A review of thermochemical conversion of microalgal biomass for biofuels: chemistry and processes
Gopalakrishnan
Kumar
ab,
Sutha
Shobana
c,
Wei-Hsin
Chen
*d,
Quang-Vu
Bach
d,
Sang- Hyoun
Kim
e,
A. E.
Atabani
f and
Jo-Shu
Chang
g
aSustainable Management of Natural Resources and Environment Research Group, Faculty of Environment and Labour Safety, Ton Duc Thang University, Ho Chi Minh City, Vietnam. E-mail: gopalakrishnankumar@tdt.edu.vn
bFaculty of Environment and Labour Safety, Ton Duc Thang University, Ho Chi Minh City, Vietnam
cDepartment of Chemistry and Research Centre, Aditanar College of Arts and Science, Virapandianpatnam, Tiruchendur, Tamil Nadu, India
dDepartment of Aeronautics and Astronautics, National Cheng Kung University, Tainan 701, Taiwan. E-mail: weihsinchen@gmail.com; chenwh@mail.ncku.edu.tw; Fax: +886-6-2389940; Tel: +886 -6-2004456
eDepartment of Environmental Engineering, Daegu university, South Korea
fEnergy Division, Department of Mechanical Engineering, Faculty of Engineering, Erciyes University, 38039, Kayseri, Turkey
gDepartment of Chemical Engineering, National Cheng Kung University, Tainan 701, Taiwan
First published on 19th October 2016
Abstract
Renewable biomass sources are organic materials, in which solar energy is stored in bio-chemical bonds, and which commonly contain carbon, hydrogen, oxygen, and nitrogen constituents, along with traces of sulfur. Renewable biomass is now considered as a crucial energy resource, which is able to meet a range of energy requirements, including generating electricity and fueling vehicles. Among all the renewable energy sources, microalgal biomass is unique, since it profitably stores solar energy. It is one of the renewable sources of carbon that can be effectively converted into expedient solid, liquid, and gaseous biofuels through different conversion techniques. In this review, thermochemical conversion technologies involving microalgal biomass are highlighted, with emphasis on the background chemistry and chemical processes. Thermochemical conversion of microalgal biomass via pyrolysis, hydrothermal liquefaction, gasification, torrefaction, and direct combustion for bioenergy production from microalgal species is discussed, though there are limited literature sources available on these technologies. The unique features of hydrothermal gasification and supercritical gasification technologies are described, with the chemical reactions involved in these processes. The decomposition pathways of the main chemical components present in the microalgal biomass, such as carbohydrates and proteins, are well elucidated with the chemical pathways. The pros and cons of direct combustion are also spotlighted.
1. Introduction
Microalgal conversion technologies to produce biofuels are gaining significant attention due to their unique features, such as the abundance of biomass worldwide, as well as its high growth rate and CO2 remediation.1 Microalgal cellular components comprise carbohydrate polymers and proteins, which are all involved in thermochemical conversion for biofuel production technology.2,3 Among thermochemical and other methods for biofuel production, technologies such as pyrolysis, hydrothermal liquefaction, gasification, torrefaction, and direct combustion, are propitious due to their higher yield and also the value added by product formation.4,5 They attract major interest with respect to industrialization with low-lipid microalgal species.6 During the last decade, mostly algal biodiesel conversion has been reviewed and also practiced for a long time; however, a recent trend has been following various new approaches to make the algal biofuels viable and applicable to large-scale operations. Very recently, thermochemical conversion has procured much attention due to its wide application and economic viability.3–8 Algal biomass conversion to fuel in the form of liquid or gas is generally classified into two categories and is identified as biochemical (photo fermentation, dark fermentation, generally with the involvement of microbes) or thermochemical (torrefaction, liquefaction, pyrolysis, gasification, and direct combustion), involving catalysts and high temperature to produce char, oil, and gas.2,3Information related to pyrolysis, hydrothermal liquefaction, gasification, torrefaction, and direct combustion is not widely available in the literature. Thus, this review/perspective focuses mainly on pyrolysis, hydrothermal liquefaction, gasification, torrefaction, and direct combustion technologies, with product decomposition at various stages. To the authors’ knowledge, no research/review has been reported on the chemical pathways of decomposition of the protein and carbohydrate components, along with recent advances in the process. So, this would bring deeper knowledge to the field of applied chemistry regarding algal biofuel technologies.
2. Thermochemical conversion technologies
Microalgal biomass is a promising feedstock for biofuel production, which involves lipid extraction for the production of biodiesel via a transesterification process.4 The microalgal residues obtained after conventional transesterification are mainly composed of proteins, carbohydrates, and a fraction of unutilized lipids, which can be employed to generate liquid and gaseous fuels, and biochar solids, depending on the temperature used.4–6 This method of conversion is an alternative progression for low-lipid or post-extraction residues of high-lipid microalgal streams. It adopts thermal decomposition and chemical reformation of the organic structures into biofuels via pyrolysis, hydrothermal liquefaction (HTL), and gasification.7This process is appropriate for both dry and wet microalgal biomass, even though it is principally considered more suitable for the processing of lignocellulosic materials of first and second-generation feedstocks than microalgal biomass.8 The wet algal feedstock must undergo dewatering, followed by cost-intensive drying steps that comprise sedimentation, flocculation, dissolved-air flotation, filtration, and centrifugation. However, HTL is particularly suitable for the conversion of wet microalgae, because it is tolerant of the high moisture content of the algal feedstock, since higher quality and yield with respect to biofuel conversion is usually achieved with HTL at lower temperatures, with a longer residence time and much higher pressure.9
The thermochemical prolytic gaseous byproducts obtained are 75–83% N2, 8.2–10.5% hydrocarbons (HC) of carbon C1–C4 (CH4–C4H10 and C3H4), 7.0–8.5% CO2, 1.2–2.5% CO, and 0.37–0.73% H2 in a nitrogen atmosphere. The TCL gaseous byproducts comprise 89% dominant N2, 4–6% CO2, 1.0–1.5% HC gases, 0.2–0.3% H2, and an insignificant quantity of CO. The higher gaseous hydrocarbons consist of N-heterocycles, substituted furans, and substituted benzenes, such as toluene, styrene, xylene, and benzene, in addition to aliphatic C-chains, which are higher in the pyrolysis runs compared to the TCL runs.9 High-performance liquid chromatography (HPLC) of the water-solubles of thermochemical conversion indicates the presence of 2.0–39.28 g L−1 formate, 3.36–16.16 g L−1 predominantly acetate, and a small quantity of propionate and ethanol. TCL water-soluble products were light yellow to brown in color and were characterized by a light smoky odor, while pyrolytic products were deep brown in color with a strong odor.9
The pyrolytic solid char obtained possesses higher energy values of 23.77–26.12 MJ kg−1 when compared to 10.98 MJ kg−1 for the solid char from TCL. Thus, the chemical energy of the original microalgal biomass was changed into valuable liquids and gaseous products by TCL, whereas in pyrolysis, most of these remain as unconverted solid char.9 The pyrolysis performed at 350 °C (Pyro350) and 3.5 °C in 1 min produced more energy-dense char, with higher volatiles and lower ash contents, compared to Pyro500.9
2.1. Pyrolysis
Pyrolysis involves the thermochemical decomposition of organic structures into an energetically useful non-condensable mixture of gases, condensable liquids, and solid residues in the absence of an oxygen/trace-oxygen atmosphere10 and in the presence/absence of a catalyst.5 Commonly, three crucial stages are involved in this process, including the removal of moisture, decomposition of organic structures, and slow disintegration of residual solids. Agrawal and Chakraborty investigated the pyrolysis of Chlorella vulgaris and described the above stages.11 Similar reports have been found for Chlorella sp., Chlorella vulgaris, Nannochloropsis gaditana, Nannochloropsis sp., Potamogeton crispus, and Sargassum thunbergii,12–16 indicating that Chlorella sp. consisted primarily of water, CO2, and H2 at high temperatures and that Nannochloropsis sp. exhibited the biochemical decomposition of organic structures, in the order of carbohydrates, proteins, and lipids. The application of catalysts in the presence of Na2CO3 may influence the decomposition of carbohydrates and proteins only, due to the breakage of hydrogen bonds by Na+ ions, which facilitates pyrolysis at low temperatures. Moreover, the pyrolytic investigation of Tetraselmis chuii, Chlorella vulgaris, Chaetoceros muelleri, D. tertiolecta, and Synechococcus reveals that they have different HHVs (higher heating values).17Non-catalytic pyrolysis (direct pyrolysis) refers to the thermal degradation of organic structures and occurs moderately at 623–973 K in the absence of catalyst. The temperature and the heating rate have a noteworthy effect on the yields, and at high temperatures, the degradation is followed by the production of pyrolytic vapors that on downstream condensation yield a dark viscous bio-oil/pyrolysis oil and a nonvolatile solid biochar.18 A slow pyrolysis process, namely, carbonization and conventional pyrolysis at heating rates of 0.1–1 K s−1 with residence times in min–hours, and fast pyrolysis via fast & flash pyrolysis at 1–200 K s−1 with a residence time of <0.5–0.5 s, have been conveniently observed.19,20 Slow pyrolysis of a microalgal stream consequently yields biochar, CH4, and CO2 gaseous products. Peng et al.21 found that the slow pyrolysis of C. protothecoides yielded bio-oil that exceeded about 40 wt%, and the yield of gaseous products increased with increasing temperature. The slow pyrolysis of Spirulina sp. yielded maximum biochar and bio-oil of <35 and <30 wt%, respectively, at an optimal temperature of about 773–823 K.22Nannochloropsis sp. indicated its main composition, which comprises long-chain carbonaceous structural units assisted by different terminal groups, with an oxygen content of 30.1 wt% and a HHV of 24.6 MJ kg−1.23
Campanella et al.24 pyrolytically studied the microalgal species Scenedesmus sp. and achieved a bio-oil yield of 16–22 wt% with a HHV of about 19 MJ kg−1. They found that, among the various pathways, viz. dehydration, deamination, direct methylation, decarboxylation, decarbonylation, cyclization, dimerization, and homolysis, there was a possibility of another path through Maillard's chemistry, which involved the thermochemical conversion of carbohydrates and proteins to yield Amadori compounds. The proposed mechanism is shown in Fig. 1.24 The carbonyl group (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) of the carbohydrate reacts with the amino group (–NH2) of the amino acid to yield Schiff-base adducts. The unstable adduct undergoes Amadori rearrangement and forms keto–enol tautomers, which, on cyclization, yield liquid Amadori compounds, such as indole, phenol, and toluidine.
O) of the carbohydrate reacts with the amino group (–NH2) of the amino acid to yield Schiff-base adducts. The unstable adduct undergoes Amadori rearrangement and forms keto–enol tautomers, which, on cyclization, yield liquid Amadori compounds, such as indole, phenol, and toluidine.
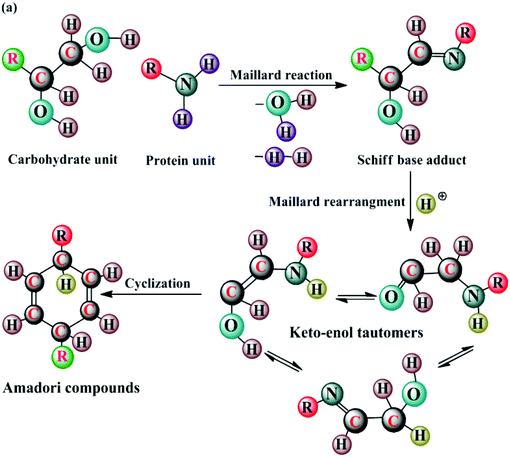 | ||
| Fig. 1 Thermochemical conversion of microalgal carbohydrates and proteins involving Maillard reaction (modified ref. 52). | ||
Fast pyrolysis is recommended, since the disadvantages of direct pyrolysis involve secondary cracking, condensation, and polymerization of the product, which decreases its HHV and high energy requirement. A fast pyrolytic investigation of the species Scenedesmus sp., Chlorella protothecoides, and M. aeruginosa showed that the bio-oil yields were 18–24 wt% with a HHV of about 29 MJ kg−1.25 Campanella and Harold26 worked on fast pyrolysis and achieved 25–30 wt% of bio-oil with a HHV of 25 MJ kg−1 in a falling solid reactor with three dissimilar atmospheres viz. nitrogen, steam, and carbon dioxide. Harman-Ware et al.27 showed that the weight ratio of bio-oil to char yielded from Scenedesmus sp. was within 3.76, via fast pyrolysis in a bench-scale fluidized bed, operated at 480 °C with a vapor residence time of 2 s, and the average total acid number for the bio-oil was 68 mg KOH g−1, which was lower than that of a bio-oil obtained from pyrolysis of wood. Miao and Wu28 examined the fast pyrolysis of heterotrophic Chlorella protothecoides microalgal feedstock and found that the bio-oil yield was about 57.9%, which was 3.4 times higher than that from autotrophic cells; they also found that heterotrophic microalgae might be better than autotrophic ones for fast pyrolysis. The bio-oil obtained was characterized by a much lower oxygen content, a higher heating value of 41 MJ kg−1, a lower density of 0.92 kg L−1, and a lower viscosity of 0.02 Pa s, compared to the corresponding values for bio-oils from autotrophic cells and wood. Table 1 shows a list of operating modes and conditions for microalgal pyrolysis, along with their bio-oil yield.
