Improving fiber alignment by increasing the planar conformation of isoindigo-based conjugated polymers
Liang
Chen
ab,
Kefeng
Zhao
ab,
Shuaijie
Chi
ab,
Jiangang
Liu
*a,
Xinhong
Yu
a and
Yanchun
Han
*a
aState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun 130022, P. R. China. E-mail: niitawh@ciac.ac.cn; ychan@ciac.ac.cn; Fax: +86-431-85262126; Tel: +86-431-85262175
bUniversity of the Chinese Academy of Sciences, No. 19A Yuquan Road, Beijing 100049, P. R. China
First published on 4th August 2016
Abstract
The macroscopic alignment of conjugated polymers with few grain boundaries is essential for carrier transport. It is well known that molecular conformation has a big role in achieving macroscopic alignment. Therefore, in this paper, a series of isoindigo-based conjugated polymers, the copolymer of isoindigo and thieno-[3,2-b]thiophene (IID–TT), isoindigo and bithiophene (IIDDT) and isoindigo and benzodithiophene (IID–BDT), were chosen to investigate the effect of molecular conformation on the fiber alignment. The long-range ordered film was obtained for IID–TT by drop-casting and solvent vapor enhanced drop-casting (SVE DC). The dichroic ratio (DR) of the IID–TT film obtained by SVE DC was 2.08 higher than that of the other two isoindigo-based conjugated polymers (IIDDT and IID–BDT), 1.07 and 1.33, respectively. The fibrils in the IID–TT film obtained by SVE DC were parallel and grown along the molecular backbones, but the fibrils of IIDDT and IID–BDT were both isotropic. The torsion angle of IID–TT (isoindigo and thieno-[3,2-b]thiophene) is 1.22°, while the torsion angle of IIDDT (isoindigo and thiophene, thiophene and thiophene) is 0.97° and 0.38° and that of IID–BDT (isoindigo and benzodithiophene) is 1.32°, respectively. The molecular conformation of IID–TT is the most coplanar among the three copolymers. The alkyl-stacking distance of IID–TT was 20.96 Å larger than that of IIDDT (19.25 Å) and IID–BDT (19.75 Å) which indicated that the interaction of the side-chain of IID–TT was the weakest among the three conjugated polymers. The DR increased with the increase in the alkyl-stacking distance. The weak interaction of the side-chain in IID–TT was beneficial for the rearrangement of molecules in parallel. The coplanar conformation could accelerate interchain assembly of the parallel molecules through π–π interaction to form parallel fibers.
1. Introduction
Conjugated polymers have attracted much attention and been extensively researched due to their good electronic properties and solution processable advantages in field-effect transistors (FETs) and solar cells.1,2 Conjugated polymers have a long, one-dimensional, rigid main chain structure with p-orbital overlaps along the main chain. Owing to these p-orbital overlaps, conjugated polymers exhibit intriguing anisotropic properties such as charge transport, absorption, and emission.3The carrier transfer in a crystalline region and through different crystalline regions both contribute to the final device performance. Increasing the crystallinity of the conjugated polymers and tuning the molecular orientation to benefit the carrier transfer could improve the charge carrier mobility.3,4 The alignment of crystalline regions in films also plays an important role in improving carrier transport. A continuous transfer pathway with a low grain boundary number and angle is preferred for carrier transport. The alignment of such pathways can decrease the amount of grains and improve the carrier mobility a lot.5 Therefore, the macroscopic alignment and assembly of conjugated polymers are essential to fully utilize their optical and electronic properties.
The alignment of conjugated polymers in films has been investigated using many fabrication methods, such as the Langmuir–Blodgett technique,6,7 mechanical rubbing,8,9 nanoimprinting,10,11 electrospinning,12 pre-patterned nano-grooved substrates,13,14 magnetic alignment,15 zone-casting,16–18 doctor-blading,19 dip coating,20 solution shearing,21 off-center spin-coating,22,23 epitaxy,24–29etc. These processing methods have demonstrated progress toward alignment and the carrier mobility has been greatly improved by the alignment of conjugated polymers. Heeger and co-workers reported a conjugated polymer alignment method using a nanogrooved substrate.13 The nanogrooved substrate was prepared by scratching the silicon wafer with a diamond lapping film. The aligned fibers of poly[4-(4,4-dihexadecyl-4H-cyclopenta[1,2-b:5,4-b′]-dithiophen-2-yl)-alt-[1,2,5]thiadiazolo[3,4-c]pyridine] (PCDTPT) were fabricated by applying the nanogrooved substrate and controlling the solvent evaporation direction and speed. The mobility of the device with the aligned film was 23.7 cm2 V−1 s−1. However, the mobility of the isotropic film was only 0.8 cm2 V−1 s−1.
Nevertheless, various processing methods have been established to accomplish a certain level of macroscopic alignment for several specific types of conjugated polymers. However, it is still elusive as to what kinds of conjugated polymers are suitable for alignment processing and what material designs are crucial for achieving well-defined macroscopic alignment. Obtaining an insight into the relationship between alignment and molecular structure is still a challenging task.
Isoindigo-based conjugated donor–acceptor copolymers have attracted much attention due to their excellent carrier mobility in field-effect transistors.30,31 In this work, we used a series of isoindigo-based conjugated polymers to investigate the role of molecular conformation of conjugated polymers in achieving alignment. The molecular conformation of IID–TT is the most coplanar and the repeating unit length of IID–TT is the shortest among the three copolymers and a long-range ordered film could be obtained for IID–TT. The coplanar conformation and the short repeating unit length could suppress the side-chain intercalation by steric hindrance to weaken the interaction of the side-chain. The weak interaction of the side-chain in IID–TT was beneficial for the rearrangement of molecules in parallel. The coplanar conformation could accelerate interchain assembly of the parallel molecules through π–π interaction to form parallel fibers.
2. Experimental
2.1 Materials
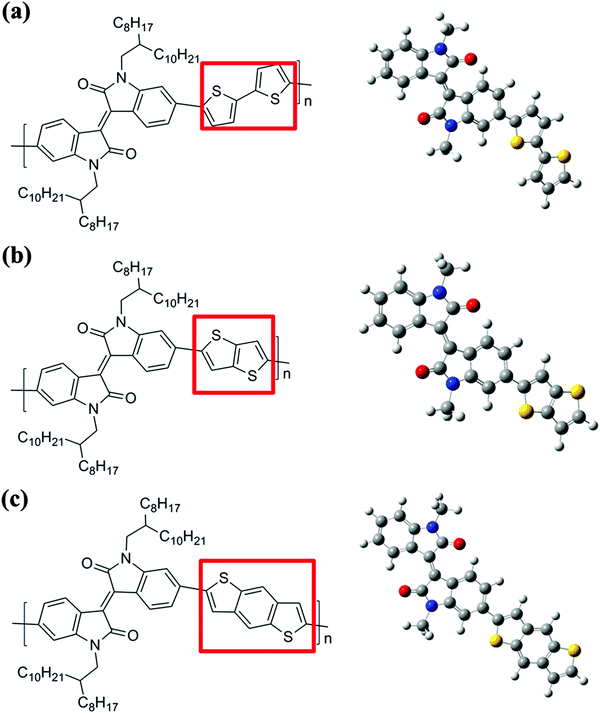
The three isoindigo-based conjugated copolymers (IIDDT, IID–TT and IID–BDT) were purchased from 1-Material Inc. and their structures are shown in Fig. 1. The molecular weight (Mn) and polydispersity index (PDI) are 28 kDa and 3.58, 26 kDa and 2.50, 23 kDa and 2.93, respectively, for IIDDT, IID–TT and IID–BDT. The solvent chloroform (CF) was purchased from Beijing Chemical, China. The glass slides as substrates were cleaned with piranha solution (70/30 v/v of concentrated H2SO4 and 30% H2O2) at 90 °C for 20 min and then rinsed with deionized water and finally blow dried with nitrogen.2.2 Sample preparation
The solutions of the three polymers were prepared at a concentration of 1 mg ml−1 in chloroform. The solutions were heated at 50 °C until they were dissolved absolutely and then placed at room temperature overnight before use. The drop-cast samples were prepared by placing 20 µl of the solution on 1.5 × 1.5 cm2 substrates. The solvent vapor enhanced drop-cast (SVE DC) samples were prepared by placing 20 µl of the solution on 1.5 × 1.5 cm2 substrates placed in covered glass Petri dishes with 3 ml CF to slow down solvent evaporation.2.3 Characterization
The macroscopic morphology of films was characterized in the polarized light mode using a Karl-Zeiss optic microscope (Germany) with a crossed polarizer and analyzer at 50× and 200× magnifications.The films for transmission electron microscopy (TEM) characterization were floated from the substrates with deionized water after being placed in an atmosphere of hydrogen fluoride vapor for several seconds and then picked up by copper grids. TEM images and selected area electron diffraction (SAED) patterns were obtained using a JEOL JEM-1011 transmission electron microscope operated at an accelerating voltage of 100 kV.
Atomic force microscopy (AFM) images were obtained using a SPA-300HV instrument with a SPI3800N controller (Seiko Instruments Inc., Japan) in tapping mode. A silicon microcantilever (spring constant 2 N m−1 and resonant frequency ≈70 kHz, Olympus, Japan) was used for the scanning.
All the UV-Vis-NIR absorption spectra and polarized UV-Vis-NIR absorption spectra were recorded by using an AvaSpect-3648 optical fiber spectrometer. Anisotropy was defined as the dichroic ratio between the maximum absorption parallel (radial direction) and perpendicular to the polarization direction.
The GIXRD patterns were obtained on a Bruker D8 Discover Reflector (Cu Kα, λ = 1.54056 Å) under 40 kV and 40 mA tube current. The X-ray profiles were recorded from 3 to 30 degrees with a step of 0.05 degrees using automatic slits.
The structural optimizations and DFT calculations were performed using the Gaussian 09 program at the B3LYP/6-31G* level of theory.
3. Results and discussion
3.1 The molecular conformation of IID–TT, IIDDT and IID–BDT
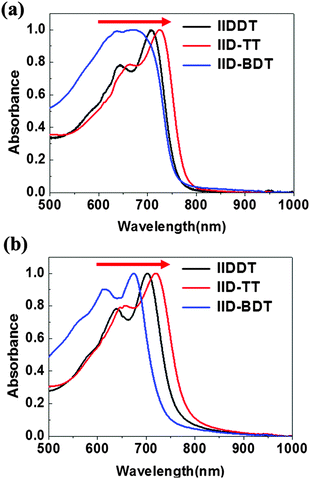
The structures of the three isoindigo-based conjugated copolymers are shown in Fig. 1. IIDDT is the copolymer of isoindigo and bithiophene (Fig. 1a). IID–TT is the copolymer of isoindigo and thieno-[3,2-b]thiophene (Fig. 1b). IID–BDT is the copolymer of isoindigo and benzodithiophene (Fig. 1c). The difference is highlighted in the red box in Fig. 1. According to the calculations using the Gaussian 09 program, the length of the donor units in the three conjugated polymers is different. The thieno-[3,2-b]thiophene (TT) unit (5.26 Å) is the shortest donor compared to the dithiophene (DT) unit (6.86 Å) and the benzodithiophene (BDT) unit (7.58 Å) and the length of IID–TT (16.88 Å) is the shortest compared to IIDDT (18.33 Å) and IID–BDT (19.17 Å). The short TT donor could make the distance of the alkyl side-chain along the molecular backbone direction short and suppress the side-chain intercalation. Suppressing the side-chain intercalation could be beneficial for weakening the interaction of the side-chain and decreasing the steric hindrance so that the backbone transformed to a more coplanar conformation. The torsion angle of the monomer was calculated using the Gaussian 09 program. The torsion angle of IID–TT (isoindigo and thieno-[3,2-b]thiophene) is 1.22°, while the torsion angle of IIDDT (isoindigo and thiophene, thiophene and thiophene) is 0.97° and 0.38° and that of IID–BDT (isoindigo and benzodithiophene) is 1.32°. Because the geometry optimization was carried out with monomers, the difference was little. The torsion angle would be enlarged with the increase in the number of the repeating units. The torsion angle of IIDDT (isoindigo and thiophene) increased to 20.73° when trimers were used to simulate each polymer backbone.32 The conformation of IID–TT was more coplanar than that of IIDDT and IID–BDT. The conformation of the three isoindigo-based conjugated copolymers was also reflected in the UV-Vis-NIR absorption spectra.The UV-Vis-NIR absorption spectra of the three conjugated polymers are shown in Fig. 2. In the UV-Vis-NIR absorption spectra, the max absorption peak and the onset of IID–TT in solution and film had a longer wavelength than the other two polymers which indicated that the bandgap of IID–TT was smaller. The smaller bandgap indicated greater π-system delocalization.33 The greater π-system delocalization suggested a more coplanar conformation.34 Although the donor units were different in the three conjugated polymers, the red-shift of IID–TT could reflect the more coplanar conformation partially. The short repeating unit length and the coplanar conformation in IID–TT could suppress the side-chain intercalation by steric hindrance to weaken the interaction of the side-chain. To investigate the role of different molecular conformations in achieving alignment, the anisotropy of the three copolymers was characterized.
3.2 Fiber anisotropy in films of IID–TT, IIDDT and IID–BDT
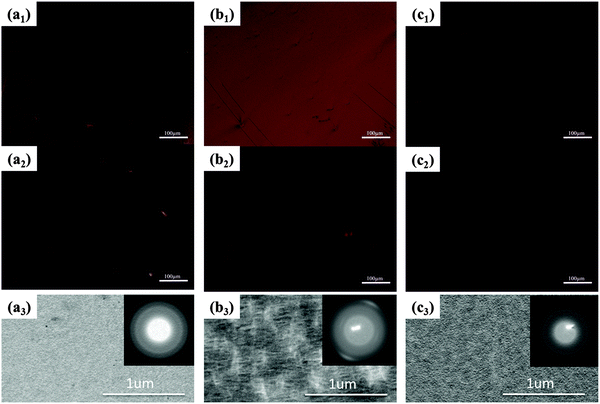
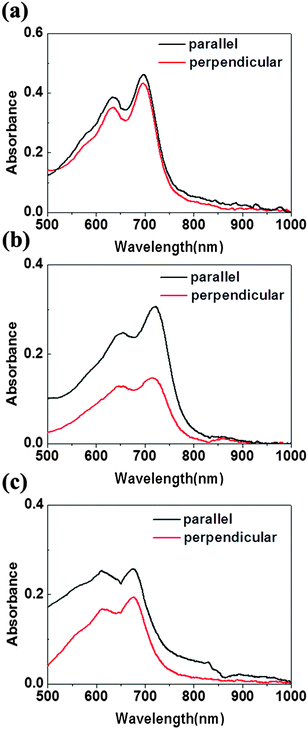
The optic microscopy images and TEM images of drop-cast IIDDT, IID–TT and IID–BDT films are shown in Fig. 3. The optic microscopy images of the IID–TT films obtained at 45° with respect to the crossed polarizer and analyzer displayed large areas of bright zones in contrast to the dark images obtained at 0° which indicated that the IID–TT films had apparent macro-optic anisotropy.35 However, the optic microscopy images of IIDDT and IID–BDT films didn't show bright zones and differences in brightness during the rotation of the samples. The TEM image of IID–TT film displayed parallel fibers and the SAED patterns displayed diffraction arcs which indicated the micro-anisotropy of the IID–TT film. There was no special micro morphology and the SAED patterns were diffraction rings in the IIDDT and IID–BDT films which indicated that the IIDDT and IID–BDT films were isotropic in the micro morphology.To quantify the difference in anisotropy of drop-cast IIDDT, IID–TT and IID–BDT films, we characterized the dichroic ratio (DR) by polarized UV-Vis-NIR absorption spectroscopy, as shown in Fig. 4. The DR was 1.47 in the IID–TT film, higher than the films of IIDDT (1.12) and IID–BDT (1.31). All the macro and micro anisotropies and the DR of the IID–TT film indicated the anisotropic long-range ordered film could be obtained for IID–TT more easily than IIDDT and IID–BDT by the simple drop-casting fabrication method. To magnify the difference in anisotropy between IIDDT, IID–TT and IID–BDT, we prepared the films of IIDDT, IID–TT and IID–BDT by solvent vapor enhanced drop-casting (SVE DC) to slow down solvent evaporation and enhance the self-assembly of conjugated polymers.
 | ||
| Fig. 4 Polarized UV-Vis absorption spectra of drop-cast IIDDT (a), IID–TT (b), IID–BDT (c) with the alignment direction parallel and perpendicular to the polarization direction. | ||
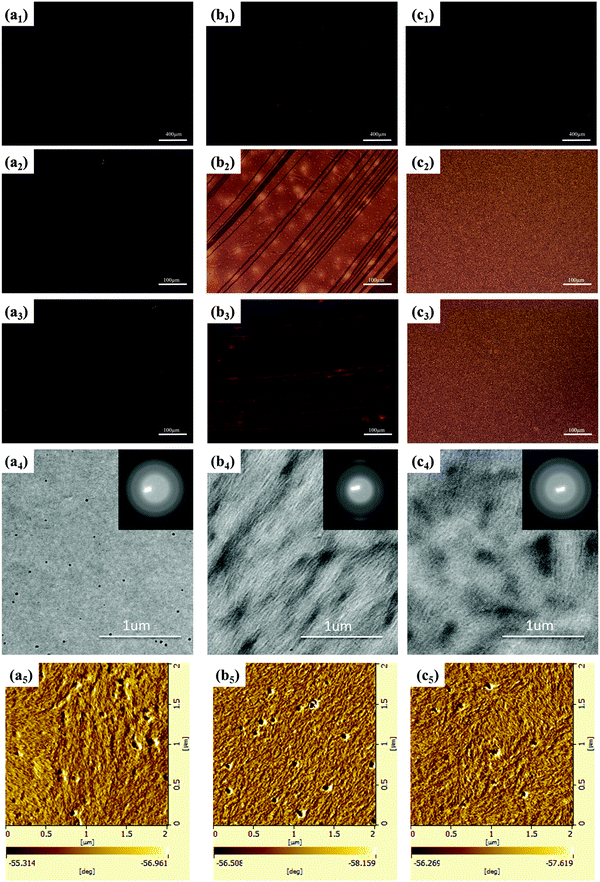
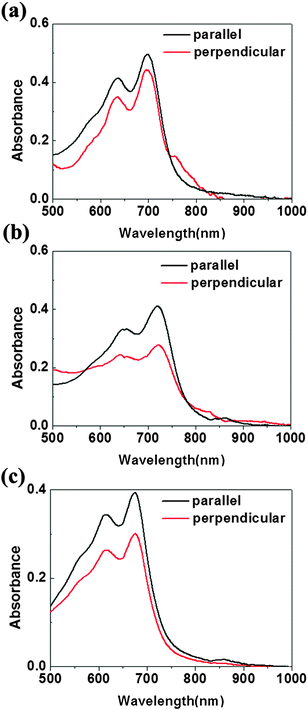
The optic microscopy images, TEM images and AFM images of IIDDT, IID–TT and IID–BDT films prepared by SVE DC are shown in Fig. 5. The optic microscopy images of IID–TT films at low magnification displayed the aligned direction along the contact line receding (the radial direction). The optic microscopy images of IID–TT films obtained at 45° with respect to the crossed polarizer and analyzer displayed large areas of bright stripe-like zones in contrast to the dark images obtained at 0° which indicated that the IID–TT films had apparent macro-optic anisotropy. The bright zones were parallel and separated by the narrow dark zones, but the narrow dark zones changed to bright zones during the rotation of the samples, which indicated that the narrow dark zones were anisotropic and the aligned direction was 45° with respect to the direction of the bright zones. The optic microscopy images of IIDDT films were still dark during the rotation of the samples and there were bright zones in the IID–BDT films but the bright zones were not continuous which indicated that the films of IIDDT and IID–BDT were isotropic. The TEM image of the IID–TT film prepared by SVE DC to enhance the self-assembly displayed obvious parallel fibers and the SAED patterns displayed diffraction arcs which indicated the micro anisotropy of the IID–TT film. There were significant differences between IID–TT and the other two polymers in morphology. In the IIDDT and IID–BDT films, there was no special micro morphology and the SAED patterns were diffraction rings which indicated that the IIDDT and IID–BDT films were isotropic in the micro morphology. The AFM image of the IID–TT film displayed parallel fibers, similar to the TEM image. The AFM images of IIDDT and IID–BDT films displayed isotropy. The DR of the films prepared by SVE DC is shown in Fig. 6. The DR was 2.08 in the IID–TT film, higher than the films of IIDDT (1.07) and IID–BDT (1.33). Compared to the drop-cast film, the DR of the IID–TT film could be improved easily by slowing down solvent evaporation to enhance the self-assembly during the SVE DC process. The DR of IIDDT and IID–BDT films prepared by SVE DC were still low and similar to the drop-cast films which indicated that it was hard to form aligned films for IIDDT and IID–BDT, while the self-assembly was enhanced. According to all the results mentioned above, the anisotropic long-range ordered film could be obtained for IID–TT more easily than IIDDT and IID–BDT by SVE DC. The research of the differences in molecular packing of the three conjugated polymers was needed to propose the origin of alignment and the role of molecular conformation of conjugated polymers in achieving alignment. The molecular packing of the three conjugated polymers was characterized to investigate the relationship between alignment and molecular conformation.
3.3 The effect of molecular conformation on fiber alignment
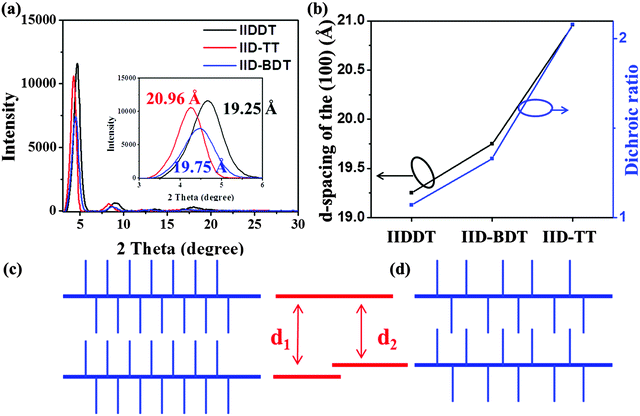
The crystalline structure of the three conjugated polymers films prepared by SVE DC was characterized by GIXRD, as shown in Fig. 7a. The peaks in out-plane GIXRD profiles indicated the alkyl-stacking (100) and there was no diffraction peak at larger than 20° (010) which indicated an edge-on molecular orientation of chains.31 The alkyl-stacking distance of IID–TT was 20.96 Å, larger than IIDDT (19.25 Å) and IID–BDT (19.75 Å), while the isoindigo in the backbone and the alkyl side-chain connected to the isoindigo were the same in the three conjugated polymers which indicated that the interaction of the side-chain of IID–TT was the weakest among the three conjugated polymers. As mentioned above, the difference of the interaction of the side-chain may arise from the different length of the donor units in the three conjugated polymers and different coplanarity in molecular conformation. The short repeating unit length and the coplanar conformation in IID–TT could suppress the side-chain intercalation by steric hindrance to weaken the interaction of the side-chain.The relationship between the alkyl-stacking distance and the DR of the three conjugated polymers prepared by SVE DC is shown in Fig. 7b. The DR increased with the increasing alkyl-stacking distance and weakening of the interaction of the side-chain. To propose the reason that weak interaction of the side-chain was beneficial for alignment, we should know the molecular packing of IID–TT in the aligned fibers. As shown in Fig. 5b4, the diffraction arcs in the SAED image represented a d-spacing of about 3.5 Å. It was identified as the (010) reflection indicating the π–π stacking direction. According to the results of out-plane GIXRD and long-range alignment coupled with SAED, the molecules have an edge-on orientation and the molecular backbones were parallel to the long axis of fibrils. The stripe-like zones were parallel to the contact line receding direction according to the optic microscopy images in Fig. 5. The crystalline structure and stripe-like alignment were similar to that of other donor–acceptor conjugated polymers.23,36,37
According to the above discussions, we could propose the formation mechanism that weak interaction of the side-chain could more easily form alignment. As shown in Fig. 7c and d, the weak interaction of the side-chain in IID–TT was beneficial for the molecules being separated from the original aggregation and rearranged into the aligned fibers during the contact line receding. The isolated edge-on molecules formed parallel fibers and stripe-like zones by directional solution-shearing along the contact line receding. Thus, the weak interaction of the side-chain in IID–TT was the reason why IID–TT could most easily form alignment among the three conjugated polymers. The coplanar conformation could accelerate interchain assembly through π–π interaction to form parallel fibers which could avoid the aligned molecules transforming to isotropic and stabilize the alignment. Thus, the short repeating unit length and the more coplanar conformation of IID–TT were helpful to weaken the interaction of the side-chain and accelerate the aligned molecules to form parallel fibers.
4. Conclusions
In this work, we investigated the effect of the molecular conformation on alignment by using a series of isoindigo-based conjugated polymers. The long–range ordered film could be obtained for IID–TT. However, IIDDT and IID–BDT were always isotopic in spite of the enhanced self-assembly. The difference in alignment of the three conjugated polymers could be attributed to the different molecular conformations. The molecular conformation of IID–TT is the most coplanar and the repeating unit length of IID–TT is the shortest among the three copolymers. The alkyl-stacking distance of IID–TT is larger than that of IIDDT and IID–BDT which indicated that the interaction of the side-chain of IID–TT is the weakest among the three conjugated polymers. The DR increased with the increase in the alkyl-stacking distance. The coplanar conformation and the short repeating unit length could suppress the tilted stacking of the alkyl side-chain to suppress the side-chain intercalation by steric hindrance to weaken the interaction of the side-chain and help the molecules to rearrange into the aligned fibers. The coplanar conformation could accelerate interchain assembly through π–π interaction to form parallel fibers and avoid the aligned molecules from transforming to isotropic and stabilize the alignment. Increasing the planar conformation could improve the fiber alignment and the fiber alignment of conjugated polymers was essential to improve the charge carrier mobility.Acknowledgements
This work was partly supported by the National Natural Science Foundation of China (21334006, 51577138, and 21474113) and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB12020300).References
- S. Holliday, J. E. Donaghey and I. McCulloch, Chem. Mater., 2014, 26, 647–663 CrossRef CAS.
- Y. Diao, L. Shaw, Z. Bao and S. C. B. Mannsfeld, Energy Environ. Sci., 2014, 7, 2145–2159 CAS.
- R. Noriega, J. Rivnay, K. Vandewal, F. P. V. Koch, N. Stingelin, P. Smith, M. F. Toney and A. Salleo, Nat. Mater., 2013, 12, 1038–1044 CrossRef CAS PubMed.
- K. J. Ihn, J. Moulton and P. Smith, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 735–742 CrossRef CAS.
- Y. K. Lan and C. I. Huang, J. Phys. Chem. B, 2009, 113, 14555–14564 CrossRef CAS PubMed.
- V. Cimrova, M. Remmers, D. Neher and G. Wegner, Adv. Mater., 1996, 8, 146–149 CrossRef CAS.
- J. Kim, S. K. McHugh and T. M. Swager, Macromolecules, 1999, 32, 1500–1507 CrossRef CAS.
- L. Biniek, N. Leclerc, T. Heiser, R. Bechara and M. Brinkmann, Macromolecules, 2013, 46, 4014–4023 CrossRef CAS.
- L. Hartmann, K. Tremel, S. Uttiya, E. Crossland, S. Ludwigs, N. Kayunkid, C. Vergnat and M. Brinkmann, Adv. Funct. Mater., 2011, 21, 4047–4057 CrossRef CAS.
- Z. J. Zheng, K. H. Yim, M. S. M. Saifullah, M. E. Welland, R. H. Friend, J. S. Kim and W. T. S. Huck, Nano Lett., 2007, 7, 987–992 CrossRef CAS PubMed.
- S. Wei, Y. Zhang, J. Liu, X. Li, Y. Wu, H. Wei, Y. Weng, X. Gao, Y. Li, S.-D. Wang and Z. Hu, Adv. Mater. Interfaces, 2015, 2, 1500153 CrossRef.
- J.-Y. Chen, C.-C. Kuo, C.-S. Lai, W.-C. Chen and H.-L. Chen, Macromolecules, 2011, 44, 2883–2892 CrossRef CAS.
- H. R. Tseng, H. Phan, C. Luo, M. Wang, L. A. Perez, S. N. Patel, L. Ying, E. J. Kramer, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Adv. Mater., 2014, 26, 2993–2998 CrossRef CAS PubMed.
- C. Luo, A. K. Kyaw, L. A. Perez, S. Patel, M. Wang, B. Grimm, G. C. Bazan, E. J. Kramer and A. J. Heeger, Nano Lett., 2014, 14, 2764–2771 CrossRef CAS PubMed.
- G. Pan, F. Chen, L. Hu, K. Zhang, J. Dai and F. Zhang, Adv. Funct. Mater., 2015, 25, 5126–5133 CrossRef CAS.
- A. Tracz, J. K. Jeszka, M. D. Watson, W. Pisula, K. Mullen and T. Pakula, J. Am. Chem. Soc., 2003, 125, 1682–1683 CrossRef CAS PubMed.
- M. J. Lee, D. Gupta, N. Zhao, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Funct. Mater., 2011, 21, 932–940 CrossRef CAS.
- Y. Su, X. Gao, J. Liu, R. Xing and Y. Han, Phys. Chem. Chem. Phys., 2013, 15, 14396–14404 RSC.
- D. Khim, H. Han, K. J. Baeg, J. Kim, S. W. Kwak, D. Y. Kim and Y. Y. Noh, Adv. Mater., 2013, 25, 4302–4308 CrossRef CAS PubMed.
- R. Z. Rogowski and A. A. Darhuber, Langmuir, 2010, 26, 11485–11493 CrossRef CAS PubMed.
- G. Giri, E. Verploegen, S. C. Mannsfeld, S. Atahan-Evrenk, H. Kim do, S. Y. Lee, H. A. Becerril, A. Aspuru-Guzik, M. F. Toney and Z. Bao, Nature, 2011, 480, 504–508 CrossRef CAS PubMed.
- Y. Yuan, G. Giri, A. L. Ayzner, A. P. Zoombelt, S. C. Mannsfeld, J. Chen, D. Nordlund, M. F. Toney, J. Huang and Z. Bao, Nat. Commun., 2014, 5, 3005 Search PubMed.
- H. Wang, L. Chen, R. Xing, J. Liu and Y. Han, Langmuir, 2015, 31, 469–479 CrossRef CAS PubMed.
- H. Dong, H. Li, E. Wang, Z. Wei, W. Xu, W. Hu and S. Yan, Langmuir, 2008, 24, 13241–13244 CrossRef CAS PubMed.
- H. Zhou, S. Jiang and S. Yan, J. Phys. Chem. B, 2011, 115, 13449–13454 CrossRef CAS PubMed.
- H. Li and S. Yan, Macromolecules, 2011, 44, 417–428 CrossRef CAS.
- H. Zhou and S. Yan, Macromol. Chem. Phys., 2013, 214, 639–653 CrossRef CAS.
- D. Sun, Y. Li, Z. Ren, M. R. Bryce, H. Li and S. Yan, Chem. Sci., 2014, 5, 3240–3245 RSC.
- L. Liu, Z. Ren, C. Xiao, B. He, H. Dong, S. Yan, W. Hu and Z. Wang, Chem. Commun., 2016, 52, 4902–4905 RSC.
- T. Lei, J. Y. Wang and J. Pei, Acc. Chem. Res., 2014, 47, 1117–1126 CrossRef CAS PubMed.
- T. Lei, Y. Cao, X. Zhou, Y. Peng, J. Bian and J. Pei, Chem. Mater., 2012, 24, 1762–1770 CrossRef CAS.
- M. S. Chen, J. R. Niskala, D. A. Unruh, C. K. Chu, O. P. Lee and J. M. J. Frechet, Chem. Mater., 2013, 25, 4088–4096 CrossRef CAS.
- H. Huang, Z. Chen, R. Ponce Ortiz, C. Newman, H. Usta, S. Lou, J. Youn, Y. Y. Noh, K. J. Baeg, L. X. Chen, A. Facchetti and T. J. Marks, J. Am. Chem. Soc., 2012, 134, 10966–10973 CrossRef CAS PubMed.
- X. Yuan, W. Zhang, L. H. Xie, J. Ma, W. Huang and W. Liu, J. Phys. Chem. B, 2015, 119, 10316–10333 CrossRef CAS PubMed.
- L. Chai, H. Zhou, X. Sun, H. Li, S. Yan and X. Yang, Chin. J. Polym. Sci., 2016, 34, 513–522 CrossRef CAS.
- Y. Xu, J. Liu, H. Wang, X. Yu, R. Xing and Y. Han, Soft Matter, 2013, 9, 9849–9856 RSC.
- H. Wang, J. Liu, Y. Xu, X. Yu, R. Xing and Y. Han, Phys. Chem. Chem. Phys., 2014, 16, 1441–1450 RSC.
| This journal is © the Partner Organisations 2017 |