Controlled release of antibody proteins from liquid crystalline hydrogels composed of genetically engineered filamentous viruses†
Toshiki
Sawada
,
Miyuki
Yanagimachi
and
Takeshi
Serizawa
*
Department of Chemical Science and Engineering, School of Materials and Chemical Technology, Tokyo Institute of Technology, 2-12-1-H121 Ookayama, Meguro-ku, Tokyo 152-8550, Japan. E-mail: serizawa@polymer.titech.ac.jp
First published on 26th August 2016
Abstract
Affinity-based controlled-release systems have attracted attention for drug delivery owing to the avoidance of burst or fast release and because they provide tunable release properties. To widen the applicability of the system, construction of universal matrices, which enable the control of drug molecule release, is essential. Herein, we constructed controlled-release systems for high molecular weight antibody proteins using genetically engineered filamentous viruses, M13 phages. Hybrid hydrogels composed of antigen HA peptide-displaying phages (HA-phages) and physically cross-linked gelatin containing anti-HA peptide antibodies (HA-antibodies) were prepared for controlled-release systems. Quantification of the antibody molecules released from the hybrid hydrogels revealed that specific interactions between the phage-displayed HA peptides and antibodies were necessary for suppression of the release. The importance of the liquid crystalline-ordered lamellar structures of the assembled phages for effective suppression was evidenced by polarized optical microscopy and quantification of dialyzed amounts of HA-antibodies. The release rates and amounts could be controlled by altering the concentration of HA-phages (i.e., the concentration of the displayed HA peptides). Furthermore, utilization of the combination of HA-phages and another antigen, FLAG peptide-displaying phages, in the hybrid hydrogel resulted in the precise, controlled release of multiple antibodies from a single hybrid hydrogel. The use of genetically engineered phages as hydrogel matrices creates attractive opportunities for the science and technology of hydrogels with controlled release.
Introduction
The development of dynamic functions of materials with nano-scaled objects is important for progress in various fields such as biomaterials, devices, and sensors.1,2 Especially, local delivery systems of drug molecules with controlled-release properties that deliver therapeutics directly to an injury site are important because the system could minimize the toxicity and unexpected side effects.3 The development of pharmaceutical technologies has experienced a remarkable evolution in recent decades to overcome these problems.4,5 Hydrogels composed of chemically or physically cross-linked three-dimensional networks are widely used as matrices for release because their high water content and soft nature render them similar to natural extracellular matrices and minimize tissue irritation and cell adhesion.5,6 Several technologies for the controlled release of drug molecules from hydrogels have emerged, including release based on mesh size,7 diffusion,8–10 degradation,11–13 and affinity.14,15 Although significant efforts have been made in the development of the various release technologies for hydrogels, an initial burst or very fast release, which is observed in many hydrogels with the capability to release macromolecular drug molecules, such as proteins, is a limiting factor for many applications both in vitro and in vivo.5,14Affinity-based release systems have emerged as attractive systems for the delivery of drug molecules because they prevent burst or fast release while providing tunable release profiles by attenuating diffusional release through transient interactions with the delivery matrices.16 The system has been essential for the delivery of proteins because proteins cannot be delivered by other controlled-release systems due to their high molecular weight and/or surface hydrophilicity.17 In the affinity-based system, binding ligands for the desired drug proteins are conjugated to the hydrogel matrix; therefore, to widen the applicability of affinity-based release hydrogel systems, the development of universal matrices that could be easily prepared for desired drug molecules is required.
Recently, genetically engineered filamentous viruses (M13 phages) have been used as a component for the construction of soft materials in various fields, such as sensors, electronics, and devices,18–23 because of their defined size and dimensions (4.5 nm width and 900 nm length, molecular weight of 16.3 MDa), ease of surface modification through genetic24 or chemical25 methods, and capability for self-assembly into regularly ordered liquid crystalline structures.26 We have developed virus-based hybrid hydrogels containing liquid crystalline-oriented phages, demonstrating the high potential applicability of phages as hydrogel components.27–29 When physically cross-linked gelatin and liquid crystalline phages were used for hydrogel formation, the gelatin molecules were segregated into the space between the phage lamellar structures.29 Because phages can be modified to specifically interact with the desired molecules through genetic engineering, phage-ordered assemblies with layered structures, such as smectic liquid crystals, could act as effective interacting sites for the molecules in the hydrogel.
When the phages in the hybrid hydrogels are designed to specifically interact with specific drug molecules, it is possible to create novel hydrogels with designable controlled-release properties. The molecular recognition capability of genetically engineered phage-displayed peptides has been utilized in materials science and engineering over the past decade.30–32 Herein, we demonstrate for the first time that liquid crystalline-oriented genetically engineered phages displaying peptides with a specific affinity for macromolecular model proteins, antibodies, can be used to regulate the release of antibodies from hybrid hydrogels (Fig. 1). This study provides a novel insight into the future use of the genetically engineered viruses as potential components of biomedical soft materials.
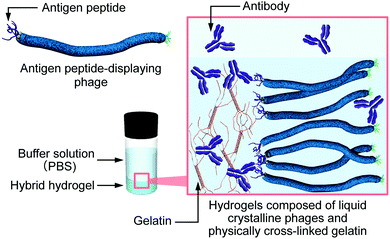 | ||
| Fig. 1 Schematic illustration of the controlled release of antibody molecules from hybrid hydrogels composed of antigen peptide-displaying phages and physically cross-linked gelatin. | ||
Results and discussion
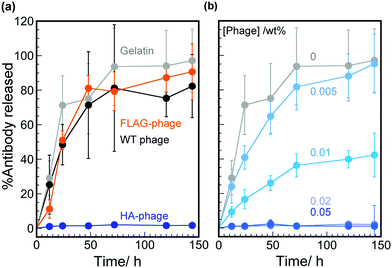
Hybrid hydrogels composed of HA peptide (sequence: YPYDVPDYA, known as a tag-peptide)-displaying phages (HA-phages) on their pIII coat protein (at the phage termini) with an affinity constant (Ka) of 1.1 × 109 M−1 for its antibody33 and gelatin molecules in the presence and absence of anti-HA peptide antibodies (HA-antibodies) were prepared. Polarized optical microscopy (POM) observation of the hydrogels was performed to investigate the effect of peptide-displaying and additional antibody molecules on the ordered structures of the phages in the hydrogels. We previously reported that the wild-type (WT) phages in the hybrid hydrogels composed of WT phages and gelatin formed liquid crystalline-oriented structures, even though the phage concentration was lower than that typically required for liquid crystalline formation.19,26 The POM images of the hybrid hydrogels containing WT or HA-phages were nearly identical (Fig. S1a and b in the ESI†), indicating that displaying peptides at the phage termini have no effect on the phage-ordered structures in the hydrogel, possibly due to the much smaller molecular weight of the HA peptide (approximately 1.1 kDa) than that of the phage. Furthermore, the addition of HA-antibody molecules to the hybrid hydrogels containing HA-phages also contributed little to the phage-ordered structure (Fig. S1c in the ESI†). Peptide-displaying and the addition of antibody molecules did not affect the hydrogel structures, demonstrating that the phage-gelatin hybrid hydrogel is suitable as a matrix for controlled release. The POM images of the gelatin hydrogels or phage solutions showed no birefringence (Fig. S1d and e in the ESI†), indicating that the liquid crystalline formation of the phages in the hydrogels was induced by mixing with gelatin, as reported previously.29 Therefore, gelatin molecules promote the formation of oriented lamellar phage structures due to the segregation of phages from gelation molecules. Additionally, the release of phage molecules from the hybrid hydrogels was quantitatively investigated. More than 99.9% of the phages remained in the hydrogel at all concentrations, demonstrating the superior properties of the matrix (Table S1 in the ESI†).The release of the HA-antibody molecules from the various types of hybrid hydrogels ([HA-antibody], 40 nM; [phage], 1.0 wt% (≈600 nM)) to phosphate buffered saline (PBS) was quantitatively investigated by an enzyme-linked immunosorbent assay (ELISA) using horseradish peroxidase (HRP)-conjugated anti-IgG antibody, as previously reported.34,35 HA-antibodies were gradually released from gelatin hydrogels in a time-dependent manner (Fig. 2a, gray plots), and the release asymptotically reached a plateau after 48 h, within experimental error. When the hybrid hydrogel composed of WT phages and gelatin molecules was used for the release, the release profile of the HA-antibodies was almost the same as that from gelatin hydrogels (Fig. 2a, black plots), suggesting that the phage molecules in the hybrid hydrogels played no role in the release. However, the release behavior was drastically changed when HA-antibodies were used to construct the hybrid hydrogels. The release ratio of HA-antibodies was approximately 1% after 144 h, indicating suppression of release (Fig. 2a, blue plots). When using FLAG peptide (peptide sequence: DYKDDDK)-displaying phages (FLAG-phages; i.e., no affinity for HA-antibodies) as a component of the hybrid hydrogels, the release of HA-antibody molecules was minimally suppressed (Fig. 2a, orange plots). Therefore, specific interactions between the phage-displayed HA peptide (antigen) at the phage termini and HA-antibodies in the hydrogel were effectively utilized for the suppression of the specific antibody release from the hydrogels.
To characterize the effect of the HA peptide on the HA-phages, the dependence of the HA-phage concentration on the release of HA-antibody molecules from the hybrid hydrogels was investigated (Fig. 2b). The release rates were dependent on the HA-phage concentration, demonstrating that the affinity of HA-phages (i.e., the concentration of HA peptides) could be used for the controlled release of HA-antibody molecules from the hybrid hydrogels. When the phage concentration was greater than 0.02 wt% (≈12 nM), the release of the antibody molecules was completely suppressed. When the phage concentration was less than 0.02 wt%, HA-antibody molecules were gradually released in a time-dependent manner. The phage can display 3–5 copies of the peptides when using the pIII displaying system,24 and the apparent concentration of HA peptides can be estimated from the phage concentration as: 12 nM phage represents 36–60 nM displayed peptide. Because the hybrid hydrogel contained 40 nM HA-antibody molecules, approximately equivalent to the number of HA peptides, nearly all the peptide molecules are thought to interact with the antibody, resulting in a complete suppression of release from the hydrogel. Affinity-based systems generally control the release of molecules by attenuating their diffusion through a matrix by reversible association-dissociation processes between the released molecules and binding ligands conjugated to the matrices.14 However, considering the dissociation rate constant of the HA-antibody for HA peptides (10−4–10−5 s−1),36,37 such suppression over 100 h cannot be explained by the normal antigen-antibody equilibrium system; therefore, the dissociation constant of the HA-antibodies decreased in the hybrid hydrogels compared to that in solution.
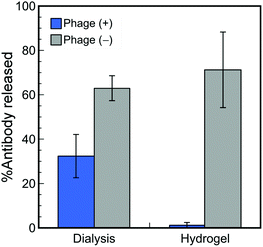
To demonstrate the effects of phage hydrogel formation on controlled-release, dialyzed amounts of HA-antibody molecules from the HA-phage solution were compared with the hybrid hydrogels. HA-antibody solutions in the presence and absence of HA-phages were dialyzed against PBS for 24 h (MWCO: 300 kDa). The amounts of HA-antibody molecules dialyzed to PBS were quantified by ELISA and compared to those of the hydrogel system (Fig. 3). The amount of HA-antibody released in the absence of the HA-phages from solution or gelatin hydrogel was within the experimental error, suggesting that the gelatin networks in the hydrogel have no effect on the release, possibly due to the larger mesh size compared to antibody molecules (Fig. S2 in the ESI†). For the HA-phages, the amount of HA-antibodies released from the mixed solution and the hydrogels was approximately 2 and 70 times smaller than without HA-phages, respectively. The effect of the HA-phages on the suppression of HA-antibody release in the mixed solution was much smaller than in the hydrogel, suggesting that HA-antibodies in the hydrogels interacted with the HA-phages more effectively than in the mixed solution. Although phages formed lyotropic liquid crystalline structures, the POM observation revealed that the hybrid hydrogels containing 0.02 wt% HA-phage showed clear birefringence, demonstrating liquid crystalline structures despite the low phage concentration (Fig. S1f in the ESI†). Based on all the observations, HA-antibodies strongly interacted with the densely positioned HA peptides displayed on the phages that formed oriented lamellar structures segregated from the gelatin molecules in the hydrogels, resulting in a highly effective suppression of release.
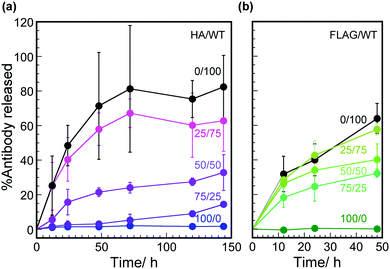
Hybrid hydrogels containing mixtures of HA-phages and WT phages with the same total concentration (0.02 wt%) were used to construct a better controlled-release system for antibody molecules (Fig. 4a). Both the release rates and total released amounts of HA-antibodies decreased with decreasing mixed percent of HA-phages. Because the total concentrations of the phages were equal, the liquid crystalline structures were considered to be almost identical. Therefore, both the molecular number of antigen peptides and the regulated phage structures were essential for the controlled release of antibody molecules from the hybrid hydrogels.
FLAG-phages and anti-FLAG peptide antibodies (FLAG-antibodies) were used instead of the HA-phage and HA-antibody, and the release of FLAG-antibody molecules was quantified by ELISA (Fig. 4b). The amounts of FLAG-antibody molecules released were slightly larger than those of the HA-antibody molecules at the same mixed ratio of phages, possibly due to the Ka value of the FLAG-phage (0.19 × 109 M−1) being smaller than that of the HA-phage. Although the Ka value of the FLAG-phage was smaller than that of the HA-phage, displayed peptide-dependent controlled release was observed. Accordingly, specific interactions between the peptides displayed on regularly assembled phages and the antibodies in the hybrid hydrogels were generally useful to control the release of antibody molecules.
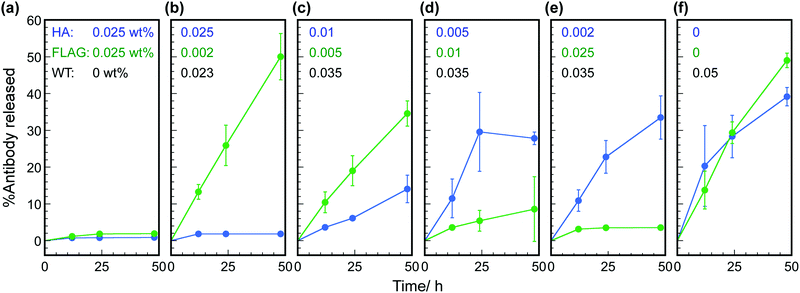
In previous sections, the effectiveness of mixing antigen peptide-displaying phages and WT phages for controlled release was demonstrated. To further expand the applicability of hybrid hydrogels, the HA- and FLAG-phages were mixed and used as matrices in the hydrogels to realize dual controlled release. WT phages were also included to adjust the total phage concentration (in this case, 0.05 wt%). Both HA- and FLAG-antibodies were loaded in each mixed hydrogel with different concentrations of phage (final concentration of each antibody: 40 nM). When two types of phages were used at a concentration of 0.025 wt%, the release of antibody molecules was effectively suppressed with respect to the single molecule release system (Fig. 5a). The amount of HA-antibody molecules released from the hydrogels increased with decreasing concentration of the HA-phages (Fig. 5b–f). The amount of FLAG-antibody released showed the same tendency. When only WT phages were used for hydrogel construction, suppression of HA- and FLAG-antibodies was rarely observed. The release behavior of these antibodies was similar to the single release systems, demonstrating orthogonal release in the same hybrid hydrogel. These results show that the hybrid hydrogels containing peptide-displaying phages that formed liquid crystalline structures are effective for the controlled release of multiple molecules with specific affinities for the displayed peptides. Therefore, similar to our genetic engineering of peptides at the phage termini, various types of drug molecules can be released from the hybrid hydrogels in a controlled manner.
Conclusions
We demonstrated that genetically engineered filamentous viruses (M13 phage) displaying antigen peptides with specific affinity for antibody proteins acted as a hydrogel matrix for the controlled release of antibody molecules. Quantification of the released amount of HA-antibody loaded in hybrid hydrogels composed of various types of phages and gelatin revealed that specific interactions between the phage-displayed HA peptide and HA-antibodies were effectively utilized for the suppression of HA-antibody release. The release rates and amounts could be controlled by the phage concentration. Phage-displayed HA peptide was effectively utilized for suppression, and an equivalent number of peptides almost completely suppressed the release. Comparison of the amount of antibody released from the hybrid hydrogels and the amount of antibody dialyzed from the phage solutions demonstrated the high suppression efficiency of the hydrogel due to the liquid crystalline-oriented lamellar structure of the phages. Mixing WT phages with HA-phages resulted in better control of the release of HA-antibodies. Combination of the antigen peptide and antibody also resulted in dual controlled release, and two types of antibody molecules were successfully released from hybrid hydrogels containing two types of phage in a controlled manner. Because various drug molecule-binding peptides have been obtained by affinity-based screening technology and rational design, various drugs can be inserted into phage surfaces for the controlled release from hybrid hydrogel systems.Experimental
Preparation of antigen peptide-displaying phages
Phages displaying antigen peptides were prepared according to a previously reported method.33 HA or FLAG peptides were genetically fused to minor coat protein 3 (pIII) of M13 phages via Gly–Gly–Gly–Ser spacers using the PhD peptide display cloning system (New England Biolabs). The prepared phages were purified by precipitation and re-dispersion in the presence of 5 w/v% poly(ethylene glycol) and 2.5 M NaCl. Each phage displayed from 3 to 5 copies of antigen peptide. The correct expression of the phages was verified by conventional DNA sequencing.Construction of hybrid hydrogels
Gelatin (500 mg) was dispersed in 9.5 mL of PBS and incubated for 1 h at ambient temperature for swelling. The dispersion was incubated for 1 h at 50 °C to dissolve completely. Then, the volume of the gelatin solution was adjusted to 10.0 mL by adding PBS. The final gelatin solution was diluted to a suitable concentration of phage solution in PBS. Then, the mixtures were incubated for 20 °C for at least 12 h.POM observation
The mixed solution of gelatin and phages was mounted onto a glass slide with a hole (hole diameter and depth of 15 and 0.6 mm, respectively). A cover glass was gently placed on the solution, and a POM (Eclipse LV100ND, Nikon) observation was performed at ambient temperature.Quantification of released antibodies from the hydrogels
PBS (300 μL) was mounted on the hydrogel prepared in a glass vial, and the vial was incubated at 25 °C. Ten microliters of the upper PBS solution was collected for quantification. Phages displaying peptides suitable for the antibody were immobilized in each well of a microtiter plate (F96 Maxisorp Nunc-Immuno Plate, Thermo Scientific) using a 2 nM solution. The collected antibody solution was mixed with 190 μL of 2 (w/v)% skimmed milk dissolved in PBS. The mixed solution (150 μL) was applied to the immobilized phages, and the mixture was incubated for 1 h at 25 °C. After washing with PBS 3 times, horseradish peroxidase (HRP)-conjugated anti-IgG antibody solution containing 2% skimmed milk was applied for 1 h at 25 °C. After washing in the same manner, the amount of antibody was estimated by measuring the fluorescence intensity of the product (QuantaBlu Fluorogenic Peroxidase Substrate Kit, Thermo Scientific, λex and λem were 360 and 465 nm, respectively) degraded by HRP. The fluorescence intensity of each well was converted to the amount of antibody molecules using a calibration curve prepared using known concentrations of antibody solution.Quantification of dialyzed antibodies from the solutions
Antibody solutions (1 mL) with or without phages were added to dialysis tubes (Float-A-Lyzer G2 Dialysis Device, MWCO: 300 kDa, Spectrumlaboratories). The solutions were dialyzed against 8 mL of PBS for 24 h. The amount of dialyzed antibody molecules was quantified using the method described in the previous section.Acknowledgements
The authors wish to thank the Division of Materials Analysis Ookayama (Tokyo Tech) for the SEM observations. This study was supported in part by a Grant-in-Aid for Young Scientists (B) from the Japan Society for the Promotion of Science (JSPS) (no. 23710122 and 15K17906), a Grant for Basic Science Research Project from The Sumitomo Foundation (no. 110427), The Asahi Glass Foundation, The Japan Prize Foundation, the Mizuho Foundation for the Promotion of Science, and The Izumi Science and Technology Foundation for T. Sawada.Notes and references
- K. Ariga, K. Minami, M. Ebara and J. Nakanishi, Polym. J., 2016, 48, 371–389 CrossRef CAS.
- K. Ariga, J. Li, J. Fei, Q. Ji and J. P. Hill, Adv. Mater., 2016, 28, 1251–1286 CrossRef CAS PubMed.
- C. Alvarez-Lorenzo and A. Concheiro, Curr. Opin. Biotechnol., 2013, 24, 1167–1173 CrossRef CAS PubMed.
- E. R. Balmayor, H. S. Azevedo and R. L. Reis, Pharm. Res., 2011, 28, 1241–1258 CrossRef CAS PubMed.
- T. Vermonden, R. Censi and W. E. Hennink, Chem. Rev., 2012, 112, 2853–2888 CrossRef CAS PubMed.
- H. Park and K. Park, Pharm. Res., 1996, 13, 1770–1776 CrossRef CAS.
- J. Siepmann and F. Siepmann, Int. J. Pharm., 2008, 364, 328–343 CrossRef CAS PubMed.
- C. Hiemstra, Z. Zhong, M. J. van Steenbergen, W. E. Hennink and J. Feijen, J. Controlled Release, 2007, 122, 71–78 CrossRef CAS PubMed.
- X. J. Loh, S. H. Goh and J. Li, Biomaterials, 2007, 28, 4113–4123 CrossRef CAS PubMed.
- R. Censi, T. Vermonden, H. Deschout, K. Braeckmans, P. di Martino, S. C. De Smedt, C. F. van Nostrum and W. E. Hennink, Biomacromolecules, 2010, 11, 2143–2151 CrossRef CAS PubMed.
- E. Ruel-Gariépy and J.-C. C. Leroux, Eur. J. Pharm. Biopharm., 2004, 58, 409–426 CrossRef PubMed.
- C. Hiemstra, Z. Zhong, S. R. Van Tomme, M. J. van Steenbergen, J. J. Jacobs, W. D. Otter, W. E. Hennink and J. Feijen, J. Controlled Release, 2007, 119, 320–327 CrossRef CAS PubMed.
- F. van de Manakker, K. Braeckmans, N. el Morabit, S. C. Smedt, C. F. van Nostrum and W. E. Hennink, Adv. Funct. Mater., 2009, 19, 2992–3001 CrossRef CAS.
- K. Vulic and M. S. Shoichet, Biomacromolecules, 2014, 15, 3867–3880 CrossRef CAS PubMed.
- T. Serizawa, H. Fukuta, T. Date and T. Sawada, Chem. Commun., 2016, 52, 2241–2244 RSC.
- N. X. Wang and H. A. von Recum, Macromol. Biosci., 2011, 11, 321–332 CrossRef CAS PubMed.
- M. van de Weert, W. E. Hennink and W. Jiskoot, Pharm. Res., 2000, 17, 1159–1167 CrossRef CAS.
- L. Wu, A. L. Lee, Z. Niu, S. Ghoshroy and Q. Wang, Langmuir, 2011, 27, 9490–9496 CrossRef CAS PubMed.
- S. Yang, W.-J. Chung, S. McFarland and S.-W. Lee, Chem. Rec., 2013, 13, 43–59 CrossRef CAS PubMed.
- N.-M. M. Courchesne, M. T. Klug, P.-Y. Y. Chen, S. E. Kooi, D. S. Yun, N. Hong, N. X. Fang, A. M. Belcher and P. T. Hammond, Adv. Mater., 2014, 26, 3398–3404 CrossRef CAS PubMed.
- N. M. Bardhan, D. Ghosh and A. M. Belcher, Nat. Commun., 2014, 5, 4918 CrossRef CAS PubMed.
- M. Pouya, S. Vesna, R. Dirk, J. F. Sandra, H. Ludger, H. Bernhard, B. Joachim and A. v. A. Peter, Langmuir, 2014, 30, 11428–11432 CrossRef PubMed.
- J. Lee, H.-E. Jin, M. S. Desai, S. Ren, S. Kim and S.-W. Lee, Nanoscale, 2015, 7, 18379–18391 RSC.
- G. P. Smith and V. A. Petrenko, Chem. Rev., 1997, 97, 391–410 CrossRef CAS PubMed.
- K. Mohan and G. A. Weiss, ACS Chem. Biol., 2016, 11, 1167–1179 CrossRef CAS PubMed.
- Z. Dogic and S. Fraden, Curr. Opin. Colloid Interface Sci., 2006, 11, 47–55 CrossRef CAS.
- T. Sawada, H. Chen, N. Shirakawa, S. Kang, J. Watanabe and T. Serizawa, Polym. J., 2014, 46, 511–515 CrossRef CAS.
- T. Sawada, S. Kang, J. Watanabe, H. Mihara and T. Serizawa, ACS Macro Lett., 2014, 3, 341–345 CrossRef CAS.
- T. Sawada, H. Otsuka, H. Yui and T. Serizawa, Polym. Bull., 2015, 72, 1487–1496 CrossRef CAS.
- M. Sarikaya, C. Tamerler, A. Jen, K. Schulten and F. Baneyx, Nat. Mater., 2003, 2, 577–585 CrossRef CAS PubMed.
- T. Serizawa, H. Matsuno and T. Sawada, J. Mater. Chem., 2011, 21, 10252–10260 RSC.
- T. Sawada, H. Mihara and T. Serizawa, Chem. Rec., 2013, 13, 172–186 CrossRef CAS PubMed.
- T. Sawada, K. Ishiguro, T. Takahashi and H. Mihara, Chem. Lett., 2011, 40, 508–509 CrossRef CAS.
- T. Sawada, T. Takahashi and H. Mihara, J. Am. Chem. Soc., 2009, 131, 14434–14441 CrossRef CAS PubMed.
- T. Sawada, K. Ishiguro, T. Takahashi and H. Mihara, Mol. BioSyst., 2011, 7, 2558–2562 RSC.
- N. R. Nygard, C. Bono, L. R. Brown, J. Gorka, K. S. Giacoletto, W. T. Schaiff, M. B. Graham, D. W. McCourt, M. Kabeer and V. L. Braciale, J. Exp. Med., 1991, 174, 243–251 CrossRef CAS PubMed.
- E. C. Nice, T. L. McInerney and D. C. Jackson, Mol. Immunol., 1996, 33, 659–670 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: POM images, remained percent of phages, SEM images. See DOI: 10.1039/c6qm00140h |
| This journal is © the Partner Organisations 2017 |