Open Access Article
Open Access ArticleSelective photocatalysis of lignin-inspired chemicals by integrating hybrid nanocatalysis in microfluidic reactors
Juan Carlos
Colmenares
 *a,
Rajender S.
Varma
*a,
Rajender S.
Varma
 b and
Vaishakh
Nair
b and
Vaishakh
Nair
 a
a
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01-224 Warsaw, Poland. E-mail: jcarloscolmenares@ichf.edu.pl
bRegional Centre of Advanced Technologies and Materials, Faculty of Science, Palacky University, Šlechtitelů 27, 783 71, Olomouc, Czech Republic. E-mail: Varma.Rajender@epa.gov
First published on 16th October 2017
Abstract
This tutorial review assesses the available and possible strategies for attaining higher selectivity and yield of value-added chemicals from lignin using nanocatalysts embedded in a photomicroreactor. The design of the photomicroreactor, the choice of photocatalysts and the techniques for assembling the catalysts in such confined spaces are crucial elements. Lignin, a predominant industrial byproduct and pollutant, has been recognized today as a rich reservoir for aromatic chemicals and a prominent resource. The conventional batch photocatalytic studies on lignin, often using dissolved lignin under alkaline conditions, often generates a wide range of valuable organic chemicals which find applications in the pharmaceutical, food processing, cosmetic and fine chemical industries. The role of photocatalysis in such lignin depolymerizations is questionable as the dissolution procedure initiates fragmentation of lignin prior to light exposure. The complexity of the lignin structure also impedes necessary and decisive information to understand the reaction mechanism during such reactions in batch photoreactors. Considering these facts, photocatalysis studies on lignin entail a thoughtful reevaluation and focus on understanding the role of photocatalysis in the product generation and authenticating the implicated reaction mechanisms. The development of a photocatalytic system for lignin depolymerization in a continuous microreactor is a superior approach for the generation of valuable products emanating from lignin depolymerization and the successful execution of such strategies can pave the way for the commercialization of bio-based chemicals.
Key learning points(1) New proof-of-concept for upgrading lignin-based chemicals via the synergistic coupling of nanophotocatalysis with microfluidic systems.(2) The use of new fabrication techniques on superior reactor materials can lower the time and operational expenses of the photomicroreactor. (3) The choice of the nanophotocatalyst and its assembly inside the photomicroreactor will affect the efficiency of the photocatalytic reaction. (4) The role of various parameters that have been eschewed during macroscale photocatalysis has to be monitored to secure better discernment for lignin photocatalysis. (5) The integration of lignin reactions in a photo microreactor can be used to carry out controlled successive experiments which have not been observed in conventional lignin photocatalysis. |
1. Introduction
Photocatalysis is one of the advanced oxidation techniques which has applications in numerous disciplines, namely sources of greener energy, medicine, chemical synthesis, and environmental technology.1 However, photocatalysis has customarily been considered as an indiscriminative process (particularly in water), because these reactions are normally regulated via a free radical mechanism (˙OH, O2−˙, HO2˙). Conventional application of photocatalysis has centered on mineralization of pollutants such as dyes, metal ions, and organic compounds, namely phenol, and often entails the use of titanium oxide (TiO2).1 Despite the widespread usefulness of TiO2 as a photocatalyst, there are two major constraints that limit its photocatalytic efficiency: (1) only 3–5% of the solar spectrum (UV region) is used efficiently for the photocatalytic process because of the large band-gap of conventional TiO2 (3.2 eV for anatase phase) and (2) fast recombination of photo-generated electron–hole pairs during TiO2 photocatalysis.2 Therefore, it has become imperative to improve the TiO2 photocatalytic efficiency by extending its absorption threshold from the UV-light region to the visible-light area which comprises a larger portion of the solar spectrum in terms of energy availability (45% of solar light) and reducing the rate of recombination of the photo-generated electron/hole pairs.2 To overcome these limitations, the development of hybrid nanocatalysts is considered a fresh approach wherein it is stated to have superior photocatalytic properties compared to conventional photocatalysts including higher visible light activity and lower recombination rate as well as a higher surface to volume ratio due to its nano size.2 Hybrid nanocatalysts could be based on titania or non-titania based materials including metal or non-metal doping, coupled with carbonaceous materials (e.g. fullerene (C60), carbon nanotubes (CNTs) and graphene), and metal oxides.3,4 The application of photocatalysis in selective organic transformations is infrequent because semiconductor photocatalysis has long been considered an inherently “nonselective” process, especially in aqueous media. Recently, different experiments on light-mediated reactions involving selective organic synthesis using homogenous photocatalysts have generated high yields of the desired products.5,6 The deoxygenation of organic molecules is an important reaction which is difficult to conduct in view of the bond strength of the carbon–oxygen bond. Rackl et al.6 carried out deoxygenation of monoallylated diols and β-amino alcohols under visible light for the synthesis of chiral tetrahydrofuran and pyrrolidine derivatives; the mechanism involved a halogen-free activation of the hydroxy group towards radical reactions suitable for 5-exo-trig/5-exo-dig cyclizations under visible-light irradiation.6 The emergence of these studies has spurred an ever-increasing interest among researchers for accomplishing selective synthesis reactions using heterogeneous photocatalysis.Nevertheless, much effort has been made to comprehend this subject culminating in identification of some reactions that can proceed with high efficiency and selectively using various heterogeneous photocatalytic systems.7,8 The heightened interest in photocatalytic selective organic transformations is well recognized as a superior alternative to conventional routes for the synthesis of fine chemicals via the choice of appropriate semiconductors and proper control of reaction conditions. In contrast to traditional protocols, photocatalytic organic synthesis features several unique advantages: (i) it is driven by sunlight as a highly renewable source of energy; (ii) it can proceed under milder conditions (room temperature and atmospheric pressure) and avoids the use of environmentally detrimental heavy metal catalysts as well as strong chemical oxidants or reducing agents; (iii) it can facilitate the design of short and efficient reaction sequences, thus minimizing side processes culminating in high selectivity.
Microreactor technology exerts a high degree of control over photochemical transformations due to its salient advantages in contrast to conventional batch processing (Fig. 1), continuous-flow operation, large specific surface area, enhanced heat- and mass-transfer rates, reduced safety hazards and the ease of increasing throughput by numbering-up to name a few. Furthermore, as shown in Fig. 1, the extremely small characteristic dimensions of microreactors ensure excellent light irradiation of the entire reaction medium thus enhancing radiation homogeneity which culminates in higher reaction selectivity and shorter reaction times.9 Such technology should lead to a better understanding of the benefits and associated risk profiles of chemicals. Performing heterogeneous photocatalysis in continuous flow microreactors has additional advantageous attributes such as ultra-low reagent and catalyst consumption leading to less waste generation, environmental friendliness, higher safety, high efficiency, portability, ease of handling of reactants, continuous product formation and catalyst separation, re-use of catalysts, and the possibility of operating under harsh conditions.9 According to the literature, the quantum yield (F) in microreactors is much higher than those encountered in batch reactors.10 This is ascribed to more homogeneous radiation distribution and the higher heat- and mass-transfer rates encountered in microreactors due to the high surface-to-volume ratio. The photonic efficiency ξ, which is defined as the number of reaction product molecules divided by the number of photons of monochromatic light incident on the reactor, is about one order of magnitude higher for microreactors (e.g. ξ = 0.0262) than for batch reactors (e.g. ξ = 0.0086–0.0042).10
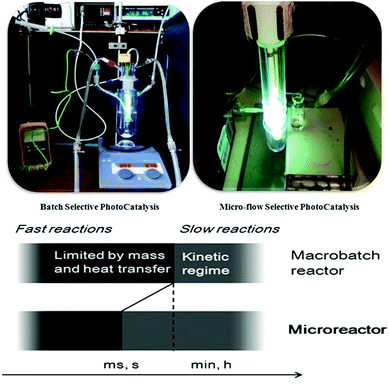 | ||
| Fig. 1 Comparison between a batch photoreactor and a continuous micro photoreactor. Adapted from ref. 9 with permission from John Wiley & Sons, Inc, Copyright 2011. | ||
Lignin accounts for approximately 40% of the total energy density of lignocellulosic biomass, making it an alluring option for fuel production.11 Current application of lignin is limited which is usually obtained as a byproduct from the paper-pulp industries and biorefineries during the extraction of cellulose pulp from lignocellulosic biomass; technology used for lignin removal has changed with time from soda and sulfite to the now more widely deployed Kraft process. In the Kraft process, lignin is separated in the form of black liquor and is generally used as a fuel for boilers to generate power for plant operations; ensuing lignin from this process is a rich source of renewable bio-based chemicals. Despite recent advances, there are currently no widespread catalytic processes for the valorization of lignin into bulk or fine chemicals.12 This fact is partly accredited to the highly complex and condensed nature of the lignin, with a prevalence of highly recalcitrant linkages/bonding motifs. Additionally, the lignin obtained from these industries has a considerable amount of sulfur content which is a well-known catalyst poison. Consequently, such industrial lignin feedstocks pose challenges for downstream catalytic valorization; literature abounds on lignin photocatalysis, wherein this industrial effluent is subjected to complete mineralization.8
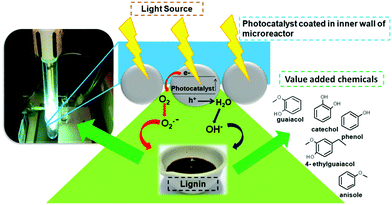
Recent fruitful studies on lignin depolymerizations to generate value-added chemicals namely guaiacol, vanillin, and phenols using thermochemical, ionic liquids and other chemical processes have altered the approach for photocatalytic studies of lignin.13 Conducting successful photocatalysis for the production of chemicals requires a molecular level understanding of the various reactions involved during photocatalytic reactions. Considering this approach, the majority of lignin photocatalysis research has been based on liquid–solid reactions using lignin as obtained from black liquor or dissolved lignin in alkaline medium. Recent studies with lignin dissolved in solvents have confirmed that prior to photocatalytic reactions, the fragmentation of lignin has occurred upon dissolution in alkaline medium. Rinaldi et al.14 observed that in the black liquor, obtained from the Kraft process, the content of β-ethers decreases drastically for both the liquor-phase (Kraft) and solid-residue lignin. Hence it is imperative to seek newer pathways to conduct controlled lignin photocatalytic reactions which can give cogent results emanating from the photocatalytic effect involved during the generation of various products. These routes would require monitoring of different reaction conditions such as lignin concentration and its purity, reaction temperature, pH at which lignin depolymerization is partially affected by the solvent, interaction of the photocatalyst with the lignin molecule, and the extent of irradiation in the photoreactor. Conventional lignin photocatalysis carried out in batch photoreactors using titania-based photocatalysts has not provided breakthrough results. As stated earlier, the use of microreactor technology here can ensure superior gains when compared to the macro-photosystems. These valuable features comprising heterogeneous photocatalysis in selective liquid phase processes, coupled with the remarkable advantages (e.g. better light utilization) of flow microreactors will have an unquestionable positive effect on the design and development of more cost-effective and sustainable processes for a green valorization of underutilized lignin-based bio-renewable wastes. This article examines various parameters namely the selection of photomicroreactors, the assembly of novel hybrid photocatalysts in a photomicroreactor and finally developing an efficient photocatalytic system for conducting lignin-based photoreactions.
2. Overcoming the obstacles of fluidic photomicroreactors in organic synthesis
Miniaturized microflow devices have become an integral part of synthetic chemistry manipulations lately.9 The small size of the integrated reaction channel (under a millimeter in at least one dimension), together with favorable heat- and mass transport, allows for precise control of reaction time, temperature, pressure and mixing. These key-features usually result in increased selectivity, conversion and yield, thus rendering microflow reactors as ‘greener’ processes for adaptation on an industrial scale.15 Importantly, in certain reactions, low temperatures (<0 °C) have to be maintained due to the generation of highly unstable intermediates requiring energy-consuming cooling devices, which severely limit its adoption on an industrial scale. As shown in Fig. 2, the Swern–Moffatt-type oxidation9 using dimethyl sulfoxide (DMSO) for the conversion of alcohols to carbonyl compounds such as aldehydes and ketones requires very low temperatures (−50 °C or below) in typical conventional batch macroreactors. Such cryogenic cooling requires a large amount of energy and results in higher costs which can be eliminated using a microflow system. The flow microreactor alternative could oxidize primary, secondary, cyclic, and benzylic alcohols, into the corresponding carbonyl compounds at room temperature in high yields and with good selectivity. In the case of polymer synthesis, the precise control of the molecular weight and molecular weight distribution is important and is achieved by controlling the reactions involving the individual monomeric units. Application of microreactors can improve the radical, cationic and anionic polymerization reactions, to synthesize structurally well-defined polymers involving end-functionalized polymers and copolymers.11 Microreactors could accomplish exothermic reactions as discerned in most of the radical polymerizations where temperature control is essential to molecular weight distribution management.9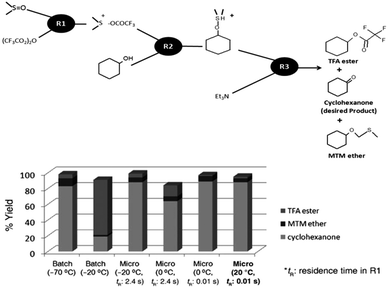 | ||
| Fig. 2 Swern–Moffatt oxidation of alcohols using a flow microreactor system. Adapted from ref. 9 with permission from John Wiley & Sons, Inc, Copyright 2011. | ||
Photocatalysis involves indirect interaction between the reactant molecule and light. The initial step of adsorption of the incident light by the catalyst or sensitizer occurs followed by the transfer of the generated electrons or energy to the acceptor molecule;9 activated molecules undergo the chemical transformation to yield the desired products. Photocatalytic studies for the selective organic synthesis in microreactors have been achieved on a large scale using homogenous catalysts like ruthenium and iridium polypyridine complexes and other organic dyes.9Scheme 1a depicts the use of tetraphenylporphyrin (TPP) for the oxidation of citronellol in a polymer-based microreactor; the reaction is carried out at lower reaction rate compared to the non-photocatalytic reaction. Similarly, in Scheme 1b, photoinduced electron transfer for the deoxygenation reaction in the synthesis of 2-deoxynucleosides has been executed with 10 mol% of a carbazole photosensitizer at 45 °C resulting in significant reduction of the reaction time in continuous flow (from 2 h to 10 min). Minimizing the exposure time of the reaction stream to the high-energy irradiation is responsible for the suppression of extensive by-product formation.9 Similarly, Nguyen et al.16 carried out deoxygenation of a series of primary and secondary alcohols involving a combination of the Garegg–Samuelsson reaction, visible light-photo redox catalysis, and flow chemistry. This group has already established a visible light mediated technique for the conversion of primary and secondary alcohols to alkyl halides as well as a visible light mediated method for the hydrodeiodination of alkyl, aryl, and alkenyl iodides using homogenous photocatalysts.16
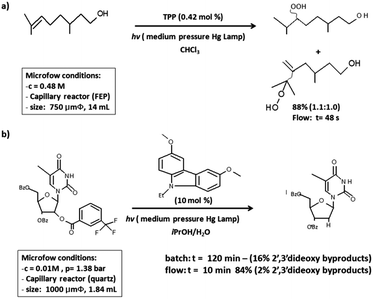 | ||
| Scheme 1 (a) Oxidation of citronellol with singlet oxygen in a capillary FEP microreactor with TPP as a photocatalyst. (b) Photocatalytic deoxygenation of protected thymidine in a capillary quartz microreactor with a carbazole as a photocatalyst. Reprinted from ref. 15 with permission from John Wiley & Sons, Inc., Copyright 2014. | ||
2.1 Heterogeneous photocatalytic reaction in a microfluidic system
Applications of TiO2-photomicroreactors are plentiful in fuel processing, waste water treatment, and air purification processes.15 Due to the excellent control over photon and mass transfer, these devices have been used to study assorted photocatalysts and their reaction kinetics. Examples in organic synthetic chemistry, however, are sporadic, as shown in the photocatalytic methylation of benzylamine (Scheme 2a) performed using a quartz microreactor deposited with a layer of TiO2;15 irradiation of the photocatalytic layer is accomplished by means of UV LED (365 nm). In batch, this reaction requires a platinum dopant that slows down the electron–hole recombination. Due to the short length scale in microreactors, the reaction is fast enough to prevent this charge recombination and no platinum is required. Consequently, better results have been acquired in a more confined microreactor that reduces the diffusion distance between the bulk solution and the photocatalytic surface (yielding 98% in a 300-micron depth reactor, compared to 70% yield in a 1000-micron depth reactor). In another example, the synthesis of L-pipecolinic acid starting from L-lysine is achieved in a pyrex microreactor with a Pt/TiO2 photocatalytic layer (Scheme 2b);15 irradiation using a high-pressure mercury lamp with a UV transmitting filter (110 mW cm−2) results in the formation of the desired compound in 52 seconds residence time (14% yield, 50% ee). Similar yields could be obtained in batch; however, it required a much longer reaction time of one hour.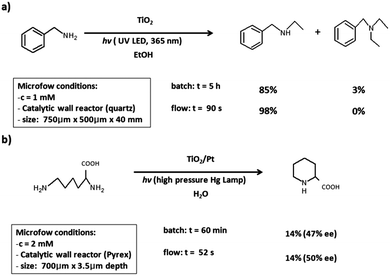 | ||
| Scheme 2 (a) Photocatalytic N-ethylation of benzylamine in a quartz microreactor with a TiO2 layer excited with 365 nm UV LEDs. (b) Synthesis of L-pipecolinic acid in a pyrex microreactor with a TiO2 layer excited with a high-pressure mercury lamp. Reprinted from ref. 15 with permission from John Wiley & Sons, Inc., Copyright 2014. | ||
2.2 Design of photomicroreactors
Photomicrochemical processes usually result in the reduction of irradiation times, enhanced selectivity and increased light efficiency, thus unambiguously demonstrating the superiority of microflow photochemistry over conventional techniques.17 One of the major differences between a photomicroreactor and a conventional microreactor is the transparency of light through the reactor walls which leads to the chemical reactions. The photomicroreactors have been fabricated using a wide range of materials, such as stainless steel, glass, polymers, ceramics, and silicon. The choice of the material is primarily based on three requirements: (1) higher transparency for the desired radiation, (2) lower light scattering through the reactor and (3) higher feasibility of the photoreaction in the microreactor.15,17 Different glass plates have been used to construct photochemical microreactors or microchannels. Glass and quartz based microreactors can act as a solid filter and, depending on the choice, different wavelengths are accessible. Quartz has a wavelength cut-off below 170 nm and can, therefore, be used for both UV- and visible light photochemical applications. They can resist chemical erosion and provide a clear observation of photoreactions inside microreactors. However, deep and anisotropic etching or micromachining is almost impossible for these glass materials and they lack compatibility with strong alkaline reaction mixtures at moderate to high reaction temperatures (erosion). A large variety of polymer capillary materials, such as polymethylmethacrylate (PMMA), polydimethylsiloxane (PDMS), fluoro polymers like perfluoroalkoxyalkane (PFA), and fluorinated ethylene propylene (FEP) have been shown to be superior in addressing these limitations.10Among the polymers, PFA and FEP are considered as prospective materials for the fabrication of photomicroreactors. The fluoro polymers have high light transmission to both UV and visible light, easy fabrication, and relatively low cost.17 These materials are highly transparent to both UV and visible light: PFA allows transmission of 91–96% for visible light (400–700 nm) and 77–91% for UV light (250–400 nm); FEP has similar properties for visible light and a bit better for UV light.15 Additionally, the materials are highly flexible and can resist strong acidic and alkaline solvents at moderate temperatures and pressures, showing their high application potential in photochemical processes. Besides their strong chemical stability, they demonstrate a higher resistance to microreactor clogging and low moisture absorbance.18 Most often these reactors are wrapped around the lamps to maximize the photon flux. One of the major challenges in using these polymer capillaries for heterogeneous photocatalysis is the deposition of the catalyst in the tubings. The chemical inert nature of the fluoro polymers renders it difficult to attain catalyst coating on to the capillary surface. The use of organic materials such as polymers or surfactants can be used as binders between the catalyst and the reactor wall. The option of performing in situ catalyst synthesis in a reactor is a new strategy that can be achieved using a synergistic combination of sonochemical and/or chemical synthesis. Recently, TiO2 suspended in water was passed though Teflon capillary tubing under ultrasound irradiation, and thin layers of commercial TiO2 could be coated in the inner wall of the tubing.19
Fabrication techniques available for making microreactors mainly include micromachining, lithography, injection molding, wet and dry etching, electrodischarge machining, laser machining, embossing, and so on.10 These conventional techniques have their limitations due to the higher cost of manufacturing, higher energy needs, assembly time, crack generation and erosion of cutting tools which restricts their widespread usage.10 The current technology for microreactor fabrication is moving towards 3-D printing technology, which is inexpensive and environmentally friendly.20,21 In this process, computer aided design (CAD) software is used to outline a 3-dimensional pattern on the surface of smooth paper using a solid ink printer or laser jet printer; organic solvents are used as a sink for printing purposes. In general, after the formation of the 3-D pattern, the polymer is replicated by a curing process to form negative relief channels.20 Finally, a negative polymeric mould is attached to a flat substrate to form a microfluidic device. Importantly, the inlet and outlet connector of the microchannel is fabricated directly by the mould, which reduces the cost of drilling and eliminates the possibilities of crack formation in the cured polymer.21
2.3 Understanding various parameters involved in photomicroreactor operation
Compatibility aspects of the reactor with the light sources and the operational solvent requirements need to be taken into consideration while designing an efficient photochemical process.15,17 Recently, light-emitting diodes (LEDs) have become more popular for application in continuous-flow photochemistry when compared to the conventional mercury or xenon lamps which require more power and heat up the reactor considerably.10 LEDs are semiconductors (PN junction) with a narrow emission spectrum that can be tailored to the required wavelength (from UV to Vis light).15 Another advantage of LEDs entails their small size which provides a means to integrate the light source on the microreactor assembly.22 In brief, the low power input of these microscale light sources, their high energy efficiency, smaller size and low cost make them ideal candidates to pair-up with microreactors. Energy losses in LEDs are minimized and only a small amount of heat is generated. Despite the small dimensions of LEDs, it is still difficult to integrate these into the interior section of microreactors. As shown in Fig. 3, the orientation of LEDs on the microreactor is decisive for controlling the effect of temperature on the chemical reaction.22 Novel nanoscale light sources are being developed that can be directly integrated into the microreactors for photocatalysis, although it remains to be verified whether these nanoscale light sources can provide enough energy for photochemical processes.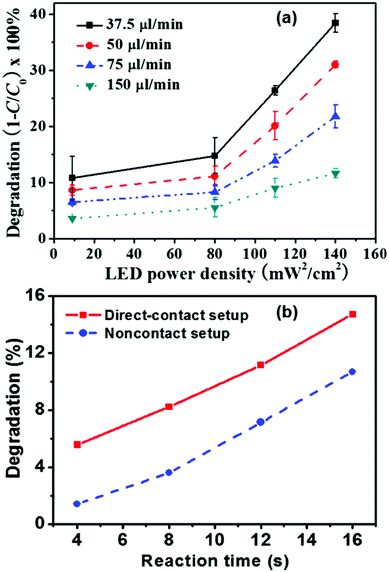 | ||
| Fig. 3 (a) Influence of the light intensity on the degradation percentage at different flow rates. (b) Change in the degradation rates due to heat from contact of the LED setup. Adapted from ref. 22 with permission from AIP Publishing LLC, Copyright 2014. | ||
Solvents for organic synthesis require the reactant molecules to be homogenously dispersed so that the reaction can be uniform throughout the reactor. Since the reactions with lignin and lignin model compounds involve organic solvents, the utmost importance should be given to its reactivity towards the microchannel. Furthermore, the solvent should be compatible with the chosen light source and the reactor material.10 While the solvent should be inert to prevent erosion of the reactor walls, it should neither be a strong light absorber, nor a quencher of the photochemical process.15 The residence time should be larger than the characteristic reaction time, thus allowing the components in the reaction medium enough time to react and reach full conversion. In fluidic microreactors, the residence time can be easily varied by controlling the flow rates or the lengths of the microchannels. Furthermore, controlling the flow rate can also prevent or reduce side reactions or decompositions caused by “over-irradiation”. In general, a comparison between batch reactors and various microreactors in terms of the Damkçhler numbers should be made while selecting appropriate reactors for photochemical transformations.23,24 The design of photoreactors is mostly based on efficiency parameters (e.g. quantum yield and photonic efficiency) whilst the kinetic aspect in a microreactor has not been exploited. The development of kinetic models and an accurate determination of their parameters are also important for accurate photoreactor modelling and optimization. It is worth mentioning here that a bigger challenge concerning the kinetic modelling of the photo-catalytic reactions is to assess the influence of the light intensity on the reaction rate.25
3. Assembly of a layered photocatalyst in a microreactor environment
The performance of a photocatalytic system is contingent on two conditions, the selection of the photocatalyst and the interaction between the reactant molecules and the photocatalyst. The combination of microreactor technology and heterogeneous photocatalysts is expected to result in an enhanced performance of photocatalytic reactions.26 This can be attributed to shorter diffusion distances and the larger surface-to-volume ratios that lead to a higher irradiation efficiency. In fact, a crucial design parameter constitutes the maximization of the photocatalytic surface, which is represented by k, the illuminated catalyst surface area per unit of liquid inside the reactor (m2 m−3). For microreactors, this value is very high (k = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667 m2 m−3) in comparison to external type annular photoreactors (k = 27 m2 m−3).9
667 m2 m−3) in comparison to external type annular photoreactors (k = 27 m2 m−3).9
3.1 Selection and synthesis of hybrid nanomaterials as photocatalysts
One of the major difficulties encountered for upscaling photocatalysis-based reactions is the availability of photocatalysts which show reactivity and productivity at lower energy conditions. The development of hybrid photocatalysts involving combinations of different materials has shown promise to deliver high reactivity along with higher selectivity (Fig. 4). The choice of using the catalysts on the nanoscale adds the advantage of having a higher surface area in the microflow system. Although a number of studies have appeared on different routes to upgrade the semiconductor oxides, they have not yet been implemented for large scale application. As stated earlier, the major problems for semiconductor photocatalysts is the higher band gap and higher recombination of photoinduced charge carriers.2 The use of metal and non-metal dopants have been well studied thus altering the basic limitations of the semiconductor catalyst.3,27 Introduction of noble metal nanoparticles like Ag, Au, and Pt on the surface of oxide photocatalysts will result in Schottky barriers at the junctions wherein the noble metal nanoparticles will act as an electron sink which can greatly enhance the efficiency of charge separation.27 Besides, for metal-oxide heterostructures, the electron transfer direction is determined by the work function of metal. Thus, platinum (Pt), which has a large work function of 5.93 eV, is one of the best candidates for constructing metal–ZnO heterostructures to improve the photocatalytic efficiency of ZnO photocatalysts. Monolayer two-dimensional (2-D) graphene (GR) is regarded as an ideal candidate as a photocatalyst carrier due to its superior mobility of charge carriers (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm2 V−1 s−1), high specific surface area (2630 m2 g−1), excellent transparency (∼97.7%) and high chemical and electrochemical stability.4,28 In recent years, GR based photocatalyst composites (Fig. 4b) have been extensively used for selective organic synthesis involving both reduction and oxidation reactions for generation of value-added compounds like methane, amines and carbonyl compounds respectively.28
000 cm2 V−1 s−1), high specific surface area (2630 m2 g−1), excellent transparency (∼97.7%) and high chemical and electrochemical stability.4,28 In recent years, GR based photocatalyst composites (Fig. 4b) have been extensively used for selective organic synthesis involving both reduction and oxidation reactions for generation of value-added compounds like methane, amines and carbonyl compounds respectively.28
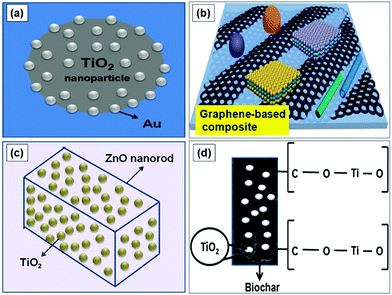 | ||
| Fig. 4 Development of hybrid nano-photocatalysts. (a) Adapted from ref. 27 with permission from Elsevier B.V., Copyright 2010, (b) adapted from ref. 4 with permission from American Chemical Society, Copyright 2015, (c) adapted from ref. 29 with permission from Elsevier B.V., Copyright 2014, (d) adapted from ref. 34 with permission from Royal Society of Chemistry, Copyright 2016. | ||
TiO2-coated ZnO nanorod arrays have been developed by He et al.29 on the inner walls of the capillaries (IWC) simply by flowing TiO2 sol into capillaries containing preformed ZnO nanorod arrays. The superior photocatalytic activity of the ZnO/TiO2 nanorod-modified capillary microchannels is attributed to the topographical morphology induced by the nanorod arrays, and the combination of two semiconductors decreasing the recombination rate of photoinduced electrons and holes. Chen et al.30 in their review on black TiO2 nanomaterials have explained the chemical and structural properties of the modified TiO2 that has shown improved optical absorption in the visible light region. Apparently, the hydrogenation treatment on TiO2 induced the oxygen vacancies and Ti3+ sites in TiO2, resulting in band-gap narrowing and the separation of photo-generated electrons and holes, which remarkably improved the photocatalytic activity of TiO2.30
The selection of a hybrid catalyst can also be based on the product selectivity required during a reaction. Lopez-Tenllado et al.31 synthesized Bi2WO6–TiO2 photocatalytic materials which are highly active and selective for the oxidation of alcohols to carbonyls; incorporation of a small amount of titania (5% molar) into Bi2WO6 considerably increased the oxidation of crotyl alcohol and higher selectivity to crotonaldehyde on bismuth tungstate. This is the result of the lower adsorption of the aldehyde on the catalyst surface which stopped further oxidation. Varma and co-workers have been in the forefront of developing hybrid photocatalysts for organic synthesis under visible light.32,33 In a recent work, they developed vanadium oxide supported on a graphitic carbon nitride (g-C3N4) surface which displayed high reactivity for selectively oxidizing methyl arenes to the corresponding aromatic aldehydes under visible light; hydrocarbons bearing benzylic methylene groups selectively get oxidized to the corresponding ketones. In another instance, the same catalyst was successfully used to study direct oxidative esterification of alcohols; the photocatalytic C–H activation in the reaction occurs with a VO@g-C3N4 catalyst.33 The expeditious reaction proceeds under neutral conditions due to the photoactive g-C3N4 surface and its strong interaction with the vanadium metal. The support stimulates the metal towards the oxidation of alcohol followed by C–H activation-esterification, while the in-built nitrogenous framework provides an adequate mild, basic environment and energy through visible light absorption.32
The use of biomaterials in developing hybrid catalysts appears to reign in the sustainability aspects to the catalyst synthesis process. Biochar is the by-product obtained during the pyrolysis of lignocellulosic biomass, which can be used as a catalyst support due to its high surface area. This carbon material is also endowed with plenty of functional groups such as hydroxyls and carboxyls which can be exploited for different reactions. The presence of catalysts in the narrow pores of the carbon support can lead to selective diffusion of reactant molecules in a size-dependent fashion leading to higher selectivity and yield.34 A titania–carbon based hybrid material has been developed via thermal treatment at 400 °C under an oxygen-deficient atmosphere; carboxylic groups adorning the char serve as the nucleation site for TiO2 formation. These conditions preserve a pure and highly crystalline anatase phase (ca. 30 nm) leading to a reduction in the electron–hole recombination rate at the carbon surface. The highly pure and crystalline TiO2 shows superior photocatalytic degradation of phenol when compared to activated carbon and grapheme oxide supports by reducing the recombination of photo-generated electron–hole pairs. A comprehensive review by Colmenares et al.34 provides key scientific insights into other TiO2/carbon materials derived from renewable and biodegradable resources emanating from biopolymeric materials such as lignin, cellulose, cellulose acetate, bacterial cellulose, bamboo, wood, starch, chitosan and agricultural residues or seafood wastes.
3.2 Assembly of photocatalysts in a photomicroreactor
The common techniques used in catalyst assembly for a microreactor involve three routes, namely packed-bed, monolithic and wall-coated type.35Fig. 5 depicts that in all the microreactor systems a catalyst support along with the catalyst is used and the requirement for the choice of the support can vary from synthetic polymers to oxides.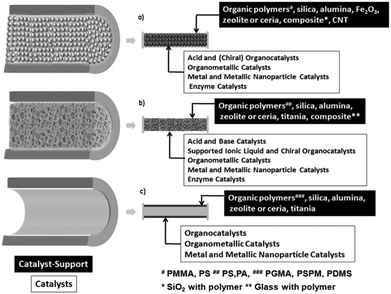 | ||
| Fig. 5 Schematic representation of the cross-section of a microchannel in (a) packed-bed, (b) monolithic and (c) wall-coated microreactors. Adapted from ref. 35 with permission from John Wiley & Sons, Inc., Copyright 2015. | ||
While considering a photo microreactor, the choice of assembly technique for the catalyst depends on the microreactor material that maximises exposure of the catalyst to the irradiating light as shown in Fig. 6; for glass and quartz-based microreactors, photocatalyst films or fibers have been used.26 The fabrication of photo microreactors uses polymer capillaries for which wall-coated catalytic microreactors are the best choice. In fixed-bed or stirred batch reactors, uncontrolled formation of hot and cold spots occurs on the catalyst surface which results in fluctuation of conversion rates and product selectivity. In contrast, for wall-coated catalytic microreactors, flow is completely laminar and the reaction takes place both in the bulk fluid and throughout the entire catalyst-coated layer in the channels, due to the short diffusion path and enhanced interfacial contact (the specific surface areas of microfluidic systems are estimated to be in the range of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m2 m−3 compared to the 100–1000 m2 m−3 attainable in traditional reactors).36
000 m2 m−3 compared to the 100–1000 m2 m−3 attainable in traditional reactors).36
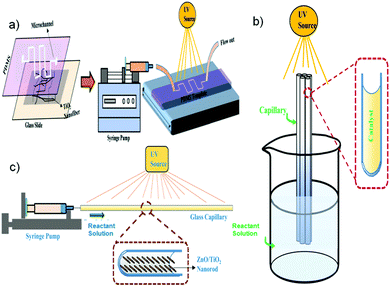 | ||
| Fig. 6 Schematic of different types of photomicroreactors.26 | ||
In a continuous process, the ideal scenario subsists when the catalyst is fixed in space and the reaction mixture is allowed to flow over it. The reaction and separation stage are thus combined in a single step, and the catalyst stays in the reactor for easy recycling. This is the main reason why the use of thin catalyst layers on the reactor walls is almost exclusively used in microstructured reactors. Wash coats, such as those applied in exhaust-gas cleaning in automobiles, as well as in monolith reactors, are often applied as a carrier on the microchannel walls including wet-chemical. In a standard form, catalyst powder or particles are introduced in a channel as miniature fixed beds. In microstructured reactors, however, beds are generally not employed as they cause a large pressure drop and change the laminar flow in the microspace. It is also observed that packed and monolithic type photo microreactors have lower optical access for monitoring and insufficient light penetration due to complicated internal arrangement of the photocatalyst in the microreactor; more complex catalyst forms are better for potential applications such as catalyst filaments, wires and membranes. Most coatings in microchannels are prepared by the wash coat route with subsequent wet-chemical impregnation. Thin film technologies such as CVD and PVD (especially sputtering) can also be used to generate compact, thin catalyst films.37 Other types of catalyst/carrier systems can be prepared via aerosol techniques, sol–gel techniques, plasma electrochemical processes, electrolysis or inkjet printing.37 The properties of wall-coated reactors lead to enhanced reaction rates and substrate conversion, with preserved product selectivity, thus allowing a greener process. Therefore, it is clear that the successful use of wall-coated catalyst microfluidic reactors for chemical transformation or energy generation would depend largely on controlling the incorporation of the catalyst onto the walls of the microchannels. These controls include:
(1) Achieving adequate mechanical stability of the coating.
(2) Controlling coating thickness, which has a great influence on the hydrodynamic flow patterns inside the microchannels due to blockage and roughness.
(3) Controlling catalyst porosity, which causes a large reduction of catalyst mass per unit area; low porosity leads to the formation of cracks and mass transfer limitations.
Finally, for wall-coated microchannels, the precise control of size and stability of the desired active metal phase in nanoparticle catalysts are critical for acquiring the immobilized catalyst surface with a higher number of exposed active sites or atoms. The availability of a large number of catalyst surface active sites provides better accessibility to reacting molecules, which can lead to superior reaction performance for a greener synthesis from both a chemistry and reactor system viewpoint, thus minimizing waste or by-product formation. Despite a few significant efforts to date, a practical and viable thin photocatalytic layer in a fluidic microreactor with sufficient efficiency, stability and low cost is yet to be demonstrated. This remarkably interesting approach of heterogeneous photocatalytic multicomponent thin layers in a microfluidic space could combine the advantages of thin layered components together with the specific internal environment of a micro reactor (e.g. high surface/volume ratio, excellent mass transfer) to overcome the drawbacks of single component photocatalysts used in conventional batch reactors.
4. Strategies for selective transformation of lignin-derived chemicals via the fusion of nanocatalysis with microfluidic photoreactors
As briefly mentioned before, the most benchmarked application of microfluidic reactors has been in performing chemical transformations on both upstream and downstream chemicals.38 Moreover, the characteristic features of microfluidic systems and nanoscale photocatalysis can be combined for conducting downstream multiphase reactions for the synthesis of value-added chemicals. Among the various opportunities, the practice of greener synthesis of fine chemicals (useful for pharmaceuticals) can be deployed to upgrade lignin-derived platform chemicals from renewable resources. Lignin is underutilized and the bulk of the produced lignin, representing about 70 million metric tons per year, is only employed as a combustible material for its high heat value.39 Due to its high aromatic content, lignin has prodigious potential to function as an alternative to non-renewable fossil resources, for the production of aromatic fine chemicals.40 However, because of its amorphous structure, lignin is highly refractory towards chemical modification, which represents a major challenge in its efficient utilization. Depolymerization techniques have been extensively used to study the complex nature of lignin and determine the range of various products that can be generated.13 Historically, phenols, substituted phenols and several aromatic and aliphatic acids have been produced from lignin, specifically the more valuable entity, vanillin.40,41 The range of phenolic compounds obtainable from catalytic reactions on lignin is fairly large (Fig. 7). Furthermore, targets may not be only restricted to products derived from coumaryl-, sinapyl- and coniferyl alcohols, but substituted catechols or functionalized styrenes generated on a larger scale could lead to newer markets.42 It is also important to understand that irrespective of the type of reactor used for photocatalysis, the significance of parameters like the choice of lignin and the solvent system deployed have to be understood which would help in determining the spectrum of products generated.4.1 Choice of lignin
The photocatalytic studies on lignin can be carried out using (1) native lignin, (2) industrial lignin and (3) lignin model compounds. Native lignin is lignin separated from the biomass using mild chemical treatments where the purity is low, while industrial lignin is of high purity and is obtained from industrial routes such as the Kraft pulping process.14 It is well documented that various separation techniques result in changes to the structure and properties of the original lignin. Furthermore, the use of such lignin requires knowledge of the composition of three monolignols present in the structure; literature precedents show that the concentration of coniferyl and sinapyl alcohols plays a major role in the selectivity and yield of phenolics.12,13 Such basic information requires extensive characterization of the material before preparing a photocatalytic set up. The knowledge of different bond linkages, functionalities and type of monolignols can be used to predict the possible product spectrum and can be helpful in the selection of the catalyst, and the solvent deployed. The use of lignin model compounds is the most sought after route to understand selective reactions involved; these organic compounds do not reveal the complete reaction implicated in lignin degradation, but do help in reducing and understanding the complexity of the lignin depolymerisation pathway.43 There are typically two types of lignin model compounds, i.e., monomers and dimers, which have been actively investigated (Fig. 8).43 A strategy to selectively break the C–C or the C–O bonds to convert lignin to useful platform chemical molecules for industry would be a significant achievement.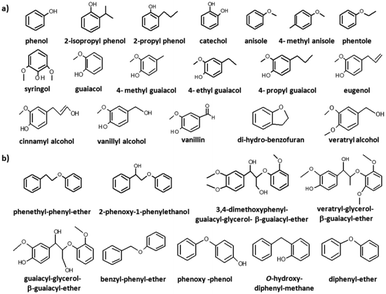 | ||
| Fig. 8 Lignin model compounds of (a) monomers and (b) dimers. Reprinted from ref. 43 with permission from Elsevier B.V., Copyright 2016. | ||
4.2 Choice of solvent
The choice of solvent is an important aspect considering that varied lignin samples have different properties. Although lignosulphonates from a sulphite process are soluble in water, the industrial Kraft lignin is not and requires higher alkaline conditions for its solubility. A vast majority of experiments on photocatalysis of lignin are conducted on Kraft lignin dissolved in alkaline solution or black liquor from the paper and pulp industries;14 clearly the highly alkaline solvent causes fragmentation thereby affecting the lignin structure (Fig. 9).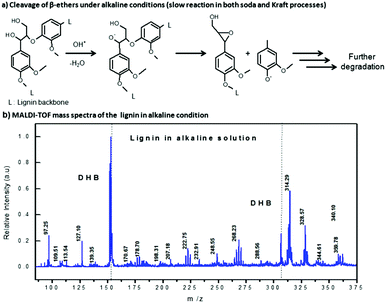 | ||
| Fig. 9 Depolymerization of lignin under alkaline conditions. Adapted from ref. 14 with permission from John Wiley & Sons, Inc., Copyright 2016. | ||
It would be appropriate to state that these studies do not delineate the actual mechanism related to photocatalysis. The uneven fragmentation of lignin molecules due to the variation in solvent pH can affect the reproducibility of the photocatalytic reaction thereby evading researchers to unveil the photocatalytic pathway. Hence, newer solvent systems for lignin dissolution have to be identified where the lignin structure is minimally affected. In a recent study, ammonia has been used as a solvent for lignin solubilisation44 which readily dissolves most varieties of lignin at room temperature and under 7–11 bar pressure; the solvent can be removed simply by releasing the pressure. The solubility of lignin in an organic solvent is dictated by the molecular weight and the aliphatic hydroxyl number of the lignin; low molecular weight lignin has the highest solubility in organic solvents while the presence of more hydroxyl groups lowers the solubility in organic solvents.45 The advantage of conducting low concentration reactions in a microreactor avoids the use of strong chemical reagents or solvents. Recent work involving a lignin–water system has been reported by Nair et al.46 wherein the generation of different water soluble organic compounds during photocatalytic experiments entails ball-milled mixtures of industrial lignin and TiO2 in a conventional batch system using UV radiation; the main organics produced during photocatalysis comprise ethyl benzene, acetovanillone, syringaldehyde, acetosyringone, vanillin, 2,6-dimethoxy benzoquinone and diisobutyl phthalate. This clearly shows that a wide range of compounds could be generated by free radical depolymerization reactions of lignin which can be mediated via active hydroxyl and superoxide radicals for the formation of the products obtained.
4.3 Lignin photocatalysis in a microreactor
From the aforementioned discussions in earlier sections, it is apparent that the depolymerization of complex materials such as lignin can be well studied in a microreactor system. The analysis of intermediate compounds obtained during the photocatalytic reaction can be easily quantified and the ensuing results can be used to establish the reaction pathways for the selective product generation. Lower dimensions and better interactions are also expected to reduce the reaction time in a microreactor. Unfortunately, there is paucity of data reported on lignin photocatalysis in a microreactor, an example being the study by Nguyen et al.47 involving lignosulphonate and lignin model compounds; photocatalytic studies were carried out at room temperature in the presence of different oxidants and the photocatalyst [Ir(ppy)2(dtbbpy)]PF6. The reaction studies on different lignin model compounds showcased the reactivity of the carbonyl portion of the molecule resulting in different yields and times of reaction (Fig. 10). The mild reaction conditions result in reductive fragmentation of the lignin model systems to generate guaiacol and β-hydroxy phenyl ketones.47 A hybrid photocatalyst can be used to achieve better photocatalysis via two means. Firstly, the generation of more electrons and holes can accelerate the lignin depolymerization reaction resulting in higher conversion. Secondly, the chemical nature of the hybrid catalyst which includes various functionalities can produce selective generation of products. Lignin dissolution in the majority of solvents can lead to the formation of dark colored solutions which can inhibit the passage of light through the reactor resulting in lower efficiency of the photocatalyst. The utility of wall-coated photomicroreactors provides more exposure of the photocatalyst to the light radiation culminating in better performance of the catalyst. The ease of controlling a microreactor system can be used for developing an in situ microreactor-analysis set up which can further help explore the underlying reaction mechanism which has been already shown for thermochemical systems.12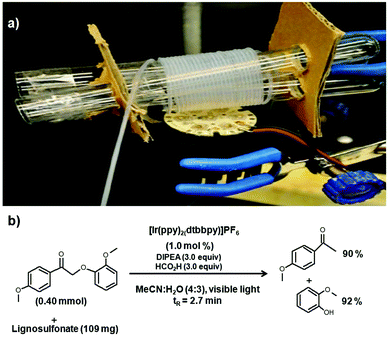 | ||
| Fig. 10 (a) Photoreaction of different lignin based compounds in a photomicroreactor with the coiled tubing exposed to a LED source. (b) Lignin photocatalytic conversion of lignosulfonate using a continuous flow reactor. Adapted from ref. 47 with permission from American Chemical Society, Copyright 2010. | ||
5. Summary and future challenges
This tutorial review summarizes most of the opportunities and main challenges pertaining to the realization of the synergistic effect of combining heterogeneous photocatalysis and microflow chemistry in the context of lignin-derived chemicals including selective transformations to high-value intermediates or/and final products for the fine chemical industry. In the long run, continuous assessments of performance determining parameters have to be put in place for photomicroreactors. The choice of photocatalyst and its stability is an important parameter which has to be scrutinized regularly after each reaction in the microreactor. The regeneration of the catalyst is an important factor for continuous usage of the system and hence the performance of the hybrid catalyst, especially the leaching of the metal ions in doped catalysts, has to be verified and monitored. Similarly, the effect of UV irradiation on the polymeric microreactor needs to be certified. Two of the major challenges in commercialization of lignin depolymerization are the diverse nature of lignin structure and its availability; the average time required for production and growth of softwoods and hardwoods is between 5 and 30 years.48 To alleviate this limitation, the bulk of research on lignin bioengineering can be directed towards angiosperms which have a shorter life cycle. The rational incorporation of chemically labile linkages into the chemical structure of lignin represents a promising area of future investigation for understanding the possible reaction pathways. The commercialization of lignin-derived products requires the upscaling of the photomicroreactor technology which could be achieved by increasing the number of reactors or an expansion of the channel size. A recent outlook from the pharmaceutical industry reveals that the following challenges continue to hinder widespread industrial adoption of flow microchemistry, namely the current lack of comprehensive regulatory guidelines, shortage of trained chemists and chemical engineers with expertise in flow chemistry, competition with existing investments and limitations in handling of solids.49 This is especially the case for potential new lignin-derived platform chemicals which are suitable targets for further upgrade via the use of fluidic photomicroreactors. The acquired knowledge base will also be of paramount importance in the future when researchers strive for the development of new technological breakthroughs with this strategy in the area of photocatalytic solar energy conversion.A more inventive approach could involve a combination of other technologies with photocatalysis which can be useful in efficient generation of organic products from lignin. The fusion of sonocatalysis with photocatalysis has been widely explored in the recent past as an advanced oxidation process (AOP) ranging from water decontamination to direct pollutant degradation.50 The combination presents some salient advantageous features such as the generation of increased amounts of radical species in aqueous solution (essentially HO2˙, O2˙− and HO˙ species under coupled UV/US irradiations) and the turbulence induced by the cavitation phenomenon, both consequences leading to the enhancement of degradation reaction rates. The ultrasonic irradiation increases turbulence in the liquid phase, decreasing mass transfer limitations and increasing the catalytically active surface area via the de-agglomeration and fragmentation of the particles.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
J. C. Colmenares and V. Nair are very grateful for the support from the National Science Centre in Poland within Sonata Bis Project no. 2015/18/E/ST5/00306.Notes and references
- J. Kou, C. Lu, J. Wang, Y. Chen, Z. Xu and R. S. Varma, Chem. Soc. Rev., 2017, 117, 1445 CrossRef CAS PubMed.
- J. Nowotny, M. A. Alim, T. Bak, M. A. Idris, M. Ionescu, K. Prince, M. Z. Sahdan, K. Sopian, M. A. M. Teridie and W. Sigmund, Chem. Soc. Rev., 2015, 44, 8424 RSC.
- N. Zhang, C. Han, Y.-J. Xu, J. J. Foley IV, D. Zhang, J. Codrington, S. K. Gray and Y. Sun, Nat. Photonics, 2016, 10, 473 CrossRef CAS.
- N. Zhang, M. Q. Yang, S. Liu, Y. Sun and Y.-J. Xu, Chem. Rev., 2015, 115, 10307 CrossRef CAS PubMed.
- D. Rackl, V. Kais, P. Kreitmeier and O. Reiser, Beilstein J. Org. Chem., 2014, 10, 2157 CrossRef CAS PubMed.
- D. Rackl, V. Kais, E. Lutsker and O. Reiser, Eur. J. Org. Chem., 2017, 2130 CrossRef CAS PubMed.
- D. Friedmann, A. Hakki, H. Kim, W. Choi and D. Bahnemann, Green Chem., 2016, 18, 5391 RSC.
- J. C. Colmenares and R. Luque, Chem. Soc. Rev., 2014, 43, 765 RSC.
- J. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331 CrossRef CAS PubMed.
- D. Cambie, C. Bottecchia, N. J. W. Straathof, V. Hessel and T. Noel, Chem. Rev., 2016, 116, 10276 CrossRef CAS PubMed.
- R. Behling, S. Valange and G. Chatel, Green Chem., 2016, 18, 1839 RSC.
- V. Nair and R. Vinu, J. Anal. Appl. Pyrolysis, 2016, 119, 31 CrossRef CAS.
- S. H. Li, S. Liu, J. C. Colmenares and Y. J. Xu, Green Chem., 2016, 18, 594 RSC.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2016, 55, 2 CrossRef PubMed.
- Y. Su, N. J. W. Straathof, V. Hessel and T. Noel, Chem. – Eur. J., 2014, 20, 10562 CrossRef CAS PubMed.
- J. D. Nguyen, B. Reiß, C. Dai and C. R. J. Stephenson, Chem. Commun., 2013, 49, 4352 RSC.
- Z. J. Garlets, J. D. Nguyen and C. R. J. Stephenson, Isr. J. Chem., 2014, 54, 351 CrossRef CAS PubMed.
- S. Kuhn, T. Noel, L. Gu, P. L. Heider and K. F. Jensen, Lab Chip, 2011, 11, 2488 RSC.
- J. C. Colmenares, E. Kuna and D. Łomot, Polish application Nr P.420175, January, 2017.
- S. Waheed, J. M. Cabot, N. P. Macdonald, T. Lewis, R. M. Guijt, B. Paull and M. C. Breadmore, Lab Chip, 2016, 16, 1993 RSC.
- M. Focke, D. Kosse, C. Muller, H. Reinecke, R. Zengerle and F. von Stetten, Lab Chip, 2010, 10, 1365 RSC.
- N. Wang, F. Tan, L. Wan, M. Wu and X. Zhang, Biomicrofluidics, 2014, 8, 1 Search PubMed.
- R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., 2011, 123, 7642 CrossRef.
- N. Kockmann, T. Kiefer, M. Engler and P. Woias, Sens. Actuators, B, 2006, 117, 495 CrossRef CAS.
- A. Visan, D. Rafieian, W. Ogieglo and R. G. H. Lammertink, Appl. Catal., B, 2014, 150–151, 93 CrossRef CAS.
- S. Das and V. C. Srivastava, Photochem. Photobiol. Sci., 2016, 15, 714 CAS.
- E. Taboada, I. Angurell and J. Llorca, J. Photochem. Photobiol., A, 2014, 281, 35 CrossRef CAS.
- M. Q. Yang and Y.-J. Xu, Phys. Chem. Chem. Phys., 2013, 15, 19102 RSC.
- Z. He, Y. Li, Q. Zhang and H. Wang, Appl. Catal., B, 2010, 93, 376 CrossRef CAS.
- X. Chen, L. Liu and F. Huang, Chem. Soc. Rev., 2015, 44, 1861 RSC.
- F. J. Lopez-Tenllado, S. Murcia-Lopez, D. M. Gómez, A. Marinas, J. M. Marinas, F. J. Urbano, J. A. Navío, M. C. Hidalgo and J. M. Gatic, Appl. Catal., A, 2015, 505, 375 CrossRef CAS.
- S. Verma, R. B. N. Baig, M. N. Nadagouda and R. S. Varma, ACS Sustainable Chem. Eng., 2016, 4, 2333 CrossRef CAS.
- S. Verma, R. B. N. Baig, M. N. Nadagouda and R. S. Varma, Green Chem., 2016, 18, 1327 RSC.
- J. C. Colmenares, R. S. Varma and P. Lisowski, Green Chem., 2016, 18, 5736 RSC.
- R. Munirathinam, J. Huskens and W. Verboom, Adv. Synth. Catal., 2015, 357, 1093 CrossRef CAS.
- G. Kolb, Chem. Eng. Process., 2013, 65, 1 CrossRef CAS.
- G. Varshney, S. R. Kanel, D. M. Kempisty, V. Varshney, A. Agrawal, E. Sahle-Demessie, R. S. Varma and M. N. Nadagouda, Coord. Chem. Rev., 2016, 306, 43 CrossRef CAS.
- K. Jahnisch, V. Hessel, H. Lowe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 CrossRef PubMed.
- D. D. Laskar, B. Yang, H. Wang and J. Lee, Biofuels, Bioprod. Biorefin., 2013, 7, 602 CrossRef CAS.
- F. G. Calvo-Flores and J. A. Dobado, ChemSusChem, 2010, 3, 1227 CrossRef CAS PubMed.
- C. Xu, R. A. D. Arancon, J. Labidid and R. Luque, Chem. Soc. Rev., 2014, 43, 7485 RSC.
- K. Barta, G. Warner, E. Beach and P. T. Anastas, Green Chem., 2014, 16, 191 RSC.
- L. Yang, K. Seshan and Y. Li, Catal. Today, 2017, 298, 276 CrossRef CAS.
- Z. Strassberger, P. Prinsen, F. van der Klis, D. S. van Es, S. Tanase and G. Rothenberg, Green Chem., 2015, 17, 325 RSC.
- J. Sameni, S. Krigstin and M. Sain, Bioresources, 2017, 12, 1548 CrossRef CAS.
- V. Nair, P. Dhar and R. Vinu, RSC Adv., 2016, 6, 18204 RSC.
- J. D. Nguyen, B. S. Matsuura and C. R. J. Stephenson, J. Am. Chem. Soc., 2014, 136, 1218 CrossRef CAS PubMed.
- Z. Guo, B. Liu, Q. Zhang, W. Deng, Y. Wang and Y. Yang, Chem. Soc. Rev., 2014, 43, 3480 RSC.
- C. A. Challener, Pharm. Technol., 2015, 39, 30 Search PubMed.
- G. Chatel, S. Valange, R. Behling and J. C. Colmenares, ChemCatChem, 2017, 9, 2615 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |