Cationic switchable lipids: pH-triggered molecular switch for siRNA delivery†
W.
Viricel
 a,
S.
Poirier
b,
A.
Mbarek
a,
R. M.
Derbali
a,
G.
Mayer
ab and
J.
Leblond
*a
a,
S.
Poirier
b,
A.
Mbarek
a,
R. M.
Derbali
a,
G.
Mayer
ab and
J.
Leblond
*a
aFaculty of Pharmacy, University of Montreal, P.O. Box 6128, Downtown Station, Montreal, QC, Canada. E-mail: jeanne.leblond-chain@umontreal.ca
bLaboratory of Molecular Cell Biology, Montreal Heart Institute, Montreal, H1T 1C8, QC, Canada
First published on 21st November 2016
Abstract
A pH-sensitive molecular switch able to change its conformation upon protonation at endosomal pH values is embedded into the structure of cationic lipidoid materials, thus conferring endosomal escape properties. Involvement of the conformational switch in the endosomal escape process was confirmed and leading material identified was able to induce efficient gene knockdown both in vitro and in vivo. The lipid nanoparticles reported here are promising for therapeutic applications and this work could serve as a template for future design of stimulus-responsive (ionic, redox, light) molecular switch for drug and gene delivery.
RNAi technology has demonstrated strong potential for therapeutics and is currently showing impressive clinical progress.1,2 Silencing gene expression may be used to treat numerous diseases such as cancer,3–5 hereditary disorders6 and infectious diseases.7 However, the strong hydrophilicity and anionic charge density of siRNA prevent their passive diffusion across cell membranes. Systemic administration of siRNA therefore requires the use of carriers able to encapsulate, protect and deliver the therapeutic cargo into the cytoplasm of target cells. Despite the many efforts made to tailor nanocarriers in order to overcome biological barriers, endosomal entrapment remains a major impediment to the in vivo therapeutic efficacy of the delivery platforms developed in the last decade.8
One of the most clinically advanced delivery vehicles for siRNA therapeutics are lipid nanoparticles (LNP).1 These siRNA delivery materials are principally composed of cationic lipids or “lipidoids”, identified through combinatorial synthesis and high throughput screening.9–11 Structure–activity analysis of these libraries suggests that using one or more tertiary amines as ionizable headgroups is essential to promoting endosomal escape and mediating meaningful gene silencing.12 At endosomal pH values, ionization of the tertiary amine alters the hydrodynamic radius of the lipid headgroup and induces an inverted non-bilayer structure, which is hypothesized to be responsible for destabilizing the endosomal membrane.13 Nevertheless, recent quantitative studies using siRNA/LNP have shown that only ∼1–2% of siRNA is successfully delivered to the cytoplasm due to endosomal entrapment or recycling to the extracellular space.14,15 This observation highlights that endosomal sequestration is still a major barrier to gene delivery and suggests that developing lipid materials equipped with an efficient endosomal escape mechanism remains highly relevant.
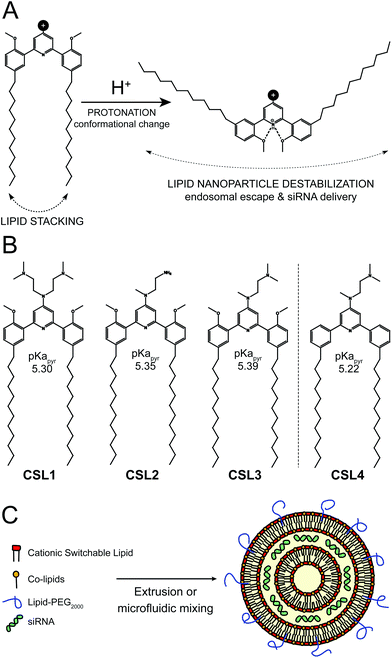
We recently reported the use of pH-dependent switchable lipids for drug delivery.16 When incorporated into liposomes, the switchable lipids triggered a fast and effective intracytoplasmic delivery of a hydrophilic cell-impermeable compound, via an endosomal escape process. Upon protonation of the central pyridine, the conformation of the lipid switches to favor intramolecular hydrogen-bonding, which changes the relative orientation of the hydrocarbon chains of the molecule, disturbing the lipid packing of the nanoparticle and prompting the release of its therapeutic cargo (Fig. 1A). This pH-sensitive mechanism triggered complete drug release in less than 15 min and was not impacted by polyethylene glycol coating. As such, these materials show great potential for gene delivery. Therefore, we decided to develop an siRNA/LNP delivery platform based on such a pH-triggered conformational switch.
The switchable lipid structure was used as a starting point for the design of cationic switchable lipids (CSL). The tricyclic pH-sensitive unit was maintained while various cationic headgroups on the 4′ position of the central pyridine ring were introduced (Fig. 1B). In silico predictions of pKapyr were used to select the headgroup structure since it previously appeared as a major determinant of in vitro behaviour.16 Among the molecules synthesized in this study, only 4-dialkylaminopyridine derived-CSL presented an in silico pKapyr value close to the endosomal pH value (∼5–6), which is required to trigger the desired conformational switch within the endosomes.
Four different CSL were synthesized (Fig. S1–S3†), all containing two alkyl chains of 12 carbons (Fig. 1B). Lipid chain lengths of 10 to 13 carbons has been reported as an advantageous specification for efficient siRNA delivery in vivo.17,18 We have already demonstrated that this chain length is compatible with the pH-triggered conformational change of the switchable lipids and effectively promotes liposome bilayer destabilization.16 Three different cationic headgroups were incorporated on the CSL: double-dimethylamine headgroup (CSL1), mono primary amine headgroup (CSL2) or mono dimethylamine headgroup (CSL3). CSL4 is the non-switchable counterpart of CSL3, used as a negative control lipid. The removal of the two methoxy moieties prevents intramolecular hydrogen bonding with the protonated pyridine, thus preventing conformational switch and LNP destabilization (Fig. S4†). When relevant, CSL3-based LNP was compared to the negative control CSL4-based LNP in order to confirm the involvement of the pH-sensitive conformational switch. LNP incorporating either one of the four CSL materials were formulated with DSPC, cholesterol and DSPE-PEG2000 as co-lipids (respectively 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37.5
37.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
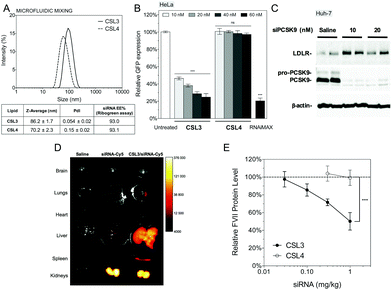
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 mol%). A hypothetical representation of a LNP is presented in Fig. 1C. At a nitrogen/phosphate molar ratio of 4 which was used for all experiments, manual extrusion yielded LNP exhibiting a hydrodynamic diameter ∼150 nm (Fig. S5A and S5B†). The siRNA encapsulation efficiency was determined to be 85–95% for all CSL, using a SYBR® Gold fluorescence assay (Fig. S5D†).
2.5 mol%). A hypothetical representation of a LNP is presented in Fig. 1C. At a nitrogen/phosphate molar ratio of 4 which was used for all experiments, manual extrusion yielded LNP exhibiting a hydrodynamic diameter ∼150 nm (Fig. S5A and S5B†). The siRNA encapsulation efficiency was determined to be 85–95% for all CSL, using a SYBR® Gold fluorescence assay (Fig. S5D†).
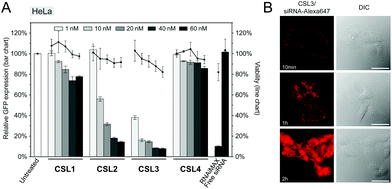
The siRNA transfection efficiency of CSL was evaluated in vitro in a HeLa/GFP model, using Lipofectamine® RNAiMAX as a positive control (Fig. 2A and S6†). Mono-substituted CSL2 and CSL3-based nanoparticles showed remarkable dose-responding knockdown efficiencies, whereas di-substituted CSL1 was unable to mediate siRNA delivery. One may hypothesize that the two dimethylamine cationic groups of CSL1 impair the conformational switch. Either steric hindrance or intramolecular hydrogen bonds between dimethylamine groups and methoxy moieties might stabilize the lipid stacking conformation and prevent the rotation of the two lipid arms of CSL1. In addition, it has been reported that too many or too strong (e.g. quaternary amines) cationic headgroups can significantly impede the cytosolic release of the siRNA payload from the cationic lipid.19–21 Interestingly, the negative control CSL4 did not show any significant transfection efficiency, suggesting that the knockdown observed with CSL2 and CSL3 could be attributed to the pH-sensitive conformational switch. No significant cytotoxicity was detected for all the CSL materials at the conditions evaluated, using a resazurin-based cell viability assay (Fig. 2A).
Time course delivery of intracytoplasmic Alexa647-siRNA delivery was assayed by live-cell fluorescence microscopy on HeLa cells, using the top performing material CSL3 (Fig. 2B). After only 1 hour, diffuse fluorescence was obvious, confirming the escape of the siRNA payload from the endocytic pathway. At the two-hour time point, the entire cytoplasms were stained with intense diffuse fluorescence. Unsurprisingly, no internalization of naked Alexa647-siRNA was observed after a two-hour incubation in the same conditions (Fig. S7†). These results confirmed the efficient uptake and fast cytoplasmic delivery of siRNA encapsulated within the CSL3-based LNP, a prerequisite for mediating potent siRNA delivery.22,23
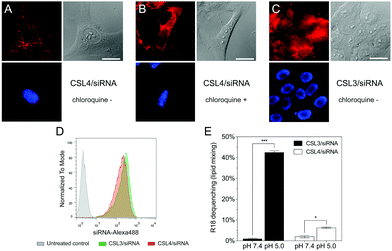
To further confirm the importance of the CSL conformational change in the endosomal escape process, we compared the cellular trafficking of CSL3 and CSL4-based LNP encapsulating Alexa647-siRNA. After 16 hours of incubation, live-cell fluorescent microscopy revealed that the CSL4 negative control formulation was mainly entrapped within the endosomes (punctate fluorescence – Fig. 3A). Disruption of the endosomal compartments by addition of chloroquine, a potent endosomolytic agent,24,25 yielded diffuse cytoplasmic fluorescence. This confirmed that endosomal entrapment was the limiting barrier to siRNA cytoplasmic delivery for CSL4-based formulation (Fig. 3B). Comparatively, the CSL3-based formulation was able to mediate endosomal escape and intracytoplasmic delivery of Alexa647-siRNA (diffuse fluorescence through the entire cells – Fig. 3C). During this assay, nearly identical cellular uptake profiles were observed for the two formulations, as assayed by flow cytometry (Fig. 3D). This suggests that the superior transfection efficiency observed with CSL3 is due to improved endosomal escape following its pH-induced conformational switch (Fig. 2A).
Endosomal escape of nanocarriers has been suggested to occur through either fusion, active transport, disruption or lysis of the endosomes.15 We conducted lipid-mixing assays with octadecyl rhodamine B (R18) labelled LNP and model unlabeled phospholipid vesicles (Fig. 3E).16,26 R18 dequenching, which is indicative of lipid mixing and thus fusogenic ability, occurred within minutes under acidic conditions with CSL3-based LNP. In contrast, CSL4-based LNP did not mediate major fusogenic activities at acidic pH. No significant dequenching was observed for either formulation at neutral pH. This confirmed that fusion ability is involved in the endosomal escape mechanism of CSL3, as previously reported for other switchable lipids.16
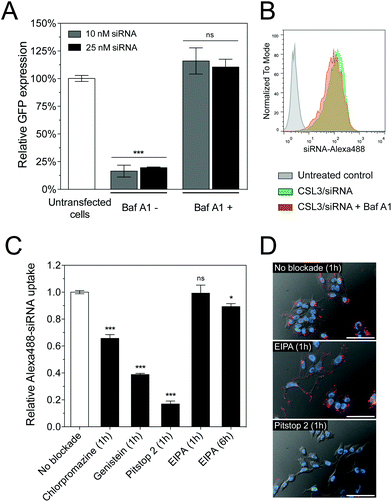
The pH of endosomes and lysosomes is controlled by acidification via vacuolar ATPases. These membrane-bound proton pumps can be inhibited using Bafilomycin A1 (Baf A1), thus blocking endosome acidification.25 We therefore hypothesized that Baf A1 could hinder the mechanism of CSL3-based LNP.27 Compared to control cells transfected without Baf A1, Baf A1-treated cells showed a complete loss of GFP knockdown (Fig. 4A). Here again, we checked that Baf A1 did not significantly impact CSL3/siRNA cellular uptake (Fig. 4B). Therefore, we confirmed that the CSL3-based formulation requires the acidification of the endosomes to trigger its conformational switch and endosomal escape, thus ensuring its transfection efficiency.
Several cellular entry pathways can result in endocytic confinement of siRNA, such as clathrin, caveolae or macropinocytosis.15 In order to better understand the cellular entry of the LNP, flow cytometry uptake assays of CSL3/siRNA-Alexa488 were performed on HeLa cells, in the presence of chemical inhibitors of endocytosis (Fig. 4C). Without inhibition, an efficient and homogenous siRNA delivery was observed (Fig. S8†). However, strong uptake inhibition was observed in the presence of clathrin and caveolae inhibitors. It was previously reported that the main uptake mechanism of LNP/siRNA in HeLa cells and hepatocytes is the clathrin-induced macropinocytosis, starting after 2 hours of incubation. This macropinocytosis allows the bulk of the LNP into the cells and is mainly responsible for the gene silencing activity.9,28 In our case, the macropinocytosis pathway (blocked using ethylisopropylamiloride – EIPA) did not seem significantly involved in LNP entry, despite a 6-hour incubation period. It appears that CSL3-based LNP were actively endocytosed via the clathrin and/or caveolae pathways. These findings were also confirmed by live-cell fluorescence microscopy (Fig. 4D).
CSL3 and CSL4 LNP/siRNA were then assessed for their in vivo potential to systemically deliver a siRNA targeting the liver-secreted coagulation factor VII.17 LNP were prepared using microfluidic mixing since this technique leads to optimized LNP and is an easily scalable process.29,30 The use of a microfluidic mixing device for LNP/siRNA assembly allowed us to reach the critical size needed to achieve efficient delivery to the hepatocytes via the 100–150 nm-sized endothelial fenestrae of the mouse liver.31,32 The C18-anchored DSPE-PEG2000 lipid was also replaced with shorter C14-anchored DMG-PEG2000, as it has been reported that a shorter aliphatic anchor in the PEG-lipid yields greater hepatic gene silencing.33 As presented in Fig. 5A, the hydrodynamic diameter of the LNP was around 70–90 nm as measured by dynamic light scattering and siRNA encapsulation efficiencies measured with a Ribogreen® fluorescent assay were 93%, which is similar to LNP obtained by manual extrusion (Fig. S5D†). LNP size remained stable for more than one week in PBS, ensuring colloidal stability before injection (Fig. S9†). CSL3-based LNP made by microfluidic mixing showed efficient dose-responding siRNA delivery in the HeLa/GFP model and still outperformed the negative control CSL4-based LNP (Fig. 5B). These results demonstrate that the pH-triggered mechanism of CSL3 is not altered either by the formulation technique or by the nature of the lipid-PEG. The gene silencing efficacy of the CSL3-based LNP was also confirmed in a hepatocyte cell line (Huh-7), targeting the therapeutic target PCSK9 protein.34 Total knockdown of PCSK9 and pro-PCSK9 proteins, as well as subsequent loss of PCSK9-induced LDLR degradation,35 demonstrate the efficient transfection ability of CSL3-based LNP with a siRNA dose as low as 10 nM (Fig. 5C).
Ex vivo fluorescence imaging studies were performed to assess the biodistribution of the CSL3-based formulation after systemic administration in male CD1 mice. CSL3/siRNA-Cy5 formulation was injected at siRNA dose of 1.5 mg kg−1. Whole-organ images (Fig. 5D) and fluorescence intensity normalized by wet-mass of organs (Fig. S10†) indicated that naked siRNA accumulate mainly in the kidneys, suggesting rapid renal clearance. On the other hand, a significant accumulation of siRNA was observed in the liver with the CSL3 nanoparticles. Although this tropism was attributed to the LNP formulation, the fluorescence in the kidneys remained high and suggested free circulating siRNA. This could be attributed either to residual siRNA in the formulation or to partial instability of LNP upon intravenous injection, as suggested by a serum stability study (Fig. S9†). Although the important liver accumulation of siRNA is a prerequisite for hepatocyte gene knockdown, it is not sufficient by itself, as it was shown that some LNP can accumulate in the liver without mediating hepatocyte gene silencing.20,36
In vivo factor VII silencing efficiency of the CSL3-based formulations was compared to that of the CSL4-based formulation (Fig. 5E). CSL3-based LNP were found to demonstrate a dose–response knockdown, resulting in significant silencing efficacy (∼50% silencing) at 1 mg kg−1. At the same dose, negative control CSL4-based LNP were unable to mediate any silencing activity. These results confirm the crucial role of the pH-sensitive conformational change of CSL3 in the in vivo transfection efficacy, in agreement with the in vitro results. The in vivo silencing efficacy of the CSL3 LNP may be further improved by lowering the molar percentage of DMG-PEG2000 in the formulation11,33 or by using combinatorial approaches to enhance the potency of the CSL3 lipid structure as well as the blood stability of the LNP (currently under investigation).
Conclusions
In summary, we report the synthesis, in vitro and in vivo efficacy, and properties of cationic switchable lipids. This proof of concept study shows that this new class of lipid materials based on a pH-triggered conformational switch is suitable for efficient siRNA delivery. Altogether, our results demonstrate that the pH-sensitive conformational switch plays a key role in the properties of CSL3-based materials, owing to its ability to promote endosomal escape. We believe that stimulus-responsive (ionic, redox, light)37 molecular switches have unique properties that may lead to significant innovations in the design of lipidoid materials in the field of gene and drug delivery. In future studies, we plan to evaluate these siRNA delivery materials in a murine metastatic breast cancer model.Acknowledgements
The National Sciences and Engineering Research Council of Canada (NSERC) is acknowledged for financial support. We thank Prof. Xavier Banquy (University of Montreal) for the use of his Nanoassemblr™ microfluidic mixing device.Notes and references
- G. Ozcan, B. Ozpolat, R. L. Coleman, A. K. Sood and G. Lopez-Berestein, Adv. Drug Delivery Rev., 2015, 87, 108–119 CrossRef CAS PubMed.
- J. E. Zuckerman and M. E. Davis, Nat. Rev. Drug Discovery, 2015, 14, 843–856 CrossRef CAS PubMed.
- K. Zhou, L. H. Nguyen, J. B. Miller, Y. Yan, P. Kos, H. Xiong, L. Li, J. Hao, J. T. Minnig, H. Zhu and D. J. Siegwart, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 520–525 CrossRef CAS PubMed.
- C. Qiu, W. Wei, J. Sun, H.-T. Zhang, J.-S. Ding, J.-C. Wang and Q. Zhang, Nanoscale, 2016, 8, 13033–13044 RSC.
- X. Xu, J. Wu, Y. Liu, M. Yu, L. Zhao, X. Zhu, S. Bhasin, Q. Li, E. Ha, J. Shi and O. C. Farokhzad, Angew. Chem., Int. Ed., 2016, 55, 7091–7094 CrossRef CAS PubMed.
- S. Crunkhorn, Nat. Rev. Drug Discovery, 2013, 12, 818–818 CrossRef CAS PubMed.
- E. P. Thi, C. E. Mire, A. C. H. Lee, J. B. Geisbert, J. Z. Zhou, K. N. Agans, N. M. Snead, D. J. Deer, T. R. Barnard, K. A. Fenton, I. MacLachlan and T. W. Geisbert, Nature, 2015, 521, 362–365 CrossRef CAS PubMed.
- K. A. Whitehead, R. Langer and D. G. Anderson, Nat. Rev. Drug Discovery, 2009, 8, 129–138 CrossRef CAS PubMed.
- K. T. Love, K. P. Mahon, C. G. Levins, K. A. Whitehead, W. Querbes, J. R. Dorkin, J. Qin, W. Cantley, L. L. Qin, T. Racie, M. Frank-Kamenetsky, K. N. Yip, R. Alvarez, D. W. Y. Sah, A. de Fougerolles, K. Fitzgerald, V. Koteliansky, A. Akinc, R. Langer and D. G. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1864–1869 CrossRef CAS PubMed.
- Y. Dong, J. R. Dorkin, W. Wang, P. H. Chang, M. J. Webber, B. C. Tang, J. Yang, I. Abutbul-Ionita, D. Danino, F. DeRosa, M. Heartlein, R. Langer and D. G. Anderson, Nano Lett., 2016, 16, 842–848 CrossRef CAS PubMed.
- Y. Sato, Y. Note, M. Maeki, N. Kaji, Y. Baba, M. Tokeshi and H. Harashima, J. Controlled Release, 2016, 229, 48–57 CrossRef CAS PubMed.
- C. A. Alabi, K. T. Love, G. Sahay, H. Yin, K. M. Luly, R. Langer and D. G. Anderson, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12881–12886 CrossRef CAS PubMed.
- M. Jayaraman, S. M. Ansell, B. L. Mui, Y. K. Tam, J. Chen, X. Du, D. Butler, L. Eltepu, S. Matsuda, J. K. Narayanannair, K. G. Rajeev, I. M. Hafez, A. Akinc, M. A. Maier, M. A. Tracy, P. R. Cullis, T. D. Madden, M. Manoharan and M. J. Hope, Angew. Chem., Int. Ed., 2012, 51, 8529–8533 CrossRef CAS PubMed.
- G. Sahay, W. Querbes, C. Alabi, A. Eltoukhy, S. Sarkar, C. Zurenko, E. Karagiannis, K. Love, D. Chen, R. Zoncu, Y. Buganim, A. Schroeder, R. Langer and D. G. Anderson, Nat. Biotechnol., 2013, 31, 653–658 CrossRef CAS PubMed.
- M. P. Stewart, A. Lorenz, J. Dahlman and G. Sahay, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016, 8, 465–478 Search PubMed.
- W. Viricel, A. Mbarek and J. Leblond, Angew. Chem., Int. Ed., 2015, 54, 12743–12747 CrossRef CAS PubMed.
- A. Akinc, A. Zumbuehl, M. Goldberg, E. S. Leshchiner, V. Busini, N. Hossain, S. A. Bacallado, D. N. Nguyen, J. Fuller, R. Alvarez, A. Borodovsky, T. Borland, R. Constien, A. de Fougerolles, J. R. Dorkin, K. N. Jayaprakash, M. Jayaraman, M. John, V. Koteliansky, M. Manoharan, L. Nechev, J. Qin, T. Racie, D. Raitcheva, K. G. Rajeev, D. W. Y. Sah, J. Soutschek, I. Toudjarska, H.-P. Vornlocher, T. S. Zimmermann, R. Langer and D. G. Anderson, Nat. Biotechnol., 2008, 26, 561–569 CrossRef CAS PubMed.
- K. A. Whitehead, J. R. Dorkin, A. J. Vegas, P. H. Chang, O. Veiseh, J. Matthews, O. S. Fenton, Y. Zhang, K. T. Olejnik, V. Yesilyurt, D. Chen, S. Barros, B. Klebanov, T. Novobrantseva, R. Langer and D. G. Anderson, Nat. Commun., 2014, 5, 4277 CAS.
- Y. J. Kwon, Acc. Chem. Res., 2012, 45, 1077–1088 CrossRef CAS PubMed.
- S. C. Semple, A. Akinc, J. Chen, A. P. Sandhu, B. L. Mui, C. K. Cho, D. W. Y. Sah, D. Stebbing, E. J. Crosley, E. Yaworski, I. M. Hafez, J. R. Dorkin, J. Qin, K. Lam, K. G. Rajeev, K. F. Wong, L. B. Jeffs, L. Nechev, M. L. Eisenhardt, M. Jayaraman, M. Kazem, M. A. Maier, M. Srinivasulu, M. J. Weinstein, Q. Chen, R. Alvarez, S. A. Barros, S. De, S. K. Klimuk, T. Borland, V. Kosovrasti, W. L. Cantley, Y. K. Tam, M. Manoharan, M. A. Ciufolini, M. A. Tracy, A. de Fougerolles, I. MacLachlan, P. R. Cullis, T. D. Madden and M. J. Hope, Nat. Biotechnol., 2010, 28, 172–176 CrossRef CAS PubMed.
- S. Itakura, S. Hama, R. Matsui and K. Kogure, Nanoscale, 2016, 8, 10649–10658 RSC.
- C. A. Alabi, K. T. Love, G. Sahay, T. Stutzman, W. T. Young, R. Langer and D. G. Anderson, ACS Nano, 2012, 6, 6133–6141 CrossRef CAS PubMed.
- D. Ma, Nanoscale, 2014, 6, 6415–6425 RSC.
- J. C. Geoghegan, B. L. Gilmore and B. L. Davidson, Mol. Ther.–Nucleic Acids, 2012, 1, e53 CrossRef PubMed.
- K. K. Hou, H. Pan, L. Ratner, P. H. Schlesinger and S. A. Wickline, ACS Nano, 2013, 7, 8605–8615 CrossRef CAS PubMed.
- L. Yao, J. Daniels, D. Wijesinghe, O. A. Andreev and Y. K. Reshetnyak, J. Controlled Release, 2013, 167, 228–237 CrossRef CAS PubMed.
- A. Wittrup, A. Ai, X. Liu, P. Hamar, R. Trifonova, K. Charisse, M. Manoharan, T. Kirchhausen and J. Lieberman, Nat. Biotechnol., 2015, 33, 870–876 CrossRef CAS PubMed.
- J. Gilleron, W. Querbes, A. Zeigerer, A. Borodovsky, G. Marsico, U. Schubert, K. Manygoats, S. Seifert, C. Andree, M. Stöter, H. Epstein-Barash, L. Zhang, V. Koteliansky, K. Fitzgerald, E. Fava, M. Bickle, Y. Kalaidzidis, A. Akinc, M. Maier and M. Zerial, Nat. Biotechnol., 2013, 31, 638–646 CrossRef CAS PubMed.
- N. M. Belliveau, J. Huft, P. J. Lin, S. Chen, A. K. Leung, T. J. Leaver, A. W. Wild, J. B. Lee, R. J. Taylor, Y. K. Tam, C. L. Hansen and P. R. Cullis, Mol. Ther.–Nucleic Acids, 2012, 1, e37 CrossRef PubMed.
- Q. Feng, J. Sun and X. Jiang, Nanoscale, 2016, 8, 12430–12443 RSC.
- S. Chen, Y. Y. C. Tam, P. J. C. Lin, M. M. H. Sung, Y. K. Tam and P. R. Cullis, J. Controlled Release, 2016, 235, 236–244 CrossRef CAS PubMed.
- E. Wisse, F. Jacobs, B. Topal, P. Frederik and B. De Geest, Gene Ther., 2008, 15, 1193–1199 CrossRef CAS PubMed.
- B. L. Mui, Y. K. Tam, M. Jayaraman, S. M. Ansell, X. Du, Y. Y. C. Tam, P. J. Lin, S. Chen, J. K. Narayanannair, K. G. Rajeev, M. Manoharan, A. Akinc, M. A. Maier, P. Cullis, T. D. Madden and M. J. Hope, Mol. Ther.–Nucleic Acids, 2013, 2, e139 CrossRef CAS PubMed.
- M. Frank-Kamenetsky, A. Grefhorst, N. N. Anderson, T. S. Racie, B. Bramlage, A. Akinc, D. Butler, K. Charisse, R. Dorkin, Y. Fan, C. Gamba-Vitalo, P. Hadwiger, M. Jayaraman, M. John, K. N. Jayaprakash, M. Maier, L. Nechev, K. G. Rajeev, T. Read, I. Röhl, J. Soutschek, P. Tan, J. Wong, G. Wang, T. Zimmermann, A. de Fougerolles, H.-P. Vornlocher, R. Langer, D. G. Anderson, M. Manoharan, V. Koteliansky, J. D. Horton and K. Fitzgerald, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 11915–11920 CrossRef CAS PubMed.
- S. Poirier, M. Mamarbachi, W.-T. Chen, A. S. Lee and G. Mayer, Cell Rep., 2015, 13, 2064–2071 CrossRef CAS PubMed.
- Y. Zhang, J. M. Pelet, D. A. Heller, Y. Dong, D. Chen, Z. Gu, B. J. Joseph, J. Wallas and D. G. Anderson, Adv. Mater., 2013, 25, 4641–4645 CrossRef CAS PubMed.
- J. Leblond and A. Petitjean, ChemPhysChem, 2011, 12, 1043–1051 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional figures. See DOI: 10.1039/c6nr06701h |
| This journal is © The Royal Society of Chemistry 2017 |