| Feedstock | Pyrolysis mode | Reactor | Operating conditions | Bio-oil yield (wt%) | |||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Heating rate (°C min−1) | Sweep gas flow rate (mL min−1) | Duration (min) | ||||
| Source ref. 108. | |||||||
| Chaetoceros muelleri | Slow | Fixed bed | 500 | 10 | 100 | 20 | 33 |
| Chlorella-like | 41 | ||||||
| Chlorella vulgaris | |||||||
| Dunaliella tertiolecta | 24 | ||||||
| Tetraselmis chuii | 43 | ||||||
| Nannochloropsis sp.(res) | 300–500 | 30 | 120 | 21–31 | |||
| Synechococcus | 500 | 100 | 20 | 38 | |||
| Spirulina platensis | 300–500 | 3.5–7.0 | 250 | 60 | 23–29 | ||
| C. protothecoides | Fast | Fluidized bed | 500 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6667 | 58 | |
| 18 | |||||||
| C. vulgaris | 400–700 | 58–72 | |||||
| C. vulgaris (res) | 500 | 53 | |||||
| M. aeruginosa | 24 | ||||||
| Scenedesmus sp. | 440 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
22 | ||||
| 480 | 55 | ||||||
| Chlorella | Catalytic (Na2CO3) | Fixed bed | 300–450 | 100–150 | 30 | 35–55 | |
| Catalytic (ZSM-5) | 400 | 250 | 29–36 | ||||
| Nannochloropsis sp.(res) | 433–644 | 30 | 120 | 21–25 | |||
| Chlorella sp. | Microwave | 650–800 | 10 | 500 | 20 | 18–27 | |
| 450–550 | 300 | 21–36 | |||||
| Chlorella vulgaris | 30 | 41–57 | |||||
| Nannochloropsis | 500 | 41–59 | |||||
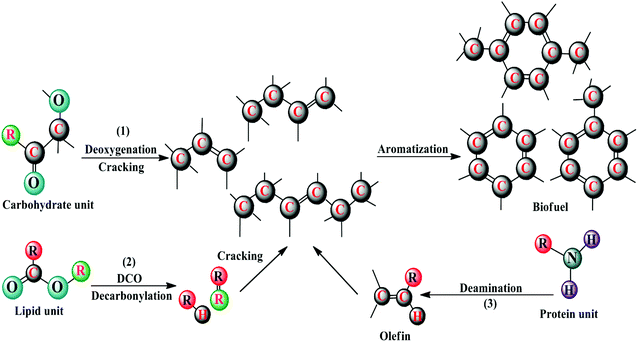
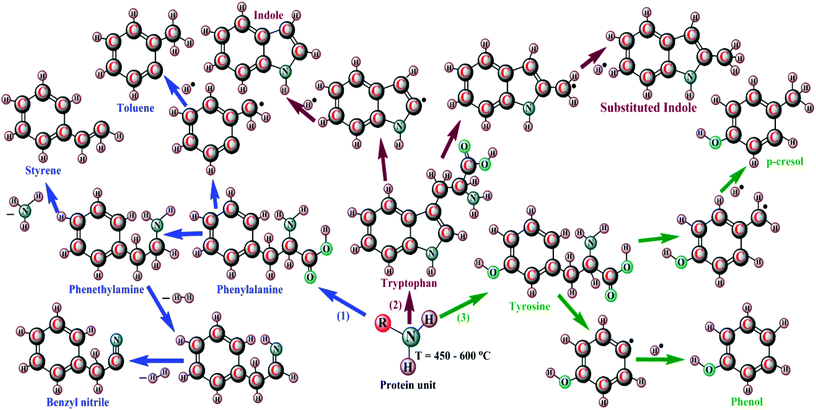
The microwave assisted pyrolysis (MAP) originally developed by Tech-in Ltd in Hainault, UK has advantages that include consistent internal heating of huge biomass particles, simplicity of control, and no necessity for agitation during the bio-oil production. Using a microwave power of 750 W, the Chlorella sp. yielded a maximum of 32 wt% of bio-oil, and using a power of 2250 W, bio-oil of about 74.9 wt% was observed with Chlorella vulgaris in the presence of activated charcoal as catalyst.29 Since the bio-oils of direct pyrolysis are acidic and viscous, they must be upgraded through a hydrogenation process for the removal of excess oxygen and alkaline contents. Furthermore, the use of a catalyst in the pyrolysis process redirects chemical reactions, such as decarbonylation, dehydration, and aromatization, which results in in situ cost-effective upgradation of bio-oil.30,31 Babich et al.30 carried out the catalytic pyrolysis of Chlorella species, with/without Na2CO3 as catalyst, and found that the catalyst promoted the gaseous yield, producing a bio-oil with low acidity and a high HHV. Campanella and Harold,26 Wang and Brown,32 Du et al.33 and Hu et al.34 investigated the bio-oil yield of Chlorella species in the presence of HZSM-5, Hβ, & HY molecular sieves and achieved high value-added aromatics of about 0.9–25.8 wt%, with a 30.1–19.5 wt% decrease in oxygen content and an improved HHV of about 24.4–32.2 MJ kg−1, while there was no change with nitrogen compounds. This could be endorsed by the Brønsted acidity and precise pore structure of the zeolites. The proposed mechanism of catalytic pyrolysis includes deoxygenation and cracking of carbohydrates (1), decarboxylation, decarbonylation, and cracking of lipids (2), and deamination & cracking of proteins (3) for the bio-oil formation, which is shown in Fig. 2, and thus involves a total of three processes.35–37 In the first process, the smaller organic moieties derived from carbohydrates of microalgal biomass residues, viz. alcohols, acids, aldehydes, and ketones, undergo deoxygenation and are then cracked into C2–C6 olefins; furthermore, these olefins experience a series of aromatization reactions to yield benzene, followed by alkylation and isomerization to produce other aromatics. In the second process, the triglycerides are thermally decomposed to heavy oxygenated hydrocarbons by decarboxylation coupled with decarbonylation, and these in turn are cracked into olefins, which are consequently converted into aromatics through a series of reactions, namely, oligomerization, cyclization, and aromatization. The third process involves the conversion of proteins to olefins and then to aromatics. In addition, the yields of different products from the catalytic pyrolysis of proteins at different temperatures are shown in Fig. 3.38 The first and second processes of this proposed mechanism indicate the formation of toluene, styrene, and nitrogenated compounds, namely, amines, benzyl nitriles, and indole from phenylalanine and tryptophan, and the third process reveals the formation of phenol and cresols through a radical mechanism from the tyrosine protein within a temperature range of about 450–600 °C.
Nannochloropsis sp. yields bio-oil with a HHV of 24.6–32.7 MJ kg−1 in the presence of HZSM-5 (H–, Fe, Ni–, Cu–) catalyst over a range of temperatures, which was observed by Pan et al.39 and the enhanced yield of organic fractions is due to Diels–Alder and condensation reactions. In the case of catalytic pyrolysis, the heteroatoms derived from the microalgal biochemical constituents can only be partially removed, and hence both these methods have their own advantages and disadvantages. Hence, to improve the quality of bio-oil, co-pyrolysis of microalgal streams with other feedstocks, such as solid wastes, coal, and polymeric materials, is given much consideration, and this does not require hydrogenation at high pressure. Moreover, the quality of the bio-oil involves the transfer of hydrogen from a co-feed of a higher H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C ratio, which effectively facilitates the deoxygenation process.40,41 The literature indicates that the co-pyrolysis of microalgal species with municipal solid waste under N2/O2 & CO2/O2 atmospheres with a blending ratio of microalgae of 10–70 wt%, and with coal, decreases the yield of char-N & increases the volatile N, while the total yield of NH3 & HCN decreases, and the formation of NH3 is inversely proportional to the temperature.
C ratio, which effectively facilitates the deoxygenation process.40,41 The literature indicates that the co-pyrolysis of microalgal species with municipal solid waste under N2/O2 & CO2/O2 atmospheres with a blending ratio of microalgae of 10–70 wt%, and with coal, decreases the yield of char-N & increases the volatile N, while the total yield of NH3 & HCN decreases, and the formation of NH3 is inversely proportional to the temperature.
2.2. Hydrothermal liquefaction
Hydrothermal liquefaction (HTL) is a promising option, since it does not need a cost-intensive dewatering and drying process for the microalgal stream. It was first described in the 1940s, followed by Shell et al. in the 1980s, with batch reactors.42 It involves the thermochemical conversion of microalgae in a slurry of solvent and is carried out at moderate temperatures of about 280–623 °C and pressures of 10–25 MPa to keep the solvent in a liquid state. Moreover, it comprises water, as an eco-friendly and low-viscosity reaction medium, which can act as an acid/base catalyst embryo, as it has a high ionic product (Kw) i.e., concentration of [H3O+] & [OH−] ions, due to the self-dissociation and high solubility of organic compounds. The reaction depends on the temperature, retention time, and composition of microalgal feedstock. Moreover, the hydrogen bonding in water molecules is weakened on heating, and consequently there is a decrease in the dielectric constant and it becomes non-polar. For instance, when the water temperature rises from 25 °C to 260 °C, there is a decrease in the dielectric constant from 76.65–13.96; thereby, a simultaneous increase in Kw was observed from 10−14 to 10−11 at a temperature just below 250 °C, which all enhances the rate of acid/base catalysis in a water medium.43 In this process, the bio-oil can be separated from the water phase easily with the addition of the organic solvents, viz. dichloro/trichloro methane, tetrahydrofuran, and n-hexane, following previously determined procedures.44–47 This involves the direct conversion of lipids, including TAGs (triacylglycerides) into fatty acids, proteins into nitrogen heterocyclics, and carbohydrates, including polysaccharides and starch, into cyclic ketones and phenols; wet biomass is converted into a liquid bio-crude in the presence/absence of a catalyst in a water medium, which can sometimes act as a reactant under critical conditions, in which the temperature falls to around 280–370 °C and the pressure is maintained at approximately 10–20 MPa.48 This is a multi-stage process (Fig. 4), which involves hydrolysis, depolymerization and repolymerization/self-condensation.49,50 Hydrothermal liquefaction (HTL) is a substitute for pyrolysis and attracts much attention, as it does not need to expend energy to dewater and dry algae, as is required for other thermochemical conversions. HTL consumes a diversity of solvents, including water as the reaction medium. In addition, products such as bio-oils, water-soluble organic polar fractions, solid and gaseous residues, and a considerable fraction of oxygen from microalgal biomass, could be removed by means of dehydration/decarboxylation (DCO). In this process, carbohydrates are quickly converted into monosaccharides with a main product, glucose, which readily isomerizes into fructose, which in turn undergoes degradation and fragmentation to yield glycolaldehydes and glyceraldehydes. In addition, coke and some volatile products, such as H2, CH4, and CO, are formed. In the case of proteins, the C–N peptide linkage between the –NH2 and –COOH structural groups hydrolyzes to yield amino acids, which in turn undergo DCO and deamination, producing HCs, –NH2, –CHO, and –COOH functional groups. Lipid molecules are hydrolyzed to form stable free fatty acids (FFAs) and glycerol, which in turn forms water-soluble fractions. Partial degradation of FFAs yields long-chain HCs. The hydrolysates of carbohydrates react with protein to form nitrogen-based heterocyclics, following Maillard's path.51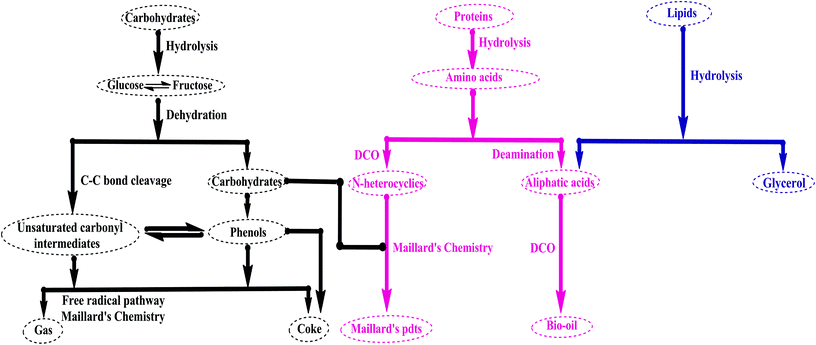 | ||
| Fig. 4 General proposed pathway of hydrothermal liquefaction (modified ref. 52 and 53). | ||
Briefly, the mechanistic pathway of bio-oil production from microalgal residues under HTL conditions reveals that the macromolecules are hydrolyzed to produce molecular chips, which include fatty acids, amino acids, and monosaccharides. Specifically, amino acids produce HCs, amines, aldehydes, and acids by DCO followed by deamination. The formed unstable intermediates, on rearrangement, form larger compounds through condensation, cyclization, and polymerization. Many researchers studied52–55 mechanistic pathways that warrant further research. Torri et al.53 proposed a mechanism for the production of bio-oil, which is a mixture of a large number of compounds, from peptides to long-chain macromolecular HCs, from Desmodesmus sp., and this comprises the pathways shown in Fig. 5. In the first step, microalgal lipids, algaenans, and some hydrophobic protein fractions, are retained in the organic solvent portion by means of solvent extraction of microalgal cellular constituents at moderately low temperatures of about 473–523 K. The cellular mass undergoes thermal degradation at temperatures below 523 K, whereby the hydrophobic proteins and carbohydrates are converted into their hydrophilic form. There is then a possibility for protein–carbohydrate–lipid interactions via several pathways. The second step includes the formation of diketopiperazines (DKP) by peptide depolymerization, and derivatives of amino acids & carbohydrates (furans) and asphaltene-like structures as a result of breakdown of proteins and cellular constituents at about 573–648 K. In addition, proteins and carbohydrates may break into small fragments (–NH2 and –CHO functionalities), even at 473 K, yielding melanoid and asphaltene-like structures. Protein degradation increases the bio-oil yield, while protein–carbohydrate-derived components amplify the nitrogen content of bio-oil. Thermal fragmentation of protein–carbohydrates yield pyrolysis-like products, viz. amino acid side chains/2-methyl cyclopentenone, and some smaller Maillard products, such as pyrroles, in the third step.
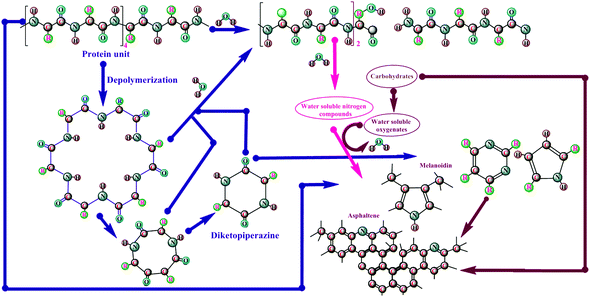 | ||
| Fig. 5 Proposed mechanism for the production of bio-oil by HTL process from microalgal biomass (modified ref. 52 and 53). | ||
Zhang et al.54 proposed a mechanical pathway for bio-oil production from microalgae under HTL conditions (Fig. 6). In the first step, the breakdown of lipids, proteins, and carbohydrates to their corresponding monomers via hydrolysis, viz. fatty acids, amino acids, glucose, fructose, xylose and phenols; in turn these monomers undergo decomposition into various intermediates. The second and third steps involve a series of reactions, in which monomers and intermediates are formed. Amines/ammonia and their corresponding keto acids are formed by amino acids on DCO (2) and deamination (3), respectively. Fatty and amino acids also form amines/ammonia by a DCO (2) mechanism. The keto and fatty acids undergo a DCO process (2) to form ketones, and the fatty acids are converted into aliphatic HCs through DCO (2). The fatty acids may experience esterification (4) and acylation (5) to yield fatty esters and amides, respectively, in the fourth and fifth steps. In the sixth step, monomers and amino acids react together via Maillard's chemistry (6) to form a nitrogenous compound, melanoidin. The seventh step involves decomposition (7) of monosaccharides to furfurals and smaller carboxylic acids. On polymerization (8), phenols and furfural derivatives form solid products. The esters are more stable, suppressing DCO as a result; there is thus a decrease in the formation of volatile products.
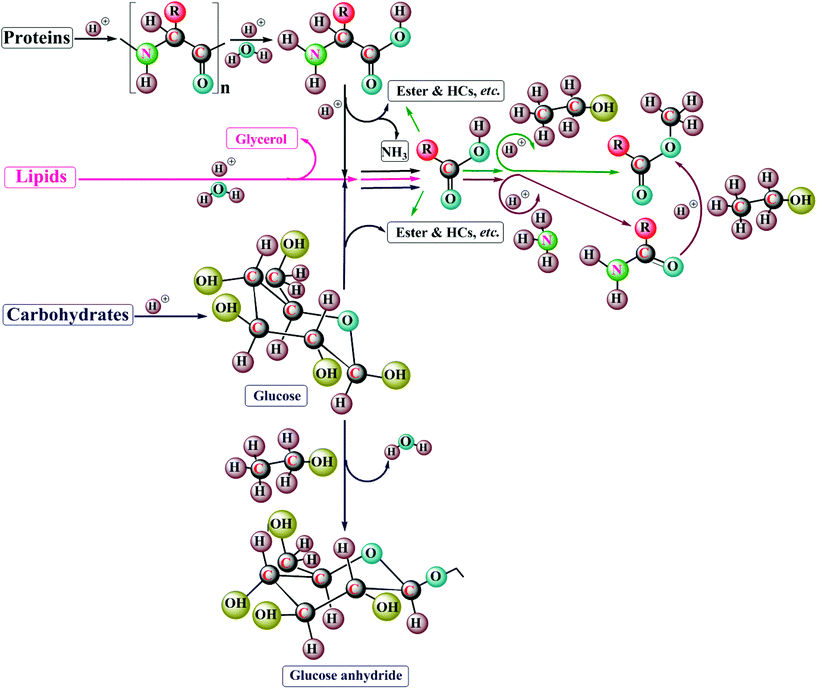 | ||
| Fig. 6 A mechanistic pathway of microalgal biomass for bio-oil production under HTL conditions (modified ref. 52 and 54). | ||
Chen et al.55 worked with sub/supercritical ethanol co-solvent–water as a weak acidic reaction medium for D. tertiolecta species to produce bio-oil under acid-catalyzed HTL conditions, and proposed a mechanical pathway (Fig. 7). This pathway involves the conversion mechanism for lipids, proteins, and carbohydrates. First, the proteins form long-chain amino acids on acidic hydrolysis; in turn, these form liquefied products via cracking, condensation, DCO, and deamination. Secondly, the carbohydrates form monosaccharides on dehydration, a part of which, on simultaneous dehydration and alcoholysis, yield glucose anhydride. Another part yields liquid products on protonation. Then, the lipid molecules, under weakly acidic conditions, form acid and glycerol on hydrolysis. Via alcoholysis and ammonolysis reaction paths, the formed acid yields ester and amide, respectively. The NH3 released from acid-catalyzed decomposition of proteins can be utilized for ammonolysis. The formed amide undergoes ethanolysis in the presence of a high ethanolic concentration to yield esters.
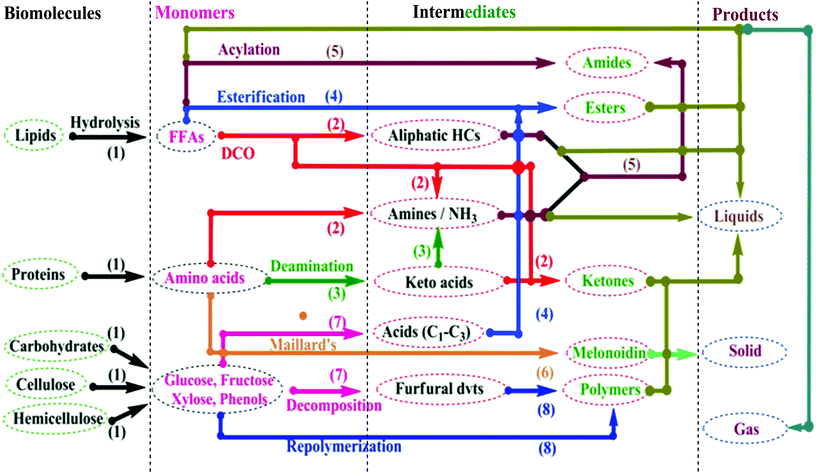 | ||
| Fig. 7 A thermochemical conversion mechanism for microalgal biomass (modified ref. 52 and 55). | ||
From Table 2, the HTL of a variety of microalgal species like B. braunii, D. tertiolecta, D. salina, C. vulgaris, N. oculata, S. platensis, and Spirulina yields more (5–30 wt%) than the initial lipid constituents. The study on B. braunii reinforces56 that the gaseous products mainly include CH4 and CO2, which is further supported by Gutierrez et al.57 Gutierrez et al. obtained 37 wt% of bio-oil with a HHV of 36 MJ kg−1 from D. tertiolecta. From low-lipid microalgal sources like Spirulina58 and Desmodesmus sp.59 it is possible to achieve bio-oil yields of 78.3 and 49 wt%, respectively, with HHVs of 22–36 MJ kg−1. Yu et al.46 obtained a low nitrogen and oxygen content bio-oil yield of 65.4 wt% and a 35.4–38.5 MJ kg−1 HHV with C. Pyrenoidosa at 553–573 K within 30–120 min. Zou et al.60 obtained a bio-oil yield of 36.9 wt% and a HHV of 26.6 MJ kg−1 with D. tertiolecta feed and a water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 at 633 K within a time period of 30 min. They ascertained that this bio-oil composition included hexadecanoic acid, palmitamide, and FAMEs with molecular formula CH1.38O0.43N0.07. Brown et al.47 and Faeth et al.61 obtained the highest bio-oil yields of 43 wt% and 66 wt%, respectively, with Nannochloropsis sp. at 623 K, using a subcritical water medium at 573–873 K within 1 minute.
10 at 633 K within a time period of 30 min. They ascertained that this bio-oil composition included hexadecanoic acid, palmitamide, and FAMEs with molecular formula CH1.38O0.43N0.07. Brown et al.47 and Faeth et al.61 obtained the highest bio-oil yields of 43 wt% and 66 wt%, respectively, with Nannochloropsis sp. at 623 K, using a subcritical water medium at 573–873 K within 1 minute.
| Hydrothermal liquefaction | Catalysts employed | Microalgal feedstock | Reaction medium | Conditions | Max. bio-oil yield (wt%) | Max. HHV (MJ kg−1) | |
|---|---|---|---|---|---|---|---|
| Temperature (K) | Holding Time (min) | ||||||
| Source ref. 26,31–34,39 and 52. | |||||||
| Noncatalytic Liquefaction | — | Chlorella pyrenoidosa | Water | 573 | 30 | 31.0 | 35.8 |
| (Direct liquefaction) | 553 | 120 | 39.4 | 35.4 | |||
| Ethanol | 443–643 | 5–120 | 64.6 | 38.9 | |||
| Desmodesmus sp. | Water | 448–723 | 5–60 | 49.0 | 36.0 | ||
| Dunaliella tertiolecta | 633 | 30 | 36.9 | 26.6 | |||
| Nannochloropsis salina | 493–648 | 30 | 46.0 | 38.1 | |||
| Nannochloropsis sp. | 473–773 | 60 | 43.0 | 39.0 | |||
| 873 | 1.0 | 66.0 | 37.0 | ||||
| Scenedesmus | 573 | 30 | 45.0 | 35.5 | |||
| 553 | 120 | 39.4 | 35.4 | ||||
| Spirulina | 573 | 30 | 32.6 | 34.7 | |||
| Ethanol | 623 | - | 35.4–45.3 | 32.6 | |||
| Water | 448–723 | 5–60 | 38.0 | 35.2 | |||
| 573 | 30 | 31.0 | 35.8 | ||||
| Ethanol | 653 | 20 | 54.0 | 38.3 | |||
| Methanol | 55.1 | 39.8 | |||||
| 1,4-Dioxane | 56.6 | 36.8 | |||||
| Water | 573 | 30 | 32.6 | 34.7 | |||
| Spirulina platensis | 623 | 60 | 39.9 | 39.9 | |||
| 623 | 41.0 | 34.6 | |||||
| Homogenous catalysis | Na2CO3 | Botryococcus braunii | 473–613 | 64.0 | — | ||
| HZSM-5/RANEY® Ni | Chlorella pyrenoidosa | Ethanol | 473–573 | 30 | 71.3 | 36.2 | |
| Na2CO3 | Chlorella vulgaris | 573 and 623 | 35.8 | 37.1 | |||
| Water | 573 and 613 | 30 and 60 | 27.3 | 37.9 | |||
| HCOOH | 623 | 27.0 | 33.2 | ||||
| H2SO4 | Dunaliella tertiolecta | Ethylene glycol | 443 | 33 | 45.0 | 28.4 | |
| Na2CO3 | Water | 43.8 | 36.0 | ||||
| 25.8 | 30.7 | ||||||
| Na2CO3 | Enteromorpha prolifera | 493–593 | 5 and 60 | 23.0 | 30.0 | ||
| Microcystis viridis | 573 and 613 | 30 and 60 | 33.0 | ||||
| HCOOH | Nannochloropsis oculata | 573 and 623 | 60 | 34.3 | 34.3 | ||
| 623 | 26.0 | 39.6 | |||||
| Porphyridium cruentum | 573 and 623 | 20.0 | 36.3 | ||||
| 623 | 27.1 | ||||||
| H2SO4 | Sargassum polycystum | Ethylene glycol | 443 | 15 | 87.7 | — | |
| KOH | Spirulina | Water | 573 and 623 | 20.0 | 39.9 | ||
| Na2CO3 | 623 | 60 | 29.0 | 36.8 | |||
| FeSO4 | Ethanol | 633 | 46.0 | 37.1 | |||
| HCOOH | Water | 623 | 60 | 29.0 | 35.1 | ||
| Ca3(PO4)2/NiO | Spirulina platensis | 573–623 | 30–60 | 34.5 | 38.4 | ||
| Heterogeneous catalysis | Co/Mo/Al2O3 | Chlorella vulgaris | 623 | 60 | 38.7 | 39.1 | |
| RANEY® Ni | Dunaliella salina | 473 | 60 | 72 | 30.1 | ||
| Ni/Al/Al2O3 | Nannochloropsis oculata | 30.0 | 38.2 | ||||
| Pt/Al/Al2O3 | 38.9 | ||||||
| Pd/C; Pt/C; Ru/C; Ni/SiO2-Al2O3;CoMo/γ-Al2O3/Zeolite | Nannochloropsis sp. | 57.0 | 38.0 | ||||
| Fe(CO)5-S | Spirulina | 573 and 613 | 30 and 60 | 78.3 | 33.0 | ||
Amongst the small bench scale studies, Jena et al.62 conducted a comparatively large-scale study on S. platensis (20 wt%) in a 1.8 L batch reactor at 623 K for 60 min, and obtained a yield of bio-oil ≈40 wt% with a HHV of 39.9 MJ kg−1. Moreover, Jazrawi et al.63 developed and Elliott et al.64 followed a continuous flow, pilot scale HTL for Chlorella and Spirulina species and accomplished 41.7 wt% bio-oil yields at 623 K for 3 min and at a moderate pressure of 20 MPa in the case of the Chlorella species. Based on the experimental data, the highest 66 wt% bio-oil yield was achieved at reaction temperatures of 573–873 K within 1 minute for Nannochloropsis sp.61 The high heteroatomic profile of bio-oils will probably lead to upgrading requirements before their application. Thus, the overall bio-oil yield depends on the cellular carbohydrate and protein profiles of the microalgal species. It also depends on the kinetics of depolymerization and the methods adopted under the HTL experimental conditions. Moreover, the bio-oil yield of different microalgal species depends on the optimal reaction parameters, higher heating values (HHV) and product distributions.47 Furthermore, both the carbon and water contents effectively determine the HHV.
An improvement in the quality of bio-oil can be achieved either by homogeneous or heterogeneous catalysis. The other methods involve the use of co-solvents/co-liquefaction for microalgal HTL. The majority of studies highlight non-catalytic and homogeneous catalytic HTL; only a few used heterogeneous catalysis (Table 2).52 The homogeneous catalyzed HTL employs alkali salts to improve gasification and the water gas shift reaction to increase the bio-oil yield; these raise the pH, inhibiting the dehydration of biomass monomers, so that the reaction involves deoxygenation and dehydration instead of DCO in the case of acid catalysis; as a result there is simple polymerization of char and tar. Dote et al.65 carried out homogeneous Na2CO3 base-catalyzed HIL for B. braunii and D. tertiolecta and observed a bio-oil yield of 28–32 wt%, with a decrease in oxygen content from 24.2 to 19.7 wt%56 at 573–613 K. Similar results56,66–68 were observed for B. braunii and D. tertiolecta with 5% Na2CO3, and the bio-oil yield followed the order, lipids > proteins > carbohydrates. In certain cases, a catalyst like FeS does not improve the yield, but alters the distribution of products, such as ethyl hexadecanoate.52 Ross et al.68 reported the effect of an alkaline catalyst that induced saponification in the case of high-lipid microalgal species. They carried out HTL with KOH and Na2CO3, as well as CH3COOH and HCOOH mixtures, as catalysts for Chlorella and Spirulina species, and found an enhanced bio-oil yield, which followed the order, Na2CO3 > CH3COOH > KOH > HCOOH. In addition, they suggested that the alkaline catalysts were suitable for HTL of high-carbohydrate-content species, and acids were appropriate for high-lipid microalgal species. Jena et al.69 used alkaline-earth [Ca3(PO4)2] and transition-metal (NiO) catalysts for the microalga S. platensis and compared the bio-oil yield with that obtained using Na2CO3, revealing that there was an increase in the bio-oil yield to 51.6 wt%, which was 29.2 wt% higher than that obtained under non-catalytic conditions. The alkaline-earth and transition-metal catalysts increased the yield of gaseous products and decreased the bio-oil production. Zhou et al.70 carried out HTL using 0.3–2.4 wt% sulphuric acid–glycol as catalyst at 393–473 K for D. tertiolecta and observed a 45.0 wt% bio-oil yield, mainly composed of FAMEs and alkanes of C17–C20 carbon chains at 443 K in 33 min. Due to cost-intensive recovery for homogeneous catalysts, the use of heterogeneous catalysts is alternatively considered, since their separation in the active state is approached simply by filtration, and they are used even for low-temperature water gasification, which is essential, as the oxygen is removed in this process.71
Duan and Savage72 liquefied Nannochloropsis sp. in the presence of six heterogeneous catalysts, viz. Pd/C, Pt/C, Ru/C, Ni/SiO2–Al2O3, CoMo/γ-Al2O3 (sulfided), and zeolites. Among these, zeolites are the most widely used catalysts. Their acidity is associated with the Si/Al ratio, demonstrating high acidity with a low ratio. A possible mechanism of cleavage of the O–H bond for dehydration of hydroxyl-containing carbohydrates on aluminosilicate zeolite is given in Scheme 1.18
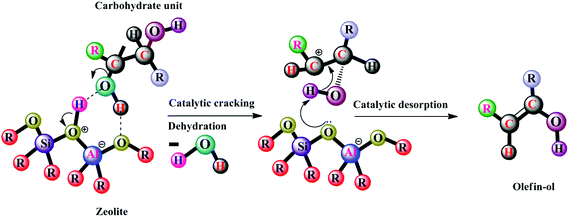 | ||
| Scheme 1 Proposed catalytic HTL pathway of carbohydrates [modified ref. 18]. | ||
The most important reactions involved in Scheme 1 are cracking and dehydration. Adsorption of the oxy-carbohydrate occurs on an acid site of aluminosilicate zeolite. This is followed by bimolecular monomeric dehydration, which yields hydroxyl-substituted olefin (olefin-ol).18 Olefin-ol, on aromatization and dehydroxylation, yields bio-oil. With oxide and transition metal catalysts, the acidity of the zeolite affects the reactivity and yields, with high acidity leading to a higher affinity towards the formation of carbon linkages and water. Pore blockage from depolymerization of carbohydrates and deamination of proteins causes deactivation of the catalyst. Zeolites form aromatics at atmospheric pressure without a requirement for H2. The final product generally has a low heating value, which is due to its low H/C and high O/C ratios. The formed olefins and aromatics increase with increasing H/Ceff ratios, suggesting that the hydrogenation of the feedstocks before the upgradation process with zeolites increases this ratio to 1.2.
The metals used play a key role in decreasing the O/C ratio, lowering the viscosity and lightening the colour, due to the promoted catalytic deoxygenation. An increase in the bio-oil yield of about 57.0 wt% was observed with a Pd/C catalyst. Ni/SiO2–Al2O3 is used for desulphurization.52 The literature revealed that NiO was used as a catalyst for both single (Spirulina) and mixed algal culture (open ponds with waste water)73 at temperatures of 573–623 K with 40.0 wt% bio-oil and a HHV of 39.0 MJ kg−1. The study52 also established that the bio-oil yield of C. vulgaris and N. oculata increased to some extent up to 10% using Ni/Co/Mo/Pt–Al2O3 heterogeneous catalysts. For a low-lipid microalgae, on HTL with RANEY® Ni/H2 and HZSM-5 catalysts, only hydrogen played a vital role in the quality and bio-oil yield, and the catalysts had no effect.54 The liquefaction reactions of Spirulina in 1-methylnaphthalene, with a smaller proportion of water in CO and Fe(CO)5, provided a conversion efficiency larger than 98.0 wt% with bio-oil, and gas and water yields were up to 83.0 wt%.59 The catalytic conversion yields bio-oil and gas composed of hydrocarbons and H2/CH4, respectively. Metal-based catalysts specifically occupy an imperative function. The recovery of the deactivated catalyst is the only inconvenience of heterogeneous catalysis.
Furthermore, water, as a single-solvent reaction medium, has some drawbacks in the HTL process, since a large portion of organic structures reside in the aqueous medium and the conversion is low; i.e. only 40% C and 35% of the feedstock were converted to bio-oil.55 Moreover, the high working temperatures (523–623 K) and pressures (10–20 MPa) consequently resulted in high O and N contents of the bio-oil, which decreased the HHV and storage stability.74 To overcome such shortcomings, organic solvents, such as 2-propanol, ethanol, and methanol, have been investigated as co-solvents, since they possess low dielectric constants to dissolve weak/non-polar reaction intermediates, leading to a higher yield of bio-oil, and they need only moderate operating circumstances.75 For example, the hydrophobic hydration of methanol–water mixtures under supercritical conditions is now attracting research in the field of chemical engineering.76,77 The literature78 showed that the low-lipid microalga C. pyrenoidosa, on HTL under sub/super-critical alcohol–water, yielded 71.3 wt% at 513 K with a HHV of 36.2 MJ kg−1 at 573 K by a deoxygenation process. Similar results were found for D. tertiolecta,75 since the sub/super-critical water presented ionic, polar non-ionic, and free radicals, and the role of alcohol as a hydrogen donor favored the production of bio-oil,79,80 which could be investigated by the isotopic tracer method.55 However, the C. pyrenoidosa species, with ethanol as a single reaction medium, yielded 9.8–84.6 wt% with a decrease in solid residue of 60.1–11.9 wt% and a HHV of 27.7–36.5 MJ kg−1 at temperatures of 443–623 K, which was due to solvent polarity. The principal components of bio-oils are FAMEs and FAEEs when ethanol and methanol are used as solvents, respectively,52 while hexadecanenitrile is the chief ingredient with the solvent 1,4-dioxan.
Currently, co-liquefaction of microalgae and coal has become a considerable attraction, since it reduces the consumption of hydrogen and the requirement for milder operations, compared to the direct liquefaction of coal. Moreover, the literature25 contained similar studies on Chlorella, Spirulina, and Littorale, with Australian Yallourn brown coal and Illinois no. 6 coal with pressurized hydrogen in 1-methylnaphthalene at 623–673 K in 60 min, using catalysts such as Fe1−xS, Fe(CO)5, and Ru3(CO)12. Under optimal conditions, D. tertiolecta and coal exhibit a synergistic effect, which not only improves bio-oil conversion (70.6 wt%) efficiently, but also improves the quantity (40.3 wt%) and quality of the bio-oil.81 In this case, microalgae can act as hydrogen donors, due to their high hydrogen content. In addition, the co-processing of microalgal biomass with waste polymer increases both the quantity and quality of the bio-oil production with a HHV of about 40 MJ kg−1, as this possesses high C and H contents. The literature82 revealed that co-liquefaction of Spirulina species and HDPE (high density polythene) with a 4/6 feed ratio in sub/super critical ethanol–water mixtures at 613 K yielded 44.8 wt% of aliphatic HC-dominant bio-oil with a HHV of 48.4 MJ kg−1. This was due to the high C and H contents and low O content. Here, microalgae could act as hydrogen receivers as they possessed a lower hydrogen content than the polymer. Table 3 shows a comparision of pyrolysis and HTL processes with microalgal species.9
| Parameters | Hydrothermal liquefaction | Slow pyrolysis |
|---|---|---|
| Source ref. 52. | ||
| Nature of feedstock | Suitable for wet feedstock (80%). Removal of moisture is done by means of bio-flocculation, dissolved air filtration & centrifugation | Suitable for dried feedstock by means of thermal methods |
| Reaction temperature (K) | 623 | 623–773 |
| Pressure (MPa) | 5–20 | atm. Press. |
| Conversion (wt%) | 93.0 | 60.0–72.0 |
| Bio-oil yield (wt%) | 40.7 | 23.8–28.5 |
| HHV (MJ kg−1) | 34.2 | 29.3–33.6 |
| Energy recovery from the original algae (%) | 67.9 | 33.9–46.7 |
| Energy consumption ratio | 0.70 | 2.11–1.56 |
| Thermal stability | High | Low |
| Storage stability | High | Low |
| Upgradation of bio-oil | Easy approach | Hard approach |
| Presence of Inorganic elements | High | Low |
| Cyclic oxygenates (wt%) | 08–12 | 16–24 |
| Low-boiling compounds (wt%) | 45–54 | 62–66 |
| Mean bio-oil mol wt (Da) | 700–1330 | 280–360 |
2.3. Gasification
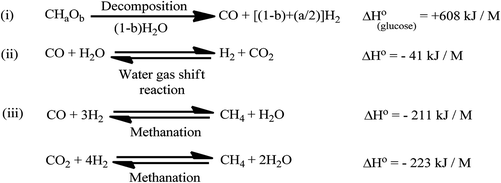
Hydrothermal gasification is the gasification of carbonaceous components in a stream of supercritical water at temperatures above 374 °C and pressures above 22.1 MPa to yield CH4. Under these experimental conditions, water possesses properties of both the gaseous and liquid states, and the viscosity and diffusivity of water are comparable to those of gases. Hydrothermal gasification is an eco-friendly conversion of microalgal biomass into biofuels, which involves the partial oxidation of microalgal biomass in steam at high temperatures, yielding a mixture of combustible gases of low calorific values (≈ 4–6 MJ kg−1) in a gasifier. The product gas constitutes CH4, CO2, and N2 and can be burned directly to produce energy to run diesel/gas turbine engines.83 The general reaction pathway is shown in Fig. 8. Hydrothermal gasification does not involve drying of biomass, even that with a high water content.5,84 It can be carried out at dissimilar temperatures and pressures/in the presence of a catalyst. Hydrothermal gasification can be non-catalytic at high temperatures (>500 °C), catalytic under sub-critical conditions (225–265 °C & 2.9–5.6 MPa)85 and low-temperature catalytic under supercritical conditions (≈ 500 °C).86 Gasification involves three main reaction pathways (Fig. 9).87 The endothermic decomposition steps of the biomass (CHaOb) include (Scheme 2) the formation of H2 and CO, followed by a water–gas shift exothermic reaction; this produces further H2 and CO2. This step is continued by means of exothermic methanation by the previously formed CO and CO2 to yield water and CH4.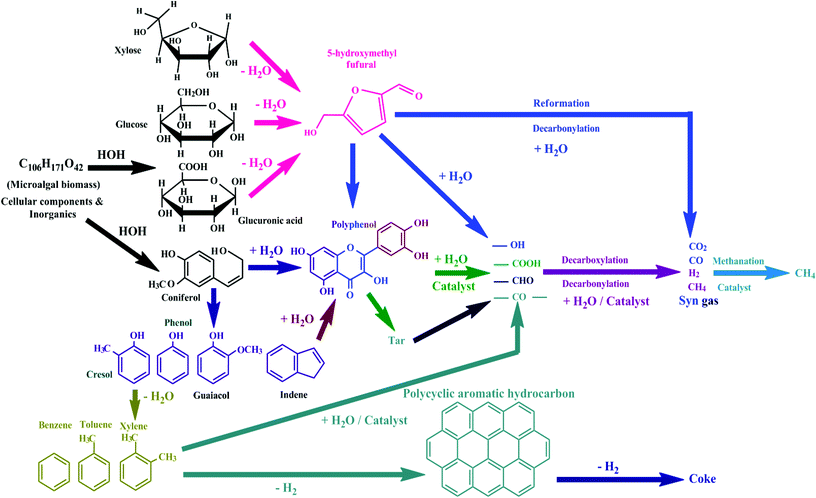 | ||
| Fig. 8 General reaction pathway of hydrothermal gasification (source ref. 84–99). | ||
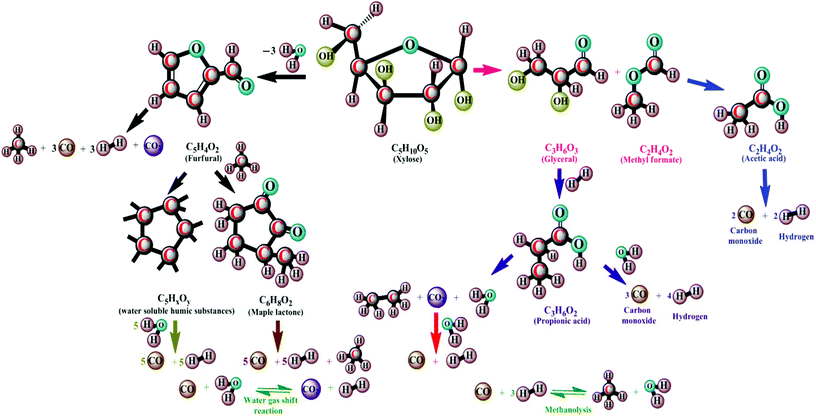 | ||
| Fig. 9 Carbohydrate decomposition mechanism in hydrothermal gasification (modified ref. 84–99). | ||
Supercritical water gasification (SCWG) is an alternative to both conventional gasification and anaerobic digestion pathways for wet biomass with/without catalysts under batch/continuous conditions into biofuels. Gassner et al.88 and van der Meijden et al.89 compared both conventional gasification and SCWG, and determined their energy efficiencies. Understanding the gasification path is somewhat challenging due to the complex structures of biomass. Moreover, the decomposition of microalgal cellular monomers was established by Kabyemela et al.90 and Matsumura et al.91
The mechanism reveals that (Fig. 9) the carbohydrate in the form of glucose/xylose isomerizes under ionic conditions to form fructose. On decomposition at temperatures of 300–400 °C, it yields acids and furfurals as intermediates under free-radical conditions.92 The smaller organic acid moieties formed then undergo decomposition by means of dehydration and decarboxylation to produce gaseous products at temperatures of 450–650 °C.93,94 The furfurals undergo polymerization under subcritical conditions into the char form. On gasification, amino acids undergo decomposition by means of hydrolysis in sub- and supercritical water at temperatures of around 250–265 °C.95 The amino acids can be converted into amino compounds and acid intermediates with the release of NH3 gas (Fig. 10). The acid intermediates on decomposition produce smaller intermediates and gases. The presence of a solid, black product is observed, in addition to liquids and gases at 500 °C.96–99 Surveys of the mechanisms are thus given in Fig. 8–10. A comparison of the thermochemical conversion of microalgal biomass with biofuel yield is given in Table 4. An investigation of methanol production from Spirulina at temperatures of around 850–1000 °C shows that at 1000 °C, a high methanol yield can be obtained.100Fig. 11 depicts the existing integration between algal cultivation and hydrothermal gasification processes.101
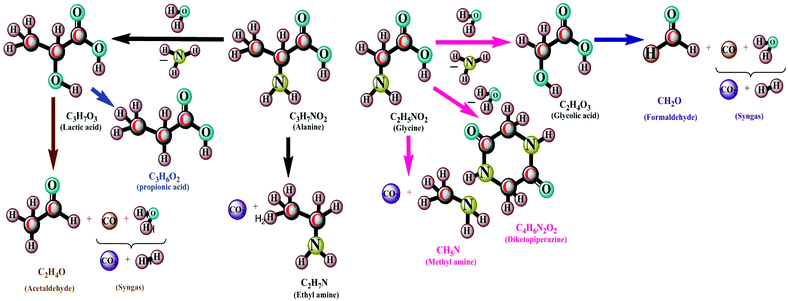 | ||
| Fig. 10 Protein decomposition mechanism in hydrothermal gasification (modified ref. 84–100). | ||
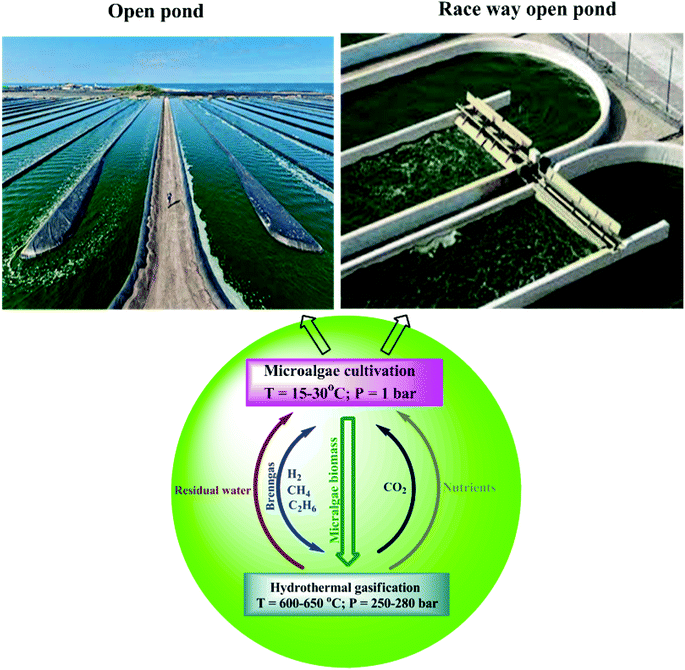 | ||
| Fig. 11 Integration between algal cultivation and hydrothermal gasification process (modified ref. 101). | ||
| Species | Scale | Conversion methods and Conditions | Yield (% dry wt) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Solid | Liquid bio-oil | Gaseous | ||||||
| Charcoal | Content | HHV (MJ kg −1) | Properties/Elemental composition | |||||
| Chlorogloeopsis fritschii | Lab-scale | Hydrothermal liquefaction, 300 °C and 350 °C (accompanied with nutrient recycling of aqueous phase) | ∼10.0 | 38.6 | O (19.0), C (66.5), H (07.2), N (06.8) | ∼13.0 | 38, 49 and 52 | |
| Chlorella protothecoides | Fast pyrolysis, heterotrophic, at 500 °C, 0.101 MPa, heating rate of 600 °C s−1, a sweep gas (N2) flow rate of 0.4 m3 h−1, a vapor residence time 2–3 s. | 11.2 | 57.2 | 41.0 | An average of low oxygen content O (11.2), C (76.2), H (11.6), a density of 0.92 kg l−1, viscosity of 0.02 Pa s (at 40 °C) | 32.0 | 16, 20, 38 and 52 | |
| Fast pyrolysis, phototrophic, at 500 °C, 0.101 MPa, heating rate of 600 °C s−1, a sweep gas (N2) flow rate of 0.4 m3 h−1, a vapor residence time 2–3 s. | 53.8 | 16.6 | 30 | O (19.4), C (62.1), H (08.8). A density of 1.06 kg l−1, viscosity of 0.10 Pa s (at 40 °C) | 32.0 | 45, 52, 53 and 61 | ||
| Fast pyrolysis, at 775 °C, 0.101 MPa, a heating rate of 10 K s−1 | 08.4 | 55.3 | 39.7 | — | 36.3 | 16 and 31 | ||
| Chlorella pyrenoidosa | Non-catalytic hydropyrolysis, temperature 310 °C, time 60 min and H2 pressure 3 MPa (a stainless steel batch) | 12.3 | 53.2 | 37.3 | Low oxygen content, O (7.6), C (72.9), H (9.8), N (9.7) | 18.5 | 31, 38, 61, 68 and 108 | |
| Chlorella sp. | Fast pyrolysis, non catalytic and catalytic using Na2CO3 catalyst, at 300–450 °C (fixed-bed reactor) | 48 & 55 at 300 °C | 55 & 40.5 at 450 °C | 27 & 33 at 400 °C & 450 °C | Low oxygen content (33.2), lower acidity, higher aromatics | 23 & 34 at 400 °C | 16, 52, 75 and 100 | |
| Pilot-scale | Microwave-assisted pyrolysis, catalyst, power of 500, 750, 1000 and 1250 W, (462–627 °C), 20 min | ∼25.0 at 750 W | 28.6 at 750 W | 30.7 at 750 W | O (16.5), C (65.4), H (7.84), N (10.3) a density of 0.98 kg L−1 (at 30 °C), a viscosity of 61.2 cSt at 750 W | 27.0 at 750 W | 45, 53, 61, 68 and 100 | |
| Chlorella vulgaris | Lab scale | Catalytic pyrolysis using H+ZSM-5 catalyst, at 500 °C (fixed-bed) | 25.7 | 52.7 | 18.6 | O (24.8), C (51.4), H (10.4), N (12.4), high hydrocarbons (∼25%) | 21.6 | 20, 31, 38 and 49 |
| Hydrothermal liquefaction, 350 °C, ∼200 bar in either pure distilled water, or 1 M base Na2CO3 or 1 M of the organic acid HCOOH (batch reactor) | ∼03.0 | ∼39.0 | 337.1 | O (14.8), C (73.6), H (10.7), N (5.9) | ∼28.0 | 16, 20, 31, 38, 45, 49, 52, 53 and 68 | ||
| Microwave-assisted pyrolysis, power of 750, 1500 and 2250 W | ∼90 Solid residues | 35.8 at 1000 W | — | — | 52.4 at 2250 W | 49 and 52 | ||
| Reactor | ∼08.0 | 46.6 | 37.5 | O (09.3), C (75.9), H (09.0), N (05.3) | ∼12.0 | 52 and 53 | ||
| Microcystis aeruginosa | Lab scale | Fast pyrolysis, phototrophic, 500 °C, 0.101 MPa, a heating rate of 600 °C min−1 at residence time of 2–3 s (in fluid bed reactor) | ∼21.0 | 24.0 | 29.0 | O (21), C (62.1), H (08.2), A density of 1.06 kg l−1, viscosity of 0.10 Pa s | ∼54.0 | 31, 38, 61, 68 and 100 |
| Nannochloropsis oculata | 07.0 | ∼37.0 | 39.0 | O (18.9), C (74.7), H (10.6), N (4.3) | ∼48.0 | 52, 61 and 68 | ||
| Porphyridium cruentum | ∼10.0 | ∼27.0 | 36.3 | O (13.3), C (72.8), H (09.1), N (05.7) | ∼15.0 | 20 and 100 | ||
| Fast pyrolysis, at 500–900 °C, heated by using a SK2-4-13 tube furnace (quartz tube reactor) | 30 at 500 °C | 91.09 (at 900 °C) | Syngas (heating based on energy consumption at 900 °C s 1.3391 (ppmv kJ) L−1 kW−1 h−1) | — | Syngas H2 emission rate 50.75 ppmv s−1 at 900 °C, CO 102 ppmv s−1 at 800 °C | 16, 20, 31, 38, 45, 49, 52, 53 and 100 | ||
| Scenedesmus dimorphus | Pressure reactors | ∼18.0 | 27.1 | 33.6 | O (12.6), C (73.0), H (08.2), N (05.7) | ∼08.0 | 20 and 52 | |
| Scenedesmus sp. | Bench scale | Fast pyrolysis, 480 °C and 100 kPa with a 2 s vapor residence time and 2 hours’ total run time (isothermal spouted bed/dynamic) | Oil/char 3.76 | 55.0 | 18.4 | An average of O (27.6), C (51.9), H (9.0), N (8.6) | — | 52 and 100 |
| Raw Scenedesmus biomass | Lab scale | Slow pyrolysis at 450 °C, reaction time of 2 h | 30.0 | 31.0 | 35.0–37.0 | O (10.5), C (72.6), H (9.0), N (6.5) | 12.0 | 16, 20, 31 and 38 |
| Raw Scenedesmus biomass | Hydrothermal liquefaction (HTL) at 300 °C, pressure ranging from 10 to 12 MPa | 07.0 | 45.0 | 33.0–40.0 | O (8.1), C (73.9), H (9.3), N (7.9) | 30.0 | 52, 75 and 100 | |
| Defatted Scenedesmus | Triplicate | 06.0 | 36.0 | O (8.2), C (72.6), H (8.9), N (10.0) | 41.0 | 52, 75 and 100 | ||
| Lab scale | 33.0 | 24.0 | O (10.5), C (72.2), H (8.9), N (7.8) | 21.0 | 52, 75 and 100 | |||
| Spirulina biomass | 30.0 | 24.0 | O (9.2), C (72.2), H (9.1), N (8.1) | 15.0 | 49 and 52 | |||
| Batch | 11.0 | 31.0 | O (9.2), C (71.2), H (9.0), N (9.2) | 35.0 | 45, 49 and 52 | |||
| Spirulina platensis | Lab-scale | Pyrolysis at 500 °C, 60 min, heating rate 7 °C min−1 (batch reactor) | 25.6 | 29.0 | 33.62 | O (6.81), C (74.7), H (10.8), N (7.13) | 28.0 | 38, 45, 49, 52 and 68 |
| Pyrolysis at 350 °C, 60 min, heating rate 3.5 °C min−1 | 39.7 | 23.0 | 29.3 | O (11.3), C (67.5), H (9.82), N (10.7) | 19.2 | 52 | ||
| Thermochemical liquefaction (TCL), at 350 °C, 60 min, heating rate 3.5 °C min−1, 2 MPa | 05.7–07.0 | 39.9–41.0 | 34.21 | O (10.1), C (73.7), H (8.90), N (6.30), TCL bio-oil better in quality and stability compared to pyrolysis oil | 22.0–23.2 | 38, 45, 49, 52 and 68 | ||
| Batch | ∼02.0 | 35.5 | 36.1 | O (11.5), C (72.7), H (08.8), N (06.3) | ∼05.0 | 52 | ||
| Spirulina sp. | ∼07.0 | ∼27.0 | 36.8 | O (10.9), C (75.4), H (10.8), N (07.0) | ∼32.0 | 49 and 52 | ||
| Blue-green algae blooms | Lab-scale | Pyrolysis, at 500 °C, particle size below 0.25 mm and sweep gas flow rate of 100 mL min−1 (fixed bed reactor) | 25.0 | 55.0 | 31.9 | O (14.5), C (67.6), H (8.95), N (7.75), high level of long-chain alkanes | 20.0 | 38, 45, 49, 52 and 100 |
2.4. Torrefaction
The aforementioned thermochemical conversion processes of microalgal biomass may be cost-intensive, and offer low efficiency due to some inherent characteristics of this biomass, which remarkably include low carbon content and high water content. These drawbacks dramatically reduce the HHV and consequently the volumetric energy density of microalgal biomass.102 In addition, the presence of a high water content increases the operating cost for dewatering or drying prior to further conversions, such as pyrolysis, gasification, and combustion. The high water content in microalgae also decreases the overall conversion efficiency due to energy lost in the form of latent heat to vaporize water during these processes.102,103Recently, torrefaction has been recognized as an effective method to upgrade biomass into more energy-dense and hydrophobic solid fuels, which can effectively overcome the aforementioned drawbacks when microalgal biomass is thermochemically converted.104 In recent years, a large number of torrefaction studies and reviews have focused on lignocellulosic biomass materials, but only a few studies employed microalgae or their residues as feedstocks.102–108 Nevertheless, it is worthy to have an overview of the torrefaction process of microalgal biomass, because the composition of lignocellulosic biomass is significantly different from that of microalgal biomass, which may show different behaviors during the torrefaction process and have different effects on the biochar products. Torrefaction is defined as a thermal pretreatment process of biomass in an oxygen-free atmosphere over a temperature range of 200–300 °C. Within this range, this can be classified into three different severities: light (200–235 °C), mild (235–275 °C), and severe (275–300 °C) torrefaction.3
A study by Wu et al.102 showed that an increase in the torrefaction temperature and residence time of Spirulina platensis increased the carbon, ash, and fixed carbon contents, as well as the HHV and hardgrove grindability index (HGI). The HHV of a microalga torrefied at 300 °C for 30 min increased from 20.46 to 25.92 MJ kg−1, and the upgraded biomass was consequently more suitable for partial replacement of the coal employed in industry. The torrefaction kinetics for the thermogravimetric analysis (TGA) indicated that the activation energy of Scenedesmus obliquus CNW-N under isothermal torrefaction was 57.52 kJ M−1, whereas it was in the range 40.14–88.41 kJ M−1 for non-isothermal torrefaction, as demonstrated by Chen et al.103 A comparative study between isothermal and non-isothermal torrefaction revealed that the latter gave more severe pretreatment of microalgae than the former at the same mean temperature. According to the study by Chen et al.3 torrefaction of microalgae involves two main stages: (1) dehydration (below 200 °C) and (2) depolymerization, decarbonization, and cracking due to the thermal decomposition of proteins and carbohydrates (above 200 °C). Furthermore, recent TGA and Fourier transform infrared (FTIR) spectra108 clearly suggested that carbohydrate is first destroyed with increasing torrefaction severity, followed by protein consumption.
Similar to lignocellulosic biomass, the most important torrefaction parameter is temperature, followed by the residence time. On the other hand, the main indicators for torrefaction include solid yield and enhancement (or energy densification) factors, which reveal the mass ratio of the remaining solid to its parent biomass, as well as the energy densification in the solid, respectively. Based on these two indicators, the energy yield of torrefaction can be obtained.106 Some microalgae contain high contents of lipids, which are valuable oil sources for biodiesel production. It can be seen in the literature that most torrefaction studies focus on the utilization of the residues from microalgae, which undergo oil-extraction to produce solid fuels. Table 5 summarizes the results from the torrefaction studies of microalgae and microalgal residues, showing the two most important indicators of the solid yield and the enhancement factor. The table reveals that the solid yield and the enhancement factor after a microalgal biomass torrefaction (for 30 min) are 51–95% and 1.03–1.46, respectively. However, the solid yield may drop to 40% if the residence time is as long as 60 min. Higher torrefaction temperatures and longer durations increase the fuel quality of torrefied products, but severe conditions consume more energy and lead to a lower energy yield.106,107 Consequently, process optimization becomes important in selecting the optimal operating conditions in terms of fuel quality and energy consumption.
| Microalgal species | Torrefaction condition | Solid yield (%) | Enhancement factor | Ref. |
|---|---|---|---|---|
| Spirulina platensis | 200 °C – 30 min | 90 | 1.07 | 98 |
| 250 °C – 30 min | 76 | 1.07 | ||
| 300 °C – 30 min | 62 | 1.27 | ||
| Spirulina platensis residue | 200 °C – 30 min | 96 | 1.05 | 98 |
| 250 °C – 30 min | 91 | 1.06 | ||
| 300 °C – 30 min | 72 | 1.18 | ||
| Chlorella vulgaris ESP-31 residue | 200 °C – 60 min | 74 | 1.14 | 101 |
| 225 °C – 60 min | 68 | 1.17 | ||
| 250 °C – 60 min | 54 | 1.34 | ||
| 275 °C – 60 min | 45 | 1.43 | ||
| 300 °C – 60 min | 40 | 1.48 | ||
| Chlamydomonas sp. JSC4 residue | 200 °C – 30 min | 94 | 1.06 | 102 |
| 225 °C – 30 min | 91 | 1.08 | ||
| 250 °C – 30 min | 82 | 1.15 | ||
| 275 °C – 30 min | 68 | 1.38 | ||
| 300 °C – 30 min | 51 | 1.46 | ||
| Chlorella vulgaris ESP-31 residue | 200 °C – 30 min | 95 | 1.03 | 103 |
| 225 °C – 30 min | 89 | 1.08 | ||
| 250 °C – 30 min | 84 | 1.11 | ||
| 275 °C – 30 min | 61 | 1.30 | ||
| 300 °C – 30 min | 57 | 1.36 |
3. Biochemical characterization of microalgae
Microalgae are considered as a suitable feedstock for third-generation renewable biofuels, owing to three plentiful major biochemical components, viz. lipids, carbohydrates, and proteins (Table 6). Solid, liquid and combustible gaseous biofuels can be obtained efficiently from microalgae through torrefaction, liquefaction, and gasification thermochemical conversion technologies, respectively; these can replace fossil fuels and stop the atmospheric greenhouse effect.3 Compared to lignocellulosic biomass, functional groups with primary elemental constituents, such as C, H, O, and N, of microalgae also compensate for the potentiality towards the production of high value-added chemicals. The conventional microalgae-to-biodiesel technology necessitates high-lipid strains, which tend to have lower biomass productivity when compared to low-lipid microalgal strains, and thereby such strains need an alternative conversion strategy. Lipids that comprise saturated and polyunsaturated fatty acids with typically 14–20 carbon units, proteins, and carbohydrates consisting of D-glucopyranose units linked via β- and/or α-glycosidic bonds, make up 7–23, 6–52, and 5–23 wt%, respectively, of the weight of microalgae for high-quality biofuel yields.3| Microalgae biomass | Elemental analysis (wt%) | Composition (dry-ash-free, wt%) | HHV (MJ kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O | S | Protein | Lipid | Carbohydrate | Othersa | ||
| a By difference, others (%) = 100 − protein − lipid − carbohydrate. b The standard nutrients condition. c The nutrients starvation condition. Source ref. 3 and 108. | ||||||||||
| Chlorella | 50.20 | 7.25 | 09.30 | 33.20 | 21.20 | |||||
| Chlorella vulgaris | 45.80 | 5.60 | 04.60 | 38.70 | 29.00 | 49.50 | 19.70 | 01.80 | 18.40 | |
| Chlorella vulgaris | 53.80 | 7.72 | 01.10 | 37.00 | 06.00 | 43.00 | 51.00 | 0.000 | 24.00 | |
| Chlorella vulgaris | 42.51 | 6.77 | 06.64 | 27.95 | 41.51 | 15.67 | 20.99 | 21.83 | 16.80 | |
| Chlorella vulgaris | 43.90 | 6.20 | 06.70 | 43.30 | 54.90 | 15.50 | 29.60 | 18.00 | ||
| Chlorella vulgaris residue | 45.04 | 6.88 | 09.79 | 29.42 | 61.24 | 05.71 | 20.34 | 12.71 | 19.44 | |
| Chlorella sorokiniana CY1 residue | 18.81 | 09.90 | 35.67 | 35.62 | 20.24 | |||||
| Chlamydomonas sp. JSC4 residue | 12.18 | 06.85 | 35.70 | 45.27 | 17.41 | |||||
| Chlamydomonas reinhardtii (wild) | 52.00 | 7.40 | 10.70 | 29.80 | 47.40 | 18.10 | 34.50 | 23.00 | ||
| Chlamydomonas reinhardtii CW15+ | 50.20 | 7.30 | 11.10 | 31.40 | 45.70 | 22.40 | 31.90 | 22.00 | ||
| Dunaliella tertiolecta | 39.00 | 5.37 | 01.99 | 53.20 | 0.62 | 61.32 | 02.87 | 21.69 | 14.12 | 14.24 |
| Hapalosiphon sp. | 47.94 | 7.44 | 06.45 | 37.58 | 0.58 | 14.75 | ||||
| Nannochloropsis oculata | 39.90 | 5.50 | 06.20 | 39.00 | 20.00 | 17.00 | 24.00 | 16.80 | ||
| Nannochloropsis oceanica | 50.06 | 7.46 | 07.54 | 34.47 | 0.47 | 19.10 | 24.80 | 22.70 | 33.40 | 21.46 |
| Nannochloropsis oceanica residue | 45.24 | 6.55 | 11.07 | 36.58 | 0.56 | 18.17 | ||||
| Spirulina platensis | 46.16 | 7.14 | 10.56 | 35.44 | 0.74 | 48.36 | 13.30 | 30.21 | 08.13 | 20.52 |
| Spirulina platensis | 45.70 | 7.71 | 11.26 | 25.69 | 0.75 | 20.46 | ||||
| Scenedesmus obliquus CNW-N | 37.37 | 5.80 | 06.82 | 50.02 | 30.38 | 04.66 | 13.41 | 51.55 | 16.10 | |
The foremost challenge in the thermochemical conversion is to synthesize the preferred products with an adequate yield and purity, since most of the heteroatoms, such as O, N, and S, from the microalgal biomass are detached, and most of the C and H atoms remain in the residue; consequently, the residual constituents are transformed into liquid fuel. So, the thermochemically produced microalgal bio-oils are characteristically similar to those from some lignocellulosic biomass, except for the existence of a smaller amount of some derivatives of aromatic compounds, due to the absence of lignin moieties. Microalgal bio-oils consist of a number of N-compounds, which were produced via degradation of proteins.3,5,6
4. Direct combustion
Incineration of microalgal biomass in the presence of air at high temperatures (>800 °C) converts the chemical energy into hot gases for heating boilers, furnaces, and steam turbines, ranging from small-scale to large-scale (100–300 MW).5,108–110 Its main disadvantage is the necessity for standardized pretreatment processes, such as sun/drum/vacuum/freeze drying, grinding, centrifugation, and settling, so it is cost-intensive. These methods are proficient in reducing the moisture content up to ≈2% and adding ≈0.5–1$ kg−1 to the cost processing of microalgal biomass. It is employed mostly for microalgal biomass with less than 50% of moisture.111,112 Moreover, its biofuel conversion efficiency is more favourable than that of coal and the efficiency can be improved if combusted along with coal.5 Only limited surveys are available on the investigation of combustion of microalgal biomass, and this requires further improvement.5,83The literature62–67 showed that the hypothetical molecular structural formula of microalgal biomass is C106H171O42N16P, which can be simply represented as C5H8O2, which is based on the mass fraction and elemental analytical report. C5H8O2 is stoichiometrically similar to acetylacetone algal biomass surrogate, so the combustion reaction, neglecting the ash content, can be given as
C5H8O2 + 6.5O2 → 5CO2 + 4H2O − 2510 kJ M−1
Moreover, it is unfeasible to burn the biomass directly in an internal combustion engine; the machinery for power production could be a Rankine engine by means of a forced convection industrial boiler. A basic model of this structure has been extended in Aspen plus™ to achieve the mass and energy flow that is essential for 1 kW continuous generation, and it would demand an area ≈20 × 20 m to output 1 kW of electricity continuously at a cost of about ≈0.95 kW h−1. This is four times the cost of modern diesel production in off-grid regions.112 Besides, the pumping of algae from a separator to a drier, along with algal solid treatment and feeding into a combustor, have not yet been automated for power production design, and so this would still necessitate noteworthy engineering input.
5. Economic feasibility
Currently, more than a few countries are promoting biofuel programs,4 since the first-generation biofuels are on the commercialization scale, second-generation biofuels are moving towards the market, and third-generation biofuels are still at the laboratory scale. It is predictable that they will all achieve improvement in the direction of the industrial scale within the next few decades. The degree of the marketplace decides the diverse techniques intended for the production of bio-fuels via thermochemical conversion of microalgal biomass, which can be coupled with discrete steps in the sequence of research, development, and innovation. At this juncture, the differences among biofuels need to be well recognized by means of analytical tools, such as energy balance and GHG emissions, considering the life cycle analysis in addition to the resource demand, job generation, and the availability of necessary technologies.4The commercialization of biofuels necessitates advanced laboratory-scale processes to yield productivities that approach the theoretically probable and scale them up without losing the concern. Since algae are cultivated in water, they require dewatering and energy-intensive drying before pyrolysis. The water used in the pyrolysis process is a source of hydrogen, and all the way through reforming of pyrolytic products is one of the cost-effective approaches.3 Bio-oil refineries should be situated in close proximity to the areas producing the algal biomass, and then the stabilized bio-oil could be transported to the refineries for conversion in the direction of transportation of the liquids, which creates a trade-off between transportation costs and economies-of-scale, which can be paid for by larger pyrolysis processes.3
Since the energy-intensive drying process is not essential, HTL is a desired conversion procedure and is particularly appropriate for producing biofuel from wet microalgal feedstock in its early stages. By comparision, the HTL biofuel is more energy-intensive, as the removal of oxygen from the algal feedstock involves a decarboxylation pathway. However, there are some advantages in the HTL process with respect to microalgal biofuel production; this is cost-competitive and so financial assistance from the Government is essential. In the case of microalgal hydrothermal gasification, there are some economically feasible basic necessities required, which comprise energy for pumping the aqueous streams, heating the reaction system to maintain its temperature at the preferred levels, and recycling of the gasification products, such as residual water, salts, and CO2, which are the nutrients for algal cultivation.
The low moisture content of torrefied microalgal biomass leads to cost-effective transportation and easy packaging, along with better quality of the torrefied biofuels. Even though, these days, a number of cost-effective torrefaction technologies are available, further research is obligatory, particularly on the reaction kinetics for designing large-scale reactors. There are limited data on the feasibility of combustion of microalgae biomass and so this necessitates further research and development; however, the overall combustion efficiency may be enhanced if it is combusted along with coal.83 In our view, with sufficient progress towards conversion technologies, the microalgal biomass could potentially be an economically workable alternative for chemical energy storage.
6. Perspectives and concluding remarks
Recently, the production of renewable biofuels from microalgal species has received substantial attention, essentially contributing to the fast growth rate of microalgae, high efficiency photosynthesis and CO2 capture, and negligible competition with food crops. Moreover, microalgae-based biofuels play an significant role in energy shortages, global warming, and climatic changes. Though there is remarkable potentiality in this field, the commercial large-scale production of microalgae-based biofuels is still in the early stages. This consequently requires sustained intensive research to step up the realistic utilization of microalgae as a promising energy feedstock and the enhancement in bio-oil production, with proficient energy-efficiency of the technologies. The thermochemical conversion technologies for microalgal feedstock are still in the early stages and the concluding remarks comprise the following significant essentials.To date, the technologies available for the cultivation, harvesting, and biofuel conversion techniques for microalgae cannot achieve cost-effective microalgal biofuel production.113,114 Even though the growth rate of microalgal biomass is fast, and the cultivation circumstances probably incorporate flue gas as a carbon source, the drying operation consumes a great deal of energy compared to other plants. Microalgal biomass productivity, land area, and water requirement are key issues throughout the cultivation and harvesting. Microalgae have the ability to absorb CO2 for their growth from cement and coal-fired power plants, and to biosorb heavy metals from industrial effluents. For their growth, about 81 g dry algae m−2 per a day needs 25% of essential energy towards bio-oil production, with a land area requirement of 6.2 million acres, 8.466 × 108 m3 saline water, and 7.914 × 109 m3 fresh water.115,116 Moreover, microalgal strains with an elevated growth rate and intense energy are vital when considering genetic engineering approaches to such species. One potential cost-effective method of diminishing the microalgal production and processing the energy cost is to merge the algal cultivation with current wastewater treatment methods.
Co-processing of microalgae with waste polymers as a hydrogen source is cost-effective, in thermochemical pyrolytic techniques. Catalysts play a critical role in the production of bio-oils from microalgae by HTL techniques. Furthermore, this technique has been focused on homogeneous/heterogeneous catalysis and on non-precious-metal-based catalysts to afford major progress toward the achievement of an essential improvement in bio-oils production. An understanding of the mechanistic pathways of algal thermochemical conversions is more significant in improving the conversion procedure and conducting experiments, particularly those involving catalytic desorption. A kinetics approach is tremendously useful in microalgal conversion techniques to design pilot-scale reactors. The special effects of a variety of microalgal feedstock constituents with working parameters that facilitate a good yield and quality of bio-oils are further examined to determine the optimal processing circumstances, as such microalgal species could be used as feedstocks under suitable reaction conditions. Direct energy conversion of microalgae via gasification and direct combustion is a propitious technology, compared to other photosynthetic conversion technologies. However, the major temperature regime involved in the process should be considered critically, along with the production of some other byproducts that would make the process economical and within the bounds of possibility.
Moreover, the consideration of the chemical composition of bio-oil is obligatory in the estimation/optimization/upgradation of the thermochemical conversion techniques, and accordingly the bio-crude characterization of the liquid products has been limited to GC-MS and micro-elemental analytical techniques. Other analytical methods are spectral studies, which can be performed with a superior quantity of samples, affording more comprehensive information concerning the composition profile of all the products.
