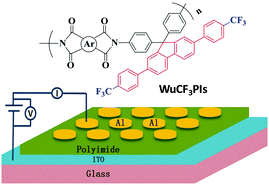
Exceptionally thermostable and soluble aromatic polyimides with special characteristics: intrinsic ultralow dielectric constant, static random access memory behaviors, transparency and fluorescence†
Yiwu
Liu‡
a,
Zhuxin
Zhou‡
a,
Lunjun
Qu
a,
Bing
Zou
b,
Zhiquan
Chen
b,
Yi
Zhang
*a,
Siwei
Liu
a,
Zhenguo
Chi
a,
Xudong
Chen
a and
Jiarui
Xu
*a
aPCFM Lab, GD HPPC Lab, Guangdong Engineering Technology Research Center for High-performance Organic and Polymer Photoelectric Functional Films, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry and Chemical Engineering, Sun Yat-sen University, Guangzhou 510275, China. E-mail: ceszy@mail.sysu.edu.cn; xjr@mail.sysu.edu.cn
bHubei Nuclear Solid Physics Key Laboratory, Department of Physics, Wuhan University, Wuhan 430072, P. R. China
First published on 12th August 2016
Abstract
A series of high-performance multifunctional polyimides with exceptional thermostability and solubility (Tg as high as 494 °C and, at the same time, well soluble in common organic solvents) were successfully designed and synthesized by introducing a typical aromatic rigid trifluoromethyl-containing moiety with special non-planar and conjugated characteristics into the polymer backbone. Additionally, these polyimides show light color (one even colorlessness) and transparency, intrinsic ultralow dielectric constant (k, k ≈ 1.93), and electrical bistability characteristics (ON/OFF ratio as high as 107, working voltage as low as 1.5 V) simultaneously. The excellent thermal stability and solubility allow them to undergo the high temperature process (over 400 °C) in the preparation of photoelectric devices (like PVD or PECVD), or the highly efficient, continuous roll-to-roll process. The as-synthesized polymers are ideal potential candidates for practical applications in the fields of ultra-large-scale integration (ULSI), high-performance polymer memory devices, flexible displays, thin film PV industries, and wearable electronics.
Introduction
Over the last few decades, different functional polymeric materials have been synthesized and have demonstrated a variety of functional properties, such as photoelectricity conversion,1 organic electroluminescence (OEL),2 piezoelectricity,3 pyroelectricity,4 resistive switching,5 low dielectric constant,6 and proton conduction.7 Many of these properties are the basis for the modern advanced technology. And the application of these functional polymeric materials in the field of new energy sources and optoelectronic devices has attracted tremendous attention, for example, in solar cells,8 fuel cells,9 organic light-emitting diodes (OLEDs),10 electrochromic (EC) devices,11 polymer memory devices,12 integrated circuits (ICs),13 flexible displays14 and wearable electronics.15 More importantly, polymer materials exhibit lightweight, easy processability, flexibility, high mechanical strength, good scalability, and three-dimensional stacking capability, which have significant advantages over inorganic or metal-oxide-based materials.16However, the preparation of polymers that combine several of these functional properties together is a synthetic challenge to both chemists and physicists, requiring sophisticated molecular and/or supramolecular designs. And more challenges are how to obtain multifunctional polymer materials while maintaining their high-performance, like thermal stability, solubility and mechanical properties. This is extremely important for the practical application of the materials. For example, most of the preparation processes in photoelectricity, like Physical Vapor Deposition (PVD) or Plasma Enhanced Chemical Vapor Deposition (PECVD) processes,17 are performed at high temperature, at 400 °C or even higher to ensure the high performance of the film. However, very few polymer materials can meet this requirement, and after functionalization, the thermostability of most of the polymers may be affected to various degrees.18
Polyimides have been widely used as the materials for electronic packaging and electrical insulation in microelectronics industries due to their excellent thermal, mechanical and dielectric properties.19 In recent years, functional polyimides and their applications have become emerging research areas, especially in the fields of high-performance interlayer dielectrics,20 polymer electronic memory devices,21 flexible displays22 and flexible photovoltaic devices.23 However, these applications are limited by the insoluble and infusible nature and dark color (yellow to brown) of the polyimides, which mainly result from the rigid backbone and the strong interchain packing, as well as the formation of a charge transfer complex (CTC) between the electron-donating diamine moiety and the electron-accepting dianhydride moiety in the polyimide molecular chain.24 In this regard, many structural modifications of the polymer backbone have been utilized to functionalize the polyimide, but usually led to the reduction of the thermal stability and mechanical properties.25 So the design and synthesis of multifunctional polyimides with transparency, solubility and high thermostability are of great interest.
In this contribution, a novel diamine monomer (WuCF3DA) with a fluorene moiety and a special spatial structure was designed and successfully introduced into the polymer to obtain a series of novel polyimides (named WuCF3PIs). The 3D chemical structure of WuCF3DA and its corresponding polyimides is shown in Fig. 1. From the perspective of chemical structures (Fig. 1a), WuCF3DA has a planar conjugated core of fluorene connected by two benzene rings; at each end of the benzene ring there is a trifluoromethyl group (–CF3) with low polarizability. From that of the geometrical structure (Fig. 1b), two phenylamine groups are connected with the conjugate fluorene plane through the carbon atom which has an sp3 hybridized orbital; the conjugated fluorene plane is almost vertical to the two phenylamine planes, and thus formed a totally non-planar structure. When such a kind of structure is introduced into the polyimide main chain (Fig. 1c), WuCF3PIs show multifunctional properties with exceptional overall properties. These polyimide films exhibit an ultralow dielectric constant (or relative permittivity, with the lowest k value of 1.93), bistable resistive switching effects (with a threshold voltage as low as 1.5 V and an ON/OFF current ratio as high as 107), as well as light color and transparency (transmittance over 85% in the visible region). More importantly, these polyimides have excellent solubility in common solvents and exceptional thermal stability, with a glass transition temperature (Tg) over 400 °C (with one up to 494 °C), 5 wt% loss temperature higher than 554 °C in N2 and 543 °C in air, and a residual higher than 65% at 800 °C in N2, indicating that they meet the requirement of high temperature processes, like PVD or PECVD, and highly efficient roll-to-roll processes, which can be used in large-area, thin-film optoelectronic devices. And as far as we know, this should be the first report of multifunctional polymers with the highest thermal stability and outstanding processability.
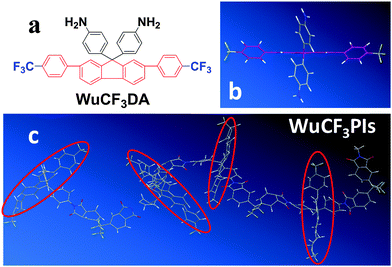 | ||
| Fig. 1 Chemical structure (a), 3D structure of diamine WuCF3DA (b) and its polyimide WuCF3PI-6F (c). | ||
Results and discussion
Synthesis and characteristics of WuCF3DA
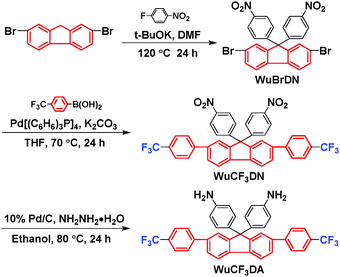
The synthesis route of WuCF3DA is described in the Experimental section and also shown in Scheme 1. Firstly, the dibromide (WuBrDN) was prepared from the reaction of 2,7-dibromofluorene with 1-fluoro-4-nitrobenzene, followed by the Suzuki reaction with 4-(trifluoromethyl)phenylboronic acid to yield the dinitro compound WuCF3DN; then WuCF3DN was reduced to form the diamine WuCF3DA, containing vertical rigid, non-planar, large conjugated fluorene moieties and low polarity trifluoromethyl groups (–CF3) in good yield (94%). The chemical structures of the synthesized intermediate products (WuBrDN and WuCF3DN) and the target diamine WuCF3DA were confirmed by 1H NMR and 13C NMR (H–H COSY, C–H QC, and C–H BC) spectroscopy, mass spectrometry, FTIR, and elemental analyses; the results are shown in the Experimental section and Fig. S1 in the ESI.† Fig. S1a and b (ESI†) illustrate the 1H and 13C NMR spectra of WuCF3DA. Assignments of each carbon and proton were assisted by the two-dimensional (2D) COSY, C–H QC and C–H BC NMR spectra shown in Fig. S1c–e in the ESI.† The spectral results were in agreement with the proposed molecular structure of WuCF3DA, and the 1H NMR spectra confirmed that the diamine was successfully synthesized by the mentioned three-step reactions as the resonance signals at around 4.95 ppm corresponded to the amino protons.The thermal properties of WuCF3DA (Table S1 in the ESI†) analyzed by TGA and DSC revealed that WuCF3DA had good thermal stability. The 5% and 10% weight-loss temperatures (Td) of WuCF3DA in N2 were 378 and 402 °C, respectively, and its glass transition temperature (Tg) was approximately 165 °C.
Due to the rigid, aromatic, non-coplanar structure and the D–π–A large conjugated characteristics, the novel diamine shows obviously photophysical properties both in the solution and solid states, as shown in Fig. 2 and Table S1 in the ESI.† The maximum UV absorption wavelength of the WuCF3DA was 314 nm in THF. When excited at its maximum absorption wavelength, the maximum PL emission of WuCF3DA in THF and in the powder state appeared at the wavelengths of 510 nm and 446 nm, respectively, exhibiting a significant blue-shift of the maximum emission for powder WuCF3DA compared to that determined in the THF solution. Similar interesting phenomena and the reason have been reported in detail in another similar diamine WuFDA system by our group.26 The special chemical structure (strong polar groups, –NH2 and –CF3) and their particular location in the molecule form a typical D–π–A molecule with strong internal charge transfer (ICT) nature. Both the ICT effect and the intermolecular hydrogen bond interaction play very important roles in the geometrical aggregation manner and the fluorescence behaviors of the WuCF3DA molecules.
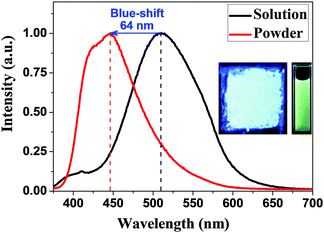 | ||
| Fig. 2 PL spectra of the WuCF3DA (10−5 M) in THF and powder (the pictures are UV irradiation excited at 365 nm). | ||
Synthesis and characteristics of WuCF3PIs
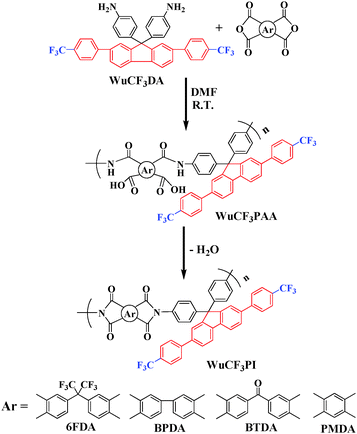
The polyimides (WuCF3PIs) were prepared in a conventional two-step procedure by the reaction of equal molar amounts of diamine WuCF3DA with commercially available aromatic dianhydride (6FDA, BPDA, BTDA and PMDA) in dimethylformamide (DMF) to form the precursor poly(amic acid)s (WuCF3PAAs). The PAAs were subsequently coated on a clean dry glass plate, followed by thermal imidization in a vacuum oven at 100 °C/1 h, 200 °C/1 h, 300 °C/1 h and 450 °C/1 h, to obtain the novel polyimides WuCF3PIs, as shown in Scheme 2.The chemical structures of WuCF3PIs were confirmed by FT-IR spectra and 1H NMR (Fig. S2 and S3 in the ESI†). The molecular weight of the WuCF3PAAs was determined by GPC, and the qualitative solubility behavior of the corresponding polyimides is summarized in Table 1. The Mw of the PAAs ranged from 46.3 × 104 to 54.2 × 104, with polydispersity indices (PDIs) ranging from 1.43 to 1.49, which indicated that the as-prepared polyimides had a very high molecular weight and a narrow molecular weight distribution.
| Polymers | GPCa | Solventb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M w × 104 | PDI | NMP | DMSO | DMAc | DMF | m-Cresol | THF | CHCl3 | |
| a With respect to polystyrene standards, with DMF as the eluent; flow rate: 1 mL min−1 and test temperature: 50 °C. b Solubility: ++: soluble at room temperature; +: partially soluble at room temperature and soluble upon heating. | |||||||||
| WuCF3PI-6F | 46.3 | 1.48 | ++ | ++ | ++ | ++ | ++ | ++ | ++ |
| WuCF3PI-BP | 54.2 | 1.43 | ++ | ++ | + | + | + | + | + |
| WuCF3PI-BT | 45.8 | 1.49 | ++ | ++ | ++ | ++ | + | + | + |
| WuCF3PI-PM | 46.5 | 1.47 | ++ | ++ | ++ | ++ | + | + | + |
The four polyimides were well soluble in polar solvents such as N-methylpyrrolidone (NMP), dimethylacetamide (DMAc), dimethyl sulfoxide (DMSO), dimethylformamide (DMF), m-cresol, tetrahydrofuran (THF), and chloroform. The enhanced solubility can be attributed to the introduction of the –CF3 groups and the large rigid conjugated fluorene moiety which is vertical to the polyimide main chain (Fig. 1c). Such structures may greatly increase the steric hindrance effect and the free volume of the polymers during aggregation, thus reducing the interactions between the polyimide macrochains. The excellent solubility makes the polymer a potential candidate for practical applications in large-scale microelectronics by spin-on or dip-coating processes.
Thermal and mechanical properties of WuCF3PIs
The thermal properties of the WuCF3PI films were investigated by TGA, DSC and DMA, and the results are shown in Table 2. The results of TGA (Fig. 3 and Fig. S4 in the ESI†) revealed that the polyimides exhibited excellent thermal stabilities with insignificant weight loss up to 550 °C in atmospheres of N2 and air. The 5% weight-loss temperatures (Td5%) were in the range of 554–596 °C in N2 and 543–580 °C in air, respectively. The amount of carbonized residue (char. yield) in N2 was higher than 65% at 800 °C. In addition, the polyimide films exhibited an extremely high Tg over 378 °C (as high as 447 °C) measured by DSC and over 406 °C (as high as 494 °C) by DMA (Fig. S5 and S6 in the ESI†). The thermal stability and Tg of the synthesized polyimides are far higher than those of the commercial polymers or other reported aromatic polymers, which allow them to undergo processing temperatures in excess of 400 °C. For example, the isothermal weight loss of the WuCF3PI-6F at 400 °C for half an hour is less than 0.1% (inset in Fig. 3). The exceptional thermal stability of the polyimides is mainly due to the rigid aromatic nature of the polymer and the special steric effects caused by the sp3 hybridized carbon atom on the fluorene group (Fig. 1c). As far as we know, a polymer with Tg close to 500 °C and at the same time well soluble in common organic solvents has not yet been reported in the literature.| Polymers | T g (°C) | T g (°C) | T d5% (°C) | T d10% (°C) | Char. yieldc (wt%) | ||
|---|---|---|---|---|---|---|---|
| N2 | Air | N2 | Air | ||||
| a Measured by DSC at a heating rate of 25 °C min−1. b Measured by DMA at 1 Hz and at a rate of 4 °C min−1. c Residual weight percentage at 800 °C in N2. | |||||||
| WuCF3PI-6F | 378 | 406 | 554 | 543 | 578 | 563 | 65 |
| WuCF3PI-BP | 413 | 465 | 596 | 580 | 620 | 600 | 71 |
| WuCF3PI-BT | 401 | 459 | 585 | 563 | 612 | 586 | 70 |
| WuCF3PI-PM | 447 | 494 | 579 | 558 | 604 | 581 | 69 |
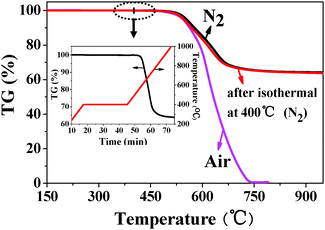 | ||
| Fig. 3 Thermal stability of the WuCF3PI-6F film in N2 and air. The inset shows the thermal stability of WuCF3PI-6F after isothermal treatment at 400 °C in N2 for 30 min. | ||
The polyimide films showed good mechanical properties as well (Table S2 in the ESI†); the tensile strength and tensile modulus were in the range of 74.3–88.3 MPa and 2.9–3.3 GPa, respectively. It is worth noticing that these mechanical properties were obtained with the as-cast film on glass plates in the lab, and better properties could be expected for the films using optimized processing procedures. The high Tg, excellent thermal stability and favorable mechanical properties of the soluble PI are most likely due to the vertical side group of the large rigid conjugated fluorene structure and the low polarity –CF3 group, and are expected to meet the requirements of high-efficiency roll-to-roll processes and high processing temperatures (higher than 400 °C) in the microelectronics industry.
Optical and fluorescence properties of WuCF3PIs
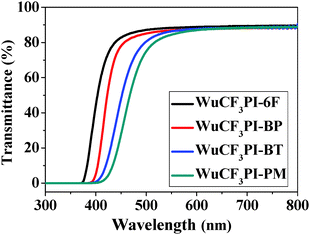
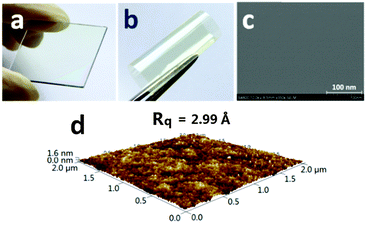
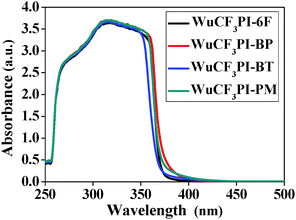
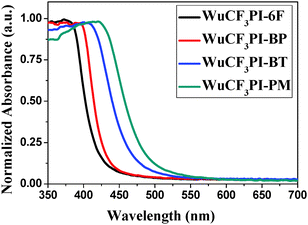
The polyimides can produce light color, near-transparent, flexible, and tough films with amorphous nature (Fig. S7 in the ESI†) via a spin-on or solution casting process. The transmission UV-vis spectra of the polyimide films observed in the visible region showed that the cut-off wavelengths (λ0) were in the range of 374–425 nm (Fig. 4). WuCF3PI-6F, for example, is almost colorless and transparent (Fig. 5a and b); the transmittance is 83.7% at 450 nm and over 90% in the visible region (black curve in Fig. 4). The film surface was smooth and compact, with a surface roughness (Rq) of 2.99 Å, which was confirmed by scanning electron microscopy (SEM, Fig. 5c) and atomic force microscopy (AFM, Fig. 5d). Compared with the commercial polyimides, such as Kapton from DuPont (deep brown) or other reported functional polyimide containing triphenylamine (TPA) moieties (dark red to deep brown),27 the improvement in color and transparency of these fluorene-containing polyimides may be due to the following reasons: firstly, the carbon atoms with sp3 hybridization in fluorene would cut off the conjugated effect of the rigid planar fluorene side chain from the polymer main chain, and thus minimize the intramolecular CTC to an extent; secondly, the vertical rigid aromatic side chain would induce a high steric hindrance, and thus effectively suppress the formation of the intermolecular CTC.The absorption spectra of the WuCF3PIs were measured in NMP solution (1 × 10−2 mg mL−1). The maximum absorption wavelength (λabsmax) of the four polyimide solutions located at around 315–319 nm (Fig. 6). These are attributed to the π–π* transition of the conjugated fluorene side-chain, due to the similar λabsmax compared to the monomer WuCF3DA (Fig. S8, λabsmax 314 nm, Table S1, ESI†). The unchanged maximum peaks indicated that the chemical structure of the polymer main chain did not affect the absorption nature of the conjugated fluorene side-chain, which is due to the carbon atoms with sp3 hybridization in fluorene that would cut off the conjugated effect of the rigid planar fluorene side chain from the polymer main chain. The absorption edge extended to about 367–373 nm, from which the band gaps (Eg, the difference between the highest occupied molecular orbital, HOMO, and the lowest unoccupied molecular orbital, LUMO) of the four polyimides were estimated at 3.36, 3.32, 3.35, and 3.38 eV, respectively (Table 3). On the other side, the absorption edges of the polyimide films extended to 418–486 nm (Fig. 7), which were red-shifted compared to the corresponding polyimides in the solution state. The different absorption behaviors of the polyimides in the solid state versus in solution indicated that the chemical and steric structure of the dianhydrides and the aggregation structure of the polymers may affect the optical properties of the polyimides.
| Polymers | In solutionsa (nm) | Films (nm) | E g | ||||
|---|---|---|---|---|---|---|---|
| λ absmax | λ absonset |
λ
PLmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
λ 0 | λ absonset |
λ
PLmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
||
| a Polymer concentration is 10−2 mg mL−1 in NMP. b Excited at the absorption maximum value in both solid and solution states. c The cut-off wavelengths (λ0) from the transmission UV-vis absorption spectra of PI films. d Calculated from the polymer solution (10−2 mg mL−1 in NMP) using the equation Eg = 1240/λonset. | |||||||
| WuCF3PI-6F | 317 | 369 | 482 | 374 | 418 | 546 | 3.36 |
| WuCF3PI-BP | 315 | 373 | 497 | 396 | 429 | 560 | 3.32 |
| WuCF3PI-BT | 315 | 370 | 503 | 412 | 465 | — | 3.35 |
| WuCF3PI-PM | 319 | 367 | 503 | 425 | 486 | — | 3.38 |
Fluorescent polyimides have attracted much attention as a new class of electronic and optical materials recently.28 In this work, as mentioned above, the fluorene moiety was an efficient fluorescent chromophore, and the synthesized WuCF3DA exhibited an intense emission peak at 510 nm in THF and 446 nm in the solid state. After the introduction of WuCF3DA into the polyimide main chain, the obtained aromatic polyimides also showed obvious fluorescence characteristics, exhibiting the maximum fluorescence emission (λPLmax) at around 482–503 nm in NMP solution. While WuCF3PI-6F and WuCF3PI-BP show λPLmax at around 546–560 nm in the film state (Fig. 8 and Table 3), respectively.
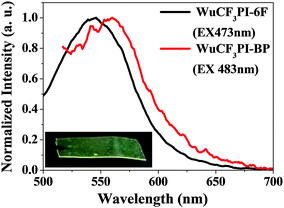 | ||
| Fig. 8 PL of the polyimide films with a thickness of ∼40 µm (the inset shows the WuCF3PI-6F film under 365 nm UV light). | ||
Dielectric properties of WuCF3PIs
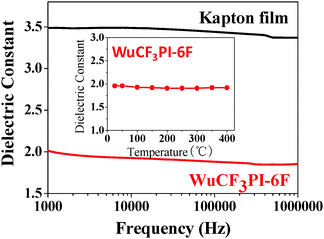
The dielectric properties of the polyimide films were measured at the frequencies between 100 Hz and 1 MHz at 20 °C and 50% relative humidity using a Solartron SI 1260 impedance/gain phase analyzer (Solartron Group Ltd, UK). A commercialized DuPont™ Kapton® polyimide film was also analysed for comparison, and the results are shown in Table S2 in the ESI.† The WuCF3PI samples showed an extremely low dielectric constant in the range of 1.93–2.27, and the WuCF3-6F film showed ultralow electrical properties, with a dielectric constant of 1.93 at 10 kHz, which is much lower than that of the Kapton film (dielectric constant of 3.49 at 10 kHz). Moreover, these outstanding dielectric properties remain stable up to 400 °C (the inset in Fig. 9), which is near the Tg of this polymer. As far as we know, full dense materials with an intrinsic k value lower than 2.0 are extremely rare in the literature.To better understand the mechanism of the exciting dielectric properties, we go further into the chemical structure and morphology of the polyimide film. As mentioned above, the polyimide films contained no nanoporous structures (Fig. 5), which spurred us to consider the chemical structure and the large free volume (sub-nanoscale) that intrinsically exist in the amorphous region of polymeric materials. The designed diamine WuCF3DA is a fluorene derivative that contains a trifluoromethyl phenyl group (–ph–CF3) (Fig. 1a). Regarding the molecular polarity, both fluorene and –CF3 groups are non-polar or low-polarity moieties, which is beneficial for lowering the molecular polarizability of the diamine monomer and the polyimide. Regarding the steric effect, the vertical rigid, non-planar, large conjugated fluorene moiety and the trifluoromethyl phenyl groups can act as a large, rigid side chain (Fig. 1b), which would prevent the macrochains from dense aggregation, or increase the free-volume size in the polyimide. Lowering the polarizability and increasing the free-volume size are both very important for reducing the intrinsic k value of the polyimide and thus are favorable approaches to the production of ultralow k materials.
Positron annihilation lifetime spectroscopy (PALS) is a useful method for studying free-volumes in materials.29 The positron lifetime spectra measured for WuCF3PI-6F and Kapton films are shown in Fig. 10. From a detailed analysis of the lifetime spectra using the PATFIT routine29 (Table 4), we found two exponential decay components (τ1 and τ2) for the Kapton film and three (τ1, τ2 and τ3) for the WuCF3PI-6F film. The shortest lifetime τ1 was 0.29 ns for Kapton and 0.25 ns for WuCF3PI-6F, which is due to the p-Ps lifetime and the free annihilation lifetime of the positrons. The second lifetime component τ2 is attributed to positron annihilation in the amorphous region, which was 0.41 ns for Kapton and 0.59 ns for WuCF3PI-6F. The long lifetime component τ3 is attributed to the pick-off annihilation of o-Ps at holes in the amorphous phase and can be used to estimate the size of local free volumes in amorphous phases by eqn (1):29
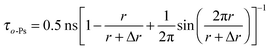 | (1) |
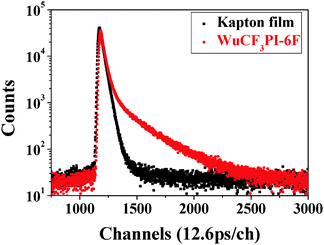 | ||
| Fig. 10 Positron lifetime spectra measured for the WuCF3PI-6F film and a DuPont™ Kapton® polyimide film. | ||
| Density (g cm−3) | τ 1 (ns) | τ 2 (ns) | τ 3 (ns) | I 1 (%) | I 2 (%) | I 3 (%) | R (Å) | k | |
|---|---|---|---|---|---|---|---|---|---|
| WuCF3PI-6F | 1.3225 | 0.25 | 0.59 | 3.26 | 44.9 | 32.6 | 22.5 | 3.80 | 1.93 |
| Kapton | 1.4406 | 0.29 | 0.41 | 34.4 | 65.6 | 3.49 |
Here the prefactor of 0.5 ns is the spin-averaged Ps annihilation lifetime, which is also observed in densely packed molecular crystals; Δr = 0.166 nm is obtained by the fitting of eqn (1) to the observed porous materials and was determined by Eldrup and Jean.29
Compared with Kapton, WuCF3PI-6F shows a long lifetime component (22.5%) of 3.26 ns, whereas the Kapton film shows no τ3 component. The mean radius (r) of the free-volume in the WuCF3PI-6F film is 3.80 Å, and the diameter of the free-volume size is approximately 7.60 Å. Compared with the commercial Kapton film, which has a linear molecular structure without a non-polar, large conjugated, rigid side chain (Fig. S9 in the ESI†), the appearance of these Ångström scale free-volumes decreases the density of the WuCF3PI-6F film by 8.20%. The density of the WuCF3PI-6F is 1.3225 g cm−3, whereas that of Kapton is 1.4406 g cm−3. As a result, the WuCF3PI-6F film shows ultralow-k properties.
Electrochemical properties of the polyimides
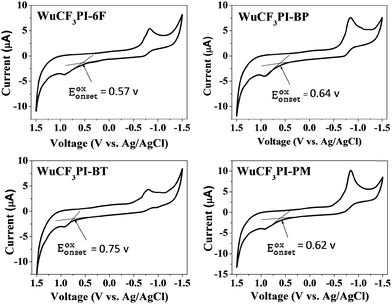
The cyclic voltammograms (CV) of the as-synthesized WuCF3PIs in NMP were measured at a scan rate of 100 mV s−1 using tetrabutylammonium perchlorate (n-Bu4NclO4, 0.1 M) as the supporting electrolyte and ferrocene as the standard (Fig. 11 and Table 5). The onset oxidation (Eoxonset) of the four PIs was observed at 0.57, 0.64, 0.75 and 0.62 V, respectively. The external ferrocene and ferrocenium (Fc/Fc+) redox standard E1/2 was measured to be 0.56 V vs. Ag/AgCl in NMP. By assuming that the HOMO level for the Fc/Fc+ standard was −4.8 eV with respect to the zero vacuum level, the HOMO energy levels for the four polyimides were determined to be −4.81, −4.88, −4.99 and −4.86 eV, respectively. Furthermore, the LUMO energy levels of the polyimides were estimated to be −1.45, −1.56, −1.64 and −1.48 eV. The HOMO and LUMO energy levels of the WuCF3PIs were comparable to those of reported functional polyimides,30 so the synthesized polyimides are expected to exhibit the similar electrical switching behavior and to be used in polymer memory devices.| Polyimide | E g (eV) | E oxonset (V) | HOMO (eV) | LUMO (eV) |
|---|---|---|---|---|
| WuCF3PI-6F | 3.36 | 0.57 | −4.81 | −1.45 |
| WuCF3PI-BP | 3.32 | 0.64 | −4.88 | −1.56 |
| WuCF3PI-BT | 3.35 | 0.75 | −4.99 | −1.64 |
| WuCF3PI-PM | 3.38 | 0.62 | −4.86 | −1.48 |
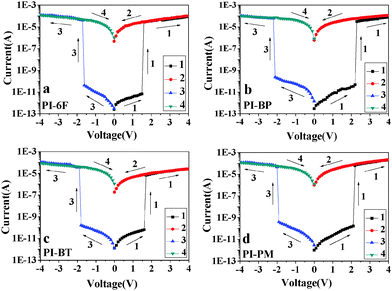
Electrical switching effects and memory performances of the ITO/WuCF3PI/Al devices
The memory effects of the WuCF3PI can be demonstrated by the current–voltage (I–V) characteristics of an ITO/WuCF3PI/Al sandwich device (Fig. 12). Fig. 13 exhibits the typical I–V curves of the electrical switching and memory devices fabricated with WuCF3PI-6F, WuCF3PI-BP, WuCF3PI-BT and WuCF3PI-PM. The four devices, with Al as the anode and ITO as the cathode, were initially in the low-conductivity (OFF) state during the first positive sweep from 0 to 4 V, and then switched to the high-conductivity (ON) state at 1.5, 2.2, 1.6 and 2.1 V with an ON/OFF current ratio in the order of 107, 106, 105 and 105, respectively. The polymeric memory devices can store data based on the ON state and OFF state response to the external applied voltages.For the case of WuCF3PI-6F (Fig. 13a), in the set process, the current jumps abruptly at Vset = 1.5 V to the ON state (“1” signal in data storage) from the OFF state (“0” signal in data storage) with an ON/OFF ratio of 107. This electronic transition from the OFF state to the ON state in the first sweep serves as the “writing” process, and the results reveal that such a memory device possesses a very low working voltage and a high ON/OFF ratio, which lead to an extremely low misreading rate for memory applications. In addition, the device could be kept in the ON state during the subsequent second sweep from 4 to 0 V. The device can be reprogrammed. After approximately 2 min, the third sweep started from the OFF state, to the ON state again with an accurate threshold voltage of −1.6 V and kept in the ON state during the fourth sweep. It suggests that the ON state could be retained for a short period of time after the removal of power and would relax to the original OFF state eventually.
Also, the device shows good stability at the OFF and ON states, where its conductivity slightly changes with the applied voltage before and after the switching behavior. Fig. 14 shows the retention times and stress tests of both the ON and OFF states of the device. Initially, the memory devices started to turn ON or OFF to a high or low conductivity state. Under a constant stress of −1 V, no obvious degradation in current was observed for both ON and OFF states for at least 2 h during the test and the WuCF3PI memory devices were stable for at least 108 continuous read pulses of −1 V. The “remanent”, yet volatile nature of the ON state, as well as the randomly accessible ON and OFF states in the ITO/WuCF3PI/Al device, is similar to the static random access memory (SRAM) characteristic.
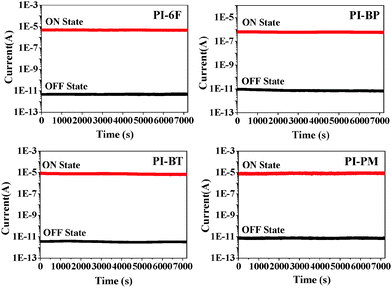 | ||
| Fig. 14 Retention time on the ON and OFF states of the ITO/WuCF3PI/Al devices under a constant readout voltage (−1 V). | ||
The mechanism of field-induced conductivity is probably similar to that of the photo-induced CT in photoconductive polyimides. To better understand the switching behavior of the WuCF3PI memory device, the electronic properties of the PIs at the ground state were studied by the use of Density Function Theory (DFT). The molecular orbital of the basic unit (BU) of the WuCF3PI-6F was calculated at the DFT/B3LYP/6-31G(d,p) level using the Gaussian 09 program package. The HOMO and the LUMOs of the BU, as well as the possible electronic transitions under excitations, are demonstrated in Fig. 15. The HOMO is located mainly on the fluorene conjugated side-chain, whereas the first LUMO is located on the dianhydride moieties, indicating that in the polyimide, the fluorene conjugated side-chains act as the electron donors and the dianhydride moieties act as the electron acceptors. Under excitations with sufficient energy, electrons in the ground state of WuCF3PI can transit to various excited states. Herein, theoretical oscillator strengths and transition contributions calculated by the time-dependent DFT (TD-DFT) method (Table 6) were used to help elucidate this process. Since extremely low oscillator strength is found in the HOMO to LUMO or LUMO1 transition, electrons have less possibility to be excited to such unoccupied orbitals. Instead, they are more inclined to jump up to LUMO3 under sufficient energy, evidenced by the much larger oscillator strength (0.9846) of this transition. Therefore, when the applied electric field reaches the switch-on voltage, some electrons at the HOMO of WuCF3PI-6F accumulate sufficient energy and transit to the LUMO3, to result in an excited state. Excitation of the donor could lead to a decrease in ionization potential and consequently promote intermolecular CT at the excited state. CT can occur indirectly from the LUMO3 within the donor to the LUMO2 or LUMO1, then to the LUMO of the acceptor, or directly from the HOMO to the LUMO2, LUMO1 and LUMO at the exited state, to form a conductive complex.
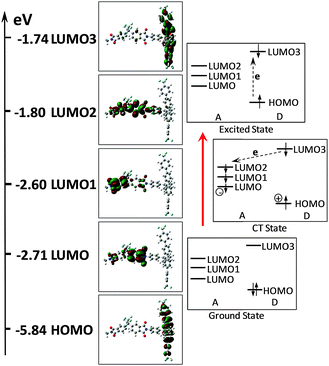 | ||
| Fig. 15 Calculated molecular orbitals, the corresponding energy levels of the basic unit (BU) of WuCF3PI-6F, and the plausible electronic transitions under an electric field. | ||
| Excited state | Oscillator strength | Orbitals | Contribution | |
|---|---|---|---|---|
| a Other prohibited transitions are not listed. | ||||
| WuCF3PI-6F | 1 | 0.0002 | HOMO → LUMO | 0.96 |
| HOMO → LUMO1 | 0.02 | |||
| 6 | 0.9846 | HOMO → LUMO2 | 0.23 | |
| HOMO → LUMO3 | 0.75 | |||
| 11 | 0.0951 | HOMO → LUMO2 | 0.74 | |
| HOMO → LUMO3 | 0.22 | |||
The calculation results correspond well to the electronic absorption spectrum of WuCF3PI-6F (Fig. 6 and Table 3). The maximum absorption peak and absorption edge at about 315 nm and 369 nm correspond to the transitions from the HOMO to LUMO3 (dominant) and LUMO2, and the HOMO to the LUMO, respectively.
Once the conductive CT states are formed under an applied electric field, they will facilitate electrical conduction under both biases and the device cannot be switched off by applying a reverse bias of the same magnitude. However, after the removal of the applied electric field for some time, the polymer will relax to its original conformation. The CT state will dissociate via back electron transfer from the dianhydride moiety to the diamine moiety as the potential barrier disappears, and consequently the SRAM characteristic could be observed in the polyimide-containing devices.
Conclusions
In summary, by introducing a novel aromatic rigid trifluoromethyl-containing moiety with special non-planar and conjugated characteristics into the polymer backbone, we have succeeded in designing and synthesizing a series of high-performance multifunctional polyimides (WuCF3PIs) with exceptional thermostability and solubility (Tg close to 500 °C and, at the same time, well soluble in common organic solvents), light color and transparency, intrinsic ultralow-k (k ≈ 1.93) and electrical bistability (ON/OFF ratio as high as 107, low working voltage as low as 1.5 V), simultaneously. Therefore the as-synthesized polymers are ideal potential candidates for practical applications in the fields of ultra-large-scale integration (ULSI), high-performance polymer memory devices, flexible displays, the thin film PV industry and wearable electronics. It is also our belief that such a design strategy is beneficial for the functionalization of polyimides and simultaneously maintaining the overall properties, and can also be extended to other novel high performance polymer systems.Experimental
Materials
2,7-Dibromofluorene, 1-fluoro-4-nitrobenzene, tetrakis(triphenyl-phosphine)palladium (Pd[P(C6H5)3]4), palladium 10% on carbon (10% Pd/C), Aliquat 336 (tricaprylylmethyl-ammonium chloride), hydrazine monohydrate (NH2NH2·H2O), 4-(trifluoro-methyl)phenyl-boronic acid, and potassium tert-butoxide (t-BuOK) were purchased from Alfa-Aesar company and were used as received. 3,3′,4,4′-Biphenyltetracarboxylic acid dianhydride (BPDA), 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BTDA), 4,4′-(hexafluoroisopropylidene)diphthalic anhydride (6-FDA) and pyromellitic dianhydride (PMDA) were purchased from National Pharmaceutical Group Chemical Reagent Co., Ltd. and they were heated at 140 °C under vacuum for 12 h prior to use. Analytical-grade dimethyl formamide (DMF) was purified by distillation under an inert nitrogen atmosphere. Tetrahydrofuran (THF) was distilled from sodium/benzophenone. All other solvents and reagents purchased from Guangzhou Dongzheng Company were of analytical grade and were used without further purification.Instrumentation
All NMR spectra were recorded on a Bruker AVANCE AV 400 spectrometer or a Varian Unity Inova 500 NB spectrometer. Samples were composed of a solution of 5–15 mg of each compound in 0.5 mL of deuterated dimethyl sulfoxide (DMSO) using tetramethylsilane (TMS) as the internal reference. Mass spectra were measured on a Thermo EI mass spectrometer (DSQ II). Elemental analysis was performed on a CHNS elemental analyzer. Infrared spectra were recorded on a BRUKER TENSOR 27 Fourier-transform infrared (FT-IR) spectrometer. Gel permeation chromatography (GPC) was performed on a Waters 717 plus autosampler and a Waters 1515 isocratic HPLC pump. Two Waters columns, connected in series, were used with DMF as the eluent and were calibrated with narrow polydispersity polystyrene standards. UV-vis absorption spectra (UV) were recorded on a Hitachi UV-vis spectrophotometer (U-3900). Fluorescence spectra (PL) were determined on a Shimadzu RF-5301PC spectrometer with slit widths of 5 nm (for polyimides) and 3 nm (for monomers). Thermogravimetric analyses (TGA) were performed using a TA thermal analyzer (Q50) under N2/air with a heating rate of 20 °C min−1 from 30 to 1000 °C; the samples were heated under flowing nitrogen/air (40 mL min−1). Differential scanning calorimetry (DSC) curves were obtained using a NETZSCH thermal analyzer (DSC 204) at a heating rate of 25 °C min−1 from 30 to 500 °C under flowing nitrogen. The glass-transition temperature (Tg) was read at the midpoint of the transition in the heat capacity and was taken from the second heating trace after rapid cooling from 500 °C at a cooling rate of 40 °C min−1. The dynamic mechanical (DMA) spectra of the samples were obtained by using NETZSCH DMA 242 equipment. The specimens were analyzed in tensile mode at a constant frequency of 1 Hz, a static force of 0.1 N, a dynamic force of 0.105 N, and a temperature range of 30–450 °C at a heating rate of 5 °C min−1. A tensile test was performed on samples cut from a 35–50 µm-thick sheet, and the test was performed using a SANS CTN6103 instrument according to GB/T16421-1996. The specimen size was 10 × 100 mm2; and the jaw separation was 50 mm. The jaw speed was first set to 2 mm min−1. When elongation reached 1 mm, the jaw speed was changed to 20 mm min−1. The morphology of the surface of WuCF3PI was studied using a field-emission scanning electron microscope (FE-SEM, Hitachi s4800, Japan) and an atomic force microscope (AFM, Bruker Mutimode 8, Germanu). The dielectric constant was measured at frequencies between 100 Hz and 1 MHz at 20 °C and 50% relative humidity using a Solartron SI 1260 impedance/gain phase analyzer in conjunction with two copper electrodes (10.2 × 10.2 mm2). The amplitude of the a.c. electric field was approximately 1 V cm−1. The samples were thin films with a size of 12 × 12 mm2. Silver paste was coated onto both surfaces of the PI film to ensure an excellent contact between the electrodes and the PI film. Positron lifetime measurements were performed as follows: two identical samples with dimensions of 1.5 × 10 × 10 mm3 were sandwiched with a 1.1 × 106 Bq 22Na positron source; the 22Na nucleus emits a 1.28 MeV γ-ray simultaneously (within a few ps) with the positron; the positron lifetime is determined from the time delay between the emission of the birth gamma (1.28 MeV) and one of the 0.511 MeV annihilation photons; lifetime measurements were conducted using a fast–fast coincidence system with a time resolution of approximately 220 ps and a channel width of 12.6 ps. We analyzed the lifetime spectra using the data processing program PATFIT. Before analyzing each spectrum, we subtracted the positron source components (392 ps/16.40%, 2.05 ns/0.70%). The variance of the fits was approximately 1.1. The film density was measured using a density balance (Mirage SD-200L, Japan) with an accuracy of 0.1 mg.Fabrication and characterization of the memory devices
The memory devices were fabricated on an indium-tin oxide (ITO)-coated glass, with the configuration of ITO/WuCF3PI/Al. The ITO glass was pre-cleaned by ultra-sonication with water, acetone, and isopropanol each for 20 min before the fabrication. A PAA solution in DMF (10 mg mL−1) was spin-coated onto a clean ITO glass at a spin speed of 2000 rpm for 45 s, and thermal imidization was performed. The thickness of the thin film was determined to be approximately 80–110 nm. Finally, an 80 nm-thick Al top electrode was thermally evaporated through the shadow mask (recorded device units of 0.2 × 0.2 mm2 in size) at a pressure of 10−7 Torr with a uniform depositing rate of 3–5 Å s−1. The electrical characterization of the memory device was performed using a Keithley 4200-SCS semiconductor parameter analyzer equipped with a Keithley 4205-PG2 arbitrary waveform pulse generator. ITO was used as the cathode, and Al was set as the anode during the voltage sweep. The probe tip was a tungsten wire (10 µm in diameter) attached to a tinned copper shaft with a point radius <0.1 µm (GGB Industries, Inc.).Acknowledgements
The financial support of the National 973 Program of China (No. 2014CB643605), the National 863 Program of China (No. 2015AA033408), the National Natural Science Foundation of China (No. 51373204, 51233008, and J1103305), the Science and Technology Project of Guangdong Province (No. 2015B090915003 and 2015B090913003), the Doctoral Fund of the Ministry of Education of China (No. 20120171130001), and the Fundamental Research Funds for the Central Universities (No. 161gzd08) is gratefully acknowledged.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 Search PubMed; Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868 CrossRef CAS PubMed; J. C. Bijleveld, A. P. Zoombelt, S. G. J. Mathijssen, M. M. Wienk, M. Turbiez, D. M. de Leeuw and R. A. J. Janssen, J. Am. Chem. Soc., 2009, 131, 16616 CrossRef PubMed; G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153 CrossRef.
- R. H. Friend, R. W. Gymer, A. B. Holmes, J. H. Burroughes, R. N. Marks, C. Taliani, D. C. C. Bradley, D. A. Dos Santos, J. L. Brédas, M. Lögdlund and W. R. Salaneck, Nature, 1999, 397, 121 CrossRef CAS; W. L. Yu, W. Huang and A. J. Heeger, Adv. Mater., 2000, 12, 828 CrossRef; D. Marsitzky, R. estberg, P. lainey, B. T. Tang, C. J. Hawker and K. R. Carter, J. Am. Chem. Soc., 2001, 123, 6965 CrossRef PubMed; C. V. Hoven, A. Garcia, G. C. Bazan and T. Q. Nguyen, Adv. Mater., 2008, 20, 3793 CrossRef; T. Lei, J. H. Dou, X. Y. Cao, J. Y. Wang and J. Pei, J. Am. Chem. Soc., 2013, 135, 12168 CrossRef PubMed; H. C. Moon, T. P. Lodge and C. D. Frisbie, J. Am. Chem. Soc., 2014, 136, 3705 CrossRef PubMed.
- Z. M. Dang, Y. Q. Lin, H. P. Xu, C. Y. Shi and J. Bai, Adv. Funct. Mater., 2008, 18, 1509 CrossRef CAS; J. J. Li, S. I. Seok, B. J. Chu, F. Dogan, Q. M. Zhang and Q. Wang, Adv. Mater., 2009, 21, 3804 CrossRef; K. Y. Lee, B. Kumar, J. S. Seo, K. H. Kim, J. I. Sohn, S. N. Cha, D. Choi, Z. L. Wang and S. W. Kim, Nano Lett., 2012, 12, 1959 CrossRef PubMed; M. Kanik, O. Aktas, H. S. Sen, E. Durgun and M. Bayindir, ACS Nano, 2014, 8, 9311 CrossRef PubMed; M. Baniasadi, J. C. Huang, Z. Xu, S. Moreno, X. Yang, J. Chang, M. A. Quevedo-Lopez, M. Naraghi and M. Minary-Jolandan, ACS Appl. Mater. Interfaces, 2015, 7, 5258 Search PubMed.
- H. Lee, R. E. Salomon and M. M. Labes, Macromolecules, 1978, 11, 171 CrossRef CAS; J. Örtegren, G. Andersson, P. Busson, A. Hult, U. W. Gedde, A. Eriksson and M. Lindgren, J. Phys. Chem. B, 2001, 105, 10223 CrossRef; Z. H. Sun, T. L. Chen, C. M. Ji, S. Q. Zhang, S. G. Zhao, M. C. Hong and J. H. Luo, Chem. Mater., 2015, 27, 4493 CrossRef.
- B. L. Hu, X. J. Zhu, X. X. Chen, L. Pan, S. S. Peng, Y. Z. Wu, J. Shang, G. Liu, Q. Yan and R. W. Li, J. Am. Chem. Soc., 2012, 134, 17408 CrossRef CAS PubMed; C. L. Tan, X. Y. Qi, Z. D. Liu, H. Li, X. Huang, L. Shi, B. Zheng, X. Zhang, L. H. Xie, Z. Y. Tang, W. Huang and H. Zhang, J. Am. Chem. Soc., 2015, 137, 1565 CrossRef PubMed; Q. D. Ling, F. C. Chang, Y. Song, C. X. Zhu, D. J. Liaw, D. S. H. Chan, E. T. Kang and K. G. Neoh, J. Am. Chem. Soc., 2006, 128, 8732 CrossRef PubMed.
- F. Hoffmann, M. Cornelius, J. Morell and M. Froba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef CAS PubMed; Z. J. Li, M. C. Johnson, M. W. Sun, T. E. Ryan, D. J. Earl, W. Maichen, J. I. Martin, S. Li, C. M. Lew, J. L. Wang, M. W. Deem, M. E. Davis and Y. S. Yan, Angew. Chem., Int. Ed., 2006, 45, 6329 CrossRef PubMed; M. A. Snyder and M. Tsapatsis, Angew. Chem., Int. Ed., 2006, 46, 7560 CrossRef PubMed; P. A. Kohl, Annu. Rev. Chem. Biomol. Eng., 2011, 2, 379 CrossRef PubMed; M. Seino, W. D. Wang, J. E. Lofgreen, D. P. Puzzo, T. Manabe and G. A. Ozin, J. Am. Chem. Soc., 2011, 133, 18082 CrossRef PubMed; S. Eslava, J. Urrutia, A. N. Busawon, M. R. Baklanov, F. I. Aldea, S. K. Maex, J. A. Martens and C. E. A. Kirschhock, J. Am. Chem. Soc., 2008, 130, 17528 CrossRef PubMed.
- L. Glasser, Chem. Rev., 1975, 75, 21 CrossRef CAS; D. Umeyama, S. Horike, M. Inukai, T. Itakura and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 12780 CrossRef PubMed; K. Miyatake, K. Fukushima, S. Takeoka and E. Tsuchida, Chem. Mater., 1999, 11, 1171 CrossRef; C. F. Kins, E. Sengupta, A. Kaltbeitzel, M. Wagner, I. Lieberwirth, H. W. Spiess and M. R. Hansen, Macromolecules, 2014, 47, 2645 CrossRef; T. Sato, Y. Hayasaka, M. Mitsuishi, T. Miyashita, S. Magano and J. Matsui, Langmuir, 2015, 31, 5174 CrossRef PubMed.
- D. Ma, M. Lv, M. Lei, J. Zhu, H. Q. Wang and X. W. Chen, ACS Nano, 2014, 8, 1601 CrossRef CAS PubMed; S. J. Liu, K. Zhang, J. M. Lu, J. Zhang, H. L. Yip, F. Huang and Y. Cao, J. Am. Chem. Soc., 2013, 135, 15326 CrossRef PubMed; C. H. Duan, K. Zhang, C. M. Zhong, F. Huang and Y. Cao, Chem. Soc. Rev., 2013, 42, 9071 RSC; M. H. Chen, J. Hou, Z. Hong, G. Y ang, S. Sista, L. M. Chen and Y. Yang, Adv. Mater., 2009, 21, 4238 CrossRef.
- P. Ngamsantivongsa, H. L. Lin and T. L. Yu, J. Membr. Sci., 2015, 491, 10 CrossRef CAS; X. F. Liao, L. Ren, D. Z. Chen, X. H. Liu and H. W. Zhang, J. Power Sources, 2015, 286, 258 CrossRef.
- Y. Shao, G. C. Bazan and A. J. Heeger, Adv. Mater., 2008, 20, 1191 CrossRef CAS; Y. Shao, X. Gong, A. J. Heeger, M. Liu and A. K. Y. Jen, Adv. Mater., 2009, 21, 1972 CrossRef.
- H. J. Yen, C. J. Chen and G. S. Liou, Adv. Funct. Mater., 2013, 23, 5307 CrossRef CAS; P. M. Beaujuge, S. V. Vasilyeva, D. Y. Liu, S. Ellinger, T. D. McCarley and J. R. Reynolds, Chem. Mater., 2012, 24, 255 CrossRef; E. Puodziukynaite, J. L. Oberst, A. L. Dyer and J. R. Reynolds, J. Am. Chem. Soc., 2012, 134, 968 CrossRef PubMed; H. J. Yen and G. S. Liou, Polym. Chem., 2012, 3, 255 RSC; H. J. Yen, H. Y. Lin and G. S. Liou, Chem. Mater., 2011, 23, 1874 CrossRef; J. J. Yen and G. S. Liou, Chem. Mater., 2009, 21, 4062 CrossRef; E. P. Knott, M. R. Graig, D. Y. Liu, J. E. Babiarz, A. L. Dyer and J. R. Reynolds, J. Mater. Chem., 2012, 22, 4953 RSC.
- T. J. Lee, C. W. Chang, S. G. Hahm, K. Kim, S. Park, D. M. Kim, J. Kim, W. S. Kwon, G. S. Liou and M. Ree, Nanotechnology, 2009, 20, 135204 CrossRef CAS PubMed; T. Kurosawa, T. Higashihara and M. Ueda, Polym. Chem., 2013, 4, 16 RSC; B. L. Hu, F. Zhuge, X. J. Zhu, S. S. Peng, X. X. Chen, L. Pan, Q. Yan and R. W. Li, J. Mater. Chem., 2012, 22, 520 RSC; C. L. Liu and W. C. Chen, Polym. Chem., 2011, 2, 2169 RSC.
- H. Ishida and H. Y. Low, Macromolecules, 1997, 30, 1099 CrossRef CAS; D. Kessler, P. J. Roth and P. Theato, Langmuir, 2009, 25, 10068 CrossRef PubMed.
- G. H. Gelinck, H. E. A. Huitema, E. V. Veenendaaal, E. Cantatore, L. Schrijnemakers, J. B. P. H. van der Putten, T. C. T. Geuns, M. Beenhakkers, J. B. Giesbers, B. H. Huisman, E. J. Meijer, E. M. Benito, F. J. Touwslager, A. W. Marsman, B. J. E. van Rens and D. M. de Leeuw, Nat. Mater., 2004, 3, 106 CrossRef CAS PubMed; P. Hosseini, C. D. Wright and H. Bhaskaran, Nature, 2014, 511, 206 CrossRef PubMed; Z. B. Wang, M. G. Helander, J. Qiu, D. O. Puzzo, M. T. Greiner, Z. M. Hudson, S. Wang, Z. W. Liu and Z. H. Lu, Nat. Photonics, 2011, 5, 753 CrossRef; B. R. Lee, E. D. Jung, J. S. Park, Y. S. Nam, S. H. Min, B. S. Kim, K. M. Lee, J. R. Jeong, R. H. Friend, J. S. Kim, S. O. Kim and M. H. Song, Nat. Commun., 2014, 5, 4840 CrossRef PubMed; M. Berggren, D. Nilsson and N. D. Robinson, Nat. Mater., 2007, 6, 3 CrossRef PubMed.
- C. L. Wan, X. K. Gu, F. Dang, T. Itoh, Y. F. Wang, H. Sasaki, M. Kondo, K. Koga, K. Yabuki, G. J. Snyder, R. G. Yang and L. Koumoto, Nat. Mater., 2015, 14, 622 CrossRef CAS PubMed; Z. T. Zhang, K. P. Guo, Y. M. Li, X. Y. Li, G. Z. Guan, H. P. Li, Y. F. Luo, F. Y. Zhao, Q. Zhang, B. Wei, Q. B. Pei and H. S. Peng, Nat. Photonics, 2015, 9, 233 CrossRef.
- S. P. Louha, I. C. Leu and M. H. Hon, Diamond Relat. Mater., 2005, 14, 1000 CrossRef CAS; H. J. Lee, E. K. Lin, H. Wang, W. L. Wu, W. Chen and E. S. Moyer, Chem. Mater., 2002, 14, 1845 CrossRef; Z. B. Wang, H. N. Wang, A. P. Mitra, L. M. Huang and Y. S. Yan, Adv. Mater., 2001, 13, 746 CrossRef; Y. Z. Zhu, T. E. Muller and J. A. Lercher, Adv. Funct. Mater., 2008, 18, 3427 CrossRef; C. M. Lew, Z. J. Li, L. Shuang, S. J. Hwang, Y. Liu, I. D. Medina, M. W. Sun, J. L. Wang, M. E. Davis and Y. S. Yan, Adv. Funct. Mater., 2008, 18, 3454 CrossRef.
- C. Yuan, K. K. Jin, K. Li, S. Diao, J. W. Tong and Q. Fang, Adv. Mater., 2013, 25, 4875 CrossRef CAS PubMed; K. Dawson, L. Zhu, L. A. Kopff, R. J. McMahon, L. Yu and M. D. Ediger, J. Phys. Chem. Lett., 2011, 2, 2683 CrossRef; R. K. Joshi, M. Yoshimura, K. Tanaka, K. Ueda, A. Kumar and N. Ramgir, J. Phys. Chem. C, 2008, 112, 13901 Search PubMed; A. Miura, S. Zhou, T. Nozaki and J. Shiomi, ACS Appl. Mater. Interfaces, 2015, 7, 13484 Search PubMed.
- S. G. Hahm, S. Choi, S. H. Hong, T. J. Lee, S. Park, D. M. Kim, W. S. Kwon, K. Kim, O. Kim and M. Ree, Adv. Funct. Mater., 2008, 18, 3276 CrossRef CAS; S. G. Hahm, S. Choi, S. H. Hong, T. J. Lee, S. Park, D. M. Kim, J. C. Kim, W. Kwon, K. Kim, M. J. Kim, O. Kim and M. Ree, J. Mater. Chem., 2009, 19, 2207 RSC.
- D. Wilson, H. D. Stenzenberger and P. M. Hergenrother, Polyimides, Chapman & Hall, London, 1990 CrossRef CAS PubMed; J. J. Ge, C. Y. Li, G. Xue, I. K. Mann, D. Zhang, F. W. Harris, S. Z. D. Cheng, S. C. Hong, X. Zhuang and Y. R. Shen, J. Am. Chem. Soc., 2001, 123, 5768 CrossRef CAS PubMed; H. Lim, W. J. Cho, C. S. Ha, S. Ando, Y. K. Kim, C. H. Park and K. Lee, Adv. Mater., 2003, 15, 1161 CrossRef.
- D. J. Liaw, K. L. Wang, Y. C. Huang, K. R. Lee, J. Y. Lai and C. S. Ha, Prog. Polym. Sci., 2012, 37, 907 CrossRef CAS; Y. W. Liu, C. Qian, L. J. Qu, Y. N. Wu, Y. Zhang, X. H. Wu, B. Zou, W. X. Chen, Z. Q. Chen, Z. G. Chi, S. W. Liu, X. D. Chen and J. R. Xu, Chem. Mater., 2015, 27, 6543–6549 CrossRef.
- T. Miteva, A. Meisel and W. Knoll, Adv. Mater., 2001, 13, 565 CrossRef CAS; M. D. Redecker, D. C. Bradley and W. W. Wu, Adv. Mater., 1999, 11, 241 CrossRef; Y. C. Kung and S. H. Hsiao, J. Mater. Chem., 2011, 21, 1746 RSC; H. T. Ban, H. Hagihara and Y. Tsunogae, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 65 CrossRef; T. Kuorosawa, M. Ueda and W. C. Chen, Macromolecules, 2010, 43, 1236 CrossRef; H. M. Colquhoun, Z. X. Zhu, C. J. Cardin, Y. Gan and M. G. B. Drew, J. Am. Chem. Soc., 2007, 129, 16163 CrossRef PubMed; Y. W. Liu, Y. Zhang, Q. Lan, S. W. Liu, Z. X. Qin, L. H. Chen, C. Y. Zhao, Z. G. Chi, J. R. Xu and J. Economy, Chem. Mater., 2012, 24, 1212 CrossRef; Y. W. Liu, Y. Zhang, Q. Lan, Z. X. Qin, S. W. Liu, C. Y. Zhao, Z. G. Chi and J. R. Xu, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1302 CrossRef; H. W. Sun, Y. Zhang, W. Yan, W. X. Chen, Q. Lan, S. W. Liu, L. Jiang, Z. G. Chi, X. D. Chen and J. R. Xu, J. Mater. Chem. C, 2014, 2, 5812 RSC; C. J. Chen, H. J. Yen, Y. C. Hu and G. S. Liou, J. Mater. Chem. C, 2013, 1, 7623 RSC.
- C. W. Chien, C. H. Wu, Y. T. Tsai, Y. C. Kung, C. Y. Lin, P. C. Hsu, H. H. Hsieh, C. C. Wu, C. M. Leu and T. M. Lee, IEEE Trans. Electron Devices, 2011, 58, 1440 CrossRef CAS.
- R. H. French, J. M. Rodriguez-Parada, M. Y. Yang, R. A. Derryberry and N. T. Pfeiffenberger, Sol. Energy Mater. Sol. Cells, 2011, 95, 2077 CrossRef CAS; D. Muhlbacher, C. J. Brabec, N. S. Sariciftci, B. V. Kotov, V. I. Berendyaev, B. M. Rumyantsev and J. C. Hummelen, Synth. Met., 2011, 121, 1609 CrossRef.
- T. Miteva, A. Meisel and W. Knoll, Adv. Mater., 2001, 13, 565 CrossRef CAS; M. Redecker, D. D. C. Bradley and W. W. Wu, Adv. Mater., 1999, 11, 241 CrossRef.
- L. M. Tao, H. X. Yang, J. G. Liu, L. Fan and S. Y. Yang, Polymer, 2009, 50, 6009 CrossRef CAS; J. G. Liu, Y. Nakamura, T. Ogura, Y. Shibasaki, S. Ando and M. Ueda, Chem. Mater., 2008, 20, 273 CrossRef; H. W. Su and W. C. Chen, J. Mater. Chem., 2008, 18, 1139 RSC; M. H. Hoang, M. H. Kim, M. J. Cho, K. H. Kim and K. N. Kim, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5064 CrossRef; Y. T. Chern and J. Y. Tsai, Macromolecules, 2008, 41, 9556 CrossRef; S. H. Hsiao, H. M. Wang, W. J. Chen, T. M. Lee and C. M. Leu, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 3109 CrossRef.
- Y. W. Liu, Y. Zhang, X. H. Wu, Q. Lan, C. S. Chen, S. W. Liu, Z. G. Chi, L. Jiang, X. D. Chen and J. R. Xu, J. Mater. Chem. C, 2014, 2, 1068 RSC.
- C. W. Chang, H. J. Yen, K. Y. Huang, J. M. Yeh and G. S. Liou, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7937 CrossRef CAS; G. S. Liou, S. H. Hsiao and Y. K. Fang, Eur. Polym. J., 2006, 42, 1533 CrossRef.
- J. H. Wu, W. C. Chen and G. S. Liou, Polym. Chem., 2016, 7, 1569–1576 RSC; K. Kanosue, T. Shimosaka, J. Wakita and S. Ando, Macromolecules, 2015, 48, 1777–1785 CrossRef CAS; J. H. Wu and G. S. Liou, Polym. Chem., 2015, 6, 5225–5232 RSC; H. J. Yen, C. J. Chen and G. S. Liou, Chem. Commun., 2013, 49, 630–632 RSC; K. Takizawa, J. Wakita, K. Sekiguchi and S. Ando, Macromolecules, 2012, 45, 4764–4771 CrossRef; J. Wakita, S. Inoue, N. Kawanishi and S. Ando, Macromolecules, 2010, 43, 3594–3605 CrossRef.
- G. Dlubek, K. Saarinen and H. M. Fretwell, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 1513 CrossRef CAS; P. Kirkegaard, N. J. Pederson and M. M. Eldrup, Risø Report M2740, Risø National Laboratory, DK-4000 Roskilde, Denmark, 1989 CrossRef; S. J. Tao, J. Chem. Phys., 1972, 56, 5499 CrossRef; M. M. Eldrup, D. Lightbody and J. N. Sherwood, Chem. Phys., 1981, 63, 51 CrossRef.
- Y. L. Liu, K. L. Wang, G. S. Huang, C. X. Zhu, E. S. Tok, K. G. Neoh and E. T. Kang, Chem. Mater., 2009, 21, 3391 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR results of WuCF3DA and WuCF3PIs; FTIR spectra of WuBrDN, WuCF3DN, WuCF3FDA and the WuCF3PIs; WAXD patterns of WuCF3PI films; UV/vis spectrum, and thermal and photophysical properties of the WuCF3DA; thermal properties and mechanical properties of the WuCF3PIs. See DOI: 10.1039/c6qm00027d |
| ‡ Theses authors contributed equally to the preparation of this work. |
| This journal is © the Partner Organisations 2017 |