Alkyl chain engineering of pyrene-fused perylene diimides: impact on transport ability and microfiber self-assembly†
Xuejun
Zhan‡
a,
Ji
Zhang‡
b,
Yanbin
Gong
a,
Sheng
Tang
a,
Jin
Tu
a,
Yujun
Xie
a,
Qian
Peng
b,
Gui
Yu
 *b and
Zhen
Li
*b and
Zhen
Li
 *a
*a
aDepartment of Chemistry, Hubei Key Lab on Organic, Polymeric Opto-Electronic Materials, Wuhan University, Wuhan, 430072, China. E-mail: lizhen@whu.edu.cn; lichemlab@163.com; Fax: +86-27-68756757; Tel: +86-27-68755363
bInstitute of Chemistry, the Chinese Academy of Sciences, Beijing 100190, China. E-mail: yugui@iccas.ac.cn
First published on 17th August 2017
Abstract
The development of new solution-processable organic π systems is significant for the development of organic electronics, especially those with high hole/electron mobilities. Conjugated bilaterally pyrene-fused perylene tetracarboxdiimide (DPPDI) is a new class of material with high mobility and large on/off ratio. In this work, in several pyrene-fused PDIs, the impact of the alkyl chain on the performance has been systematically investigated, including the alkyl type (linear/branch), the alkyl chain length, and the position of the branching point. For the first time, the “antenna effect” has been observed in small molecules. Interestingly, the synthesized DPPDIs can form microfibers, indicating their tendency to form well-organized self-assembled structures. Moreover, a pyrene-fused PDI bearing a 5-decylpentadecyl N-alkyl chain group demonstrates an improved mobility of up to 1.38 cm2 V−1 s−1, which is the highest hole mobility value among pyrene derivative-based FETs through solution processing.
Introduction
Due to the attractive features of flexibility and low cost, organic electronic materials have gained broad attention from both academia and industry.1–4 Organic field effect transistors (OFETs), important components of organic electronics, have witnessed lots of breakthroughs, partially due to their potential applications in radio frequency identification (RFID) tags, flexible displays, electronic papers, sensors and so forth. Thanks to the great efforts of scientists, the mobility of some organic materials could even be comparable to those of inorganic materials.5–8 However, so far, most of the good OFETs with high motilities are based on high quality crystals,9–11 which require special processing12 or even complicated device fabrication,13,14 causing some obstacles for their possible commercialization. In this sense, it is still a hot topic to develop solution-processable new organic π systems with high hole/electron mobilities through intelligent molecular design.Generally, there are two main approaches to get organic semiconductors with high mobility.15 One is the synthesis of planar molecules with new conjugation structures, and another is alkyl chain engineering. While more attention has been paid to the construction of new π-conjugated backbones regardless of the encountered synthetic challenges, much less interest has been focused on alkyl chain engineering, although the peripheric alkyl chains of aromatic cores have been proved to largely affect the device, as a result of their impact on the molecular arrangement in thin films.16–20 As a typical example (Chart S1, ESI†), for the thienoacene-based molecules, Takimiya and co-workers developed a series of 2,7-dialkyl substituted benzothieno[3,2-b][1]-benzothiophene derivatives for solution-processable OFETs.21 Accompanying the length of the linear alkyl chain varying from 5 to 14, a maximum mobility of 2.75 cm2 V−1 s−1 (n = 13) was achieved, which was more than six times that of 0.43 cm2 V−1 s−1 (n = 5). Zhu et al. also reported some interesting results on naphthalene diimide (NDI)-based small molecule semiconductors.22 By just changing the branching position in the branched chain, the electron mobility could be enhanced by almost one order of magnitude. Marks et al. prepared a series of oligothiophenes decorated with fluoroalkyl chains,23 which exhibited totally different transporting ability in comparison with molecules substituted by alkylated counterparts. Actually, for various aromatic cores with special arrangement in films, the effects of the chain length, alkyl chain type (linear or branched ones), and even the bifurcation point, exist, however, with some difference.8,15 Thus, more research is still needed to deeply understand the structure–property relationship, possibly providing some guidance for rational molecular design.
Recently, we have focused on the expansion of the aromatic core of perylene tetracarboxdiimide (PDI) through the Scholl reaction. The synthesized pyrene-fused PDI derivatives have emerged as a new kind of organic semiconductors, with the mobility beyond 1.0 cm2 V−1 s−1 accompanied by a high on/off ratio of 108 in air.24 Prompted by the good performance, in this paper, a series of pyrene-fused PDIs modified by linear alkyl chain or branched chains with different lengths were synthesized, for further systematic investigation.
Experimental results showed that a minor change in the alkyl chain could induce distinctly different properties, due to the existence of the odd–even effect, “antenna” effect and so forth. Excitingly, the maximum mobility could be improved to 1.38 cm2 V−1 s−1, which is the highest hole mobility value among pyrene derivatives through solution processing. Herein, we would like to present the synthesis, and thermal, photophysical, and electrochemical properties, as well as device performance in detail.
Results and discussion
Synthesis
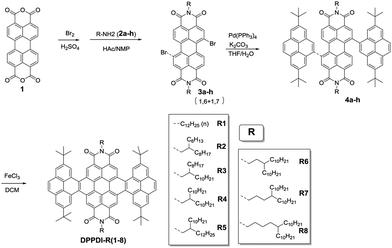
The eight bilaterally benzannulated PDIs with various side chains were prepared through similar synthetic routes (Scheme 1). A bromination reaction and the subsequent reaction with different amines (2a–h) yielded 3a–h from the commercially available perylene-3,4:9,10-tetracarboxylic acid bisanhydride (1). Palladium(0)-catalyzed Suzuki cross-coupling reactions were applied to afford pyrene-substituted compound 4a–h, which were treated with ferric chloride in dichloromethane (DCM) to produce pyrene-fused compounds DPPDI-R(1–8) with satisfactory yields.25,26 Among them, DPPDI-R1 bore a linear dodecyl chain, while DPPDI-R2, DPPDI-R3, DPPDI-R4, and DPPDI-R5 possessed two-branched N-alkyl chains of C10,8, C12,8, C12,10, and C14,10, respectively, with the carbon atom numbers of 18–24. Actually, DPPDI-R2, DPPDI-R3, DPPDI-R4, and DPPDI-R5 were designed to investigate the influence of side-chain length on the device performance. Otherwise, DPPDI-R6, DPPDI-R7, and DPPDI-R8 containing the three-, four-, and five-branched N-alkyl chains of C13,10, C14,10, and C15,10, respectively, were also studied to make a comparison with DPPDI-R4 (two-branched), so as to enable the investigation of the effect of the branching point. All of these compounds were easily purified by column chromatography, and fully characterized by 1H and 13C NMR, MALDI-TOF mass spectrometry, and elemental analysis. Due to their peripheric long alkyl chains, the DPPDIs showed good solubility in common organic solvents such as DCM, chloroform, and tetrahydrofuran (THF). Also, because of the good thermal and chemical stability of the PDI core, all these PDI derivatives are stable to ambient oxygen, moisture, and light.Thermal properties
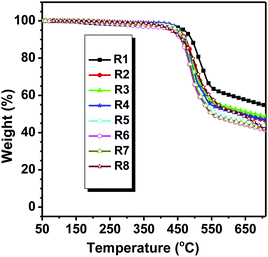
Good thermal stability of the planar compound is beneficial to the process and operation stability of opto-electronic devices.14 To study the influence of the alkyl chain on the thermal stability, the thermal properties of all the pyrene-fused compounds were investigated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). As depicted in Fig. 1 and Table 1, all the PDI derivatives were thermally stable with Td (corresponding to 5% weight loss) values in the range of 430–470 °C. Compared to other counterparts with branched N-alkyl chains, DPPDI-R1 decorated by a linear alkyl chain showed better stability. The Td values of DPPDI-R2, DPPDI-R3, DPPDI-R4, and DPPDI-R5 were almost the same, indicating that the alkyl chain length nearly has no influence on the stability. However, it did show obvious influence, when the branched alkyl chains adopted different branching point. Analyzing the Td values of DPPDI-R4, DPPDI-R6, DPPDI-R7, and DPPDI-R8 carefully, the thermal stability became poor along with the increased distance between the branching point and the conjugated core. Similar to those reported in the literature,19 this could be ascribed to the steric hindrance effect induced by the branching point and the resultant molecular arrangement.| Compound | T d (°C) | T g (°C) | T m (°C) | λ max,onset (nm) | E g (eV) | E HOMO (eV) | E LUMO (eV) | λ max,abs | λ em | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvc (nm) | Filmg (nm) | Solvc (nm) | Filmg (nm) | ||||||||
| a 5% weight loss temperature measured by TGA under N2. b Determined by DSC curves under N2. c Observed from absorption spectra in CH2Cl2 solution, 10 μM. d Band-gap estimated from the optical absorption band edge of the solution. e Calculated from the onset oxidation potentials of the compounds. f Estimated using empirical equations ELUMO = EHOMO + Eg. g On glass. | |||||||||||
| DPPDI-R1 | 470 | 292 | 411 | 659 | 1.88 | −5.44 | −3.56 | 409 | 414 | 640 | 662 |
| DPPDI-R2 | 457 | 226 | 391 | 656 | 1.89 | −5.46 | −3.57 | 409 | 414 | 639 | 661 |
| DPPDI-R3 | 452 | 168 | 345 | 656 | 1.89 | −5.47 | −3.58 | 409 | 415 | 641 | 657 |
| DPPDI-R4 | 455 | 203 | 329 | 658 | 1.88 | −5.45 | −3.57 | 409 | 414 | 639 | 658 |
| DPPDI-R5 | 453 | 159 | 304 | 655 | 1.89 | −5.45 | −3.56 | 409 | 414 | 639 | 659 |
| DPPDI-R6 | 436 | 190 | 325 | 658 | 1.88 | −5.47 | −3.59 | 409 | 414 | 640 | 658 |
| DPPDI-R7 | 449 | 144 | 327 | 657 | 1.89 | −5.44 | −3.55 | 409 | 414 | 640 | 660 |
| DPPDI-R8 | 430 | — | 300 | 658 | 1.88 | −5.46 | −3.58 | 409 | 415 | 641 | 660 |
From the DSC curves, the phase transition temperatures and melt points could be obtained (Fig. S1 and S2, ESI†). DPPDI-R1 with a linear alkyl chain showed higher Tg and Tm (292 °C and 411 °C, respectively), indicating the rigid structure of the central aromatic core. With branched alkyl chains, DPPDI-R2, DPPDI-R3, DPPDI-R4, and DPPDI-R5 possessed Tg values of 226, 168, 203 and 159 °C, respectively. Although all these four compounds had two-branched alkyl chains, the discontinuous change of Tg suggested that DPPDI-R4 should have distinct properties. Otherwise, in DPPDI-R4, DPPDI-R6, DPPDI-R7, and DPPDI-R8, the Tg values decreased along with the extension of the branched point. It is worth mentioning that the melting points of DPPDI-R4, DPPDI-R6, DPPDI-R7, and DPPDI-R8 were also discontinuous as the alkyl chains got longer. This could be caused by the odd–even oscillation effect.19 Generally, a highly packed structure requires more energy for melting, thus from the melt points, we could get some structure-relationships as discussed in the following sections.
Optical properties
To investigate the possible influence of the alkyl chain on optical properties, absorption and fluorescence spectra were obtained. Fig. 2a and b show their absorption spectra in diluted DCM solutions and thin solid films. In solutions, all pyrene-fused compounds exhibited similar absorptions, in which, the bands around 400 and 475 nm could be respectively attributed to the absorption of pyrene and the perylene bisimide unit.24 The typical shoulder bands between 550 and 650 nm, mainly caused by the HOMO–LUMO transition, could also be observed, indicating that the alkyl chains nearly have no influence in diluted solutions. However, the case became totally different in the spin-coated films. The shifts of the absorption peaks, along with the relative absorption intensity, varied when various alkyl chains were adopted. However, the absorption of the film did not exhibit an obvious tailing phenomenon, possibly due to the shielding effect of the tert-butyl groups on pyrene units towards π–π interactions, which, actually, could also weaken the difference present for the solution and film state.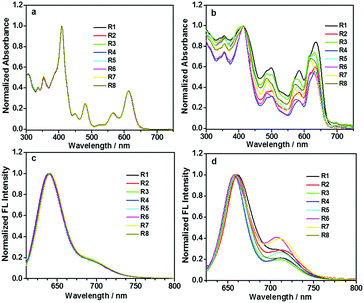 | ||
| Fig. 2 Normalized absorption (a and b) and fluorescence (c and d) spectra of DPPDI-R in CH2Cl2 solutions (10 μM) or in thin films on glass. | ||
All the eight pyrene-fused molecules exhibited red emission under the irradiation of light with an appropriate wavelength, both in diluted DCM solutions and thin films (Fig. 2c and d). Similar to their absorption behavior discussed earlier, the fluorescence spectra were almost identical in the solution state, with the emission peak located at 640 nm along with a relatively weak shoulder peak at around 700 nm. However, in films, some impact of the alkyl chain could be observed. Moderate shifts of the fluorescence peaks along with the relative emission intensity located at 640 and 700 nm varied, when various alkyl chains were adopted. Based on the above-discussed results, it is obvious that the different alkyl chains did not alter the molecular electronic structure, but affected the packing status in films.
Electrochemical properties
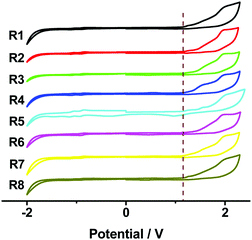
Cyclic voltammetry (CV) measurements were carried out to investigate the electrochemical properties of these bilaterally fused PDI derivatives. The highest occupied molecular orbital (HOMO) energy levels were estimated from the onset oxidation potentials according to the equation: HOMO = −(4.8 + Eox) eV, while the lowest unoccupied molecular orbital (LUMO) energy levels were obtained from optical band-gap energies (estimated from the onset wavelengths of the UV absorptions) and HOMO values. As shown in Fig. 3 and Table 1, owing to the enlarged planar π system, the DPPDIs showed a band-gap energy of 1.89 eV, while the HOMO and LUMO energy levels were around −5.46 and −3.57 eV, respectively. Regardless of the change of the alkyl chains, the energy levels of the DPPDI family are almost the same, further confirming that the alkyl chains do not alter the electronic structure of the molecules.15 Based on this, the optimized structures and calculated energy levels of the DPPDI core decorated with methyl groups were also obtained (Fig. 4) through density functional theory (DFT) calculations (B3LYP/6-31g*). As demonstrated in the optimized structures, the molecular backbone adopted a twisted structure. One pyrene unit twisted out of the plane of the central PDI (63.5°), while the other was almost planar with a smaller twist angle of 21.3°. As shown in Fig. 4b, its HOMO and LUMO energy levels were −5.32 eV and −3.00 eV, respectively, resulting in a larger bandgap in comparison with the optical one.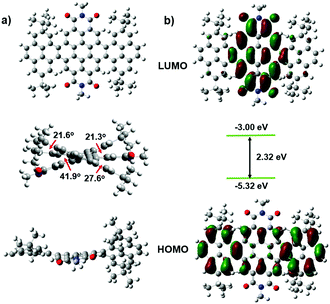 | ||
| Fig. 4 DFT-optimized molecular conformations (a), spatial distributions and energy levels of frontier molecular orbitals (b) of DPPDI-R. The alkyl chains have been clarified as methyl groups. | ||
Micro–nano structure assembly
To get more information about the influence of the alkyl chain on the solid-state packing structures, we tried to culture single crystals of these synthesized molecules, but failed. However, when dissolving the bilaterally pyrene-fused PDIs in chloroform and slowly diffusing the solvents, microfibers of DPPDI-R1 and DPPDI-R3 could be formed. Fig. 5 shows the bright-field and fluorescence images of these microfibers. In the bright-field photos (Fig. 5a and b), the microfibers were of green color with some needle-like branches. More interestingly, the fibers emitted red luminescence similar to the corresponding thin films. Their microfiber-forming ability indicated that DPPDIs with big π systems are able to form well-organized self-assembly structures, thus being beneficial for the enhancement of the carrier mobilities in OFETs.27 And the prominent self-assembly ability of DPPDI-R1 and DPPDI-R3 still proved that different alkyl chains could influence the arrangement of the molecules.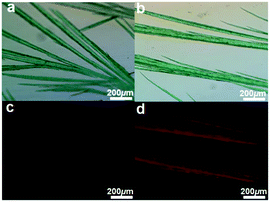 | ||
| Fig. 5 Micro–nano structure of DPPDI-R1 (a and c) and DPPDI-R3 (b and d): (up) bright field images and (down) fluorescence images. | ||
Device performance
To further investigate the influence of the alkyl chain on mobility, spin-coated OFET devices with bottom-gate/bottom-contact (BG/BC) configuration were fabricated. Before the deposition of the organic semiconductors, source and drain electrodes made of gold were pre-prepared on the SiO2/Si substrates. Octyltrichlorosilane (OTS) treatment was performed on the gate dielectrics, which were placed in a vacuum oven with OTS at a temperature of 120 °C to form an OTS self-assembled monolayer. Then the thin film was spin-coated on the OTS-modified SiO2/Si substrates from the chloroform solutions. The films were annealed at different temperatures, and the optimized temperatures were the same (160 °C). Even in air, most of the pyrene-fused compounds showed good stability, and the OFET characteristics of the devices were determined at room temperature in air (Fig. 6 and Table 2).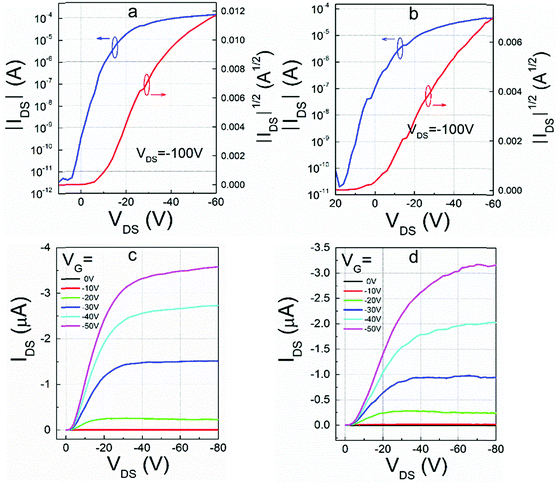 | ||
| Fig. 6 Representative transfer and output characteristics of (a and c) DPPDI-R1 and (b and d) DPPDI-R6. | ||
| Compound | μ avg, rt. (cm2 V−1 s−1) | μ avg, rt. (cm2 V−1 s−1) | μ max (cm2 V−1 s−1) | lg(Ion/Ioff) | T annealing (°C) |
|---|---|---|---|---|---|
| a Characterized under room temperature without thermal annealing. b Average values of hole mobilities from more than eight devices tested after annealing. | |||||
| DPPDI-R1 | — | 0.37 | 0.64 | 3–4 | 160 |
| DPPDI-R2 | — | 0.022 | 0.032 | 6 | 160 |
| DPPDI-R3 | 1.5 × 10−4 | 0.10 | 0.13 | 6 | 160 |
| DPPDI-R4 | — | 0.011 | 0.029 | 5–6 | 160 |
| DPPDI-R5 | — | 0.89 | 1.13 | 7 | 160 |
| DPPDI-R6 | 9.9 × 10−5 | 0.19 | 0.24 | 6–7 | 160 |
| DPPDI-R7 | — | 0.43 | 0.54 | 6–7 | 160 |
| DPPDI-R8 | — | 1.16 | 1.38 | 6–7 | 160 |
Except for the output characteristics of DPPDI-R4, all the transfer and output curves could be obtained (Fig. 6 and Fig. S4, ESI†). Without thermal annealing, DPPDI-R3 and DPPDI-R6 exhibited hole mobilities of 1.5 × 10−4 and 9.9 × 10−5 cm2 V−1 s−1, respectively. Similar to Würthner's work,28 regardless of several attempts, the n-type transport ability could not be found and even lower values were found for other PDI derivatives, indicating that the transport ability was derived from the pyrene units instead of the PDI unit with electron-withdrawing ability. Combined with reports in the literature,29 the PDI unit mainly played a role in the formation of the well-organized structure. Excitingly, after the thermal annealing at the optimized temperature of 160 °C, DPPDI-R1 decorated with linear alkyl chains could show much higher hole mobility, and the maximum value could be 0.64 cm2 V−1 s−1, with an average one of 0.37 cm2 V−1 s−1 though with a relatively low on/off ratio of 103–104 (Table 2).
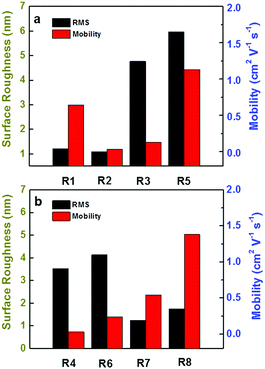
Once substituted by branched alkyl chains, the on/off ratio of the devices could increase to 105–107, much higher than that of the linear one. When decorated by the branched alkyl chain of 2-hexyldecyl instead of the linear dodecyl group, the maximum mobility decreased to 0.032 cm2 V−1 s−1, with an average mobility of 0.022 cm2 V−1 s−1, disclosing the significant effect of alkyl chain engineering. With the same type of two-branched N-alkyl chains, DPPDI-R3, DPPDI-R4 and DPPDI-R5, showed the maximum hole mobilities of 0.13 cm2 V−1 s−1, 0.029 cm2 V−1 s−1 and 1.13 cm2 V−1 s−1, respectively. Though fluctuating and discontinuous, the mobility values could show an increasing tendency accompanied by an increase in alkyl chain length, when the carbon numbers of the “antenna” were considered (Scheme 2). It seems that an asymmetric antenna was more favorable for the mobility when compared to DPPDI-R4 with C10 at both antennae of the branched chain. Actually, to the best of our knowledge, this unexpected and interesting “antenna effect” has never been reported in solution-processable small molecules decorated by alkyl chains, without any other element besides carbon and hydrogen.8,17 In this sense, it was not reasonable to investigate the influence of side-chain length on device performance when DPPDI-R4 was compared with other DPPDIs with two-branched alkyl chains. As shown in Scheme 2, for DPPDIs with the same branching point, the mobilities exhibited an increasing tendency along with an increase of alkyl chains.
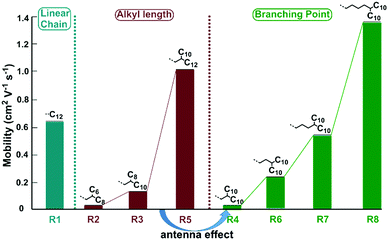 | ||
| Scheme 2 The impact of alkyl chain type (linear or branched), alkyl chain length, and branching point of the branched alkyl chains. | ||
For DPPDI-R4, DPPDI-R6, DPPDI-R7, and DPPDI-R8 possessing the two-, three-, four-, and five-branched N-alkyl chains of C12,10, C13,10, C14,10, and C15,10, respectively, the change in mobilities with the outreach of the branching point can be seen in Table 2 and Scheme 2. An obvious increasing tendency could be observed along with the extension of the branching point. Meanwhile, DPPDI-R8 exhibited a high mobility up to 1.38 cm2 V−1 s−1, with an on/off ratio of 107. To the best of our knowledge, this result is the highest hole mobility among pyrene derivatives through solution processing.5 The result was different from the literature reports,22 in which, the three-branched one was the best choice. This disclosed that the optimized alkyl chain could be different in accordance with the changed aromatic skeleton. Also, it was expected that the transport ability of DPPDIs could be further enhanced through rational molecular design with longer alkyl chains.30
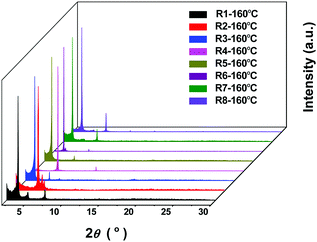
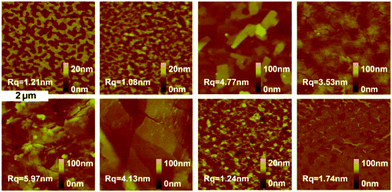
To further understand the above impact on the device performance, thin film morphologies of the eight molecules, spin-coated on the OTS modified SiO2/Si, were studied by X-ray diffraction (XRD) and atomic force microscopy (AFM). As shown in Fig. S5 (ESI†), no obvious crystallinity was found in the pristine films without the thermal annealing treatment, while crystallinity appeared in thin films at Tannealing = 160 °C (Fig. 7). More concretely, the XRD pattern of DPPDI-R1 showed three peaks located at 3.56°, 4.89° and 7.17°, which corresponded to a d-spacing of 1.36 nm. The film of DPPDI-R2 exhibited peaks located at 4.95°, 5.52°, and 5.81°, showing very poor crystallinity for carrier transport.31 When the branched alkyl chain was 2-octyldodecane (DPPDI-R3), the molecular packing became better, and a peak (3.23°) corresponding to the d-spacing of 1.50 nm appeared. In comparison with DPPDI-R1 decorated by a linear chain, the branched alkyl chain of DPPDI-R3 could induce larger d-spacing, deriving from the steric hindrance effect. From the XRD patterns, we could find that the packing styles of DPPDI-R2 and DPPDI-R4 are similar, which seems to be unfavorable for the transfer of holes. The XRD diffraction peaks of DPPDI-R5 were 2.96° and 6.01°, corresponding to a larger d-spacing of 1.63 nm in comparison with those of DPPDI-R1 and DPPDI-R3. So the improved hole mobility of DPPDI-R5 indicated that the mobility can also be impacted by other factors such as the quality of the spin-coated film, inter-plane arrangement, defects, and so on. As shown in the XRD patterns of DPPDI-R4, DPPDI-R6, DPPDI-R7 and DPPDI-R8, the diffraction peaks shifted to smaller angles (corresponding to larger d-spacing value) with stronger intensity along with the outreach of the branching point. For the annealed film of DPPDI-R8, a series of diffraction peaks located at 3.23°, 4.75°, 6.48°, 9.73° and 13.04° could be observed, indicating that a highly ordered arrangement was favorable for carrier transport in the film.24
The AFM images of DPPDIs with different morphologies are shown in Fig. 8, in which, different scanning ranges and height scale bars were chosen to show the morphology more clearly. The films of DPPDI-R2 and DPPDI-R4 exhibited poor crystallinity, possibly accounting for their poor hole transport ability. DPPDI-R3, DPPDI-R5, and DPPDI-R6 exhibited larger grains, resulting in a relatively higher surface roughness (RMS) of 4.77 nm, 5.97 nm and 4.13 nm, respectively. Also, the film overall showed better continuous morphologies with lower RMS values along with the outreach of the branching point. DPPDI-R8 demonstrated a continuous film with fewer defects, which should be favorable for the transfer of carriers. Actually, Pei et al. have reported that linear chain modified BN-TTNs exhibited RMS-related mobilities: films of BN-TTNs with larger RMS values exhibited low mobilities or no FET characteristics, while lower RMS values induced relatively higher mobilities.15 However, for the pyrene-fused PDI derivatives, this rule was partially applicable. As shown in Fig. 9, for the three DPPDIs with two-branched alkyl chains, compounds with higher mobility exhibited higher RMS values. When the branching point outreached, the mobility showed an increasing tendency while the RMS values exhibited discontinuous fluctuation. These results further prove that the influencing factors are multiple,32–35 and a certain effect can play different roles in different systems.
Conclusions
In summary, a series of pyrene-fused PDI derivatives were rationally designed and successfully obtained. The impact of the alkyl chain type (linear/branch), alkyl chain length, and branching point position on the electronic structure, solid-state packing, and OFET performance have been systematically investigated. Besides the unexpected “antenna effect,” the synthesized DPPDIs could form microfibers, indicating the tendency to form well-organized self-assembled structures. DPPDI-R8 exhibits a high mobility up to 1.38 cm2 V−1 s−1, which is the highest value of the hole mobility among pyrene derivatives through solution processing. The experimental results confirm that the alkyl chain can largely adjust the transport ability of the corresponding DPPDIs, as a result of the modified molecular arrangement instead of its electronic structure.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the National Science Foundation of China (no. 21325416, 51573140) for financial support.Notes and references
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789 CAS
.
- J. Y. Kim, T. Yasuda, Y. S. Yang and C. Adachi, Adv. Mater., 2013, 25, 2666 CrossRef CAS PubMed
.
- J. Wu, W. Pisula and K. Müllen, Chem. Rev., 2007, 107, 718 CrossRef CAS PubMed
.
- B. Kan, Q. Zhang, M. Li, X. Wan, W. Ni, G. Long, Y. Wang, X. Yang, H. Feng and Y. Chen, J. Am. Chem. Soc., 2014, 136, 15529 CrossRef CAS PubMed
.
- C. Wang, H. Dong, W. Hu, Y. Liu and D. Zhu, Chem. Rev., 2012, 112, 2208 CrossRef CAS PubMed
.
- W. Jiang, Y. Li and Z. Wang, Acc. Chem. Res., 2014, 47, 3135 CrossRef CAS PubMed
.
- J. E. Anthony, A. Facchetti, M. Heeney, S. R. Marder and X. Zhan, Adv. Mater., 2010, 22, 3876 CrossRef CAS PubMed
.
- J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, J. Am. Chem. Soc., 2013, 135, 6724 CrossRef CAS PubMed
.
- C. Reese and Z. Bao, Adv. Mater., 2007, 19, 4535 CrossRef CAS
.
- A. K. Tripathi, M. Heinrich, T. Siegrist and J. Pflaum, Adv. Mater., 2007, 19, 2097 CrossRef CAS
.
- Z. Liang, Q. Tang, J. Xu and Q. Miao, Adv. Mater., 2011, 23, 5514 CrossRef CAS PubMed
.
- Y. Shirai, S. Takami, S. Lasmono, H. Iwai, T. Chikyow and Y. Wakayama, J. Polym. Sci., Part B: Polym. Phys., 2011, 49, 1762 CrossRef CAS
.
- L. Li, W. Hu, L. Chi and H. Fuchs, J. Phys. Chem. B, 2010, 114, 5315 CrossRef CAS PubMed
.
- Y. Wen, Y. Liu, Y. Guo, G. Yu and W. Hu, Chem. Rev., 2011, 111, 3358 CrossRef CAS PubMed
.
- X. Wang, F. Zhuang, X. Zhou, D. Yang, J. Wang and J. Pei, J. Mater. Chem. C, 2014, 2, 8152 RSC
.
- H. B. Akkerman, S. C. B. Mannsdeld, A. P. Kaushik, E. Verploegen, L. Burnier, A. P. Zoombelt, J. D. Saathoff, S. Hong, S. A. Evrenk, X. Liu, A. A. Guzik, M. F. Toney, P. Clancy and Z. Bao, J. Am. Chem. Soc., 2013, 135, 11006 CrossRef CAS PubMed
.
- T. Lei, J. Wang and J. Pei, Chem. Mater., 2014, 26, 594 CrossRef CAS
.
- T. Lei, J. Dou and J. Pei, Adv. Mater., 2012, 24, 6457 CrossRef CAS PubMed
.
- C. Zeng, C. Xiao, R. Xin, W. Jiang, Y. Wang and Z. Wang, RSC Adv., 2016, 6, 55946 RSC
.
- J. Zhang, L. Tan, W. Jiang, W. Hu and Z. Wang, J. Mater. Chem. C, 2013, 1, 3200 RSC
.
- K. Takimiya, S. Shinamura, I. Osaka and E. Miyazaki, Adv. Mater., 2011, 23, 4347 CrossRef CAS PubMed
.
- F. Zhang, Y. Hu, T. Schuettfort, C. Di, X. Gao, C. R. McNeill, L. Thomsen, S. C. B. Mannsfeld, W. Yuan, H. Sirringhaus and D. Zhu, J. Am. Chem. Soc., 2013, 135, 2338 CrossRef CAS PubMed
.
- A. Facchetti, M. Mushrush, M. H. Yoon, G. R. Hutchison, M. A. Ratner and T. J. Marks, J. Am. Chem. Soc., 2004, 126, 13859 CrossRef CAS PubMed
.
- X. Zhan, J. Zhang, S. Tang, Y. Lin, M. Zhao, J. Yang, H. Zhang, Q. Peng, G. Yu and Z. Li, Chem. Commun., 2015, 51, 7156 RSC
.
- A. C. Bédard, A. Vlassova, A. C. Hernander-Perez, A. Bessette, G. S. Hanan, M. A. Heuft and S. K. Collins, Chem. – Eur. J., 2013, 19, 16295 CrossRef PubMed
.
- W. Jiang, Y. Li, W. Yue, Y. Zhen, J. Qu and Z. Wang, Org. Lett., 2010, 12, 228 CrossRef CAS PubMed
.
- R. Li, W. Hu, Y. Liu and D. Zhu, Acc. Chem. Res., 2010, 43, 529 CrossRef CAS PubMed
.
- S. L. Suraru, U. Zschieschang, H. Klauk and F. Würthner, Chem. Commun., 2011, 47, 11504 RSC
.
- A. P. H. J. Schenning, J. V. Herrikhuyzen, P. Jonkheijm, Z. Chen, F. Würthner and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 10252 CrossRef CAS PubMed
.
- J. Quinn, C. Guo, A. Chan, Y. He, E. Jin and Y. Li, J. Mater. Chem. C, 2015, 3, 11937 RSC
.
- W. Liu, Y. Zhou, Y. Ma, Y. Cao, J. Wang and J. Pei, Org. Lett., 2007, 9, 4187 CrossRef CAS PubMed
.
- X. Gao and Z. Zhao, Sci. China: Chem., 2015, 58, 947 CrossRef CAS
.
- Y. Gong, X. Zhan, Q. Li and Z. Li, Sci. China: Chem., 2016, 59, 1623 CrossRef CAS
.
- L. Chai, H. Zhou, X. Sun, H. Li, S. Yan and X. Yang, Chin. J. Polym. Sci., 2016, 34, 513 CrossRef CAS
.
- Y. Zheng, J. Wang and J. Pei, Sci. China: Chem., 2015, 58, 937 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthetic procedures, DSC curves, UV calculation, NMR spectra of the compounds, HRMS (MALDI-TOF) spectra of the compounds, and device fabrication and characterizations. See DOI: 10.1039/c7qm00268h |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |