to the batch process.
In 2006, Braga and co-workers demonstrated for the first time how the addition of small amounts of solvent to the powder mixture precursors could effectively improve the crystallisation of the compounds and accelerate the synthesis.115 They synthesized the CuCl2(dace) (where dace is the trans-1,4-diaminocyclohexane) one-dimensional coordination polymer by grinding the starting materials for 5–10 minutes in the presence of water or DMSO which was then removed by thermal and vacuum treatment. In 2009, Friščić and Fábián demonstrated the ability to selectively and quantitatively build different metal–organic architectures by simply changing the amount and type of solvent using the same starting materials. In this specific work, they presented the formation of four coordination polymers and two porous structures by grinding zinc oxide and fumaric acid in the presence of different types of solvents.116 In 2010, Klimakow et al. synthesized the well-known HKUST-1 and its benzenetribenzoate-based analogue MOF-14 via the LAG approach. The MOFs were obtained by grinding the copper acetate monohydrate salt with the corresponding organic linkers for 25 minutes. Acetic acid was formed as a by-product, which blocked the micropores and consequently resulted in a smaller BET surface area compared to other synthetic approaches.117 James and co-workers showed that by adding small amounts of liquid by-products, generated via the SFG method, before the mechanical process, that the synthesis could be accelerated.118 By adding small amounts of acetic acid into the precursor mixtures, the formation of Cu(INA)2 MOF was dramatically accelerated, while for HKUST-1, due to the lower solubility of the trimesic acid, no improvement was reported. In 2010, the same group studied by X-ray diffraction the structural properties of [Zn2(fma)2(bipy)], where fma corresponds to fumaric acid and bipy to 4,4′-bipyridine, prepared by mechanosynthesis (see Fig. 5b).119 The acetic acid and H2O by-products occluded in the pores were removed by thermal treatment and the interpenetrated structure was refined using Rietveld methods.
In 2015, Prochowicz et al. described the “SMART” (SBU-based Mechanochemical Approach for pRecursor Transformation) strategy for the synthesis of IRMOFs.120 The successful mechanochemical synthesis was performed by mixing pre-assembled oxo-zinc amidate clusters with terephthalic acid in the presence of microlitres of N,N-diethylformamide (DEF) over 60 minutes. Additionally, the study showed the importance of the acid–base relationship between reagents in this type of approach.
Recent work by Friščić and co-workers presented the synthesis of UiO-66 and UiO-66-NH2 at gram scale by adding different amounts of N,N-dimethylformamide (DMF) and methanol to the solid mixtures of the reactants as well as exposing the powder mixture to methanol vapours at 45 °C for 3 days and 1 week respectively.121 The best BET surface area obtained for UiO-66 was 1020 m2 g−1 with 75 minutes grinding, and 945 m2 g−1 for UiO-66-NH2 after 90 minutes grinding, both in the presence of methanol, for the materials exposed to methanol vapours.
The third mechanochemical methodology, ILAG, was demonstrated to be highly efficient for the synthesis of pillared-layered MOFs. For example, the zinc pillared material based on terephthalic acid and dabco (1,4-diazabicyclooctane) was synthetised after 45 minutes reaction by adding catalytic amounts of an alkali metal or ammonium nitrate salt into the mixture (Fig. 5c).122 Using the same starting materials, but replacing the ammonium nitrate with sulphates, yielded the same pillared-layered structure but on a hexagonal grid. A second example was presented, showing the mechanochemical ILAG approach for the room-temperature synthesis of ZIF-8, using zinc oxide as the starting material and stoichiometric amounts of ammonium salts.123 In this case the use of salts enabled synthesis with imidazole enabling the selective ZIF topology formation by changing the type of ammonium salt and adjusting the reaction times.
Mechanochemistry is a versatile method that allows the synthesis of most of the common MOF structures, however so far all examples described a production at less than one gram scale. As an alternative, extrusion techniques have been explored for the up-scaling of MOF production under solvent-free conditions. Extrusion is one of the major continuous manufacturing processes used in industries such as food, metallurgy, plastics and pharmaceuticals and has shown very promising results for the synthesis and shaping of MOFs. In 2015, James and co-workers showed the synthesis of HKUST-1, ZIF-8 and aluminium fumarate MOF with twin-screw extrusion (TSE) at the gram scale. Fig. 5d shows the TSE used for the synthesis of MOFs which consists of a feed port where the MOF precursors were introduced into a heatable barrel containing the screw and an exit port which a die can be attached to shape the final material. HKUST-1 was synthesized by extruding copper hydroxide and trimesic acid in the presence of methanol. The extrudate was stirred in ethanol and dried at 150 °C for 2 hours yielding a N2 BET surface area of 1738 m2 g−1. In the case of ZIF-8 the synthesis was performed by a single screw extrusion (SSE) where the zinc carbonate and the 2-methylimidazole ligand were extruded at 200 °C without the addition of any solvent. In this case, the activation was carried out by stirring the material in methanol and drying the material at 150 °C yielding a N2 BET surface area of 1738 m2 g−1. A last example was obtained by introducing a mixture of aluminium sulphate, sodium hydroxide and fumaric acid into the twin extruder at 150 °C. In this case the by-product was removed by washing the extrudates with water and N2 BET surface area obtained was 1010 m2 g−1. Extrusion is an efficient way to produce MOFs solvent free with high with very promising space-time-yield (STY) (see Fig. 5e). Kilogram scale production could be achieved by using a large-scale equipment and paired with a more detailed knowledge and understanding of the MOF synthesis by this methodology.
Beyond the use of SD in these applications, the local heating of micro- and submicrometer droplets that occurs during the SD process can also be used to conduct chemical reactions. Thus far, this concept has mainly been utilized for discovering and isolating metastable phases of materials that can be only reached thanks to the fast drying conditions of the SD method.131
In 2013, Maspoch et al. expanded this concept to the synthesis of supramolecular materials and, in particular, MOFs.132 The main principle of the process was based on the fast drying of atomized microdroplets of a solution that contains the MOF precursors (Fig. 6a and b). Thus, the process starts with atomization of a solution of the MOF precursors into a spray of microdroplets. This step is accomplished by simultaneously injecting one or more solutions, at a certain rate, (hereafter, feed rate) and compressed air or nitrogen gas, at another certain rate (hereafter, flow rate). Thus, each precursor droplet contacts – and is suspended by – a gas stream heated to a certain temperature (hereafter, inlet temperature), causing the solvent to be heated and evaporated and inducing the MOF precursors (e.g. metal ions and organic ligands) to react forming MOF nanoparticles inside each droplet. At this moment, the newly formed MOF nanoparticles accumulate and merge into compact or hollow spherical MOF superstructures/beads while the solvent is fully evaporated. These MOF superstructures/beads are finally collected inside a collector located at the end of the spray drier instrument.
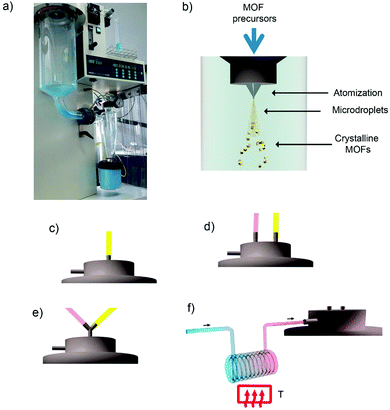 | ||
| Fig. 6 Spray-drying method for the production of MOFs. (a) Photograph of the spray-dryer while it is used to fabricate HKUST-1. (b) Schematic illustration of the spray-drying synthesis of MOFs. The MOF precursor solution can be introduced into the spray drier using a: (c) two-fluid nozzle; (d) three-fluid nozzle; (e) T-junction; and (f) continuous flow rector coupled to a two-fluid nozzle. (© Springer Nature and The Royal Society of Chemistry, adapted with permission from ref. 132 and 134). | ||
Table 1 lists all MOFs – together with the optimized conditions and yields – that have been synthesized using the SD method so far. Besides the optimization of the synthesis parameters such as type of reagents/solvents, feed/flow rates and inlet temperature, a very important aspect that needs to be carefully selected when one wants to synthesize a specific MOF by SD is how the precursor solution is introduced into the spray drier (Table 2). To date, there are four major modes for introducing the MOF precursor solution: (i) use of a two-fluid nozzle (Fig. 6c); (ii) use of a three-fluid nozzle (Fig. 6d); (iii) use a T-junction (Fig. 6e); and (iv) use a continuous flow coupled to a reactor (Fig. 6f).
| MOF | Substrates | Solvent | Electrolyte | BET surface area (m2 g−1) | Ref. |
|---|---|---|---|---|---|
| Abbreviations: MTBS, methyltributylammonium methyl sulfate; MTBAMS, methyltributylammonium methyl sulphate; BMIM, 1-butyl-3-methylimidazole; FTO, fluorine doped tin oxide; BE, bipolar electrochemistry. | |||||
| Anodic deposition | |||||
| HKUST-1 | Copper electrode | MeOH | — | 1820 | 50 |
| Copper electrode | EtOH:H2O | MTBS | — | 57 | |
| Copper mesh | EtOH:H2O | — | — | 59 | |
| Copper electrode | EtOH:H2O | MTBS | 1440 | 58 | |
|
Copper electrode
Copper mesh |
EtOH:H2O, organic solvents | MTBS | — | 61 | |
| Copper electrode | EtOH, EtOH:H2O | MTBS | 1309 | 63 | |
| ZIF-8 | Zinc electrode | AcN:H2O | MTBS | 1600 | 58 |
| Zinc electrode | DMF:H2O | MTBS | 1730 | 65 | |
| ZIF-4 | Zinc electrode | DMF:H2O | MTBAMS | 75 | 65 |
| ZIF-14 | Zinc electrode | DMF:H2O | MTBAMS | 598 | 65 |
| ZIF-7 | Zinc electrode | DMF:H2O | MTBAMS | 358 | 65 |
| ZIF-64 | Cobalt electrode | DMF:H2O | MTBAMS | 1521 | 65 |
| MIL-53(Al) | Aluminium electrode | DMF:H2O | KCl or NaCl | 1200 | 58 |
| MIL-100(Al) | Aluminium electrode | EtOH:H2O | — | 969 | 58 |
| MIL-100(Fe) | Iron electrode | EtOH:H2O | MTBS | — | 64 |
| Tb-BTC | Terbium foil | EtOH:H2O | MTBS | — | 66 |
| Gd-BTC | Gadolinium foil | EtOH:H2O | MTBS | — | 66 |
| MOF-5 | Zinc plate | EtOH:H2O | NH4F | — | 67 |
| Zinc plate and titanium plate | DMF | BMIM | 914.7 | 68 and 69 | |
| MOF | Substrates | Method | Ref. |
|---|---|---|---|
| Cathodic deposition | |||
| MOF-5 | FTO | Cathodic | 70 |
| UiO-66 | Zirconium foil | Anodic and cathodic | 76 |
| MOF-5 | FTO | Cathodic | 71 |
| MOF-5/(Et2NH2)2Zn3(BDC)4 | FTO | Cathodic | 72 |
| Others | |||
| ZIF-8 | Zinc wire | BE | 77 |
| UiO-66 | FTO | Electrophoretic deposition | 74 |
| HKUST-1 | FTO | Electrophoretic deposition | 73 |
| Glass | Galvanic displacement | 75 | |
| Porous stainless steel | Electrophoretic deposition | 73 | |
| Cu bead | BE | 77 | |
| MIL-53 | FTO | Electrophoretic deposition | 74 |
| NU-1000 | FTO | Electrophoretic deposition | 74 |
| MOF | Metal salt/ligand/solvent | Feed rate (mL min−1) | Inlet T (°C) | Yield (%) | BET (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|
| Abbreviations: BTC, trimesic acid; BDC, 1,4-benzenedicarboxylic acid; BPTC, biphenyl-3,3′,5,5′-tetracarboxylic acid; BTB, 1,3,5-tris(4-carboxyphenyl)benzene; DHBDC, 2,5-dihydroxyterephthalic acid; DMF, dimethylformamide; EtOH, ethanol; MeOH, methanol; MiM, 2-methyl imidazole; NDC, naphthalenedicarboxylic acid. | ||||||
| Two fluid nozzle | ||||||
| HKUST-1 | Cu(NO3)2·2.5H2O/BTC/DMF:EtOH:H2O | 4.5 | 180 | 70 | 1260 | 132 |
| Cu-BDC | Cu(NO3)2·2.5H2O/BDC/DMF | 4.5 | 180 | 70 | 543 | 132 |
| NOTT-100 | Cu(NO3)2·2.5H2O/BPTC/DMF:H2O | 4.5 | 180 | 54 | 1140 | 132 |
| MOF-14 | Cu(NO3)2·2.5H2O/BTB/DMF:EtOH:H2O | 4.5 | 180 | 30 | — | 132 |
| Zn-MOF-74 | Zn(NO3)2·6H2O/DHBDC/DMF:H2O | 4.5 | 180 | 50 | — | 132 |
| Mg-MOF-74 | Mg(NO3)2·6H2O/DHBDC/DMF:EtOH:H2O | 4.5 | 180 | 35 | — | 132 |
| Ni-MOF-74 | Ni(NO3)2·6H2O/DHBDC/DMF:EtOH:H2O | 4.5 | 180 | 40 | — | 132 |
| MIL-88B | FeCl3/2-amino-BDC/DMF:MeOH:H2O | 4.5 | 180 | 27 | — | 132 |
| Three fluid nozzle | ||||||
| ZIF-8 | Zinc acetate/MiM/H2O | 4.5 | 180 | 10 | 941 | 132 |
| Cu-PB | Cu(NO3)2/K3Co(CN)6/H2O | 4.5 | 180 | 20 | 617 | 132 |
| SIFSIX-3-Co | CoSiF6/pyrazine/MeOH | 2.4 | 85 | 44 | 136 | |
| SIFSIX-3-Ni | NiSiF6/pyrazine/MeOH | 2.4 | 85 | — | 136 | |
| SIFSIX-3-Cu | CuSiF6·H2O/pyrazine/MeOH | 2.4 | 85 | 55 | 136 | |
| SIFSIX-3-Zn | ZnSiF6·xH2O/pyrazine/MeOH | 2.4 | 85 | 57 | 136 | |
| SIFSIX-1-Zn | ZnSiF6·xH2O/4,4′-bipyridine/MeOH | 2.4 | 85 | 40 | 1300 | 136 |
| TIFSIX-1-Cu | Cu(NO3)2·2.5H2O/4,4′-bipyridine/MeOH | 2.4 | 130 | 79 | 1650 | 136 |
| T junction | ||||||
| MIL-88A | FeCl3/fumaric acid/DMF:MeOH:H2O | 4.5 | 180 | 40 | — | 132 |
| MOF-5 | Zinc acetate/BDC/DMF | 4.5 | 180 | 60 | 1215 | 132 |
| IRMOF-3 | Zinc acetate/2-amino-BDC/DMF | 4.5 | 180 | 70 | — | 132 |
| MPM-1-TIFSIX | TiF6·(NH4)2/Cu(NO3)2·2.5H2O/H2O:MeCN | 2.4 | 150 | 74 | 805 | 136 |
| MOF | Metal salt/ligand/solvent | Feed rate (mL min−1) | T 1 (°C) | Inlet T (°C) | Yield (%) | BET (m2 g−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Continuous flow | |||||||
| UiO-66 | ZrCl4/BDC/DMF:H2O | 2.4 | 115 | 180 | 70 | 1106 | 134 |
| UiO-66-NH2 | ZrCl4/2-NH2-BDC/DMFH2O | 2.4 | 115 | 180 | 67 | 752 | 134 |
| UiO-66-NH2 | ZrOCl2/2-amino-BDC/DMF:H2O | 2.4 | 90 | 180 | 83 | 1150 | 211 |
| UiO-66-NO2 | ZrCl4/2-nitro-BDC/acetic acid:H2O | 2.4 | 115 | 180 | 62 | 679 | 134 |
| UiO-66-Br | ZrCl4/2-bromo-BDC/DMF:H2O | 2.4 | 115 | 180 | 68 | 527 | 134 |
| UiO-66-(OH)2 | ZrCl4/2,5-dihydroxy-BDC/DMF:H2O | 2.4 | 115 | 180 | 81 | 401 | 134 |
| UiO-66-acetamido | ZrCl4/2,5-dihydroxy-BDC/DMF:H2O | 2.4 | 115 | 180 | 51 | 586 | 134 |
| UiO-66-1,4-NDC | ZrCl4/1,4-NDC/DMF:H2O | 2.4 | 115 | 180 | 45 | 431 | 134 |
| UiO-66-2,6-NDC | ZrCl4/2,6-NDC/DMF:H2O | 2.4 | 115 | 180 | 49 | 557 | 134 |
| Fe-BTC/MIL-100 | Fe(NO3)3·9H2O/BTC/DMF | 2.4 | 135 | 180 | 78 | 1039 | 134 |
| Ni8(OH)4(H2O)2(L)6 | Ni(CH3COO)2·4H2O/1H-pyrazole-4-carboxylic acid/DMF:H2O | 2.4 | 100 | 180 | 60 | 377 | 134 |
The use of two-fluid nozzle is the simplest process. It is based on the preparation of a homogeneous solution or suspension that contains all MOF precursors, which is then injected through a two-fluid nozzle.132 This two-fluid nozzle allows the simultaneous injection of this precursor solution at a certain feed rate and compressed air or nitrogen gas at another certain flow rate. In general, this method is very useful to synthesize MOFs that are built up from mononuclear metal ions or smaller metal clusters or secondary building-units (SBUs). An archetypical class of MOFs that can be fabricated using this approach is the large family of MOFs constructed from Cu(II) paddlewheel units and polycarboxylate linkers. For example, HKUST-1 (also known as Cu-BTC or Basolite™ C300) can be synthesized by spray-drying a solution of Cu(NO3)2·2.5H2O and trimesic acid (H3BTC) (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) in DMF, ethanol and water (1
2 molar ratio) in DMF, ethanol and water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a feed rate of 4.5 mL min−1, a flow rate of 336 mL min−1 and an inlet temperature of 180 °C. They could be obtained as hollow spherical MOF superstructures (size: 2.4 ± 0.4 μm) or nanoparticles (size: 75 ± 28 nm) (Fig. 7a).
1) with a feed rate of 4.5 mL min−1, a flow rate of 336 mL min−1 and an inlet temperature of 180 °C. They could be obtained as hollow spherical MOF superstructures (size: 2.4 ± 0.4 μm) or nanoparticles (size: 75 ± 28 nm) (Fig. 7a).
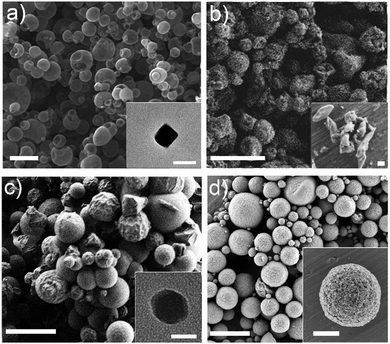 | ||
| Fig. 7 SEM and TEM images of several MOFs synthesized by spray-drying. (a) Hollow spherical superstructures of HKUST-1 synthesized using a two-fluid nozzle. Inset shows a TEM image of a single HKUST-1 nanoparticle. (b) Spherical superstructures of MIL-88A synthesized using a T-junction. Inset shows a SEM image of MIL-88A particles. (c) Superstructures of ZIF-8 synthesized using a three-fluid nozzle. Inset shows a TEM image of a single ZIF-8 nanoparticle. (d) Compact superstructures/beads of UiO-66 synthesized using a continuous flow reactor coupled to a two-fluid nozzle. Inset shows a SEM image of a single bead. Scale bars: 10 μm (c), 5 μm (a and d), 2 μm (b, inset d), 200 nm (inset b), and 50 nm (inset a and c). (© Springer Nature and The Royal Society of Chemistry, reprinted with permission from ref. 132 and 134). | ||
Second and third routes for introducing the MOF precursors inside the spray drier instrument are very similar.132 They are based on using multi-fluid nozzles, to independently atomize the solutions containing the MOF precursors, or additional channels, to independently inject them. Both approaches enable mixing of the precursor solutions just before they are heated into the atomized droplets. In the first approach, mixing occurs inside the drying chamber, thanks to the coalescence of the atomized droplets, whereas in the second one, mixing is done through a connector inserted before the two-fluid nozzle. Using either variation decreases the probability that unwanted species or micrometre-sized MOFs will form in the precursor solution before it is spray-dried. They also enable the use of reagents (e.g. bases) to accelerate MOF formation, thus increasing yields and purities and enabling the synthesis of new hollow MOF superstructures and related nanocrystals. To date, both approaches have allowed the synthesis of several MOFs, including MIL-88A,132 ZIF-8132,133 and Fe-BTC/MIL-100133 (Fig. 7b and c).
In the last approach, the MOF precursor solution is passed through a continuous-flow reactor just before the entrance of the spray dryer.134 This process begins by injecting the precursor solution into a continuous coil flow reactor encased in a thermostatic oil tank, where it is heated at a certain temperature (T1) to promote the SBU formation and nucleation. Here, the residence time of the precursor solution in the coil flow reactor is controlled by the rate of the pump (the feed rate). Since the outlet flow of the reactor is connected directly to the nozzle of the spray-dryer, the pre-heated solution is automatically injected into the spray-drier at the same feed rate. The solution is then atomised using a two-fluid nozzle, and is dried at a certain inlet temperature and flow rate, such that the MOF growth is confined to the atomised microdroplets.
In most of the cases, this last continuous process enables the collection of dried MOFs shaped in the form of compact micrometre superstructures/beads instead of the hollow ones usually obtained in the first three strategies. This difference is attributed to the formation, inside the reactor, of a suspension containing a primary nucleus. In a general spray-drying process, the atomised droplets are exposed to hot air, the solvent evaporates and consequently, the droplet surface shrinks. During this process, hollow superstructures are formed when there is a non-linear change in precursor concentration at the droplet: specifically, it causes the formation of an impermeable shell and the generation of gas at the core. However, in this latter case, uniform precursor concentration and droplet temperature are reached, owing to the presence of the uniformly-distributed nuclei in the droplet. The rate at which the nucleus can be brought to the surface by diffusion is lower than the rate at which the nucleus can grow during the drying–evaporation process. This difference favours a linear change in precursor concentration and temperature at the droplet, and consequently, drives the formation of dense superstructures.
The main advantage of this last SD approach is that it allows the synthesis of MOFs assembled from high-nuclearity SBUs. Indeed, numerous members of the family of UiO-66 (e.g. UiO-66-NH2, UiO-66-Br, etc.) as well as Fe-BTC/MIL-100 and [Ni8(OH)4(H2O)2(L)6]n (where L = 1H-pyrazole-4-carboxylic acid) series were synthesized using the resulting spray-drying continuous flow-assisted synthesis. For example, UiO-66 was synthesized using ZrCl4 and BDC as reagents, DMF and H2O as solvents, an initial concentration of 0.1 M for both reagents, a final molar ratio (Zr/BDC/H2O/DMF) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 135, a T1 of 115 °C; an inlet temperature of 180 °C, and a flow rate of 336 mL min−1. Under these optimized conditions, in which the amount of water, the feed rate and the coil temperature were found to be very important, UiO-66 was fabricated with a space-time yield of 19.6 kg m−3 day−1 (Fig. 7d).
135, a T1 of 115 °C; an inlet temperature of 180 °C, and a flow rate of 336 mL min−1. Under these optimized conditions, in which the amount of water, the feed rate and the coil temperature were found to be very important, UiO-66 was fabricated with a space-time yield of 19.6 kg m−3 day−1 (Fig. 7d).
Lastly, the innovations of using SD in the MOF field have been centered on the use of new chemistries to build and/or modify MOFs;135,136 the synthesis of multivariate or multimetallic MOFs;132,137 and the mixture of MOFs with other materials to make composites.132,138 With this aim, the use of SD has been extended to the synthesis of porous materials that are not based on coordination bonds but on hydrogen bonds.136 For instance, MPM-1-TIFSIX, a porous material based on the hydrogen-bonded assembly of [Cu2(ade)4(TiF6)2] (ade = adenine) paddlewheels (Fig. 8a), was synthesized by spray-drying an aqueous solution of Cu(NO3)2·2.5(H2O) and TiF6(NH4)2 along with a solution of adenine in water/acetonitrile mixture using a 2-fluid nozzle and an inlet temperature of 150 °C. Moreover, SD was also very recently found to be a fast method to post-synthetically modify MOFs using conventional covalent chemistry (Fig. 8b).135 To perform this modification, a suspension of pre-synthesized MOF crystals are spray-dried together with the desired reagent. With this simple method, two MOFs, the amine-terminated UiO-66-NH2 and the aldehyde-terminated ZIF-90, were rapidly post-synthetically modified with aldehydes and amines, respectively, using the well-known Schiff-base condensation reaction and achieving conversion efficiencies up to 20% and 42%, respectively. Moreover, it was demonstrated that the aldehyde groups of ZIF-90 could be cross-linked using a diamine molecule with a conversion efficiency of 70%.
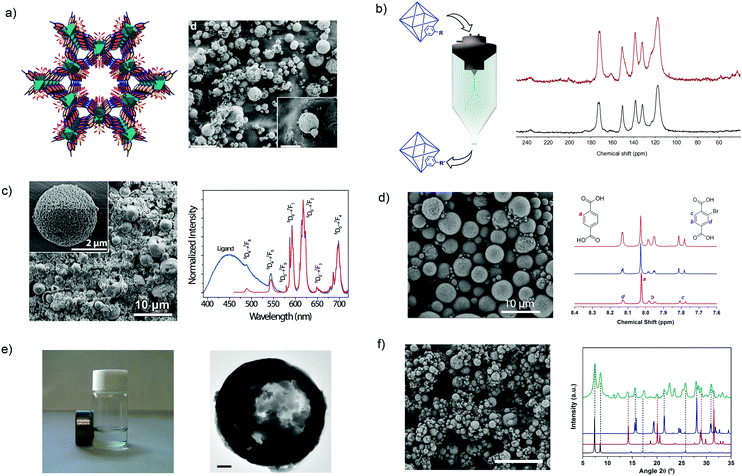 | ||
Fig. 8 Spray-drying method for building and/or modifying MOFs. (a) Crystal structure and SEM image of MPM-1-TIFSIX. Scale bar: 20 μm and inset: 5 μm. (b) Schematic illustration of the post-synthetic modification of MOFs using spray-drying and 13C MAS-NMR spectra that confirms the formation of the CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N imine group. (c) SEM image of a multi-metallic lanthanide-based MOF and excitation spectra of it. Scale bar: 10 μm and inset: 2 μm. (d) SEM image of a multi-variate UiO-66 and NMR spectra confirming that both BDC and BDC-Br are forming the UiO-66 structure. Scale bar: 10 μm. (e) HKUST-1 coupled with magnetic nanoparticles. Scale bar: 200 nm. (f) SEM image of UiO-66 coupled with CaCl2 and XRD pattern of the composite material showing the presence of both components. Scale bar: 20 μm. (© Springer Nature and The Royal Society of Chemistry, reprinted with permission from ref. 132, 134–137 and 139). N imine group. (c) SEM image of a multi-metallic lanthanide-based MOF and excitation spectra of it. Scale bar: 10 μm and inset: 2 μm. (d) SEM image of a multi-variate UiO-66 and NMR spectra confirming that both BDC and BDC-Br are forming the UiO-66 structure. Scale bar: 10 μm. (e) HKUST-1 coupled with magnetic nanoparticles. Scale bar: 200 nm. (f) SEM image of UiO-66 coupled with CaCl2 and XRD pattern of the composite material showing the presence of both components. Scale bar: 20 μm. (© Springer Nature and The Royal Society of Chemistry, reprinted with permission from ref. 132, 134–137 and 139). | ||
Another advantage of SD as a synthetic method in the MOF field is the possibility to synthesize multi-metallic and multi-variate MOFs. From an experimental point of view, the synthesis of these multi-component MOFs does not require technological changes. Its main principle is based on mixing different metal ions or organic linkers in the MOF precursor solution that is spray-dried. With this approach, Wang et al. showed the synthesis of lanthanide-based MOF nanoparticles in which the ratio of Tb(III)/Eu(III) was controlled (Fig. 8c).137 They proved that the resulting MOF nanoparticles could be used as promising nanothermometers with high detection sensibilities, spatial resolutions and short acquisition times. Similarly, multi-variate UiO-66s were synthesized by mixing different ratios of two (benzenedicarboxylic acid and 2-bromobenzenedicarboxylic acid) or three (benzenedicarboxylic acid, 2-aminobenzenedicarboxylic acid and 2-bromobenzenedicarboxylic acid) organic linkers in the MOF precursor solution (Fig. 8d).134 The resulting UiO-66 materials showed tunable pore surface area. For example, the surface area decreased with increasing equivalents of 2-bromobenzenedicarboxylic acid: 818 m2 g−1 for 0.6; 678 m2 g−1 for 1.3; and 570 m2 g−1 for 2.3.
Finally, SD is also a very simple and fast method to produce MOF-based composites. As above, these MOF-based composites can be created by just mixing other materials – pre-synthesized or their precursors for in situ synthesis – in the MOF precursor solution. With this basic idea, Maspoch et al. demonstrated that different substances such as magnetic inorganic nanoparticles (Fig. 8e),132 inorganic salts (NaCl, CaCl2 and LiCl)132,139 (Fig. 8f) and fluorescent molecules132 can be combined with MOFs, thereby creating different types of composite materials that combine the intrinsic properties of MOFs and these other materials. Finally, the same authors showed that SD method can be also used to combine MOFs with organic polymers.138 In this specific case, pre-synthesized HKUST-1 nanocrystals were encapsulated into polystyrene spheres to improve the hydrolytic stability of HKUST-1.
| Method | MOF | Residence time | Temperature (°C) | STY (kg m−3 day−1) | SA BET (m2 g−1) |
|---|---|---|---|---|---|
| MF | HKUST-1140 | — | RT | — | 620 |
| HKUST-1141 | 1 min | 90 | 5.8 | 1105 | |
| MOF-5141 | 3 min | 120 | — | 3185 | |
| IRMOF-3141 | 3 min | 120 | — | 2428 | |
| UiO-66141 | 15 min | 140 | — | 1509 | |
| MIL-88b137 | 4 min | 95 | — | — | |
| ZIF-8143 | 15 s | RT | 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1770 | |
| Ce-BDC142 | 30 s | 230 | — | — | |
| UiO-66144 | 0.44–2.2 min | 120 | — | 922–1206 | |
| PFR | HKUST-1149 | 1.2 min | 85 | 4533 | 1805 |
| UiO-66149 | 10 min | 130 | 1186 | 672 | |
| NOTT-400149 | 15 min | 85 | 1078 | 741 | |
| Al-Fum150 | 1 min | 65 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 159 |
1054 | |
| HKUST-1148 | 5 min | 60 | — | 1673 | |
| MIL-53(Al)147 | 20 min | 250 | 1021 | 919 | |
| MIL-53(Al)147 | 20 min | 250 | 1300 | 1010 | |
| STA-12(Cd)151 | 5–20 min | 70 | — | 134 | |
| ZIF-8146 | <5 s | 100 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 625 |
1806 | |
| ZIF-8146 | <5 s | 100 | — | 1780 | |
| CAU-13151 | 20 min | 130 | 3049 | 401 | |
| CPO-27145 | <5 s | 300 | 1501 | 1030 | |
| HKUST-1147 | 20 min | 250 | 730 | 1554 | |
| HKUST-1145 | <5 s | 300 | 4399 | 1950 | |
| UiO-66151 | 45 min | 120 | 428 | 1263 | |
| STA-12151 | 20 min | 70 | 428 | 134 | |
| HKUST-1153 | 1 min | 360 W | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1550 | |
| MIL-53(Al)153 | 4 min | 200 W | 3618 | 1376 | |
| UiO-66153 | 7 min | 200 W | 7204 | 1052 | |
| MOF-74(Ni)152 | <1 s | 150 | 2160 | 840 | |
| CSTR | UiO-66154 | 8–40 h | 100–120 | — | 810 |
| MOF-5155 | 5 h | 140 | 1000 | 2302 | |
In 2011, Ameloot and co-workers were first to show that microfluidics could be used for the synthesis of metal–organic materials.140 They synthesized metal–organic crystals in a micro-scale reactor, in which the reagent phases were injected into an immiscible carrier fluid, causing the spontaneous formation of droplets where the reaction occurs (Fig. 9b). In this case, the immiscibility of the water and oil phases was exploited as a template for the controlled formation of hollow metal–organic copper trimesate HKUST-1 microcapsules. The authors described the crystallisation process as a dynamic on-going process of nucleation and crystal growth that resulted in the formation of crystalline MOF membranes with a uniform wall thickness.
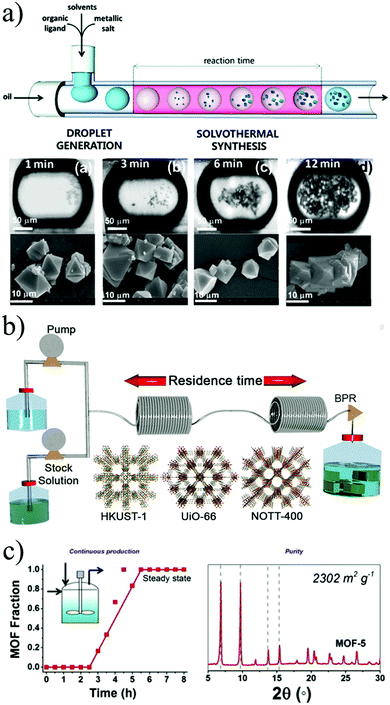 | ||
| Fig. 9 Flow chemistry methods for the production of MOFs. (a) Schematic representation of a continuous flow microfluidic device for producing MOF crystals (top). Optical and SEM images of HKUST-1 crystals obtained via the microfluidic approach at different residences time. (b) Schematic representation showing the continuous flow synthesis of HKUST-1, UiO-66 and NOTT-400 of MOFs. (c) Reaction profile of the solvothermal synthesis of MOF-5 crystals with its corresponding X-ray pattern diffraction and BET surface area value. (© The American Chemical Society, Springer Nature and Elsevier, reprinted with permission from ref. 141, 149 and 155). | ||
Two years later, Faustini et al. reported the solvothermal and hydrothermal synthesis of MOFs and MOF-composite superstructures using oil microdroplets as a reactors.141 Four representative MOF structures, copper trimesate HKUST-1, zinc terephthalate MOF-5, zinc aminoterephthalate IRMOF-3 and zirconium terephthalate UiO-66, were synthesised, yielding substantially faster kinetics in comparison to the conventional batch processes (see Fig. 9a). In addition, they reported the possibility of creating MOF heterostructures using imidazolate frameworks (ZIFs) in a two-step process. Firstly, the iron oxide precursor solution and the oil phase were injected and reacted in a microreactor at 80 °C for 2 minutes. Then, the resulting iron oxide particles were transported downstream to a second microreactor, where they merged and reacted with a mixture of ZIF-8 precursor (zinc nitrate and 2-methylimidazolate in methanol, and polystyrenesulphonate). This lead to the creation of core–shell Fe3O4@ZIF-8 composite superstructures.
The same year, Coronas and co-workers demonstrated the feasibility of the droplet-based microfluidic approach for the crystallisation of the iron fumarate MIL-88B MOF. In this study, they confirmed that the size of the resulting crystals was dependent of the temperature and residence time. They observed a continuous increase in particle size with average sizes increasing from 90 to 900 nm with higher residence times and/or higher temperatures.
In addition, D'Arras and co-workers demonstrated the possibility of synthesizing new structures using microsystems.142 They reported the structure of a new cerium(III)–terephthalate MOF which was synthesized flow-type reactor at high temperature and pressure with a very short residence time.
The latest MR studies were reported by Polyzoidis et al. and Tai et al., where they synthesized ZIF-8 and UiO-66 nanoparticles respectively in a PFA (perfluoroalkoxy) microreactor showing that by varying the residence time and the molar ratio of the reactants. They were able to modify the size and shape of the final crystals from a few nanometres to several micrometres.143,144 These two last examples demonstrate that microfluidic systems are ideal for reaction optimisation and screening experiments within the laboratory. However, in order to synthesize large quantities of MOFs, it is better to use reactors with larger channel dimensions, as these are more suitable for large volumetric throughput.
Moving to the PFR reactors the first work was reported in 2012 by Gimeno-Fabre et al. showed the synthesis of HKUST-1 and Ni-CPO-27 in a counter current mixing reactor where the MOF precursors were mixed with a preheated supercritical water stream at high pressures.145 The high temperatures were used in order to increase the rate of crystal growth, with a limitation in that heating beyond 300 °C could lead to the formation of metal oxides as a waste-product. Three years later, the same reactor was used to demonstrate the large scale production of ZIF-8 and the control of the size and shape of the crystals by adding ammonium hydroxide or trimethylamine in the reaction mixture.146 The STY obtained in this process was 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 625 kg m−3 day−1 and with a surface area of 1800 m2 g−1. Retaining the use of supercritical water and an ethanol stream, Bayliss et al. developed a system to produce MIL-53(Al) and HKUST-1 under continuous flow conditions obtaining a STY of 1300 kg m−3 day−1 and 730 kg m−3 day−1, respectively.147 These last two methods produced high quality materials with high STY, however high temperatures and pressures were still required, which increase the overall cost of the process and could limit the practicality of the technique at industrial scale.
625 kg m−3 day−1 and with a surface area of 1800 m2 g−1. Retaining the use of supercritical water and an ethanol stream, Bayliss et al. developed a system to produce MIL-53(Al) and HKUST-1 under continuous flow conditions obtaining a STY of 1300 kg m−3 day−1 and 730 kg m−3 day−1, respectively.147 These last two methods produced high quality materials with high STY, however high temperatures and pressures were still required, which increase the overall cost of the process and could limit the practicality of the technique at industrial scale.
In 2013 Chang et al. reported the proof-of-concept mesoscale flow production of HKUST-1 using 5 minutes as a residence time and with a surface area of 1673 m2 g−1.148 The particle size of the MOF could be adjusted by changing the relative ratios of the solvents and reaction temperatures from 150 nm to 4 μm. To demonstrate the versatility and efficacy of flow reactors to produce MOFs, Rubio-Martinez and co-workers used a PFA reactor to synthesize the copper trimesate HKUST-1, the zirconium terephthalate UiO-66 and the scandium biphenyl-tetracarboxylate NOTT-400, all with different reaction requirements (see Fig. 9).149 The materials were obtained in 5, 10 and 15 minutes, respectively, without loss in yield or product quality. It was demonstrated that the results could be up-scaled 30-fold using a bench-top reactor, allowing a production rate greater than a kilogram per day and a STY of 4533 kg m−3 day−1 using a bench-top reactor. The successful up-scaling of this process was demonstrated in a second publication where the production of the aluminium fumarate MOF was proved in 4 different stainless-steel tubular flow reactors: a 10 mL coil tubing at laboratory scale, two intermediate stages with 107 mL and 374 mL reactor volume, and a pilot-scale 1.394 L reactor, delivering unprecedented production rates and STYs (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 159 kg m−3 day−1) while maintaining the product quality.150 To our best knowledge, this is the highest reported value of STY for a MOF produced by continuous methods. Additionally, the reactor design used in this work demonstrated the possibility to readily translate reaction parameters from the laboratory scale to pilot scale without any re-optimization of the reaction conditions, while maintaining the STY values within the same range.
159 kg m−3 day−1) while maintaining the product quality.150 To our best knowledge, this is the highest reported value of STY for a MOF produced by continuous methods. Additionally, the reactor design used in this work demonstrated the possibility to readily translate reaction parameters from the laboratory scale to pilot scale without any re-optimization of the reaction conditions, while maintaining the STY values within the same range.
The last work using PFR comes from Stock and co-workers who presented the synthesis of UiO-66, CAU-13 and STA-12 – a new cadmium phosphonate network using a 16 mL PTFE reactor yielding a STY of 428 kg m−3 day−1 and 3049 kg m−3 for UiO-66 and CAU-13, respectively.151 One year later, the same was group reported the water-based synthesis the zirconium fumarate and UiO-66-NH2 starting from a slurry of the starting solutions.
In a slightly different reactor design, two recent works reported the combination of microwave assisted heating with a PFR system. The first study from 2015 by Albuquerque et al. reported on a system where the microwave reactor was attached to the flow reactor in order to accelerate the nucleation of the MOFs and to improve the reproducibility of the synthesis.152 The MOF precursors of MOF-74(Ni) were introduced first into the nucleation zone that consisted of a microwave reactor and consequently the material was introduced into a PFA coil for 8 min to growth the final crystals. As a result, they obtained a better crystallinity in a shorter reaction time and achieved a STY of 2160 kg m−3 day−1. The second work by Taddei et al. presented the synthesis of UiO-66, HKUST-1 and MIL-53(Al) in a 6.2 and 53 mL PTFE flow reactors heated by microwave.153 The materials were obtained in 7, 1 and 4 minutes of residence time, while maintaining the product quality and resulting in high STY of 7204 kg m−3 day−1, 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 kg m−3 day−1 and 3618 kg m−3 day−1 for UiO-66, HKUST-1 and MIL-53(Al) respectively.
800 kg m−3 day−1 and 3618 kg m−3 day−1 for UiO-66, HKUST-1 and MIL-53(Al) respectively.
The use of stirred CSTR was showcased by two groups, synthesizing NH2-UiO-66 and MOF-5. The first work in 2013 by Schoenecker and co-workers who synthesized the amine-functionalised UiO-66 in DMF by convention heating.154 In this system the MOF precursors were pumped into a pre-mixing tank over 15 min and then introduced into a 2 litre flow crystallisation reactor over 8 to 40 hours during which time small aliquots of the intermediate product were collected at different times to bulk reaction kinetics. The product obtained had good crystallinity but the BET surface area and the yield were below the values reported in batch. A later study by McKinstry et al. presented the synthesis of MOF-5 in a CSTR at atmospheric pressure obtaining the desired quality and with a STY of 1000 kg m−3 day−1 (see Fig. 9c).155
3. Downstream processes
After any MOF synthesis, careful processing is required to obtain the final functional material. Directly after the synthesis, the product slurry needs to be washed with the reaction solvent to remove any unreacted and by-products, e.g. using a centrifuge or a Buchner filter. Subsequently, an activation process is required to remove guest molecules, trapped within the framework, to obtain the expected surface area of the structure. Depending on the MOF structure, these two stages can be the most time-limiting stages of the process and become hugely significant in the large-scale production. The last stages of the process consist in drying and shaping the MOFs as well as a heat activation step before testing. Fig. 10 shows a diagram of the typical downstream processes for the synthesis of a MOF.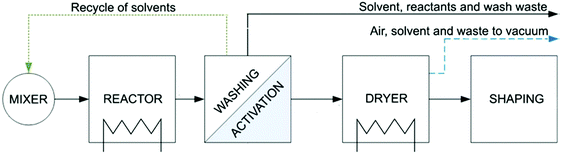 | ||
| Fig. 10 Continuous MOF process. Schematic representation of the different stages of the continuous process for MOFs production: synthesis, washing, activation, drying and shaping. | ||
Despite promising advances in MOF synthesis, there are still challenges remaining related to the downstream processing. On the laboratory scale these processes are well established and sufficient to obtain milligram amounts of quality materials. However, these conventional downstream methods are not well-suited to high production rates. The first stage, the washing and separation of the small crystals from the mother liquid still is a major obstacle for the large scale production MOFs. There are many well established types of equipment for solid–liquid separation such as centrifuges, cyclones, settling chambers, classifiers or filters, in addition to the direct evaporation of the mother liquor. However, the small size of the MOF particles, their low concentration in the solvent, as well as their density approaching that of the solvent (due to the high porosity), makes separation via most conventional methods inefficient or expensive at an industrial scale.156
Once the MOF slurry has been cleaned of the excess linkers and by-products, the activation stage is the next step of the process in order to obtain the highest porosity and BET surface area of the framework. Several strategies exist to remove the unreacted and solvent molecules trapped in the pores of the MOFs without collapsing the framework.157 The most common procedure is a simple heating of the MOF to certain temperature under vacuum. Each MOF has its optimal protocol in order to obtain the highest surface area but generally the temperature should be between the boiling point of the solvent and the decomposition temperature of the structure. However, in most cases this strategy leads to a lower surface area, or to a collapse of the structure due to the high surface tension and capillary forces imposed on the structure by the liquid-gas phase transition of the trapped solvent molecules. An alternative strategy is to exchange the solvent used for the synthesis with one that has a lower-boiling point such as methanol, chloroform or acetone prior to heating the sample under vacuum. This strategy is laborious as generally most MOFs require soaking for a long period of time to ensure that the new solvent infiltrates. For example, in the case of MOF-74 and UiO-66 both MOFs require a soaking in daily refreshed methanol for 3 and 7 days respectively to ensure the complete removal of DMF (solvent used for the synthesis and washing stage) from the pores.36,158 Some frameworks, such as ZIF-8 and MIL-53(Al) require a solvent exchange process with methanol with an additional thermal treatment, 300 °C for 2 hours and 330 °C for 72 hours respectively to obtain the BET surface areas of 1630 m2 g−1 and 1590 m2 g−1, respectively.31,159
An attractive substitute for the solvent exchange method is the use of supercritical CO2. This relatively new strategy consists of exchanging the synthetic solvent for a one that is miscible with liquid CO2 such as ethanol or methanol and then subsequently exchanging this second one for liquid CO2 at high pressure and temperature for several hours. The difference here is that the CO2 supercritical phase eliminates surface tension and capillary forces making this activation method much milder than the conventional and solvent exchange methods. There are several MOFs that have been effectively activated with this strategy. For example, MOF-200 and MOF-210, where a simple solvent exchange followed by pore evacuation under vacuum was not effective, were successful activated, without losing the porosity, by a full solvent exchange with liquid CO2.4 The surface areas obtained were 4530 and 6240 m2 g−1, respectively. Another example is the supercritical CO2 activation of bio-MOF-100 were the DMF solvated samples were soaked in ethanol for 48 hours, and a an exchange/activation with CO2 liquid over a period of 8 hours yielded a BET surface area of 4300 m2 g−1.160 In a variant of this method the sample is placed in a column and the supercritical CO2 flows through the sample instead of using static CO2 exchange. This method was presented by Koh and co-workers who activated UMCM-9 microporous coordination polymers via supercritical CO2 flow activation yielding a surface area BET of 4970 m2 g−1.161
Later novelties on the activation have been based on freeze-drying activation techniques which uses thermal cycling and the vacuum sublimation of solvents (benzene and cyclohexane) at low temperatures to avoid the impact of capillary forces on porous structures in order to control the stability of frameworks and improve its porosity.162 Recently Rubio-Martinez et al. presented for the first time the use of megasonics as an alternative strategy for the simultaneous separation and activation of MOF crystals.163 Its operating principle is based on the application of high frequency ultrasound to the MOF solution, leading to the separation of the solid MOF particles from the solvent. Additionally, the megasonic treatment leads to an activation by the simultaneous removal of occluded reagents from the MOF crystals. This one-step process showed an improvement of up to 47% the surface area of the final product compared to conventional methods. The method removes one stage from the downstream processing and is readily scalable and thus capable of producing commercially usable product at a large scale.
Shaping of MOF powders produced in any of the fabrication methods explained above is also mandatory for using them in real industrial applications. For instance, extruded or compact MOFs in the form of beads, pellets and monolithic bodies are required if MOFs want to be used for gas separation and storage applications. In methane storage (e.g. Adsorbed Natural Gas or ANG), for example, it is of utmost importance to fill the storage tanks with the largest amount of adsorbent; a condition that can only be achieved if MOF powders are densely packed. Furthermore, powdered MOFs are usually more difficult to be handled and can potentially contaminate pipes during charge/discharge cycles. For other applications such as functional textiles,164 alternative shaping and/or integration methods that process MOFs into paper sheets,165 fibers,166 membranes,167,168 foams169 or coatings170 are also needed (Fig. 11).
 | ||
| Fig. 11 Examples of shaped MOFs. (a) Functional textiles; (b) paper sheets; (c) pellets; (d) extruded monolith; (e) fibers; (f) membrane; (g) foams; and (h) granules. (© Nature Publishing group, American Chemical Society, Royal Society of Chemistry, Elsevier with permission from ref. 163–165, 167, 180 and 184). | ||
Considering the potential adsorption-related applications of MOFs, most of the efforts done in shaping MOFs have been dedicated to their densification. The objective of densification is to pack the maximum amount of an active MOF on a certain volume without losing its integrity and adsorption capacity. Dailly et al. calculated that doubling the density of HKUST-1 powder would result in an adsorbent with performances comparable of those of the state-of-the-art carbons at intermediate pressures (30–100 bars).171 However, despite the high industrial importance of shaping MOF powders, the interest of academic research groups to face this problematic is quite recent. In fact, the structuration of MOFs into shaped bodies was initiated by some companies (mainly, BASF), which tend to keep these shaping processes as in-house know-how or are only disseminated in patents. In this context, BASF published the first patent application concerning “shaped bodies containing metal–organic frameworks” in 2002. This patent was centered in the fabrication of MOF-2 and MOF-5 pellets using an eccentric press. In this process, both MOFs were mixed with graphite that acts as a binder improving their mechanical strength.172
Pelletization under pressure is probably the most common method used for densifying MOFs. In this process, a fine powder is pressed at a certain pressure to give pellets that can be crushed or fractionized by sieving. In some cases, before MOF powder is pressed, it can be blended with a binder to improve the cohesion between crystals and their mechanical strength. There are however two factors of this method that tend to affect the final adsorption properties of the pellet shaped MOFs. From one side, the pressure applied can crush the structure of the MOF due to its low mechanical stability. From the other side, the use of binders can dilute the porous powder and/or cause pore blockage, resulting in a reduced performance per unit mass (or volume) of the adsorbent.
As shown in Table 4, the influence of the pressure applied during pelletization of MOF powders was studied in some representative MOFs. However, systematic studies that correlate the pelletization conditions with the resulting adsorption properties of MOFs are very limited, and some discrepancies can be found. A general tendency when compressed pellets are processed is a decrease of the BET surface area and porosity of MOFs. This is specially the case for HKUST-1 that is mechanically fragile. For HKUST-1, it has been described that a significant loss of its BET surface area occurs at moderate pressures. Ahn et al. reported a loss of 50% of its BET surface area when the applied pressure was around 10 MPa,173 whereas Bazer-Bachi et al.174 and Peterson et al.175 found a similar decrease when pressures of 80 MPa and 70 MPa were applied, respectively. Other MOFs have shown better mechanical resistance. For example, UiO-66 and analogues, which are known for their high mechanical and thermal stability owing to their 12-fold connected clusters in the three spatial directions, were shown better stability during the compression process. Here, Peterson et al. observed a loss lower than 10% of the BET surface area of UiO-66 when it was pelletized under a pressure of 70 MPa.175 Dietzel et al. also reported the total conservation of the BET surface area of CPO-27 after tableting it at 100 MPa, whereas its porous character totally disappears at 1 GPa.176 In the case of ZIF-8, the BET surface area was well preserved until a pressure of 700 MPa. Above this pressure, different reports revealed some discrepancies. While Bazer-Bachi et al. observed a loss lower than 20% of its BET surface area,174 Chapman et al. observed a loss of 50% at pressures above 1 GPa.177 This difference can be attributed to non-reported parameters, such as the pressure increase rate and the dwell time, which can dramatically influence the integrity of the final pellet.
| MOF | Pressure | Binder | Property | Ref. |
|---|---|---|---|---|
| Pellet | ||||
| HKUST-1 | — | Alox C and graphite | Very high adsorption capacity for CO2 | 178 |
| 70 and 700 MPa | Without binder |
50% decrease of the BET surface area (after 700 MPa)
Maintained ammonia removal capacity |
175 | |
| 0.24–40 MPa | Cellulose ester, K15M | 76% decrease of the BET surface area (after 40 MPa) | 174 | |
| 3–35 MPa | Without binder | 50% decrease of the BET surface area (after 10 MPa) | 173 | |
| MIL-53(Al) | 1–8 bar | Polyvinyl alcohol |
Below 5 bar, constant selectivity
Above 5 bar, selectivity decreased |
179 |
| UiO-66 | — | Graphite |
22% decrease of the BET surface area
Suitable material for o-xylene over p- and m-xylene separation at low concentrations |
212 |
| 70 and 700 MPa | Without binder |
Maintained BET surface area
16% decrease in octane loadings (after 700 MPa) |
175 | |
| ZIF-8 | 398–1432 MPa | Cellulose ester, K15M |
10% decrease of the BET surface area (after 1432 MPa)
No change in catalytic reactivity |
174 |
| SIM-1 | 40–398 MPa | Cellulose ester, K15M | 28% decrease of the BET surface area (after 398 MPa) | 174 |
| CPO-27-Ni | 0.1–1 GPa | Without binder | Maintained methane storage capacity | 176 |
| MOF | Binder | Property | Ref. |
|---|---|---|---|
| Foam | |||
| MIL-101(Cr) | Ni foam | Decrease in hydrogen storage capacities (19%) | 182 |
| UiO-66 | Polyurethane | Maintaining more than 70% of the adsorption capacity for benzene and n-hexane | 184 |
| UiO-66-NH4, Mg-MOF-74, HKUST-1, ZIF-8 | Carboxymethylcellulose | — | 185 |
| HKUST-1@Fe3O4 | Carboxymethylcellulose | High catalytic activity in C–H oxidation | 185 |
| Extrusion | |||
| HKUST-1 |
Silres MSE 100
Culmial MHPC 20 |
70% decrease of the BET surface area | 180 |
| Zr-MOF | Sucrose and water | 50% decrease of the BET surface area | 182 |
Binders are sometimes utilized during this pelletization process. As state above, they basically serve to improve the cohesion between MOF crystals and their mechanical stability. Some tested binders include graphite,178 polyvinyl alcohol (PVA)179 and cellulose ester.174 To our knowledge, however, there is no a systematic, rational study on the influence of the nature and concentration of these binders on the BET surface area and general properties of MOFs during their pelletization.
The presence of binders is also necessary in other shaping processes, including foaming, extrusion, granulation and cake crushing. In all these procedures, MOF powders are initially dispersed in a solvent/binder mixture. The choice of the binder gives a certain texture and property to the mixture, which is then manipulated in different ways to obtain the desired MOF shapes. For example, in the extrusion process, the MOF solvent/binder mixture forms a paste that can then be extruded to induce shaping of the MOF into different morphologies (Table 4). Following this latter method, Kaskel et al. mixed HKUST-1 crystals with a silicone resin and a plasticizer to form a paste that was subsequently extruded into monolithic HKUST-1 strings in a ram extruder. In this case, the decrease of the BET surface area was significant (70% of its initial BET surface area) due to the presence of binders and heating conditions, but the performance of the extruded monolith was higher than the monolith obtained by in situ synthesis of HKUST-1 in cordierite honeycombs.180 The extrusion method was also used by Ren et al. to prepare UiO-66 spherical pellets (with a BET surface area of 674 m2 g−1 that corresponds to 50% of its initial value) from a paste made of UiO-66 and 10 wt% sucrose/H2O mixture using a granulator.181
Starting from the binder-solvent–MOF mixture, monolithic MOF foams can also be formed (Table 4). In this case, the nature of the binder tends to form macroporous foam-type solids in which the MOF can be entrapped. Similar MOF foams can also be produced by synthesizing the MOF in the presence of pre-formed foam. Using both approaches, foams with MIL-101,182 HKUST-1183 and UiO-66184 were prepared. For example, Wang et al. synthesized a foam monolith composed of HKUST-1@Fe3O4-MF (MF means magnetic fluid) by dispersing HKUST-1@Fe3O4-MF particles in an aqueous carboxymethylcellulose solution. The treatment with acetonitrile and finally drying led to the formation of monolithic foams with high catalytic activity for C–H oxidation.185
Other shaping processes require the formation of a dried MOF cake that is crushed. Here, the MOF powder is mixed with a certain amount of a binder (typically, polyvinyl alcohol (PVA)) and the resulting mixture is dissolved in a solvent forming a paste. This paste is then dried, crushed and sieved into the wanted particle size fraction. Denayer et al. used this method to prepare MIL-53(Al) pellets using PVA as the binder. As revealed by N2 adsorption isotherms, the MIL-53(Al) pellets showed a loss of BET surface area of 32% but maintained good CH4/CO2 selectivity capacities.179 Finally, other methodologies have also started to be explored to incorporate MOFs into fibers186 and papers, and shape them into alginate-based spherical beads187 or ceramic beads.188
These few examples, indicate that the shaping of MOFs for specific applications is still in an embryonic stage and strong efforts have still to be dedicated to the rational study of this process if we want to be able to access to the real commercial applications. It is also clear that, in adsorption-related applications, this shaping process must respect the relatively low thermal, chemical and mechanical stability of MOFs so that their adsorption capacities are mostly preserved. For other applications, however, this latter condition is not so important. In catalysis, for example, Bazer-Bachi et al. observed that, even though pelletization of ZIF-8 decreased its adsorption properties, it did not change its catalytic activity.174
4. Perspectives and commercial developments
Since the first patent filed in 1995 and assigned to the Nalco Chemical Company, commercialisation of MOFs progressed gradually until the first MOF-based products released in 2016 by MOF Technologies and Numat Technologies (see Fig. 12).189,190 MOF production at scale is now underway which will help secure customer confidence and open the door for other MOF-based products. However, the growing market will push the MOF suppliers for further cost-efficiency, reproducibility and environmental sustainability to remain competitive. Here is a brief summary of companies working with MOFs in production, technology development and retail to date. | ||
| Fig. 12 Commercially available MOF-based products released in 2016. (a) NT-7815 micro-adsorbent for extending the storage life and quality of many fruits and vegetables by Decco Post-Harvest and MOF Technologies and (b) ION-X gas storage tank for storing speciality gases used in the electronics manufacturing industry by Numat Technologies. (© The Springer Nature reprinted with permission from ref. 191). | ||
MOF Technologies was founded in 2012 based on patented mechanochemical manufacturing technology invented at the Queen's University of Belfast.47 This innovative process allows the production of MOFs using little or no solvents. Solvent-free synthesis has advantages in both waste and energy management. Solvent waste is a major issue in the chemical industry. The energy required to initiate reaction can sometimes be reduced using mechanical energy rather than thermal energy. Recently this method has been configured for continuous production through extrusion which is scalable.
It is unknown how many MOFs can be manufactured using mechanochemical synthesis, however MOF Technologies offer a wide catalogue for direct purchase. At the end of 2016 they sold around 100 kg of MOFs from their catalogue: magnesium formate, Cu-BTC (HKUST-1), ZIF-8, Al(OH) fumarate, ZIF-67, Mg-MOF-74 and Zn-SIFSIX-pyrazine. See Table 5 for a summary of MOFs for sale from each manufacturer.
| MOF | Manufacturer |
|---|---|
| Al(OH) fumarate | MOF Apps |
| MOF Technologies | |
| CAU-10 | ProfMOF |
| Cu-BTC | BASF |
| MOF Apps | |
| MOF Technologies | |
| Fe-BTC | BASF |
| Magnesium formate | BASF |
| MOF Technologies | |
| Mg-MOF-74 | MOF Technologies |
| MIL-100 | KRICT |
| MOF Apps | |
| MIL-101-NH2 | MOF Apps |
| MIL-53 | BASF |
| MIL-68 | MOF Apps |
| MOF-177 | BASF |
| PCN-250(Fe) | Framergy |
| UiO-66 series | Inven2 |
| MOF Apps | |
| ProfMOF | |
| ZIF-67 | MOF Apps |
| MOF Technologies | |
| ZIF-8 | BASF |
| MOF Apps | |
| MOF Technologies | |
| STREM Chemicals Inc. | |
| Zn-SIFSIX-pyrazine | MOF Technologies |
MOF Technologies were the first to announce a MOF-based commercial product available through fruit and vegetable supplier Decco Worldwide Post-Harvest Holdings. The product has been registered with the U.S. Environmental Protection Agency under the proprietary name NT-7815 (EPA reference: 2792-79). NT-7815 is described as a micro-adsorbent delivering 0.7 wt% of 1-MCP, which is a gas that blocks postharvest ethylene responses, extending the storage life and quality of many fruits and vegetables. MOF Technologies has not released any details regarding the MOF incorporated within the product. With the announcement of the new product, MOF Technologies have expanded their production facility capable of producing 15 kg h−1 in preparation for full scale between 5 and 10 tonnes per year from 2018 depending on which MOF.191
NuMat Technologies established in 2013, have also released a MOF-based product called ION-X based on a proprietary set of MOFs for storing gases such as arsine, phosphine and boron trifluoride for the electronics industry.192 The company is setting up a facility in Asia that will receive MOF-filled tanks from the United States. NuMat has a partnership with one of the top gas companies in Asia who will fill the tanks with gas which will then be distributed to customers. Asia which contains most of the major manufacturers of electronics in the world and therefore this position will likely offer direct access to this market which is 70% of the total demand. NuMat will lead the initial production of the proprietary set of MOFs for ION-X and have explored multiple manufacturing methods including flow, mechanochemical, solvothermal and others.
MOF Apps, founded in 2013, are the exclusive licensee for UiO-66 and the zirconium-based family of MOFs. With a focus on MOF Application Services, the company aims to bring research and industry together to identity and develop commercially viable application opportunities in the areas of gas storage, industrial cooling, toxic gas protection and healthcare. MOF Apps develops and offers integrated solutions using MOFs which are cost-competitive and which outperform state-of-the art systems. MOF Apps have sold the most amount of MOF to a leading vehicle manufacturer in August 2015 to test as adsorbed natural gas fuel platform.193
ProDia is a Horizon 2020 project funded through the European commission. It is a consortium of over 15 parties focused on the development of reliable production methods of nanoporous materials and their applications. Pilot-scale production of up to 100 kg will be led by Johnson Matthey for water-based synthesis, MOF Technologies for mechanosynthesis and Axel One for spray-drying synthesis.194
ProfMOF founded in 2015 by a group of scientists at the University of Olso, Inven2 and Kongsberg, focused on the commercialization of the MOF-material. ProfMOF prefer water-based and continuous flow production of MOFs. Prof Norbert Stock, inventor of CAU series and advisor for ProfMOF, has developed the water-based synthesis method for some of the zirconium MOFs and CAUs series.43,195,196 The ProfMOF catalogue includes: CAU-10, UiO-66, UiO-66-ADC, UiO-66-FA, UiO-66-BDC, UiO-66-BDC-NH2, UiO-66-BDC-COOH and UiO-66-BPDC/UiO-67.197
STREM Chemicals Inc. has become a distributor of MOFs manufactured in agreement with various MOF companies including KRICT, Inven2 and Framergy. Their catalogue includes: (CuI)4(DABCO)2, (CuI)4(C6H14N2)2, C6H12N4(CuCN)5, PCN-250(Fe) CONEKTIC™ F250 by Framergy, MIL-100(Fe) KRICT F100 by KRICT, ZIF-8 and UiO-66 by Inven2.198
Sigma-Aldrich is a distributor of MOFs supplied by BASF under the product names Basolite® and Basosiv™. Fig. 13 shows the total number of academic publications that reference these products. The actual number of sales or quantities is unavailable and therefore these numbers represent the minimum. According to this data, Sigma-Aldrich has made at least a total of 1198 sales for research purposes. Their catalogue includes: Cu-BTC Basolite® C300 by BASF, MIL-53 Basolite® A100 by BASF, Fe-BTC Basolite® F300 by BASF, ZIF-8 Basolite® Z1200 by BASF, MOF-177 Basolite® Z377 by BASF, Mg-Formate Basosiv™ M050 by BASF and Al-fumarate Basolite® A520 by BASF (no longer available).199
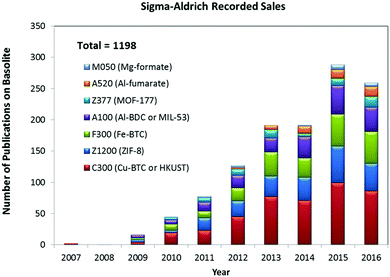 | ||
| Fig. 13 Number of publications referring to the Basolite® and Basosiv™ products supplied by Sigma-Aldrich. | ||
Pseudo-startup MOFWORX from CSIRO Australia is commercializing MOFs based on patented flowchem manufacturing technology together with a diverse material and application-based portfolio. The group have built a reactor called Mindi (the aboriginal name for a mythological serpent that spits out white powder) that is capable of 10 kg h−1 production. The company aims to become a product development house for MOF-based technologies supported by their own manufacturing capability.200
Other companies are working towards the commercialization of MOFs such as the MOF company, MOFGen, Framergy, ACSYNAM and Promethean particles.201–204 MOFGen are developing nanoporous materials for materials for a number of applications including medical devices, wound-healing and consumer healthcare. Framergy own the license for PCN-250 which can be used for natural gas capture and storage, and currently sold through STREM Chemical Inc. Promethean particles have commissioned a continuous flow reactor based on super critical water capable of producing 1000 tonnes per year. The company has focused on nanoparticle production for inks electronics industry but are capable of shifting to MOF production if the market becomes more attractive.
5. Conclusions and future priorities
The last two decades have seen great progress in the field of metal organic frameworks both in the discovery of new structures and the development of new functional properties of these nanomaterials. However, a crucial pre-requisite for accessing this potentiality in real world applications is the ability to routinely synthesize these materials in large quantities with high efficiency. It is only within the last five years that interest has arisen in the scientific community to develop novel synthetic methods and explore the scale up the synthesis of MOFs, focusing on economically viable strategies that do not rely on expensive or rare raw materials and also consider safety and environmental issues.In this review, the advances in several synthetic methodologies were discussed. These new synthetic methods allow an unprecedented level of control over the reaction conditions, which in turn lead to a better control over particle size morphology, and reproducibility between batches. However, there are still a few issues remaining before MOF production reaches the level of a mature commercial technology. The solvent free approach and water-based synthesis are the most likely strategies to succeed in becoming economically and environmentally feasible for large ton scale production. Aside from the development of synthesis routes, another critical area is downstream processing, as the conventional downstream methods used at the laboratory scale are not well-suited to high production rates. This means that large scale application of MOFs will be limited by their commercial availability and thus most likely also the sustainability of the synthesis procedure until these methods are further developed. This future research, which will involve researchers of many different fields, will certainly introduce metal–organic materials up to their use for real practical applications.
Efforts in MOF commercialization have lead to the creation of several spin off companies, and the two new MOF markets recently released open a new and exciting time.
Acknowledgements
M. R. M., M. R. H. and A. T. acknowledge the financial support from CSIRO Mineral Resources and Manufacturing business units. M. R. H. acknowledges FT13000345 for funding support. C. A., I. I. and D. M. acknowledge the financial support from MINECO-Spain through projects PN MAT2012-30994, 2014-SGR-80, EU FP7 ERC-Co 615954, and European Union's Horizon 2020 research and innovation programme under grant agreement no. 685727.References
- B. F. Hoskins and R. Robson, Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments, J. Am. Chem. Soc., 1989, 111, 5962–5964 CrossRef CAS.
- B. F. Hoskins and R. Robson, Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the zinc cyanide and cadmium cyanide structures and the synthesis and structure of the diamond-related frameworks [N(CH3)4][CuIZnII(CN)4] and CuI[4,4′,4′′,4′′′-tetracyanotetraphenylmethane]BF4·xC6H5NO2, J. Am. Chem. Soc., 1990, 112, 1546–1554 CrossRef CAS.
- O. M. Yaghi and H. Li, Hydrothermal Synthesis of a Metal–Organic Framework Containing Large Rectangular Channels, J. Am. Chem. Soc., 1995, 117, 10401–10402 CrossRef CAS.
- H. Furukawa, et al., Ultrahigh Porosity in Metal–Organic Frameworks, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- M. Kondo, T. Yoshitomi, H. Matsuzaka, S. Kitagawa and K. Seki, Three-Dimensional Framework with Channeling Cavities for Small Molecules: {[M2(4,4′-bpy)3(NO3)4]·xH2O}n (M = Co, Ni, Zn), Angew. Chem., Int. Ed. Engl., 1997, 36, 1725–1727 CrossRef CAS.
- S. Kitagawa, R. Kitaura and S. Noro, Functional Porous Coordination Polymers, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- G. Férey, et al., A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- C. Serre, et al., Very Large Breathing Effect in the First Nanoporous Chromium(III)-Based Solids: MIL-53 or CrIII(OH)·{O2C−C6H4CO2}·{HO2C−C6H4−CO2H2}, J. Am. Chem. Soc., 2002, 124, 13519–13526 CrossRef CAS PubMed.
- J. R. Long and O. M. Yaghi, The pervasive chemistry of metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1213–1214 RSC.
- T. M. McDonald, et al., Cooperative insertion of CO2 in diamine-appended metal–organic frameworks, Nature, 2015, 519, 303–308 CrossRef CAS PubMed.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Selective gas adsorption and separation in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1477 RSC.
- L. E. Kreno, et al., Metal–Organic Framework Materials as Chemical Sensors, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- F.-Y. Yi, D. Chen, M.-K. Wu, L. Han and H.-L. Jiang, Chemical Sensors Based on Metal–Organic Frameworks, ChemPlusChem, 2016, 81, 675–690 CrossRef CAS.
- Y. Liu, Z. U. Wang and H.-C. Zhou, Recent advances in carbon dioxide capture with metal–organic frameworks, Greenhouse Gases: Sci. Technol., 2012, 2, 239–259 CrossRef CAS.
- S. R. Miller, et al., Biodegradable therapeutic MOFs for the delivery of bioactive molecules, Chem. Commun., 2010, 46, 4526–4528 RSC.
- L. J. Murray, M. Dincă and J. R. Long, Hydrogen storage in metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1294–1314 RSC.
- J. A. Mason, M. Veenstra and J. R. Long, Evaluating metal–organic frameworks for natural gas storage, Chem. Sci., 2014, 5, 32–51 RSC.
- J.-R. Li, J. Sculley and H.-C. Zhou, Metal–Organic Frameworks for Separations, Chem. Rev., 2012, 112, 869–932 CrossRef CAS PubMed.
- B. V. Voorde, B. de Bueken, J. Denayer and D. D. Vos, Adsorptive separation on metal–organic frameworks in the liquid phase, Chem. Soc. Rev., 2014, 43, 5766–5788 RSC.
- Y. He, R. Krishna and B. Chen, Metal–organic frameworks with potential for energy-efficient adsorptive separation of light hydrocarbons, Energy Environ. Sci., 2012, 5, 9107–9120 CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Carbon Dioxide Capture: Prospects for New Materials, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- K. Sumida, et al., Carbon Dioxide Capture in Metal–Organic Frameworks, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed.
- P. Horcajada, et al., Metal–Organic Frameworks in Biomedicine, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- P. Horcajada, et al., Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging, Nat. Mater., 2009, 9, 172–178 CrossRef PubMed.
- K. Konstas, et al., Methane storage in metal organic frameworks, J. Mater. Chem., 2012, 22, 16698–16708 RSC.
- S. Anderson and R. Newell, Prospects for Carbon Capture and Storage Technologies, Annu. Rev. Environ. Resour., 2004, 29, 109–142 CrossRef.
- M. E. Boot-Handford, et al., Carbon capture and storage update, Energy Environ. Sci., 2013, 7, 130–189 Search PubMed.
- O. M. Yaghi, Novel crystalline metal–organic microporous materials, 1997 Search PubMed.
- A. Rabenau, The Role of Hydrothermal Synthesis in Preparative Chemistry, Angew. Chem., Int. Ed. Engl., 1985, 24, 1026–1040 CrossRef.
- A. T. Çolak, G. Pamuk, O. Z. Yeşilel and F. Yüksel, Hydrothermal synthesis and structural characterization of Zn(II)- and Cd(II)-pyridine-2,3-dicarboxylate 2D coordination polymers, {(NH4)2[M(μ-pydc)2]·2H2O}n, Solid State Sci., 2011, 13, 2100–2104 CrossRef.
- T. Loiseau, et al., A Rationale for the Large Breathing of the Porous Aluminum Terephthalate (MIL-53) Upon Hydration, Chem. – Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- H. Deng, et al., Large-Pore Apertures in a Series of Metal–Organic Frameworks, Science, 2012, 336, 1018–1023 CrossRef CAS PubMed.
- T. M. McDonald, et al., Capture of Carbon Dioxide from Air and Flue Gas in the Alkylamine-Appended Metal–Organic Framework mmen-Mg2(dobpdc), J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed.
- J. H. Cavka, et al., A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability, J. Am. Chem. Soc., 2008, 130, 13850–13851 CrossRef PubMed.
- L. Valenzano, et al., Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory, Chem. Mater., 2011, 23, 1700–1718 CrossRef CAS.
- M. J. Katz, et al., A facile synthesis of UiO-66, UiO-67 and their derivatives, Chem. Commun., 2013, 49, 9449 RSC.
- D. Feng, et al., Zirconium-Metalloporphyrin PCN-222: Mesoporous Metal–Organic Frameworks with Ultrahigh Stability as Biomimetic Catalysts, Angew. Chem., Int. Ed., 2012, 51, 10307–10310 CrossRef CAS PubMed.
- D. Feng, et al., Kinetically tuned dimensional augmentation as a versatile synthetic route towards robust metal–organic frameworks, Nat. Commun., 2014, 5, 5723 CrossRef CAS PubMed.
- M. Gaab, N. Trukhan, S. Maurer, R. Gummaraju and U. Müller, The progression of Al-based metal–organic frameworks – From academic research to industrial production and applications, Microporous Mesoporous Mater., 2012, 157, 131–136 CrossRef CAS.
- D. Farrusseng, S. Aguado and C. Pinel, Metal–Organic Frameworks: Opportunities for Catalysis, Angew. Chem., Int. Ed., 2009, 48, 7502–7513 CrossRef CAS PubMed.
- M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Luminescent metal–organic frameworks, Chem. Soc. Rev., 2009, 38, 1330 RSC.
- N. Stock and S. Biswas, Synthesis of Metal–Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites, Chem. Rev., 2012, 112, 933–969 CrossRef CAS PubMed.
- H. Reinsch, S. Waitschat, S. M. Chavan, K. P. Lillerud and N. Stock, A Facile ‘Green’ Route for Scalable Batch Production and Continuous Synthesis of Zirconium MOFs, Eur. J. Inorg. Chem., 2016, 4490–4498 CrossRef CAS.
- Y. Sun and H.-C. Zhou, Recent progress in the synthesis of metal–organic frameworks, Sci. Technol. Adv. Mater., 2015, 16, 054202 CrossRef PubMed.
- U. Mueller, et al., Method for Electrochemical Production of a Crystalline Porous Metal Organic Skeleton Material, 2006 Search PubMed.
- J. S. Chang, S. H. Jhung and J. S. Hwang, A Synthesis Method of Porous Organic-Inorganic Hybrid Materials, 2006 Search PubMed.
- S. L. James, A. Lazuen-Garay and A. Pichon, Use of grinding in chemical synthesis, 2006 Search PubMed.
- D. Maspoch, et al., Method for the preparation of metal organic frameworks, 2016 Search PubMed.
- M. Rubio-Martinez, et al., Production of metal–organic frameworks, 2016 Search PubMed.
- U. Mueller, et al., Metal–organic frameworks—prospective industrial applications, J. Mater. Chem., 2006, 16, 626–636 RSC.
- M. Taddei, D. A. Steitz, J. A. van Bokhoven and M. Ranocchiari, Continuous-Flow Microwave Synthesis of Metal–Organic Frameworks: A Highly Efficient Method for Large-Scale Production, Chem. – Eur. J., 2016, 22, 3245–3249 CrossRef CAS PubMed.
- W.-J. Li, M. Tu, R. Cao and R. A. Fischer, Metal–organic framework thin films: electrochemical fabrication techniques and corresponding applications & perspectives, J. Mater. Chem. A, 2016, 4, 12356–12369 CAS.
- H. Al-Kutubi, J. Gascon, E. J. R. Sudhölter and L. Rassaei, Electrosynthesis of Metal–Organic Frameworks: Challenges and Opportunities, ChemElectroChem, 2015, 2, 462–474 CrossRef CAS.
- M. Dincă and M. Li, Methods for electrochemically induced cathodic deposition of crystalline metal–organic frameworks, 2014 Search PubMed.
- N. Campagnol, et al., On the electrochemical deposition of metal–organic frameworks, J. Mater. Chem. A, 2016, 4, 3914–3925 CAS.
- P. Schäfer, M. A. van der Veen and K. F. Domke, Unraveling a two-step oxidation mechanism in electrochemical Cu-MOF synthesis, Chem. Commun., 2016, 52, 4722–4725 RSC.
- R. Ameloot, et al., Patterned Growth of Metal–Organic Framework Coatings by Electrochemical Synthesis, Chem. Mater., 2009, 21, 2580–2582 CrossRef CAS.
- A. Martinez Joaristi, J. Juan-Alcañiz, P. Serra-Crespo, F. Kapteijn and J. Gascon, Electrochemical Synthesis of Some Archetypical Zn2+, Cu2+, and Al3+ Metal Organic Frameworks, Cryst. Growth Des., 2012, 12, 3489–3498 CAS.
- T. R. C. Van Assche, et al., Electrochemical synthesis of thin HKUST-1 layers on copper mesh, Microporous Mesoporous Mater., 2012, 158, 209–213 CrossRef CAS.
- J. Gascon, S. Aguado and F. Kapteijn, Manufacture of dense coatings of Cu3(BTC)2 (HKUST-1) on α-alumina, Microporous Mesoporous Mater., 2008, 113, 132–138 CrossRef CAS.
- T. R. C. Van Assche, et al., On controlling the anodic electrochemical film deposition of HKUST-1 metal–organic frameworks, Microporous Mesoporous Mater., 2016, 224, 302–310 CrossRef CAS.
- T. R. C. Van Assche, et al., Electrochemical synthesis of thin HKUST-1 layers on copper mesh, Microporous Mesoporous Mater., 2012, 158, 209–213 CrossRef CAS.
- M. Hartmann, et al., Adsorptive Separation of Isobutene and Isobutane on Cu3(BTC)2, Langmuir, 2008, 24, 8634–8642 CrossRef CAS PubMed.
- N. Campagnol, et al., High pressure, high temperature electrochemical synthesis of metal–organic frameworks: films of MIL-100 (Fe) and HKUST-1 in different morphologies, J. Mater. Chem. A, 2013, 1, 5827 CAS.
- S. D. Worrall, et al., Electrochemical deposition of zeolitic imidazolate framework electrode coatings for supercapacitor electrodes, Electrochim. Acta, 2016, 197, 228–240 CrossRef CAS.
- N. Campagnol, E. R. Souza, D. E. De Vos, K. Binnemans and J. Fransaer, Luminescent terbium-containing metal–organic framework films: new approaches for the electrochemical synthesis and application as detectors for explosives, Chem. Commun., 2014, 50, 12545–12547 RSC.
- W.-J. Li, J. Lü, S.-Y. Gao, Q.-H. Li and R. Cao, Electrochemical preparation of metal–organic framework films for fast detection of nitro explosives, J. Mater. Chem. A, 2014, 2, 19473–19478 CAS.
- H. M. Yang, et al., Electrochemical synthesis of flower shaped morphology MOFs in an ionic liquid system and their electrocatalytic application to the hydrogen evolution reaction, RSC Adv., 2014, 4, 15720–15726 RSC.
- H. Yang, et al., In situ electrochemical synthesis of MOF-5 and its application in improving photocatalytic activity of BiOBr, Trans. Nonferrous Met. Soc. China, 2015, 25, 3987–3994 CrossRef CAS.
- M. Li and M. Dincă, Reductive Electrosynthesis of Crystalline Metal–Organic Frameworks, J. Am. Chem. Soc., 2011, 133, 12926–12929 CrossRef CAS PubMed.
- M. Li and M. Dincă, On the Mechanism of MOF-5 Formation under Cathodic Bias, Chem. Mater., 2015, 27, 3203–3206 CrossRef CAS.
- M. Li and M. Dincă, Selective formation of biphasic thin films of metal–organic frameworks by potential-controlled cathodic electrodeposition, Chem. Sci., 2014, 5, 107–111 RSC.
- H. Zhu, H. Liu, I. Zhitomirsky and S. Zhu, Preparation of metal–organic framework films by electrophoretic deposition method, Mater. Lett., 2015, 142, 19–22 CrossRef CAS.
- I. Hod, et al., Directed Growth of Electroactive Metal–Organic Framework Thin Films Using Electrophoretic Deposition, Adv. Mater., 2014, 26, 6295–6300 CrossRef CAS PubMed.
- R. Ameloot, et al., Patterned film growth of metal–organic frameworks based on galvanic displacement, Chem. Commun., 2010, 46, 3735 RSC.
- I. Stassen, et al., Electrochemical Film Deposition of the Zirconium Metal–Organic Framework UiO-66 and Application in a Miniaturized Sorbent Trap, Chem. Mater., 2015, 27, 1801–1807 CrossRef CAS.
- S. Yadnum, et al., Site-Selective Synthesis of Janus-type Metal–Organic Framework Composites, Angew. Chem., 2014, 126, 4082–4086 CrossRef.
- I. Bilecka and M. Niederberger, Microwave chemistry for inorganic nanomaterials synthesis, Nanoscale, 2010, 2, 1358–1374 RSC.
- M. Gharibeh, G. A. Tompsett, K. S. Yngvesson and W. C. Conner, Microwave synthesis of zeolites: effect of power delivery, J. Phys. Chem. B, 2009, 113, 8930–8940 CrossRef CAS PubMed.
- N. A. Khan and S. H. Jhung, Synthesis of metal–organic frameworks (MOFs) with microwave or ultrasound: Rapid reaction, phase-selectivity, and size reduction, Coord. Chem. Rev., 2015, 285, 11–23 CrossRef CAS.
- A. Hoz, Á. de la Díaz-Ortiz and A. Moreno, Microwaves in organic synthesis. Thermal and non-thermal microwave effects, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- S.-H. Jhung, J.-H. Lee and J.-S. Chang, Microwave Synthesis of a Nanoporous Hybrid Material, Chromium Trimesate, Bull. Korean Chem. Soc., 2005, 26, 880–881 CrossRef CAS.
- S. H. Jhung, et al., Microwave Synthesis of Chromium Terephthalate MIL-101 and Its Benzene Sorption Ability, Adv. Mater., 2007, 19, 121–124 CrossRef CAS.
- Z. Ni and R. I. Masel, Rapid Production of Metal–Organic Frameworks via Microwave-Assisted Solvothermal Synthesis, J. Am. Chem. Soc., 2006, 128, 12394–12395 CrossRef CAS PubMed.
- J.-S. Choi, W.-J. Son, J. Kim and W.-S. Ahn, Metal–organic framework MOF-5 prepared by microwave heating: Factors to be considered, Microporous Mesoporous Mater., 2008, 116, 727–731 CrossRef CAS.
- K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal−Organic Frameworks for Imaging and Drug Delivery, J. Am. Chem. Soc., 2009, 131, 14261–14263 CrossRef CAS PubMed.
- N. A. Khan and S.-H. Jhung, Facile Syntheses of Metal–organic Framework Cu(BTC)2(H2O)3 under Ultrasound, Bull. Korean Chem. Soc., 2009, 30, 2921–2926 CrossRef CAS.
- D.-W. Jung, D.-A. Yang, J. Kim, J. Kim and W.-S. Ahn, Facile synthesis of MOF-177 by a sonochemical method using 1-methyl-2-pyrrolidinone as a solvent, Dalton Trans., 2010, 39, 2883–2887 RSC.
- N. A. Khan, I. J. Kang, H. Y. Seok and S. H. Jhung, Facile synthesis of nano-sized metal–organic frameworks, chromium-benzenedicarboxylate, MIL-101, Chem. Eng. J., 2011, 166, 1152–1157 CrossRef CAS.
- E. Haque and S. H. Jhung, Synthesis of isostructural metal–organic frameworks, CPO-27s, with ultrasound, microwave, and conventional heating: Effect of synthesis methods and metal ions, Chem. Eng. J., 2011, 173, 866–872 CrossRef CAS.
- E. Haque, N. A. Khan, J. H. Park and S. H. Jhung, Synthesis of a Metal–Organic Framework Material, Iron Terephthalate, by Ultrasound, Microwave, and Conventional Electric Heating: A Kinetic Study, Chem. – Eur. J., 2010, 16, 1046–1052 CrossRef CAS PubMed.
- T. Chalati, P. Horcajada, R. Gref, P. Couvreur and C. Serre, Optimisation of the synthesis of MOF nanoparticles made of flexible porous iron fumarate MIL-88A, J. Mater. Chem., 2011, 21, 2220–2227 RSC.
- J.-H. Park, S.-H. Park and S.-H. Jhung, Microwave-Syntheses of Zeolitic Imidazolate Framework Material, ZIF-8, J. Korean Chem. Soc., 2009, 53, 553–559 CrossRef CAS.
- P. Silva, A. A. Valente, J. Rocha and F. A. Almeida Paz, Fast Microwave Synthesis of a Microporous Lanthanide−Organic Framework, Cryst. Growth Des., 2010, 10, 2025–2028 CAS.
- S. M. F. Vilela, et al., Multifunctional micro- and nanosized metal–organic frameworks assembled from bisphosphonates and lanthanides, J. Mater. Chem. C, 2014, 2, 3311–3327 RSC.
- P. P. Bag, X.-S. Wang and R. Cao, Microwave-assisted large scale synthesis of lanthanide metal–organic frameworks (Ln-MOFs), having a preferred conformation and photoluminescence properties, Dalton Trans., 2015, 44, 11954–11962 RSC.
- W. Liang and D. M. D'Alessandro, Microwave-assisted solvothermal synthesis of zirconium oxide based metal–organic frameworks, Chem. Commun., 2013, 49, 3706–3708 RSC.
- M. Taddei, et al., Efficient microwave assisted synthesis of metal–organic framework UiO-66: optimization and scale up, Dalton Trans., 2015, 44, 14019–14026 RSC.
- W. Liang, et al., Defect engineering of UiO-66 for CO2 and H2O uptake – a combined experimental and simulation study, Dalton Trans., 2016, 45, 4496–4500 RSC.
- A. L. Garay, A. Pichon and S. L. James, Solvent-free synthesis of metal complexes, Chem. Soc. Rev., 2007, 36, 846–855 RSC.
- G. Kaupp, Solid-state molecular syntheses: complete reactions without auxiliaries based on the new solid-state mechanism, CrystEngComm, 2003, 5, 117–133 RSC.
- T. Friščić, Supramolecular concepts and new techniques in mechanochemistry: cocrystals, cages, rotaxanes, open metal–organic frameworks, Chem. Soc. Rev., 2012, 41, 3493–3510 RSC.
- P. Balaz, Mechanochemistry in Nanoscience and Minerals Engineering, Springer, 2008 Search PubMed.
- V. V. Boldyrev, Mechanochemistry and mechanical activation of solids, Solid State Ionics, 1993, 63, 537–543 CrossRef.
- A. Stolle, T. Szuppa, S. E. S. Leonhardt and B. Ondruschka, Ball milling in organic synthesis: solutions and challenges, Chem. Soc. Rev., 2011, 40, 2317–2329 RSC.
- D. E. Crawford and J. Casaban, Recent Developments in Mechanochemical Materials Synthesis by Extrusion, Adv. Mater., 2016, 28, 5747–5754 CrossRef CAS PubMed.
- S. L. James, et al., Mechanochemistry: opportunities for new and cleaner synthesis, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- D. Braga and F. Grepioni, Making crystals from crystals: a green route to crystal engineering and polymorphism, Chem. Commun., 2005, 3635–3645, 10.1039/B504668H.
- T. Friščić, New opportunities for materials synthesis using mechanochemistry, J. Mater. Chem., 2010, 20, 7599–7605 RSC.
- A. Pichon, A. Lazuen-Garay and S. L. James, Solvent-free synthesis of a microporous metal–organic framework, CrystEngComm, 2006, 8, 211–214 RSC.
- A. Pichon and S. L. James, An array-based study of reactivity under solvent-free mechanochemical conditions—insightsand trends, CrystEngComm, 2008, 10, 1839–1847 RSC.
- S. Tanaka, K. Kida, T. Nagaoka, T. Ota and Y. Miyake, Mechanochemical dry conversion of zinc oxide to zeolitic imidazolate framework, Chem. Commun., 2013, 49, 7884–7886 RSC.
- N. K. Singh, M. Hardi and V. P. Balema, Mechanochemical synthesis of an yttrium based metal–organic framework, Chem. Commun., 2013, 49, 972–974 RSC.
- K. Leng, Y. Sun, X. Li, S. Sun and W. Xu, Rapid Synthesis of Metal–Organic Frameworks MIL-101(Cr) Without the Addition of Solvent and Hydrofluoric Acid, Cryst. Growth Des., 2016, 16, 1168–1171 CAS.
- D. Braga, et al., Simple and Quantitative Mechanochemical Preparation of a Porous Crystalline Material Based on a 1D Coordination Network for Uptake of Small Molecules, Angew. Chem., Int. Ed., 2006, 45, 142–146 CrossRef CAS PubMed.
- T. Friščić and L. Fábián, Mechanochemical conversion of a metal oxide into coordination polymers and porous frameworks using liquid-assisted grinding (LAG), CrystEngComm, 2009, 11, 743–745 RSC.
- M. Klimakow, P. Klobes, A. F. Thünemann, K. Rademann and F. Emmerling, Mechanochemical Synthesis of Metal−Organic Frameworks: A Fast and Facile Approach toward Quantitative Yields and High Specific Surface Areas, Chem. Mater., 2010, 22, 5216–5221 CrossRef CAS.
- W. Yuan, et al., Study of the mechanochemical formation and resulting properties of an archetypal MOF: Cu3(BTC)2 (BTC = 1,3,5-benzenetricarboxylate), CrystEngComm, 2010, 12, 4063–4065 RSC.
- K. Fujii, et al., Direct structure elucidation by powder X-ray diffraction of a metal–organic framework material prepared by solvent-free grinding, Chem. Commun., 2010, 46, 7572–7574 RSC.
- D. Prochowicz, et al., A mechanochemical strategy for IRMOF assembly based on pre-designed oxo-zinc precursors, Chem. Commun., 2015, 51, 4032–4035 RSC.
- K. Užarević, et al., Mechanochemical and solvent-free assembly of zirconium-based metal–organic frameworks, Chem. Commun., 2016, 52, 2133–2136 RSC.
- T. Friščić, et al., Ion- and Liquid-Assisted Grinding: Improved Mechanochemical Synthesis of Metal–Organic Frameworks Reveals Salt Inclusion and Anion Templating, Angew. Chem., Int. Ed., 2010, 49, 712–715 CrossRef PubMed.
- P. J. Beldon, et al., Rapid Room-Temperature Synthesis of Zeolitic Imidazolate Frameworks by Using Mechanochemistry, Angew. Chem., Int. Ed., 2010, 49, 9640–9643 CrossRef CAS PubMed.
- S. R. Percy, Improvement in drying and concentrating liquid substances by atomizing, 1872 Search PubMed.
- R. P. Patel, M. P. Patel and A. M. Suthar, Spray drying technology: an overview, Indian J. Sci. Technol., 2009, 2, 44–47 Search PubMed.
- D. Charalampopoulos and R. A. Rastall, Prebiotics and Probiotics Science and Technology, Springer, 2009 Search PubMed.
- K. Okuyama, M. Abdullah, I. Wuled Lenggoro and F. Iskandar, Preparation of functional nanostructured particles by spray drying, Adv. Powder Technol., 2006, 17, 587–611 CrossRef CAS.
- J. Thiele, et al., Early development drug formulation on a chip: Fabrication of nanoparticles using a microfluidic spray dryer, Lab Chip, 2011, 11, 2362 RSC.
- B. Rivas-Murias, et al., Spray drying: An alternative synthesis method for polycationic oxide compounds, J. Phys. Chem. Solids, 2011, 72, 158–163 CrossRef CAS.
- R. Sun, Y. Lu and K. Chen, Preparation and characterization of hollow hydroxyapatite microspheres by spray drying method, Mater. Sci. Eng., C, 2009, 29, 1088–1092 CrossRef CAS.
- K. Okuyama, M. Abdullah, I. Wuled Lenggoro and F. Iskandar, Preparation of functional nanostructured particles by spray drying, Adv. Powder Technol., 2006, 17, 587–611 CrossRef CAS.
- A. Carné-Sánchez, I. Imaz, M. Cano-Sarabia and D. Maspoch, A spray-drying strategy for synthesis of nanoscale metal–organic frameworks and their assembly into hollow superstructures, Nat. Chem., 2013, 5, 203–211 CrossRef PubMed.
- A. Garcia Marquez, et al., Green scalable aerosol synthesis of porous metal–organic frameworks, Chem. Commun., 2013, 49, 3848 RSC.
- L. Garzón-Tovar, et al., A spray-drying continuous-flow method for simultaneous synthesis and shaping of microspherical high nuclearity MOF beads, React. Chem. Eng., 2016, 1, 533–539 Search PubMed.
- L. Garzón-Tovar, S. Rodríguez-Hermida, I. Imaz and D. Maspoch, Spray Drying for Making Covalent Chemistry: Postsynthetic Modification of Metal–Organic Frameworks, J. Am. Chem. Soc., 2017, 139, 897–903 CrossRef PubMed.
- V. Guillerm, et al., Continuous One-Step Synthesis of Porous M-XF6-based Metal–Organic and Hydrogen-Bonded Frameworks, Chem. – Eur. J., 2017 DOI:10.1002/chem.201605507.
- Z. Wang, et al., Lanthanide-Organic Framework Nanothermometers Prepared by Spray-Drying, Adv. Funct. Mater., 2015, 25, 2824–2830 CrossRef CAS.
- A. Carné-Sánchez, et al., Protecting Metal–Organic Framework Crystals from Hydrolytic Degradation by Spray-Dry Encapsulating Them into Polystyrene Microspheres, Adv. Mater., 2015, 27, 869–873 CrossRef PubMed.
- L. Garzón-Tovar, J. Pérez-Carvajal, I. Imaz and D. Maspoch, Composite Salt in Porous Metal–Organic Frameworks for Adsorption Heat Transformation, Adv. Funct. Mater., 2017 DOI:10.1002/adfm.201606424.
- R. Ameloot, et al., Interfacial synthesis of hollow metal–organic framework capsules demonstrating selective permeability, Nat. Chem., 2011, 3, 382–387 CrossRef CAS PubMed.
- M. Faustini, et al., Microfluidic Approach toward Continuous and Ultra-Fast Syn-thesis of Metal–Organic Framework Crystals and Hetero-Structures in Confined Microdroplets, J. Am. Chem. Soc., 2013, 14619–14626 CrossRef CAS PubMed.
- L. D'Arras, et al., Fast and Continuous Processing of a new sub-micronic Lanthanide-based Metal–Organic Framework, New J. Chem., 2014, 38, 1477–1483 RSC.
- A. Polyzoidis, T. Altenburg, M. Schwarzer, S. Loebbecke and S. Kaskel, Continuous microreactor synthesis of ZIF-8 with high space–time-yield and tunable particle size, Chem. Eng. J., 2016, 283, 971–977 CrossRef CAS.
- S. Tai, et al., Facile preparation of UiO-66 nanoparticles with tunable sizes in a continuous flow microreactor and its application in drug delivery, Microporous Mesoporous Mater., 2016, 220, 148–154 CrossRef CAS.
- M. Gimeno-Fabra, et al., Instant MOFs: continuous synthesis of metal–organic frameworks by rapid solvent mixing, Chem. Commun., 2012, 48, 10642–10644 RSC.
- A. S. Munn, P. W. Dunne, S. V. Y. Tang and E. H. Lester, Large-scale continuous hydrothermal production and activation of ZIF-8, Chem. Commun., 2015, 51, 12811–12814 RSC.
- P. A. Bayliss, et al., Synthesis of metal–organic frameworks by continuous flow, Green Chem., 2014, 16, 3796 RSC.
- K.-J. Kim, et al., High-rate synthesis of Cu–BTC metal–organic frameworks, Chem. Commun., 2013, 49, 11518 RSC.
- M. Rubio-Martinez, et al., Versatile, High Quality and Scalable Continuous Flow Production of Metal–Organic Frameworks, Sci. Rep., 2014, 4, 5443 CrossRef CAS PubMed.
- M. Rubio-Martinez, et al., Scalability of Continuous Flow Production of Metal–Organic Frameworks, ChemSusChem, 2016, 9, 938–941 CrossRef CAS PubMed.
- S. Waitschat, M. T. Wharmby and N. Stock, Flow-synthesis of carboxylate and phosphonate based metal–organic frameworks under non-solvothermal reaction conditions, Dalton Trans., 2015, 44, 11235–11240 RSC.
- G. H. Albuquerque, et al., Gas–liquid segmented flow microwave-assisted synthesis of MOF-74(Ni) under moderate pressures, CrystEngComm, 2015, 17, 5502–5510 RSC.
- M. Taddei, D. A. Steitz, J. A. van Bokhoven and M. Ranocchiari, Continuous-Flow Microwave Synthesis of Metal–Organic Frameworks: A Highly Efficient Method for Large-Scale Production, Chem. – Eur. J., 2016, 22, 3245–3249 CrossRef CAS PubMed.
- P. M. Schoenecker, G. A. Belancik, B. E. Grabicka and K. S. Walton, Kinetics study and crystallization process design for scale-up of UiO-66-NH2 synthesis, AIChE J., 2013, 59, 1255–1262 CrossRef CAS.
- C. McKinstry, et al., Scalable continuous solvothermal synthesis of metal organic framework (MOF-5) crystals, Chem. Eng. J., 2016, 285, 718–725 CrossRef CAS.
- G. D. Ulrich, Chemical engineering process design and economics: a practical guide, Process Publishing, 2004 Search PubMed.
- J. E. Mondloch, O. Karagiaridi, O. K. Farha and J. T. Hupp, Activation of metal–organic framework materials, CrystEngComm, 2013, 15, 9258–9264 RSC.
- L. J. Wang, et al., Synthesis and Characterization of Metal–Organic Framework-74 Containing 2, 4, 6, 8, and 10 Different Metals, Inorg. Chem., 2014, 53, 5881–5883 CrossRef CAS PubMed.
- K. S. Park, et al., Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- J. An, et al., Metal-adeninate vertices for the construction of an exceptionally porous metal–organic framework, Nat. Commun., 2012, 3, 604 CrossRef PubMed.
- K. Koh, J. D. V. Oosterhout, S. Roy, A. G. Wong-Foy and A. J. Matzger, Exceptional surface area from coordination copolymers derived from two linear linkers of differing lengths, Chem. Sci., 2012, 3, 2429–2432 RSC.
- Y.-P. He, Y.-X. Tan and J. Zhang, Comparative Study of Activation Methods on Tuning Gas Sorption Properties of a Metal–Organic Framework with Nanosized Ligands, Inorg. Chem., 2012, 51, 11232–11234 CrossRef CAS PubMed.
- M. Rubio-Martinez, et al., Scalable simultaneous activation and separation of metal–organic frameworks, RSC Adv., 2016, 6, 5523–5527 RSC.
- M. Yu, et al., Covalent immobilization of metal–organic frameworks onto the surface of nylon—a new approach to the functionalization and coloration of textiles, Sci. Rep., 2016, 6, 22796 CrossRef PubMed.
- B. Garai, A. Mallick and R. Banerjee, Photochromic metal–organic frameworks for inkless and erasable printing, Chem. Sci., 2016, 7, 2195–2200 RSC.
- Y. Wu, et al., Electrospun fibrous mats as skeletons to produce free-standing MOF membranes, J. Mater. Chem., 2012, 22, 16971 RSC.
- S. Qiu, M. Xue and G. Zhu, Metal–organic framework membranes: from synthesis to separation application, Chem. Soc. Rev., 2014, 43, 6116–6140 RSC.
- Y. Zhang, et al., Constructing Free Standing Metal Organic Framework MIL-53 Membrane Based on Anodized Aluminum Oxide Precursor, Sci. Rep., 2014, 4, 4947 CrossRef PubMed.
- J. Ren, H. W. Langmi, B. C. North and M. Mathe, Review on processing of metal–organic framework (MOF) materials towards system integration for hydrogen storage: Review on processing of MOF materials towards system integration, Int. J. Energy Res., 2015, 39, 607–620 CrossRef CAS.
- Z. Wang, et al., Monolithic, Crystalline MOF Coating: An Excellent Patterning and Photoresist Material, ChemNanoMat, 2015, 1, 338–345 CrossRef CAS.
- M. Beckner and A. Dailly, Adsorbed methane storage for vehicular applications, Appl. Energy, 2015, 149, 69–74 CrossRef CAS.
- M. Ulrich, L. Lobree, M. Hesse Michael, Y. Omar and E. Mohamed, Shaped bodies containing metal–organic frameworks, 2003 Search PubMed.
- J. Kim, S.-H. Kim, S.-T. Yang and W.-S. Ahn, Bench-scale preparation of Cu3(BTC)2 by ethanol reflux: Synthesis optimization and adsorption/catalytic applications, Microporous Mesoporous Mater., 2012, 161, 48–55 CrossRef CAS.
- D. Bazer-Bachi, L. Assié, V. Lecocq, B. Harbuzaru and V. Falk, Towards industrial use of metal–organic framework: Impact of shaping on the MOF properties, Powder Technol., 2014, 255, 52–59 CrossRef CAS.
- G. W. Peterson, et al., Effects of pelletization pressure on the physical and chemical properties of the metal–organic frameworks Cu3(BTC)2 and UiO-66, Microporous Mesoporous Mater., 2013, 179, 48–53 CrossRef CAS.
- M. Tagliabue, et al., Methane storage on CPO-27-Ni pellets, J. Porous Mater., 2011, 18, 289–296 CrossRef CAS.
- K. W. Chapman, G. J. Halder and P. J. Chupas, Pressure-Induced Amorphization and Porosity Modification in a Metal−Organic Framework, J. Am. Chem. Soc., 2009, 131, 17546–17547 CrossRef CAS PubMed.
- S. Cavenati, C. A. Grande, A. E. Rodrigues, C. Kiener and U. Müller, Metal Organic Framework Adsorbent for Biogas Upgrading, Ind. Eng. Chem. Res., 2008, 47, 6333–6335 CrossRef CAS.
- V. Finsy, et al., Separation of CO2/CH4 mixtures with the MIL-53(Al) metal–organic framework, Microporous Mesoporous Mater., 2009, 120, 221–227 CrossRef CAS.
- P. Küsgens, A. Zgaverdea, H.-G. Fritz, S. Siegle and S. Kaskel, Metal–Organic Frameworks in Monolithic Structures: Rapid Communications of the American Ceramic Society, J. Am. Ceram. Soc., 2010, 93, 2476–2479 CrossRef.
- J. Ren, et al., A more efficient way to shape metal–organic framework (MOF) powder materials for hydrogen storage applications, Int. J. Hydrogen Energy, 2015, 40, 4617–4622 CrossRef CAS.
- J. Ren, T. Segakweng, H. W. Langmi, B. C. North and M. Mathe, Ni foam-immobilized MIL-101(Cr) nanocrystals toward system integration for hydrogen storage, J. Alloys Compd., 2015, 645, S170–S173 CrossRef CAS.
- U. Betke, et al., Micro-Macroporous Composite Materials: SiC Ceramic Foams Functionalized With the Metal Organic Framework HKUST-1, Chem. Ing. Tech., 2016, 88, 264–273 CrossRef CAS.
- M. L. Pinto, S. Dias and J. Pires, Composite MOF Foams: The Example of UiO-66/Polyurethane, ACS Appl. Mater. Interfaces, 2013, 5, 2360–2363 CAS.
- Y. Chen, et al., Shaping of Metal–Organic Frameworks: From Fluid to Shaped Bodies and Robust Foams, J. Am. Chem. Soc., 2016, 138, 10810–10813 CrossRef CAS PubMed.
- J. Zhao, et al., Highly Adsorptive, MOF-Functionalized Nonwoven Fiber Mats for Hazardous Gas Capture Enabled by Atomic Layer Deposition, Adv. Mater. Interfaces, 2014, 1, 1400040 CrossRef.
- A. I. Spjelkavik, D. Aarti, S. Divekar, T. Didriksen and R. Blom, Forming MOFs into Spheres by Use of Molecular Gastronomy Methods, Chem. – Eur. J., 2014, 20, 8973–8978 CAS.
- S. Aguado, J. Canivet and D. Farrusseng, Facile shaping of an imidazolate-based MOF on ceramic beads for adsorption and catalytic applications, Chem. Commun., 2010, 46, 7999 RSC.
- MOF Technologies Announces World's First Commercial Application of Metal Organic Framework Technology by Decco Worldwide at MOF 2016, MOF Technologies, 2016.
- 2016, R. T. O. MOFs offer safer toxic gas storage, Chemistry World available at: http://https://www.chemistryworld.com/news/mofs-offer-safer-toxic-gas-storage-/1017610.article, accessed: 8th February 2017.
- Fundamentals and Applications of Advanced Porous Materials – CSIRO. Fundamentals and Applications of Advanced Porous Materials – CSIRO available at: http://https://events.csiro.au/Events/2016/June/23/Advanced-Porous-Materials.
- Frameworks for commercial success, Nat. Chem., 2016, 8, 987 CrossRef PubMed.
- MOFapps – News, available at: http://mofapps.com/news.html, accessed: 8th February 2017.
- ProDIA, available at: http://prodia-mof.eu/, accessed: 27th March 2017.
- A. C. Dreischarf, M. Lammert, N. Stock and H. Reinsch, Green Synthesis of Zr-CAU-28: Structure and Properties of the First Zr-MOF Based on 2,5-Furandicarboxylic Acid, Inorg. Chem., 2017, 56, 2270–2277 CrossRef CAS PubMed.
- S. Waitschat, H. Reinsch and N. Stock, Water-based synthesis and characterisation of a new Zr-MOF with a unique inorganic building unit, Chem. Commun., 2016, 52, 12698–12701 RSC.
- Discover our MOF products|ProfMOF, available at: http://profmof.com/discover-our-mof-products/, accessed: 8th February 2017.
- MOF, available at: http://https://secure.strem.com/catalog/family/MOF/, accessed: 8th February 2017.
- Metal Organic Frameworks, available at: http://www.sigmaaldrich.com/technical-documents/articles/materials-science/metal–organic-frameworks.html, accessed: 8th February 2017.
- Home, available at: http://https://www.mofworx.com/, accessed: 8th February 2017.
- The MOF company, http://themofcompany.com.
- Framergy, available at: http://www.framergy.com/, accessed: 8th February 2017.
- ACSYNAM, available at: http://https://acsynam.com/, accessed: 8th February 2017.
- Promethean to Showcase Manufacturing Capability with MOF production, 2016, available at: http://www.prometheanparticles.co.uk/promethean-to-showcase-manufacturing-capability-with-mof-production/, accessed: 8th February 2017.
- U. Mueller, et al., Organometallic Framework Material, Preparation and Use, 2006 Search PubMed.
- S. James, A. Lazuen-Garay and A. Pichon, Chemical Synthesis, 2007 Search PubMed.
- M. Koch and G. Jonschker, Monolithic Materials for Gas Stores, 2010 Search PubMed.
- M. D. Allendorf and P. J. Hesketh, Method and apparatus for detecting an analyte, 2011 Search PubMed.
- U.-H. Lee, et al., Method for Preparing Porous Organic-Inorganic Hybrid Materials, 2014 Search PubMed.
- M. Schroder, M. Poliakoff, P. Bayliss and E. Velilla, Metal–Organic Frameworks, 2014 Search PubMed.
- Unpublished work.
- M. A. Moreira, et al., Reverse Shape Selectivity in the Liquid-Phase Adsorption of Xylene Isomers in Zirconium Terephthalate MOF UiO-66, Langmuir, 2012, 28, 5715–5723 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2017 |