Thus, microalgal feedstocks proficiently construct renewable bio-fuels and attract research attention due to their fast growth rate, tolerancy level towards greenhouse gases, and heavy-metal biosorption from industrial effluents and waste water sources. Moreover, many studies showed that employing thermochemical techniques is related to the oil upgrading process.
Acknowledgements
This work was supported in part by the Korea Research Fellowship Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning (Grant No: 2016H1D3A1908953). The financial assistance to one of the authors (GK) from Ton Duc Thang University, Vietnam is gratefully acknowledged. This research also received funding in part from the Ministry of Science and Technology under grant numbers MOST 102-2221-E-006-288-MY3 and 105-3113-E-042A-001, Taiwan, R.O.C. The authors would like to acknowledge Erciyes University, Kayseri, Turkey for the financial support under FOA-2015-5817 and FOA-2015-5790 projects (BAP, Bilimsel Araştırma Projeleri).References
- G. Kumar, G. Zhen, T. Kobayashi, P. Sivagurunathan, S. H. Kim and K. Q. Xu, Impact of pH control and heat pre-treatment of seed inoculum in dark H2 fermentation: A feasibility report using mixed microalgae biomass as feedstock, Int. J. Hydrogen Energy, 2016, 41, 4382–4392 CrossRef CAS.
- M. R. Diaz Rey, M. Cortes-reyes, C. Herrera, M. A. Larrubia, N. Amaedo, M. Laborde and L. J. Alemany, Hydrogen rich gas production from algae biomass by low temperature catalytic gasification, Catal. Today, 2015, 257, 177–184 CrossRef CAS.
- W. H. Chen, B. J. Lin, M. Y. Huang and J. S. Chnag, Thermochemical Conversion of microalgal biomass into biofuels: A review, Bioresour. Technol., 2015, 184, 314–327 CrossRef CAS PubMed.
- T. Mutanda, D. Ramesh, S. Karthikeyan, S. Kumari, A. Anandraj and F. Bux, Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production, Bioresour. Technol., 2011, 102, 57–70 CrossRef CAS PubMed.
- N. Pragya, K. K. Pandey and P. K. Sahoo, A review on harvesting, oil extraction and biofuels production technologies from microalgae, Renewable Sustainable Energy Rev., 2013, 24, 159–171 CrossRef CAS.
- K. G. Wang, R. C. Brown, S. Homsy, L. Martinez and S. S. Sidhu, Fast pyrolysis of microalgae remnants in a fluidized bed reactor for bio-oil and biochar production, Bioresour. Technol., 2013, 127, 494–499 CrossRef CAS PubMed.
- A. Demirbas, Mechanisms of liquefaction and pyrolysis reactions of biomass, Energy Convers. Manage., 2000, 41, 633–646 CrossRef CAS.
- D. Chiaramonti, M. Prussi, M. Buffi, D. Casini and A. M. Rizzo, Thermochemical conversion of microalgae: Challenges and opportunities, Energy Procedia, 2015, 75, 819–826 CrossRef CAS.
- U. Jena and K. C. Das, Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae, Energy Fuels, 2011, 25, 5472–5482 CrossRef CAS.
- D. R. Vardon, B. K. Sharma, G. V. Blazina, K. Rajagopalan and T. J. Strathmann, Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis, Bioresour. Technol., 2012, 109, 178–187 CrossRef CAS PubMed.
- A. Agrawal and S. Chakraborty, A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis, Bioresour. Technol., 2013, 128, 72–80 CrossRef CAS PubMed.
- A. M. Rizzo, M. Prussi, L. Bettucci, I. M. Libelli and D. Chiaramonti, Characterization of microalga Chlorella as a fuel and its thermogravimetric behavior, Appl. Energy, 2013, 102, 24–31 CrossRef CAS.
- L. S. Silva, D. L. Gonzalez, A. M. G. Minguillan and J. L. Valverde, Pyrolysis, combustion and gasification characteristics of Nannochloropsis gaditana microalgae, Bioresour. Technol., 2013, 130, 321–331 CrossRef PubMed.
- D. M. Li, L. M. Chen, S. L. Chen, X. W. Zhang, F. J. Chen and N. H. Ye, Comparative evaluation of the pyrolytic and kinetic characteristics of a macroalga (Sargassum thunbergii) and a freshwater plant (Potamogeton crispus), Fuel, 2012, 96, 185–191 CrossRef CAS.
- S. P. Zou, Y. L. Wu, M. D. Yang, C. Li and J. M. Tong, Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer, Bioresour. Technol., 2010, 101, 359–365 CrossRef CAS PubMed.
- A. Marcilla, A. G. Siurana, C. Gomis, E. Chapuli, M. C. Catala and F. J. Valdes, Characterization of microalgal species through TGA/FTIR analysis: Application to Nannochloropsis sp., Thermochim. Acta, 2009, 484, 41–47 CrossRef CAS.
- S. Grierson, V. Strezov, G. Ellem, R. Mcgregor and J. Herbertson, Thermal characterisation of microalgae under slow pyrolysis conditions, J. Anal. Appl. Pyrolysis, 2009, 85, 118–123 CrossRef CAS.
- T. Dickerson and J. Soria, Catalytic fast pyrolysis: A Review, Energies, 2013, 6, 514–538 CrossRef CAS.
- E. Suali and R. Sarbatly, Conversion of microalgae to biofuel, Renewable Sustainable Energy Rev., 2012, 16, 4316–4342 CrossRef CAS.
- L. Brennan and P. Owende, Biofuels from microalgae–a review of technologies for production, processing, and extractions of biofuels and co-products, Renewable Sustainable Energy Rev., 2010, 14, 557–577 CrossRef CAS.
- W. M. Peng, Q. Y. Wu and P. G. Tu, Pyrolytic characteristics of microalgae as renewable energy source determined by thermogravimetric analysis, J. Appl. Phycol., 2000, 12, 147–152 CrossRef CAS.
- K. Chaiwong, T. Kiatsiriroat, N. Vorayos and C. Thararax, Study of bio-oil and bio-char production from algae by slow pyrolysis, Biomass Bioenergy, 2013, 56, 600–606 CrossRef CAS.
- P. Pan, C. W. Hu, W. Y. Yang, Y. S. Li, L. L. Dong, L. F. Zhu, D. M. Tong, R. W. Qing and Y. Fan, The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils, Bioresour. Technol., 2010, 101, 4593–4599 CrossRef CAS PubMed.
- A. Campanella, R. Muncrief, M. P. Harold, D. C. Griffith, N. M. Whitton and R. S. Weber, Thermolysis of microalgae and duckweed in a CO2-swept fixed-bed reactor: bio-oil yield and compositional effects, Bioresour. Technol., 2012, 109, 154–162 CrossRef CAS PubMed.
- N. Ikenaga, C. Ueda, T. Matsui, M. Ohtsuki and T. Suzuki, Co-liquefaction of micro algae with coal using coal liquefaction catalyst, Energy Fuels, 2001, 15, 350–355 CrossRef CAS.
- A. Campanella and M. P. Harold, Fast pyrolysis of microalgae in a falling solids reactor: effects of process variables and zeolite catalysts, Biomass Bioenergy, 2012, 46, 218–232 CrossRef CAS.
- A. E. Harman-Ware, T. Morgan, M. Wilson, M. Crocker, J. Zhang, K. Liu, J. Stork and S. Debolt, Microalgae as a renewable fuel source: fast pyrolysis of Scenedesmus sp., Renewable Energy, 2013, 60, 625–632 CrossRef CAS.
- X. Miao and Q. Wu, High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides, J. Biotechnol., 2004, 110, 85–93 CrossRef CAS PubMed.
- A. Marcilla, L. Catala, J. C. G. Quesada, F. J. Valdes and M. R. Hernandez, A review of thermochemical conversion of microalgae, Renewable Sustainable Energy Rev., 2013, 27, 11–19 CrossRef CAS.
- I. V. Babich, M. V. D. Hulst, L. Lefferts, J. A. Moulijn, P. O. Connor and K. Seshan, Catalytic pyrolysis of microalgae to high quality liquid bio-fuels, Biomass Bioenergy, 2011, 35, 3199–3207 CrossRef CAS.
- P. T. Williams and N. Nugranad, Comparison of products from the pyrolysis and catalytic pyrolysis of rice husks, Energy, 2000, 25, 493–513 CrossRef CAS.
- K. G. Wang and R. C. Brown, Catalytic pyrolysis of microalgae for production of aromatics and ammonia, Green Chem., 2013, 15, 675–681 RSC.
- Z. Y. Du, X. C. Ma, Y. Li, P. Chen, Y. H. Liu, X. Y. Lin, H. W. Lei and R. S. Ruan, Production of aromatic hydrocarbons by catalytic pyrolysis of microalgae with zeolites: Catalyst screening in a pyroprobe, Bioresour. Technol., 2013, 139, 397–401 CrossRef CAS PubMed.
- Z. F. Hu, X. Q. Ma and C. X. Chen, A study on experimental characteristic of microwave-assisted pyrolysis of microalgae, Bioresour. Technol., 2012, 107, 487–493 CrossRef CAS PubMed.
- A. G. Gayubo, A. T. Aguayo, A. Atutxa, R. Aguado and J. Bilbao, Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. I. Alcohols and phenols, Ind. Eng. Chem. Res., 2004a, 43, 2610–2618 Search PubMed.
- A. G. Gayubo, A. T. Aguayo, A. Atutxa, R. Aguado, M. Olazar and J. Bilbao, Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. H. Aldehydes, ketones, and acids, Ind. Eng. Chem. Res., 2004b, 43, 2619–2626 Search PubMed.
- J. D. Adjaye and N. N. Bakhshi, Production of Hydrocarbons by Catalytic Upgrading of a Fast pyrolysis bio-Oil comparative catalyst performance and reaction pathways, Fuel Process. Technol., 1995, 45, 185–202 CrossRef CAS.
- Z. Y. Du, Y. C. Li, X. Q. Wang, Y. Q. Wan, Q. Chen, C. G. Wang, X. Y. Lin, Y. H. Liu, P. Chen and R. Ruan, Microwave-assisted pyrolysis of microalgae for biofuel production, Bioresour. Technol., 2011, 102, 4890–4896 CrossRef CAS PubMed.
- P. Pan, C. W. Hu, W. Y. Yang, Y. S. Li, L. L. Dong, L. F. Zhu, D. M. Tong, R. W. Qing and Y. Fan, The direct pyrolysis and catalytic pyrolysis of Nannochloropsis sp. residue for renewable bio-oils, Bioresour. Technol., 2010, 101, 4593–4599 CrossRef CAS PubMed.
- V. I. Sharypov, N. G. Beregovtsova, B. N. Kuznetsov, L. Membrado, V. L. Cebolla, N. Marin and J. V. Weber, Co-pyrolysis of wood biomass and synthetic polymers mixtures. Part III: Characterisation of heavy products, J. Anal. Appl. Pyrolysis, 2003, 67, 325–340 CrossRef CAS.
- C. X. Chen, X. Q. Ma and Y. He, Co-pyrolysis characteristics of microalgae Chlorella vulgaris and coal through TGA, Bioresour. Technol., 2012, 117, 264–273 CrossRef CAS PubMed.
- P. E. Savage, Algae under pressure and in hot water, Science, 2012, 338, 1039–1040 CrossRef CAS PubMed.
- A. V. Bandura and S. N. Lvov, The ionization constant of water over wide ranges of temperature and density, J. Phys. Chem. Ref. Data, 2006, 35, 15–30 CrossRef CAS.
- S. S. Toor, H. Reddy, S. Deng, J. Hoffmann, D. Spangsmark and L. B. Madsen, Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions, Bioresour. Technol., 2013, 131, 413–419 CrossRef CAS PubMed.
- G. A. Laura, T. Cristian, S. Chiara, V. S. Jaapjan, F. Daniele, S. R. A. Kersten and D. W. F. Brilman, Hydrothermal Treatment (HTT) of Microalgae: Evaluation of the Process as Conversion method in algae Biorefinery concept, Energy Fuels, 2012, 26, 642–657 CrossRef.
- G. Yu, Y. H. Zhang, L. Schideman, T. Funk and Z. C. Wang, Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae, Energy Environ. Sci., 2011, 4, 4587–4595 CAS.
- T. M. Brown, P. G. Duan and P. E. Savage, Hydrothermal liquefaction and gasification of microalga Nannochloropsis sp., Energy Fuels, 2010, 24, 3639–3646 CrossRef CAS.
- P. Biller and A. B. Ross, Potential yields and properties of oil from the hydrothermal liquefaction of microalgae with different biochemical content, Bioresour. Technol., 2011, 102, 215–225 CrossRef CAS PubMed.
- X. K. Pei, X. Z. Yuan, G. M. Zeng, H. J. Huang, J. Y. Wang, H. Li and H. N. Zhu, Co-liquefaction of microalgae and synthetic polymer mixture in sub- and supercritical ethanol, Fuel Process. Technol., 2012, 93, 35–44 CrossRef CAS.
- S. Yin, R. Dolan, M. Harris and Z. Tan, Subcritical hydrothermal liquefaction of cattle manure to bio-oil; Effects of conversion parameters on bio-oil yield and characterization of bio-oil, Bioresour. Technol., 2010, 101, 3657–3664 CrossRef CAS PubMed.
- S. K. Hoekman, A. Broch, C. Robbins, E. Ceniceros and M. Natarajan, Review of biodiesel composition, properties and specifications, Renewable Sustainable Energy Rev., 2012, 16, 143–169 CrossRef CAS.
- Y. Chen, W. Yulong, H. Derun, L. Chun, M. P. Harold, W. Jianlong and Y. Mingde, Thermochemical conversion of low-lipid microalgae for the production of liquid fuels: challenges and opportunities, RSC Adv., 2015, 5, 18673–18701 RSC.
- C. Torri, L. G. Alba, C. Samorì, D. Fabbri and D. W. F. Brilman, Hydrothermal treatment (HTT) of microalgae: Detailed molecular characterization of HTT oil in view of htt mechanism elucidation, Energy Fuels, 2012, 26, 658–671 CrossRef CAS.
- J. X. Zhang, W. T. Chen, P. Zhang, Z. Y. Luo and Y. H. Zhang, Hydrothermal liquefaction of Chlorella pyrenoidosa in sub- and supercritical ethanol with heterogeneous catalysts, Bioresour. Technol., 2013, 133, 389–397 CrossRef CAS PubMed.
- Y. Chen, Y. L. Wu, P. L. Zhang, D. R. Hua, M. D. Yang, C. Li, Z. Chen and J. Liu, Direct liquefaction of Dunaliellatertiolecta for bio-oil in sub/supercritical ethanol–water, Bioresour. Technol., 2012, 124, 190–198 CrossRef CAS PubMed.
- S. Sawayama, S. Inoue, Y. Dote and S. Y. Yokoyama, CO2 fixation and oil production through microalga, Energy Convers. Manage., 1995, 36, 729–731 CrossRef CAS.
- A. Gutierrez, R. K. Kaila, M. L. Honkela, R. Slioor and A. O. I. Krause, Hydrodeoxygenation of guaiacol on noble metal catalysts, Catal. Today, 2009, 147, 239–246 CrossRef CAS.
- T. Matsui, A. Nishihara, C. Ueda, M. Ohtsuki, N. Ikenaga and T. Suzuki, Liquefaction of micro- algae with iron catalyst, Fuel, 1997, 76, 1043–1048 CrossRef CAS.
- G. A. Laura, T. Cristian, S. Chiara, V. S. Jaapjan, F. Daniele, S. R. A. Kersten and D. W. F. Brilman, Hydrothermal treatment (HTT) of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept, Energy Fuels, 2012, 26, 642–657 CrossRef.
- S. P. Zou, Y. L. Wu, M. D. Yang, C. Li and J. M. Tong, Pyrolysis characteristics and kinetics of the marine microalgae Dunaliellatertiolecta using thermogravimetric analyzer, Bioresour. Technol., 2010, 101, 359–365 CrossRef CAS PubMed.
- J. L. Faeth, P. J. Valdez and P. E. Savage, Fast hydrothermal liquefaction of Nannochloropsis sp. to produce biocrude, Energy Fuels, 2013, 27, 1391–1398 CrossRef CAS.
- U. Jena, K. C. Das and J. R. Kastner, Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis, Bioresour. Technol., 2011, 102, 6221–6229 CrossRef CAS PubMed.
- C. Jazrawi, P. Biller, A. B. Ross, A. Montoya, T. Maschmeyer and B. S. Haynes, Pilot plant testing of continuous hydrothermal liquefaction of microalgae, Algal Res., 2013, 2, 268–277 CrossRef.
- D. C. Elliott, T. R. Hart, A. J. Schmidt, G. G. Neuenschwander, L. J. Rotness, M. V. Olarte, A. H. Zacher, K. O. Albrecht, R. T. Hallen and J. E. Holladay, Process development for hydrothermal liquefaction of algae feedstocks in a continuous flow reactor, Algal Res., 2013b, 2, 445–454 Search PubMed.
- Y. Dote, S. Sawayama, S. Inoue, T. Minowa and S. Yokoyama, Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction, Fuel, 1994, 73, 1855–1857 CrossRef CAS.
- S. P. Zuo, Y. L. Wu, M. D. Yang, I. Kaleem, C. Li and J. M. Tong, Production and characterization of bio oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake, Energy, 2010, 35, 5406–5411 CrossRef.
- A. Banerjee, R. Sharma, Y. Chisti and U. C. Banerjee, Botryococcus braunii: a renewable source of hydrocarbons and other chemicals, Crit. Rev. Biotechnol., 2002, 22, 245–270 CrossRef CAS PubMed.
- A. B. Ross, P. Biller, M. Kubacki, H. Li, A. L. Langton and J. Jones, Hydrothermal processing of microalgae using alkali and organic acids, Fuel, 2010, 89, 2234–2243 CrossRef CAS.
- U. Jena, K. C. Das and J. R. Kastner, Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis, Appl. Energy, 2012, 98, 368–375 CrossRef CAS.
- D. Zhou, L. Zhang, S. C. Zhang, H. B. Fu and J. M. Chen, Hydrothermal liquefaction of macroalgae Enteromorpha prolifera to bio-oil, Energy Fuels, 2010, 24, 4054–4061 CrossRef CAS.
- S. S. Toor, L. Rosendahl and A. Rudolf, Hydrothermal liquefaction of biomass: A review of subcritical water technologies, Energy, 2011, 36, 2328–2342 CrossRef CAS.
- P. G. Duan and P. E. Savage, Hydrothermal liquefaction of a microalga with heterogeneous catalysts, Ind. Eng. Chem. Res., 2011, 50, 52–61 CrossRef CAS.
- U. Jena, N. Vaidyanathan, S. Chinnasamy and K. C. Das, Evaluation of microalgae cultivation using recovered aqueous co-product from thermochemical liquefaction of algal biomass, Bioresour. Technol., 2011, 102, 3380–3387 CrossRef CAS PubMed.
- G. W. Roberts, M. O. P. Fortier, B. S. M. Sturm and S. M. S. Williams, Promising pathway for algal biofuels through wastewater cultivation and hydrothermal conversion, Energy Fuels, 2013, 27, 857–867 CrossRef CAS.
- C. B. Xu and T. Etcheverry, Hydro-liquefaction of woody biomass in sub- and super-critical ethanol with iron-based catalysts, Fuel, 2008, 87, 335–345 CrossRef CAS.
- S. Lamanna and R. Cannistraro, Effect of ethanol addition upon the structure and the cooperativity of the water H bond network, Chem. Phys., 1996, 213, 95–110 CrossRef.
- J. H. Lee and N. R. Foster, Oxidation of methanol in supercritical water, J. Ind. Eng. Chem., 1999, 5, 116–122 CAS.
- P. G. Duan, B. B. Jin, Y. P. Xu, Y. Yang, X. J. Bai, F. Wang, L. Zhang and J. Miao, Thermo-chemical conversion of Chlorella pyrenoidosa to liquid biofuels, J. Bioresour. Technol., 2013, 133, 197–205 CrossRef CAS PubMed.
- J. Gao, Supercritical hydration of organic compounds. The potential of mean force for benzene dimer in supercritical water, J. Am. Chem. Soc., 1993, 115, 6893–6895 CrossRef CAS.
- X. Z. Yuan, H. Li, G. M. Zeng, J. Y. Tong and W. Xie, Sub- and supercritical liquefaction of rice straw in the presence of ethanol–water and 2-propanol–water mixture, Energy, 2007, 32, 2081–2088 CrossRef CAS.
- R. L. Yang, Y. Chen, Y. L. Wu, D. R. Hua, M. D. Yang, C. Li, Z. Chen and J. Liu, Production of liquid fuel via coliquefaction of coal and Dunaliella tertiolecta in a sub-/supercritical water–ethanol system, Energy Fuels, 2013, 27, 2619–2627 CrossRef CAS.
- X. K. Pei, X. Z. Yuan, G. M. Zeng, H. J. Huang, J. Y. Wang, H. Li and H. N. Zhu, Co-liquefaction of microalgae and synthetic polymer mixture in sub- and supercritical ethanol, Fuel Process. Technol., 2012, 93, 35–44 CrossRef CAS.
- I. Rawat, R. R. Kumar, T. Mutanda and F. Bux, Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production, Appl. Energy, 2011, 88, 3411–3424 CrossRef CAS.
- Z. Shen, J. X. Z. Zhou and Y. Zhang, The production of acetic acid from microalgae under hydrothermal conditions, Appl. Energy, 2011, 88, 3444–3447 CrossRef CAS.
- R. D. Cortright, R. R. Davda and J. A. Dumesic, Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water, Nature, 2002, 418, 964–967 CrossRef CAS PubMed.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Froling, M. J. J. Antal and J. W. Tester, Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies, Energy Environ. Sci., 2008, 1, 32–65 CAS.
- S. Cheah, W. S. Jablonski, J. L. Olstad, D. L. Carpenter and K. D. Barthelemy, et al., Effects of thermal pretreatment and catalyst on biomass gasification efficiency and syngas composition, Green Chem., 2016 10.1039/c6gc01661h.
- M. Gassner, F. Vogel, G. Heyen and F. Marechal, Optimal process design for the polygeneration of SNG, power and heat by hydrothermal gasification of waste biomass: Process optimization for selected substrates, Energy Environ. Sci., 2011, 4, 1742–1758 CAS.
- C. M. van der Meijden, H. J. Veringa and L. P. L. M. Rabou, The production of synthetic natural gas (SNG): A comparison of three wood gasification systems for energy balance and overall efficiency, Biomass Bioenergy, 2010, 34, 302–311 CrossRef CAS.
- B. M. Kabyemela, T. Adschiri, R. M. Malaluan and K. Arai, Glucose and fructose decomposition in subcritical and supercritical water: Detailed reaction pathway, mechanisms, and kinetics, Ind. Eng. Chem. Res., 1999, 38, 2888–2895 CrossRef CAS.
- Y. Matsumura, S. Yanachi and T. Yoshida, Glucose decomposition kinetics in water at 25 MPa in the temperature range of 448–673 K, Ind. Eng. Chem. Res., 2006, 45, 1875–1879 CrossRef CAS.
- A. Kruse and A. Gawlik, Biomass conversion in water at 330–410 °C and 30–50 MPa. Identification of key compounds for indicating different chemical reaction pathways, Ind. Eng. Chem. Res., 2003, 42, 267–279 CrossRef CAS.
- T. M. Aida, N. Shiraishi, M. Kubo, M. Watanabe and R. L. Smith, Reaction kinetics of D-xylose in sub- and supercritical water, J. Supercrit. Fluids, 2010, 55, 208–216 CrossRef CAS.
- A. K. Goodwin and G. L. Rorrer, Reaction rates for supercritical water gasification of xylose in a micro-tubular reactor, Chem. Eng. J., 2010, 163, 10–21 CrossRef CAS.
- D. Klingler, J. Berg and H. Vogel, Hydrothermal reactions of alanine and glycine in sub- and supercritical water, J. Supercrit. Fluids, 2007, 43, 112–119 CrossRef CAS.
- G. J. Dileo, M. E. Neff, S. Kim and P. E. Savage, Supercritical water gasification of phenol and glycine as models for plant and protein biomass, Energy Fuels, 2008, 22, 871–877 CrossRef CAS.
- K. Nath and D. Das, Hydrogen from biomass, Curr. Sci., 2003, 85, 265–271 CAS.
- J. Gil, J. Corella, M. P. Aznar and M. A. Caballero, Biomass gasification in atmospheric and bubbling fluidized bed: effect of the type of gasifying agent on the product distribution, Biomass Bioenergy, 1999, 17, 389–403 CrossRef CAS.
- A. Abuadala, I. Dincer and G. Naterer, Exergy analysis of hydrogen production from biomass gasification, Int. J. Hydrogen Energy, 2010, 35, 4981–4990 CrossRef CAS.
- A. Hirano, K. Hon-Nami, S. Kunito, M. Hada and Y. Ogushi, Temperature effect on continuous gasification of microalgal biomass: theoretical yield of methanol production and its energy balance, Catal. Today, 1998, 45, 399–404 CrossRef CAS.
- Wikimedia Commons, Photobioreactor PBR 500 P IGV Biotech., 2016 [cited 2016 20th January], Available from: https://commons.wikimedia.org/wiki/File:Photobioreactor_PBR_500_P_IGV_Biotech.jpg.
- K. T. Wu, C. J. Tsai, C. S. Chen and H. W. Chen, The characteristics of torrefied microalgae, Appl. Energy, 2012, 100, 52–57 CrossRef CAS.
- W. H. Chen, Z. Y. Wu and J. S. Chang, Isothermal and non-isothermal torrefaction characteristics and kinetics of microalga Scenedesmus obliquus CNW-N, Bioresour. Technol., 2014, 155, 245–251 CrossRef CAS PubMed.
- W. H. Chen, M. Y. Huang, J. S. Chang and C. Y. Chen, Thermal decomposition dynamics and severity of microalgae residues in torrefaction, Bioresour. Technol., 2014, 169, 258–264 CrossRef CAS PubMed.
- J. K. Mwangi, W. J. Lee, L. M. Whang, T. S. Wu, W. H. Chen and J. S. Chang, Microalgae oil: algae cultivation and harvest, algae residue torrefaction and Diesel engine emissions tests, Aerosol Air Qual. Res., 2015, 15, 81–98 CAS.
- W. H. Chen, M. Y. Huang, J. S. Chang, C. Y. Chen and W. J. Lee, An energy analysis of torrefaction for upgrading microalga residue as a solid fuel, Bioresour. Technol., 2015, 185, 285–293 CrossRef CAS PubMed.
- W. H. Chen, M. Y. Huang, J. S. Chang and C. Y. Chen, Torrefaction operation and optimization of microalga residue for energy densification and utilization, Appl. Energy, 2015, 154, 622–630 CrossRef CAS.
- Y. C. Chen, W. H. Chen, B. J. Lin and J. S. Chang, Ong HC. Impact of torrefaction on the composition, structure and reactivity of a microalga residue, Appl. Energy, 2016, 181, 110–119 CrossRef CAS.
- W. H. Chen, J. H. Peng and X. T. Bi, A state-of-the-art review of biomass torrefaction, densification and applications, Renewable Sustainable Energy Rev., 2015, 44, 847–866 CrossRef CAS.
- P. McKendry, Energy production from biomass (part 2): conversion technologies, Bioresour. Technol., 2002, 83, 47–54 CrossRef CAS PubMed.
- H. Goyal, D. Seal and R. Saxena, Bio-fuels from thermochemical conversion of renewable resources: a review, Renewable Sustainable Energy Rev., 2008, 12, 504–517 CrossRef CAS.
- W. Palz, J. Coombs and D. O. Hall, Energy from biomass: 3rd E.C.Conference, Commission of European Communities, Taylor and Francis, 1985, p. 159.
- L. Xia, H. Ge, X. Zhou, D. Zhang and C. Hu, Photoautotrophic outdoor two-stage cultivation for oleaginous microalgae Scenedesmus obtusus XJ-15, Bioresour. Technol., 2013, 144, 261–267 CrossRef CAS PubMed.
- D. Lopez Barreiro, W. Prins, F. Ronsse and W. Brilman, Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects, Biomass Bioenergy, 2013, 53, 113–110 CrossRef CAS.
- A. Raheem, W. A. K. G. Wan Azlina, Y. H. Taufiq Yap, M. K. Danquah and R. Harun, Thermochemical conversion of microalgal biomass for biofuel production, Renewable Sustainable Energy Rev., 2015, 49, 990–999 CrossRef CAS.
- S. P. Cuellar Bermudez, J. S. Garcia Perez, B. E. Rittmann and R. Parra Saldivar, Photosynthetic bioenergy utilizing CO2: an approach on flue gases utilization for third generation biofuels, J. Cleaner Prod., 2015, 98, 53–65 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |