Highly stable n-type thermoelectric materials fabricated via electron doping into inkjet-printed carbon nanotubes using oxygen-abundant simple polymers†
Shohei
Horike
 *a,
Tatsuya
Fukushima
*a,
Tatsuya
Fukushima
 a,
Takeshi
Saito
a,
Takeshi
Saito
 b,
Takuya
Kuchimura
a,
Yasuko
Koshiba
b,
Takuya
Kuchimura
a,
Yasuko
Koshiba
 a,
Masahiro
Morimoto
a,
Masahiro
Morimoto
 ac and
Kenji
Ishida
ac and
Kenji
Ishida
 *a
*a
aDepartment of Chemical Science and Engineering, Graduate School of Engineering, Kobe University, 1-1 Rokkodai-cho, Nada-ku, Kobe 657-8501, Japan. E-mail: horike@crystal.kobe-u.ac.jp; kishida@crystal.kobe-u.ac.jp
bNanomaterials Research Institute, National Institute of Advanced Science and Technology, 1-1-1 Higashi, Tsukuba 305-8565, Japan
cGraduate School of Science and Engineering for Research, University of Toyama, 3190 Gofuku, Toyama 930-8555, Japan
First published on 5th October 2017
Abstract
Single-walled carbon nanotubes (SWCNTs) are important candidates for flexible and non-toxic thermoelectric (TE) energy-harvesting devices because they have large Seebeck coefficients, good flexibility, and inkjet printability onto plastic substrates. Here we describe the successful n-type conversion of intrinsic p-type SWCNTs by polymer–dopant charge transfer. The negative Seebeck coefficients of the polymer-doped SWCNTs were strongly related to the highest occupied molecular orbital levels of the polymer, demonstrating that the polymers were electron donors for the nanotubes and that the doping level could be controlled by modifying the functional groups. The n-type SWCNTs obtained using oxygen-abundant polymers, such as poly(vinyl alcohol) and poly(vinyl acetate), exhibited the largest negative Seebeck coefficients and high stability under ambient conditions lasting for at least 3 weeks. Printed and folded p- and n-type SWCNTs on flexible substrates showed efficient TE voltage improvements. Our findings enable the easy, low-cost preparation of air-stable n-type SWCNTs, permitting the exploitation of SWCNTs as flexible and eco-friendly TE materials.
Design, System, ApplicationConverting intrinsic p-type single-walled carbon nanotubes (SWCNTs) into air-stable n-type materials is a challenging issue for flexible thermoelectric (TE) power modules. This study investigates a series of vinyl polymers as metal-free, nontoxic donor materials for the stable charge-type conversion of SWCNTs. TE charge-carrier analyses revealed that the polymer–dopant charge transfer successfully converted the SWCNTs into n-type materials. The negative Seebeck coefficients of the functionalized SWCNTs were correlated with the highest occupied molecular orbital (HOMO) levels of the dopant polymers. We found that doping polymers with negatively charged oxygen atoms containing lone pairs of electrons and ambient HOMO levels resulted in the largest Seebeck coefficient with excellent stability. The HOMO level calculation offers a simple but useful general principle for designing and screening new potential donor materials. Further, the stability of the induced n-type nature was attributed to the polymer dopants acting as oxygen impurity barriers and charge delocalization structures. Finally, printing and folding these SWCNTs on flexible substrates were demonstrated as a specific example for implementing charge-carrier-controlled SWCNTs in TE modules and improving the dimensional voltage output. |
Introduction
The supply of electrical power to embedded and wearable web-connected monitoring devices is an important problem. Battery replacements and power transmission by wiring are unrealistic because future systems will utilize trillions of sensors.1 For automatic sensor operation over extended periods without additional maintenance, energy-harvesting technologies have emerged as promising candidates.2–4 Thermoelectric (TE) conversion devices, which generate electrical power from temperature differences, have particularly high potential because temperature gradients are common in human life.TE materials based on organic and nanoscale carbon materials are attractive because they are flexible, non-toxic, lightweight, and versatile in processing; these advantages are uncommon among commercially available inorganic crystalline materials.5–7 Single-walled carbon nanotube (SWCNT)-based TE materials are emerging as energy-harvesting platforms because they show relatively large Seebeck coefficients and charge carrier mobilities8–11 as well as flexibility and printability onto plastic substrates.12 The latter two properties are critical for the close attachment of TE materials to curved-surface heat sources, which enables the efficient harvest of human body heat, an unused but bountiful thermal energy source.
TE devices should be modularized to contain many p- and n-type TE legs connected electrically in series and thermally in parallel; this device architecture enables the direct addition of TE voltage from each leg subjected to the same temperature difference.13,14 Therefore, both p- and n-type materials are needed to develop practical TE devices. Pristine SWCNTs and composites thereof with π-conjugated polymers have been actively investigated as suitable materials for flexible p-type TE devices; however, converting them to air-stable n-type components remains challenging.
Holes are the major charge carriers in SWCNTs, as electrons are withdrawn by oxygen upon exposure to air.15,16 A common technique to alter the major carrier species from holes to electrons is charge transfer between SWCNTs and electron donors physically adsorbed onto nanotubes.17,18 Alkali metal donors decompose into cations and electrons available for injection into the conduction bands of the nanotubes, achieving n-type conduction and negative Seebeck coefficients; however, n-type alkali metal-modified SWCNTs suffer from easy auto-oxidation and hole injection from oxygen impurities.19 Nonoguchi et al. recently coordinated SWCNTs with crown ether-based metal cations along with electron donors to stabilize n-type SWCNTs.20 Although the n-type SWCNTs were stable in air, the toxicity of crown ethers including metal cations is unknown. The use of metal-free compounds is preferred for future eco-friendly power supplies; molecular materials containing only carbon, hydrogen, oxygen, and nitrogen are extremely desirable. Among them, amine compounds such as butylamine and poly(ethyleneimine) (PEI) demonstrated success as Lewis-base molecular dopants, in which lone pairs of electrons on nitrogen atoms were partially injected into nanotube conduction bands.21–23 However, the electron transport behavior of these amine-doped SWCNTs gradually reverted to p-type upon exposure to air.22,23 Although amines remain as state-of-the-art molecular donors for nanotubes, the stability of the n-type conversion is inadequate.20,22,23 Thus, inexpensive molecular dopants with versatility, stability, and biocompatibility must be explored.
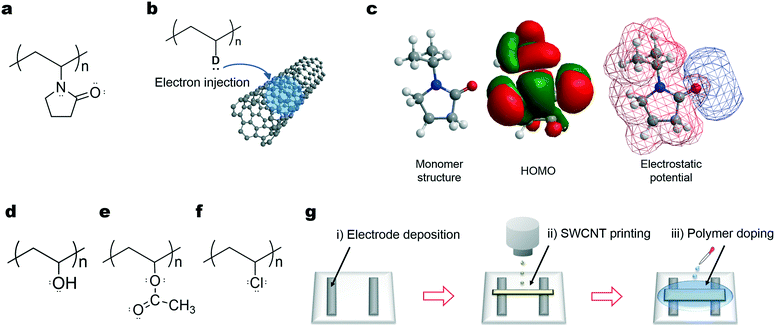
Here, we explore metal- and amine-free electron donors with simple chemical structures for SWCNTs. During this exploration, we considered the electron doping mechanism of SWCNTs using amine-based poly(vinyl pyrrolidone) (PVP), a recently reported nanotube electron donor.24,25 PVP contains a tertiary amine with a five-membered carbon ring and a double-bonded oxygen atom, as shown in Fig. 1a. According to previous reports, the nitrogen atom of the amine group with a lone pair of electrons exhibits electron donation. To verify that this atom participates in charge transfer, we calculated the highest occupied molecular orbital (HOMO) levels and electrostatic potential on the PVP monomer unit, because negatively charged atoms that contain lone pairs and contribute to the HOMO could provide the main contribution to the electron transfer according to frontier molecular orbital theory26 (as schematically depicted in Fig. 1b). The oxygen atom is negatively charged, while the nitrogen atom of the amine group shows a relatively positive electrostatic potential, although each atom contributes to the HOMO, as shown in Fig. 1c. The nitrogen atom surrounded by the five-membered ring may be also sterically hindered from interacting with SWCNTs; therefore, the oxygen atom, rather than the nitrogen atom, is considered the main contributor to electron transfer into SWCNTs by PVP doping. Considering this, along with the easy photodegradation of amine compounds,27 amines were discarded as potential electron donors, and we focused on candidate electron donors containing negatively charged oxygen atoms with lone pairs of electrons. We found that simple, versatile, chemically stable, low-cost, safe, and common materials containing oxygen atoms can be used as effective and stable electron donors for SWCNTs, promoting the future widespread and long-term use of TE power in residential environments.
Results and discussion
N-type doping using oxygen-abundant polymers
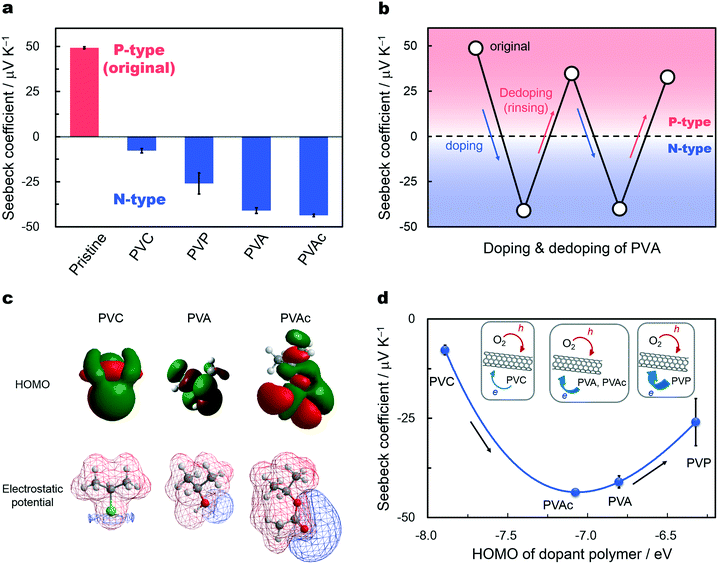
Polymer compounds are desirable as dopants that utilize the flexibility, light weight, wet-processability, and mass-production capacities of SWCNTs. From this perspective, we used two versatile and well-known polymers, poly(vinyl alcohol) (PVA) and poly(vinyl acetate) (PVAc), which contain abundant oxygen atoms with lone pairs in their functional groups. We also utilized PVP and poly(vinyl chloride) (PVC) to compare the contributions of the functional groups to the electron transfer into SWCNTs. The molecular structure of each compound is depicted in panels a and d–f of Fig. 1, including the lone pairs. These compounds have simple chemical structures with identical carbon main chains, excluding the functional groups, thus providing the ability to systematically examine their electron donating ability (namely the reduction power or Lewis basicity to SWCNTs), as is depicted in Fig. 1b. Polymer doping is conducted by spin-coating or drop-casting each solution onto inkjet-printed SWCNT thin films (Fig. 1g). Inkjet printing is a high-speed on-demand fabrication process that prepares thin films of the desired area and thickness without using excessive solvents,28–30 allowing the production of TE modules with precision and low cost. After doping, the polarity (p- or n-type) of the doped SWCNTs was evaluated through TE charge-carrier determination. The TE measurement provided information on the major carriers of the material; positive and negative Seebeck coefficients (calculated as the output voltage for a defined temperature difference) indicate p- and n-type polarities using holes and electrons as major charge carriers, respectively.31Fig. 2a shows the Seebeck coefficients of the films at 300 K in air before and after polymer doping. The coefficient of the original SWCNT thin film is approximately +50 μV K−1, matching previously reported values20,24,25 and indicating the p-type nature of the pristine SWCNTs from the hole injection by oxygen impurities. By polymer doping, the positive Seebeck coefficient is successfully converted into a negative value. Negative coefficients indicate n-type materials; the change in sign indicates the alteration of the major charge carriers of SWCNTs from holes to electrons via polymer electron injection. Charge-carrier injection is supported by Raman spectrometry,32 where the G− band frequency of metallic SWCNTs displays a measureable upshift after polymer doping (Fig. S1, ESI†). Piao et al. recently reported that PVA did not behave as an electron donor for SWCNTs, while PVP donated electrons.25 Meanwhile, our buckypapers showed negative conversions of the Seebeck coefficients after immersion in a PVA aqueous solution, as well as in a PVP solution of DMF (Fig. S2, ESI†). The difference between our results is currently unclear, but it might be due to some experimental differences including the remaining surfactants, the SWCNT synthesis method, and the degree of polymerization of the dopant polymers.
Interestingly, the PVA doping and dedoping processes are reversible for SWCNTs, as shown in Fig. 2b. The original p-type SWCNTs are converted to n-type upon PVA doping and then made positive again after rinsing PVA with deionized water. This also demonstrates the effective electron donation of PVA. To the best of our knowledge, this is the first time that PVA is revealed to be an effective electron donor for SWCNTs.
The n-type SWCNTs exhibit different Seebeck coefficients depending on the dopant polymers, with coefficients ranging from approximately −7 to −43 μV K−1, as shown in Fig. 2a. The PVA- and PVAc-doped SWCNTs exhibit large Seebeck coefficients of approximately −40 μV K−1, which approach the magnitude of the original positive coefficients and match the values of the PEI-functionalized SWCNTs previously reported.23 Furthermore, these dopant polymers require neither toxic solvents such as benzene nor halogenated solvents for dissolution and are thus suitable for future eco-friendly and biocompatible devices.
We next studied the dopant-polymer dependence of the Seebeck coefficient values, predicting a relationship with the electron-donating ability of each functional group. Considering that highly negatively charged atoms with lone pairs that contributed to the HOMO dominated the electron transfer contribution to the nanotubes, as described in the above discussion of PVP, the electrostatic potentials and HOMO levels were selected as parameters for comparing electron donation. To verify these selections and to explain the differences in the Seebeck coefficients, we calculated the HOMO levels and electrostatic potentials of the dopant polymers on their monomer units, similar to the calculations for PVP, as shown in Fig. 2c. Each compound is polarized between the carbon main chain and the functional groups. The atoms with lone pairs, such as the chlorine of PVC, the hydroxyl oxygen of PVA, and the double-bonded acetoxy oxygens of PVAc, are highly negatively charged, as can be understood based on electronegativity. Further, these atoms contribute to the HOMO, as shown in Fig. 2c, indicating that they are the main contributors to electron donation into the SWCNTs. The negative Seebeck coefficients obtained are plotted against the HOMO levels of these atoms in Fig. 2d. These parameters show an excellent correspondence; the negative Seebeck coefficient first increases (from the PVC- to PVAc-doped SWCNT) and then decreases upon PVP-doping with increases in the HOMO levels. The relationship is systematic enough to explain the differences in the Seebeck coefficients of the SWCNTs and the electron donation capacities of the dopant polymers. Higher HOMO levels correspond to stronger electron donation by the dopant polymers. The Seebeck coefficient values are determined by the balance between the amounts of electrons and holes introduced by the functional groups of the dopant polymers and oxygen impurities,24,25 as depicted in the insets of Fig. 2d. The electron donation of PVC is relatively weak; the injected electrons mainly combine with the original holes, converting the positive coefficient into a negative value close to zero. Under doping with PVA and PVAc, the negative Seebeck coefficients reach larger magnitude values approaching −40 μV K−1, recovering the absolute value of the coefficients to levels equivalent to that of the original p-type SWCNTs (+50 μV K−1). This is caused by the injection of a greater amount of electrons than in PVC-doping, possibly achieving an electron density commensurate with that of holes in the original SWCNTs. The decrease in the magnitude of the negative coefficient with PVP-doping (to approximately −25 μV K−1) relates to a complex behavior in which the Seebeck coefficient and charge-carrier density are complementary.33,34 The increased injection of electrons therefore decreases the Seebeck coefficient under PVP doping. The HOMO level and electrostatic potential calculations reveal the electron donation ordering of PVC < PVAc < PVA < PVP and illustrate that the molecules with negatively charged oxygen atoms and ambient HOMO levels are critical for adequate n-type doping. This supports the use of polymers with negatively charged oxygen-containing functional groups as new candidate electron donors to realize high-performance n-type TE legs. When a new class of dopant molecules must be determined based on their constituent atoms and molecular structures, the simple HOMO and electrostatic potential calculations offer a useful benchmark for screening dopants.
Stability and TE performance of n-type SWCNTs
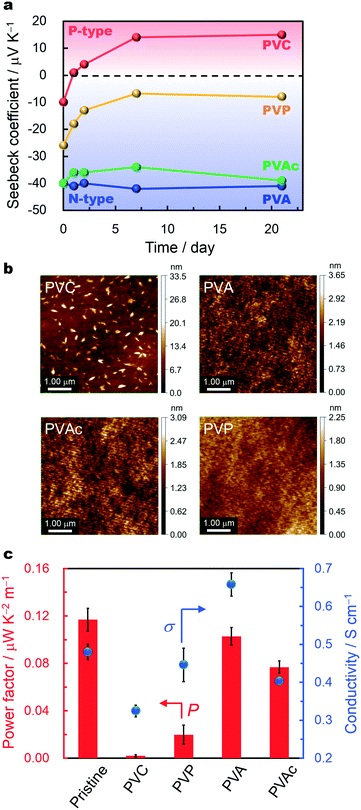
Fig. 3a shows the time dependence of the Seebeck coefficients in ambient air after polymer doping. The Seebeck coefficient of the PVC-doped SWCNTs reverts from negative to positive after 1 day. Although PVP is regarded as a relatively stable electron donor,24 its air stability is low, and the PVP-doped SWCNT shows a distinct decrease in the magnitude of the negative coefficient. For the PVA- and PVAc-doped SWCNTs, the negative Seebeck coefficients remain stable in ambient air for at least 3 weeks.The charge type of SWCNTs is well known to be quite sensitive to oxygen.35,36 Thus, the significant differences in stability are mainly attributed to auto-oxidation by oxygen impurities in air22,23 and the polymer stabilities themselves. To verify these sources, we estimate the amounts of oxygen transmitted through the dopant polymers from the oxygen transmission coefficients (OTCs) and the polymer film thicknesses, as summarized in Table 1. Although the film thickness differs according to the polymer type, the oxygen transmission through the films can be determined using the OTC values from a previous study.37 The oxygen transmission amount through PVA is several orders of magnitude less than that through PVC. The difference in the stabilities of the PVC- and PVA-doped SWCNTs can therefore be attributed to the suppression of auto-oxidation by PVA, which acts as both an electron donor and oxygen barrier layer disturbing the further adsorption of oxygen impurities. The OTC is determined by the primary structures of molecules; larger polarities tend to correspond to smaller OTCs, and hydrogen bonding may strongly relate to the transmission. From this perspective, PVA is suitable because it offers adequate electron donation and oxygen blockage by hydrogen bonding. Although the OTC value of PVP is unknown to the best of our knowledge, the photodegradation of amine compounds destabilizes the PVP-doped SWCNTs. Atomic force microscope (AFM) images of the dopant-polymer surfaces in Fig. 3b indicate that the surfaces of the polymers are quite smooth, excluding that of PVC. The poor stability of the n-type SWCNT doped with PVC may also be related to the surface roughness, as the rough surface may cause a greater amount of oxygen transmission owing to its large surface area.
| Dopant polymer | PVC | PVA | PVAc | PVP |
|---|---|---|---|---|
| Thickness [μm] | 7.6 | 2.6 | 5.7 | 8.5 |
| Oxygen transmission coefficient [cm3 cm m−2 per day per Pa] | 3.2 × 10−6 | 4.0 × 10−11 | 2.2 × 10−5 | N/A |
| Oxygen transmission amount [cm3 per day] | 8.8 × 10−9 | 3.7 × 10−14 | 4.5 × 10−8 | N/A |
The PVAc-doped SWCNTs show considerable stability, despite an oxygen transmission amount equivalent to that of the PVC-doped materials. The mechanism underlying this stability remains unclear; however, resonance-induced charge delocalization in the acetoxy groups may contribute. Nonoguchi et al. recently reported the stabilization of n-type SWCNTs by adsorbing crown ether-coordinated metal cations, in which the positive charges of the center metal cations were delocalized in the ether rings. The positive charge may have stabilized the conducting electrons delocalized in the expanded sp2 framework of the SWCNTs by providing charge balance, namely by the hard and soft acids and bases (HSAB) concept.20 Electron transfer from PVAc to the SWCNTs leaves the acetoxy group of PVAc with a minimal positive charge. Here, we expect that some resonant structure of the single- and double-bonded oxygens induces the delocalization of the positive charge, and thereby stabilizes the conducting electrons injected into the SWCNTs by good charge matching, with long-term retention of the negative Seebeck coefficient. With this analysis, we determine some qualities of stable electron donors, including superior oxygen impurity blocking performances and resonant positive-charge structures.
For energy harvesting applications, the TE power factor P:
| P = S2σ | (1) |
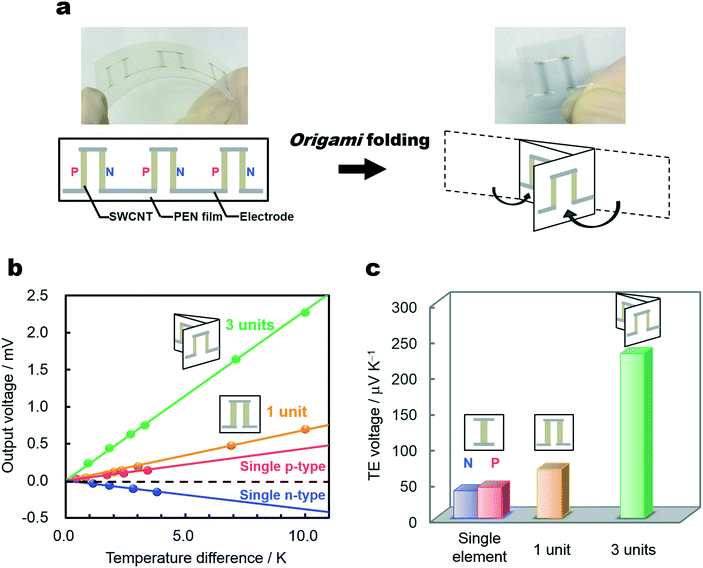
To demonstrate the improvement in TE voltage by connecting p- and n-type SWCNTs, we designed TE modules on a flexible 25 μm-thick polyethylene naphthalate (PEN) film. SWCNTs and electrodes were deposited by inkjet printing directly onto the PEN film, and PVA was doped by drop-casting, creating a module comprising three units of p- and n-type SWCNTs, as shown in Fig. 4a. We fold this module into three; this device configuration, called an origami TE module, can improve the dimensional voltage output, and the applicability of this configuration is unique to thin, flexible materials. Fig. 4b shows the output voltage versus temperature difference obtained from single elements and modules. All the devices exhibit linear increases in output voltage versus temperature difference, suggesting adequate TE powering from these device configurations. The TE voltages, obtained from the slopes of Fig. 4b, are summarized in Fig. 4c. The Seebeck coefficients of the single p- and n-type SWCNTs are measured at approximately +43 and −38 μV K−1. Connecting more units causes a monotonic increase in the TE voltage with the addition of each Seebeck coefficient, confirming that the TE voltage from each p- and n-type element is added to improve the overall TE performance of the device. The maximum output voltage observed in the three-unit module is approximately 2.2 mV at the temperature difference of 10 K, selected as a plausible temperature difference between the human body and ambient air; the voltage matches those of previously reported TE buckypapers.13 The output voltages can be further enhanced by connecting additional TE legs. The process of inkjet printing and film folding is faster, cheaper, and lower in waste production than the connection of buckypapers. We expect that simple, non-toxic, versatile, flexible, and stable dopant polymers, along with the demonstrated fabrication processes, can be extended to increase the practicality of energy harvesting technologies in the near future.
Conclusions
Oxygen-abundant polymers with structural simplicity, non-toxicity, and chemical stability were explored as potential electron donors. SWCNTs functionalized by these polymers exhibited good n-type TE performances with excellent air stability. Highly negatively charged atoms with lone pairs of electrons dominantly participated in the charge transfer, and the negative Seebeck coefficients of the functionalized SWCNTs were correlated with the HOMO levels of the dopant polymers. These results clearly indicated the possibility of screening electron-donor materials based on simple chemical calculations of the properties of the constituent atoms. The stability of the induced n-type nature was attributed to the polymer dopants acting as oxygen impurity barriers and charge delocalization structures. To exploit the flexibility and inkjet printability of the materials and to improve the dimensional voltage output, origami TE modules composed of p- and n-type SWCNT thin films were fabricated and characterized through TE measurements. The TE module showed increasing voltage with increasing TE units, indicating the additive behavior of the voltages generated by the elements.The present study was performed with varied functional groups and a fixed carbon main chain; we expect that the concept demonstrated in this work could be extended to the exploration of further effective dopants, as well as the development of more appropriate doping methods for enhancing the n-type TE power factors of SWCNTs in the near future.
Experimental section
Preparation of SWCNT thin films and polymer doping
SWCNTs comprising a mixture of semiconducting and metallic nanotubes with a mean diameter of 1 nm were synthesized by the enhanced direct-injection pyrolytic synthesis method and were ultrasonically dispersed in deionized water using the nonionic surfactant poly(oxyethylene)4 laurylether. Nickel electrodes of 30 nm in thickness and gaps of 9 mm were vapor-deposited onto quartz glass substrates measuring 20 × 20 mm. After 90 s of oxygen plasma irradiation under vacuum (YHS-R, SAKIGAKE-semiconductor) to render the substrate surface hydrophilic, the aqueous dispersion of SWCNTs was directly deposited by inkjet printing (Pulse Injector/Cluster Technology, inkjet nozzle diameter of 25 μm) between the electrodes at room temperature in air, followed by vacuum annealing at 453 K for 90 min to evaporate any remaining water and surfactant molecules. The dimensions of all the SWCNT thin films were set to 12 mm (length) × 2 mm (width) × 50 nm (thickness). The SWCNT thin films were then covered with the dopant polymers. The number-averaged degree of polymerization (Mn) of each compound was 1100 for PVC, 500 for PVA and PVAc, and 220 for PVP. The films were prepared by facile spin-coating or drop-casting of solutions of each polymer in N,N-dimethylformamide (PVC, PVAc, and PVP) or deionized water (PVA). The concentration of all solutions was 10 wt%. Dedoping of PVA was carried out by immersing the PVA-coated device in deionized water for 1 h. The thicknesses of the polymers were determined using a step meter (XP-200, AMBiOS TECHNOLOGY). The surface morphology of the polymers was observed via AFM in air (JSPM-5200, JEOL). The origami TE module was fabricated on a PEN film with dimensions of 60 mm (length) × 20 mm (width) × 25 μm (thickness). The surface of the PEN film was treated with oxygen plasma, similar to the quartz glass substrates. The inkjet-printing of silver electrodes was performed using silver ink (L-Ag1TeH, ULVAC) using the same setup as for SWCNT printing. Then, the electrodes were annealed at 423 K for 60 min in air to improve their conductivity. Next, the SWCNT thin films were inkjet-printed using the same procedure as that on the quartz glass substrates, followed by annealing at 423 K for 3 h under vacuum. Finally, PVA doping was conducted by drop-casting the PVA solution in deionized water onto the SWCNT thin films, and the module was gently folded.Thermoelectric and Raman spectroscopic measurements
All Seebeck measurements were performed in air by reading the TE voltages from the SWCNT thin films and the origami TE modules using a semiconductor parameter analyzer (B1500A, Keysight Technologies). Linear temperature gradients in the in-plane directions were applied to the SWCNT thin films, with the low-temperature side held constant at 300 K. The Seebeck coefficients were then obtained from the slopes of the TE voltages and temperature differences. The stability of the Seebeck coefficients was evaluated after storing the devices at 300 K in ambient air. The electrical conductivities were measured on the same devices at 300 K in air (B1500A, Keysight Technologies), and the power factors were calculated from these parameters. Raman spectrometry was performed on the same SWCNT/dopant polymer films at room temperature in air using an excitation laser with a wavelength of 532.36 nm (NRS-7100, JASCO). The nanotubes were mixtures with several chirality values. The (11, 2) and (9, 3) metallic nanotubes were in resonance with the applied laser wavelength.Molecular calculations
All molecular calculations were performed via density functional theory using the Gaussian 3.0 package at the B3LYP/6-31G* levels on the monomer units, namely 2-chloropropane for PVC, propane-2-ol for PVA, isopropyl acetate for PVAc, and N-propan-2-ylpyrrolidin-2-one for PVP after optimizing the molecular structures using Molecular Mechanics Program 2 using the Chem & Bio 3D package.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported in part by a Grant-in-Aid for a JSPS Research Fellow (No. 17J00903).References
- R. Bogue, Sens. Rev., 2014, 34, 137 CrossRef.
- N. S. Hudak and G. G. Amatucci, J. Appl. Phys., 2008, 103, 101301 CrossRef.
- T. Krupenkin and J. A. Taylor, Nat. Commun., 2011, 2, 448 CrossRef PubMed.
- Y. Qin, X. Wang and Z. L. Wang, Nature, 2009, 451, 809 CrossRef PubMed.
- K. P. Pernstich, B. Rössner and B. Batoll, Nat. Mater., 2008, 7, 321 CrossRef CAS PubMed.
- G.-H. Kim, L. Shao, K. Zhang and K. P. Pipe, Nat. Mater., 2013, 12, 719 CrossRef CAS PubMed.
- S. Horike, M. Misaki, Y. Koshiba, M. Morimoto, T. Saito and K. Ishida, Appl. Phys. Express, 2016, 9, 081301 CrossRef.
- Q. Yao, L. Chen, W. Zhang, S. Liufu and X. Chen, ACS Nano, 2010, 4, 2445 CrossRef CAS PubMed.
- C. A. Hewitt, A. B. Kaiser, S. Roth, M. Craps, R. Czerw and D. L. Carroll, Appl. Phys. Lett., 2011, 98, 183110 CrossRef.
- A. Javey, J. Guo, Q. Wang, M. Lundstorm and H. Dai, Nature, 2003, 424, 654 CrossRef CAS PubMed.
- K. Xiao, Y. Liu, P. Hu, G. Yu, X. Wang and D. Zhu, Appl. Phys. Lett., 2003, 83, 150 CrossRef CAS.
- K. Suemori, S. Hoshino and T. Kamata, Appl. Phys. Lett., 2013, 103, 153902 CrossRef.
- C. A. Hewitt, A. B. Kaiser, S. Roth, M. Craps, R. Czerw and D. L. Carroll, Nano Lett., 2006, 12, 1307 CrossRef PubMed.
- S. LeBlanc, Sustainable Mater. Technol., 2014, 1–2, 26 CrossRef.
- D. Kang, N. Park, J. Ko, E. Bae and W. Park, Nanotechnol., 2005, 16, 1048 CrossRef CAS.
- A. Javey, R. Tu, D. B. Farmer, J. Guo, R. G. Gordon and H. Dai, Nano Lett., 2005, 2, 345 CrossRef PubMed.
- L. Grigorian, G. U. Sumanasekera, A. L. Loper, S. Fang, J. L. Allen and P. C. Eklund, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, R4195(R) CrossRef.
- D. L. Duong, I. H. Lee, K. K. Kim, J. Kong, S. M. Lee and Y. H. Lee, ACS Nano, 2010, 4, 5430 CrossRef CAS PubMed.
- T. Takenobu, T. Takano, M. Shiraishi, Y. Murakami, M. Ata, H. Kataura, Y. Achiba and Y. Iwasa, Nat. Mater., 2003, 2, 683 CrossRef CAS PubMed.
- Y. Nonoguchi, M. Nakano, T. Murayama, H. Hagino, S. Hama, K. Miyazaki, R. Matsubara, M. Nakamura and T. Kawai, Adv. Funct. Mater., 2016, 26, 3021 CrossRef CAS.
- J. Kong and H. Dai, J. Phys. Chem. B, 2001, 105, 2890 CrossRef CAS.
- M. Shim, A. Javey, N. W. S. Kam and H. Dai, J. Am. Chem. Soc., 2001, 123, 11512 CrossRef CAS PubMed.
- D. D. Freeman, K. Choi and C. Yu, PLoS One, 2012, 7, e47822 CAS.
- Y. Nonoguchi, K. Ohashi, R. Kanazawa, K. Ashiba, K. Hata, T. Nakagawa, C. Adachi, T. Tanase and T. Kawai, Sci. Rep., 2013, 3, 3344 CrossRef PubMed.
- M. Piao, M. R. Alam, G. Kim, U. Dettlaff-Weglikowska and S. Roth, Phys. Status Solidi B, 2012, 249, 2353 CrossRef CAS.
- K. Fukui, T. Yonezawa and H. Shingu, J. Chem. Phys., 1952, 20, 722 CrossRef CAS.
- Y. Chen, C. Hu, X. Hu and J. Qu, Environ. Sci. Technol., 2009, 43, 2760 CrossRef CAS PubMed.
- H. Sirringhaus, T. Kawase, R. H. Friend, T. Shimoda, M. Inbasekaran, W. Wu and E. P. Woo, Science, 2000, 15, 2123 CrossRef.
- H. Okimoto, T. Takenobu, K. Yanagi, Y. Miyata, H. Shimotani, H. Kataura and Y. Iwasa, Adv. Mater., 2010, 22, 3981 CrossRef CAS PubMed.
- H.-Y. Tseng, B. Purushothaman, J. Anthony and V. Subramanian, Org. Electron., 2011, 12, 1120 CrossRef CAS.
- Y. Sun, P. Sheng, C. Di, F. Jiao, W. Xu, D. Qiu and D. Zhu, Adv. Mater., 2012, 24, 932 CrossRef CAS PubMed.
- A. Das, A. K. Sood, A. Govindaraj, A. M. Saitta, M. Lazzeri, F. Mauri and C. N. R. Rao, Phys. Rev. Lett., 2007, 99, 136803 CrossRef PubMed.
- H. Ohta, S. Kim, Y. Mune, T. Mizoguchi, K. Nomura, S. Ohta, T. Nomura, Y. Nakanishi, Y. Ikuhara, M. Hirano, H. Hosono and K. Koumoto, Nat. Mater., 2007, 6, 129 CrossRef CAS PubMed.
- A. D. LaLonde, Y. Pei and J. Snyder, Energy Environ. Sci., 2011, 4, 2090 CAS.
- K. Bradley, S.-H. Jhi, P. G. Collins, J. Hone, M. L. Cohen, S. G. Louie and A. Zettl, Phys. Rev. Lett., 2000, 85, 4361 CrossRef CAS PubMed.
- P. G. Collins, K. Bradley, M. Ishigami and A. Zettl, Science, 2000, 287, 1801 CrossRef CAS PubMed.
- M. Salame, PMSE Prepr., 1967, 8, 137 CAS.
- G. J. Snyder and E. S. Toberer, Nat. Mater., 2008, 7, 105 CrossRef CAS PubMed.
- S. Horike, M. Misaki, Y. Koshiba, T. Saito and K. Ishida, Jpn. J. Appl. Phys., 2016, 55, 03DC01 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Raman spectra; Seebeck coefficients of buckypapers. See DOI: 10.1039/c7me00063d |
| This journal is © The Royal Society of Chemistry 2017 |