An ambipolar azaacene as a stable photocathode for metal-free light-driven water reduction†
Pei-Yang
Gu‡
a,
Zilong
Wang‡
a,
Fang-Xing
Xiao
b,
Zongqiong
Lin
a,
Rongbin
Song
a,
Qing-Feng
Xu
c,
Jian-Mei
Lu
*c,
Bin
Liu
*b and
Qichun
Zhang
*ad
aSchool of Materials Science and Engineering, Nanyang Technological University, Singapore 639798, Singapore. E-mail: qczhang@ntu.edu.sg
bSchool of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore 637459, Singapore. E-mail: liubin@ntu.edu.sg
cCollege of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou 215123, P. R. China. E-mail: lujm@suda.edu.cn
dDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore
First published on 23rd August 2016
Abstract
Present photoelectrochemical (PEC) cells for water splitting are based on inorganic electrodes. For future large-scale applications, electrodes that are metal-free, of low cost, and with sustainable availability are crucial. Herein, we report a new ambipolar larger azaacene (DQNDN) as a single-active-element-based photocathode in PEC cells with a current density of 0.13 mA cm−2 at −0.13 V versus RHE.
Light-driven water splitting to produce renewable hydrogen fuel has been considered as an attractive technology to address energy issues.1–7 Among various strategies to convert solar energy into chemical energy,8–14 employing photoelectrochemical (PEC) cells is believed to be one of the most promising approaches because these inexpensive and simple devices require only semiconductor-based photoelectrodes, water, and sunlight.15–17 As the heart of PEC cells, semiconductor-based photoelectrodes should possess strong absorption ability in the visible region, efficient charge separation and transport capability, excellent stability against corrosion, and suitable reduction and oxidation potentials for water splitting.18–20 Until now, the semiconductors as active elements in photoelectrodes have been dominated by inorganic materials.21–23 Although their efficiency and stability are pretty high, their ability for large-scale processing as well as their cost has posed a limitation for their practical applications. Thus, searching for new photoelectrode materials with suitable energy levels and inexpensive large-scale fabrication is highly desirable.
As the counterpart of inorganic semiconductors, organic conjugated materials have been widely employed as active elements in optoelectronic devices such as solar cells,24,25 light-emitting diodes,26,27 field-effect transistors,28,29 and memory devices.30,31 However, their application as photoelectrodes in metal-free PEC cells is rare.32,33 Until now, only a few polymers (BBL) (poly(benzimidazobenzophenanthroline)34 and carbon nitride35,36) and some small molecules (porphyrins, perylenediimides or their hybrids37) have been demonstrated as promising photoelectrodes in PEC cells. Employing ambipolar conjugated molecules as single active elements in photocathodes for light-driven metal-free water reduction is unprecedented. More importantly, compared with polymer-based photoelectrodes, small molecules could offer several advantages including monodispersity, reliable synthetic reproducibility, and adjustable structure packing features.
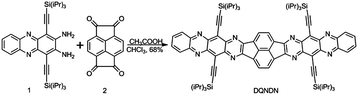
We and other groups have demonstrated that azaacenes can be employed as promising organic semiconductors for many applications38,39 and their charge transport properties can range from p-, ambipolar, to n-type.40–42 Considering the balance of dynamic charge transport during PEC processing, ambipolar azaacenes could be good candidates for photoelectrodes. In this report, we successfully synthesized a new azaacene 6,11,18,23-tetrakis((triisopropylsilyl)ethynyl)diquinoxalino[g,g′]naphtha[1′′,8′′:3,4,5;4′′,5′′:3′,4′,5′]-dicyclopenta[1,2-b:1′,2′-b′]diquinoxaline (DQNDN, Scheme 1), which has been confirmed to show ambipolar transport properties through Mott–Schottky measurements. We believe that the as-prepared azaacene may possess the following advantages: (1) DQNDN may display strong absorption in the visible region due to its large planarity and longer conjugation length; (2) DQNDN may exhibit efficient charge separation and transport properties because large azaacenes usually possess good electron mobility; and (3) DQNDN may possess suitable reduction and oxidation potentials for water splitting. These favourable facts strongly encouraged us to investigate DQNDN as a potential photocathode in PEC cells.
The synthetic route to prepare DQNDN is shown in Scheme 1 and the detailed synthesis can be found in the ESI.† The intermediate compounds 1 and 2 were prepared according to literature procedures.43,44DQNDN was obtained as a brown-black powder in 68% yield through a one-step condensation reaction between 1 and 2 using acetic acid/chloroform as a mixed solvent. Although four triisopropylsilyl (TIPS) groups have been introduced to attach onto the backbone, the solubility of DQNDN is still poor and it is insoluble in common solvents. It can only dissolve in trifluoroacetic acid (TFA) owing to the protonation of nitrogen atoms. The thermal stability of DQNDN was evaluated by thermogravimetric analysis under a nitrogen atmosphere. As shown in Fig. S4 (ESI†), DQNDN displays very good thermal stability with an onset decomposition temperature of ∼405 °C (considering the 5% weight-loss temperature).
The nanostructured DQNDN was fabricated by slowly dropping ethanol into DQNDN/TFA solution with rapid stirring. The as-prepared precipitation was centrifuged and washed with ethanol, deionized water, and ethanol, respectively. Scanning electron microscopy analysis (SEM, Fig. 1) reveals that nanofibers (diameter: 100–200 nm) can evenly coat on the surface of fluorine-doped tin oxide (FTO) glass. The normalized solid-state absorption spectrum of the nanostructured DQNDN film is shown in Fig. S5 (ESI†), which possesses typical azaacene finger peaks (280, 360, 585 nm). The peak at 850 nm might come from its aggregation.45 Overall, the solid-state absorption spectrum of the nanostructured DQNDN film covers the whole visible spectrum region, suggesting that it should be a good light-absorbing material, which might be a good candidate for photoelectrodes in PEC cells.
 | ||
| Fig. 1 SEM images of nanostructured DQNDN nanofibers. At (a) low resolution and (b) high resolution. | ||
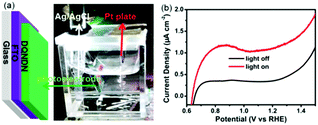
As shown in Fig. 2a, the PEC behaviour of DQNDN was measured under a 300 W xenon lamp (Newport) coupled to an AM 1.5 G filter in a three-electrode electrochemical system with a Ag/AgCl electrode as a reference, a Pt plate as a counter electrode, and the nanostructured DQNDN film as a working electrode. A 0.5 M Na2SO4 aqueous solution was used as the electrolyte. In our previous research, azaacenes have already been demonstrated as photoanodes in PEC cells.46,47 As a new member of the azaacene family, DQNDN was also tested as a photoanode in PEC cells. The linear scan voltammetry (LSV, Fig. 2b) and the corresponding current density–potential curves (Fig. S7a, ESI†) in the dark and under light irradiation display the largest current density of 0.81 μA cm−2 at 0.87 V versus the reversible hydrogen electrode (RHE), supporting the occurrence of PEC reactions. To confirm the real performance of the PEC cells based on the nanostructured DQNDN film, the time course of the photocurrent density was measured at a potential of 1.23 V versus the RHE, shown in Fig. S7b (ESI†). At the beginning, a fast current density decay was found, followed by a slight current density decrease, suggesting that the charge transfer and extraction are not optimal (the adjustment of PEC cell components may further improve the performance). The time course of the photocurrent density (∼30 nA cm−2) has also been measured at a potential of 0.61 V versus the RHE (Fig. S8, ESI†) for comparison with our previous work,46,47 and these results are similar. To further reveal the charge transport behaviour of the nanostructured DQNDN film, Mott–Schottky measurements were performed in 0.5 M Na2SO4 aqueous solution. As shown in Fig. S9 (ESI†), the positive slope displays the n-type behaviour of the nanostructured DQNDN film, which is consistent with our previous PEC analysis.46,47 Besides the n-type behaviour, the Mott–Schottky measurement also provides us with a negative slope (p-type behaviour), suggesting that the nanostructured DQNDN film could also be utilized as a photocathode in PEC cells.
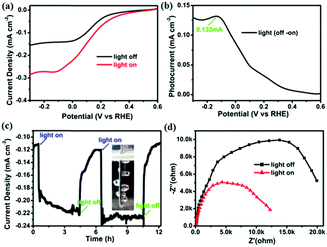
As shown in Fig. 3a and b, the LSV and the corresponding current density–potential curve exhibit the largest current density of 0.13 mA cm−2 at −0.13 V versus RHE, indicating that the nanostructured DQNDN film can be used as a good photocathode in PEC cells. To further understand the photocathode behaviours of the nanostructured DQNDN film, the time course of the photocurrent density was measured at a potential of −0.20 V versus RHE in Fig. 3c. The photocurrent density closely matches that of the LSV curve and exhibits almost no change over repeated cycles with stability for at least 12 h, which clearly suggests that the nanostructured DQNDN film is stable as a photocathode and unstable as a photoanode.
We also carried out electrochemical impedance spectroscopy (EIS) measurements of DQNDN at −0.20 V versus RHE (this condition is similar to the photocurrent measurements) to characterize the charge transport properties between the photoelectrode and the electrolyte. The as-obtained results are presented as Nyquist plots in Fig. 3d, where the x- and y-axes are the real part (Z′) and the negative of the imaginary part (−Z′′) of the impedance. Compared with the result attained under dark conditions, the diameter of the semicircle in the Nyquist plots decreases under light irradiation, indicating that the charge transport resistance of the nanostructured DQNDN film at the interface domain of the electrode is remarkably decreased under light irradiation than that in the dark, which is in agreement with the increase of the photocurrent upon light irradiation.
Conclusions
In summary, we have demonstrated that a large azaacene DQNDN can be employed as both a photoanode and a photocathode in PEC cells due to its ambipolar properties. Although the performance of DQNDN nanofibers as a photoanode is poor and unstable with a current density of 0.81 μA cm−2 at 0.87 V versus RHE, the performance of the nanostructured DQNDN film as a photocathode is very efficient and stable with a current density of 0.13 mA cm−2 at −0.13 V versus RHE. Our results clearly suggest that ambipolar organic small molecules could be promising candidates for highly efficient photocathodes in metal-free PEC cells for water reduction.Acknowledgements
Q. Z. acknowledges financial support from AcRF Tier 1 (RG133/14 and RG 13/15). Q. Z. also thanks the support from the Open Project of State Key Laboratory of Supramolecular Structure and Materials (Grant number: sklssm201630), Jilin University, China.Notes and references
- L. Z. Wu, B. Chen, Z. J. Li and C. H. Tung, Acc. Chem. Res., 2014, 47, 2177–2185 CrossRef CAS PubMed.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- N. Guijarro, M. S. Prévot, X. Yu, X. A. Jeanbourquin, P. Bornoz, W. Bourée, M. Johnson, F. Le Formal and K. Sivula, Adv. Energy Mater., 2016, 6, 1501949 CrossRef.
- M. G. Walter, E. L. Warren, J. R. McKone, S. W. Boettcher, Q. Mi, E. A. Santori and N. S. Lewis, Chem. Rev., 2010, 110, 6446–6473 CrossRef CAS PubMed.
- J. D. Blakemore, R. H. Crabtree and G. W. Brudvig, Chem. Rev., 2015, 115, 12974–13005 CrossRef CAS PubMed.
- X. Yu, A. Shavel, X. An, Z. Luo, M. Ibanez and A. Cabot, J. Am. Chem. Soc., 2014, 136, 9236–9239 CrossRef CAS PubMed.
- W. Li, D. He, S. W. Sheehan, Y. He, J. E. Thorne, X. Yao, G. W. Brudvig and D. Wang, Energy Environ. Sci., 2016, 9, 1794–1802 CAS.
- T. Yu, Y. Zeng, J. Chen, Y. Y. Li, G. Yang and Y. Li, Angew. Chem., Int. Ed., 2013, 52, 5631–5635 CrossRef CAS PubMed.
- D. Kang, T. W. Kim, S. R. Kubota, A. C. Cardiel, H. G. Cha and K. S. Choi, Chem. Rev., 2015, 115, 12839–12887 CrossRef CAS PubMed.
- J. R. Swierk and T. E. Mallouk, Chem. Soc. Rev., 2013, 42, 2357–2387 RSC.
- Z. Ji, M. He, Z. Huang, U. Ozkan and Y. Wu, J. Am. Chem. Soc., 2013, 135, 11696–11699 CrossRef CAS PubMed.
- E. S. Kim, N. Nishimura, G. Magesh, J. Y. Kim, J. W. Jang, H. Jun, J. Kubota, K. Domen and J. S. Lee, J. Am. Chem. Soc., 2013, 135, 5375–5383 CrossRef CAS PubMed.
- M. Gratzel, Nature, 2001, 414, 338–344 CrossRef CAS PubMed.
- J. R. Swierk, D. D. Méndez-Hernández, N. S. McCool, P. Liddell, Y. Terazono, I. Pahk, J. J. Tomlin, N. V. Oster, T. A. Moore, A. L. Moore, D. Gust and T. E. Mallouk, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 1681–1686 CrossRef CAS PubMed.
- Z. Li, W. Luo, M. Zhang, J. Feng and Z. Zou, Energy Environ. Sci., 2013, 6, 347–370 CAS.
- T. Hisatomi, J. Kubota and K. Domen, Chem. Soc. Rev., 2014, 43, 7520–7535 RSC.
- (a) J. Li, J. Miao, G. Long, J. Zhang, Y. Li, R. Ganguly, Y. Zhao, Y. Liu, B. Liu and Q. Zhang, J. Mater. Chem. C, 2015, 3, 9877–9884 RSC; (b) W.-W. Xiong, J. Miao, K. Ye, Y. Wang, B. Liu and Q. Zhang, Angew. Chem., Int. Ed., 2015, 54, 546–550 CAS.
- J. Liu, T. Hisatomi, M. Katayama, T. Minegishi, J. Kubota and K. Domen, J. Mater. Chem. A, 2016, 4, 4848–4854 CAS.
- K. Sivula and R. van de Krol, Nat. Rev. Mater., 2016, 1, 15010 CrossRef.
- J. Li, X. Gao, B. Liu, Q. Feng, X. B. Li, M. Y. Huang, Z. Liu, J. Zhang, C. H. Tung and L. Z. Wu, J. Am. Chem. Soc., 2016, 138, 3954–3957 CrossRef CAS PubMed.
- F. E. Osterloh, Chem. Soc. Rev., 2013, 42, 2294–2320 RSC.
- Y. Li, L. Zhang, A. Torres-Pardo, J. M. Gonzalez-Calbet, Y. Ma, P. Oleynikov, O. Terasaki, S. Asahina, M. Shima, D. Cha, L. Zhao, K. Takanabe, J. Kubota and K. Domen, Nat. Commun., 2013, 4, 2566 Search PubMed.
- L. Wu, S. Y. Chen, F. J. Fan, T. T. Zhuang, C. M. Dai and S. H. Yu, J. Am. Chem. Soc., 2016, 138, 5576–5584 CrossRef CAS PubMed.
- (a) Y.-J. Cheng, S.-H. Yang and C.-S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed; (b) T. M. Clarke and J. R. Durrant, Chem. Rev., 2010, 110, 6736–6767 CrossRef CAS PubMed.
- (a) W. Chen, X. Yang, G. Long, X. Wan, Y. Chen and Q. Zhang, J. Mater. Chem. C, 2015, 3, 4698–4705 RSC; (b) Q. Zhang, J. Xiao, Z. Y. Yin, H. M. Duong, F. Qiao, F. Boey, X. Hu, H. Zhang and F. Wudl, Chem. – Asian J., 2011, 6, 856–862 CrossRef CAS PubMed; (c) P.-Y. Gu, N. Wang, A. Wu, Z. Wang, M. Tian, Z. Fu, X. W. Sun and Q. Zhang, Chem. – Asian J., 2016, 11, 2135–2138 CrossRef CAS PubMed.
- (a) A. C. Grimsdale, K. Leok Chan, R. E. Martin, P. G. Jokisz and A. B. Holmes, Chem. Rev., 2009, 109, 897–1091 CrossRef CAS PubMed; (b) S. Scholz, D. Kondakov, B. Lussem and K. Leo, Chem. Rev., 2015, 115, 8449–8503 CrossRef CAS PubMed.
- (a) P.-Y. Gu, Y. Zhao, J.-H. He, J. Zhang, C. Wang, Q.-F. Xu, J.-M. Lu, X. W. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 3030–3035 CrossRef CAS PubMed; (b) G. Li, Y. Zhao, J. Li, J. Cao, J. Zhu, X. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 196–203 CrossRef CAS PubMed; (c) L. Xu, Y. Zhao, G. Long, Y. Wang, J. Zhao, D. Li, J. Li, R. Ganguly, Y. Li, H. Sun, X. W. Sun and Q. Zhang, RSC Adv., 2015, 5, 63080–63086 RSC; (d) J. Li and Q. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 28049–28062 CrossRef PubMed; (e) J. Li, Y. Zhao, J. Lu, G. Li, J. Zhang, Y. Zhao, X. Sun and Q. Zhang, J. Org. Chem., 2015, 80, 109–113 CrossRef CAS PubMed.
- (a) J. Zaumseil and H. Sirringhaus, Chem. Rev., 2007, 107, 1296–1323 CrossRef CAS PubMed; (b) M. Mas-Torrent and C. Rovira, Chem. Rev., 2011, 111, 4833–4856 CrossRef CAS PubMed.
- (a) J. Zhang, C. Wang, G. Long, N. Aratani, H. Yamada and Q. Zhang, Chem. Sci., 2016, 7, 1309–1313 RSC; (b) J. Zhang, P. Gu, G. Long, R. Ganguly, Y. Li, N. Aratani, H. Yamada and Q. Zhang, Chem. Sci., 2016, 7, 3851–3856 RSC; (c) C. Wang, J. Zhang, G. Long, N. Aratani, H. Yamada, Y. Zhao and Q. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6292–6296 CrossRef CAS PubMed; (d) W. Chen, J. Zhang, G. Long, Y. Liu and Q. Zhang, J. Mater. Chem. C, 2015, 3, 8219–8224 RSC.
- (a) N. G. Kang, B. Cho, B. G. Kang, S. Song, T. Lee and J. S. Lee, Adv. Mater., 2012, 24, 385–390 CrossRef CAS PubMed; (b) J. Y. Seok, S. J. Song, J. H. Yoon, K. J. Yoon, T. H. Park, D. E. Kwon, H. Lim, G. H. Kim, D. S. Jeong and C. S. Hwang, Adv. Funct. Mater., 2014, 24, 5316–5339 CrossRef CAS.
- (a) C. Wang, P. Gu, B. Hu and Q. Zhang, J. Mater. Chem. C, 2015, 3, 10055–10065 RSC; (b) C. Wang, B. Hu, J. Wang, J. Gao, G. Li, W.-W. Xiong, B. Zou, M. Suzuki, N. Aratani, H. Yamada, F. Huo, P. S. Lee and Q. Zhang, Chem. – Asian J., 2015, 10, 116–119 CrossRef PubMed; (c) C. Wang, J. Wang, P. Li, J. Gao, S. Y. Tan, W. Xiong, B. Hu, P. S. Lee, Y. Zhao and Q. Zhang, Chem. – Asian J., 2014, 9, 779–783 CrossRef CAS PubMed.
- A. Guerrero, M. Haro, S. Bellani, M. R. Antognazza, L. Meda, S. Gimenez and J. Bisquert, Energy Environ. Sci., 2014, 7, 3666–3673 CAS.
- F. Fumagalli, S. Bellani, M. Schreier, S. Leonardi, H. C. Rojas, A. Ghadirzadeh, G. Tullii, A. Savoini, G. Marra, L. Meda, M. Grätzel, G. Lanzani, M. T. Mayer, M. R. Antognazza and F. Di Fonzo, J. Mater. Chem. A, 2016, 4, 2178–2187 CAS.
- P. Bornoz, M. S. Prevot, X. Yu, N. Guijarro and K. Sivula, J. Am. Chem. Soc., 2015, 137, 15338–15341 CrossRef CAS PubMed.
- J. Bian, L. Xi, C. Huang, K. M. Lange, R.-Q. Zhang and M. Shalom, Adv. Energy Mater., 2016, 6, 1600263–1600268 CrossRef.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS PubMed.
- T. Abe, K. Nagai, S. Kabutomori, M. Kaneko, A. Tajiri and T. Norimatsu, Angew. Chem., Int. Ed., 2006, 45, 2778–2781 CrossRef CAS PubMed.
- J. Li and Q. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 28049–28062 Search PubMed.
- U. H. Bunz, Acc. Chem. Res., 2015, 48, 1676–1686 CrossRef CAS PubMed.
- Q. Miao, Adv. Mater., 2014, 26, 5541–5549 CrossRef CAS PubMed.
- G. J. Richards, J. P. Hill, T. Mori and K. Ariga, Org. Biomol. Chem., 2011, 9, 5005–5017 CAS.
- A. Mateo-Alonso, Chem. Soc. Rev., 2014, 43, 6311–6324 RSC.
- B. D. Lindner, J. U. Engelhart, O. Tverskoy, A. L. Appleton, F. Rominger, A. Peters, H. J. Himmel and U. H. Bunz, Angew. Chem., Int. Ed., 2011, 50, 8588–8591 CrossRef CAS PubMed.
- L. Zhu, Z.-S. Fu, H.-J. Pan, W. Feng, C. Chen and Z.-Q. Fan, Dalton Trans., 2014, 43, 2900–2906 RSC.
- M. M. Payne, S. R. Parkin and J. E. Anthony, J. Am. Chem. Soc., 2005, 127, 8028–8029 CrossRef CAS PubMed.
- Z. Wang, J. Miao, G. Long, P. Gu, J. Li, N. Aratani, H. Yamada, B. Liu and Q. Zhang, Chem. – Asian J., 2016, 11, 482–485 CrossRef CAS PubMed.
- G. Li, J. Miao, J. Cao, J. Zhu, B. Liu and Q. Zhang, Chem. Commun., 2014, 50, 7656–7658 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, synthesis of DQNDN, and fabrication and measurement of PEC cells. See DOI: 10.1039/c6qm00113k |
| ‡ Dr P.-Y. Gu and Z. Wang contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |