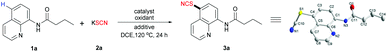Copper-catalyzed C5-selective thio/selenocyanation of 8-aminoquinolines†
Jichao
Chen
a,
Tianyu
Wang
a,
Tong
Wang
*ab,
Aijun
Lin
a,
Hequan
Yao
a and
Jinyi
Xu
*a
aState Key Laboratory of Natural Medicines and Department of Medicinal Chemistry, China Pharmaceutical University, 24 Tong Jia Xiang, Nanjing 210009, P. R. China. E-mail: jinyixu@china.com
bBeijing National Laboratory for Molecular Sciences, CAS Key Laboratory of Molecular Recognition and Function, Institute of Chemistry, Chinese Academy of Sciences, 100190 Beijing, P. R. China. E-mail: wangtong@iccas.ac.cn
First published on 26th October 2016
Abstract
Copper-catalyzed direct C5-position thio/selenocyanation of quinolines using commercially available, inexpensive KSCN/SeCN as the thio/selenocyanation reagent was developed, which had good tolerance toward various aliphatic or aromatic 8-aminoquinoline derivatives. Importantly, the synthetic application of this protocol led to some useful compounds.
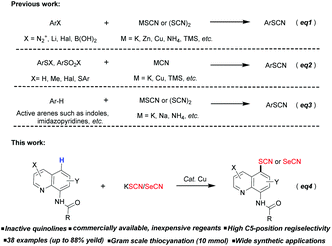
Organic thiocyanates are a class of important compounds with diverse bioactivities such as antibacterial, antifouling and antitumor activities.1 Meanwhile, the thiocyanate can be handily transformed to other common functional groups such as disulfides, thioethers, thiocarbamates and sulfonylcyanides.2 Although many methods have been established to synthesize alkyl thiocyanates, the construction of aryl thiocyanates has not been fully developed. Generally, the thiocyanate group can be introduced to arenes by the traditional cross-coupling of thiocyanate sources with active aryl species such as aryl halides, aryl diazonium salt (Sandmeyer reaction), aryl metal reagents or arylboronic acids (Scheme 1, eq1).2a,b,3 The cyanation of thiophenols, disulfides or aryl sulfonates is another approach for synthesizing aryl thiocyanates (eq2).2a,b,4 Undoubtedly the aforementioned methods have made great synthesis contributions, whereas the toxicity and high cost of the reagent, unavailable starting materials, or undesired byproducts could limit their wide applications. Compared with those that feature prefunctionalized arenes, the direct aryl thiocyanation seems more attractive. In fact, this strategy has been applied to the thiocyanation of some active heteroaromatics such as indoles and imidazopyridines (eq3).5 However, to the best of our knowledge, the direct thiocyanation of inactive arenes has rarely been reported.
Quinolines are important structural motifs widely present in bioactive compounds, pharmaceuticals and natural products.6 They often serve as ligands for coordination chemistry or directing groups for various metal-catalyzed reactions.7 They are also used as fluorescent probes for the detection of Zn2+ and Fe3+ or imaging fluorescence in living cells.8 Great progress has been made in the 2-, 3-, 4- and 8-position modifications of quinolines,9 and the C5-position functionalization of 8-aminoquinolines has recently received increasing attention.10 Stahl and co-workers initially reported the Cu-catalyzed C5-chlorination of 8-aminoquinoline.10a After that, Fe-catalyzed allylation,10b Cu-mediated/catalyzed halogenation,10c,d chalcogenation,10e sulfonylation,10f–k amination,10l trifluoromethylation,10m selenylation,10n and Co-catalyzed nitration10o have also been reported on the C5 position independently. Very recently, Wang and co-workers realized the Ni-catalyzed C5-position difluoroalkylation of 8-aminoquinoline.10p
Given the importance of quinolines and thiocyanates, it is of great interest to combine the two ones into a single entity. Herein, we would like to report a general and efficient protocol for the preparation of quinoline aryl thiocyanates on the C5 position, using commercially available and inexpensive KSCN as the thiocyanation reagent (Scheme 1, eq4). Moreover, this protocol was expanded to the corresponding selenocyanation of quinolines (eq4).
Initially, N-(quinolin-8-yl)butanamide (1a) and KSCN (2a) were selected as the substrates to optimize the reaction conditions. Fortunately, after several trials, when the reaction was performed in 1,2-dichloroethane (DCE) using K2S2O8 (2.0 equiv.) as an oxidant in the presence of a catalytic amount of CuI (20 mol%), N-(quinolin-5-thiocyanate-8-yl)butanamide (3a) was obtained in 62% yield (Table 1, entry 1). The structure of 3a was unambiguously confirmed using X-ray crystallography analysis. The control experiment reveals that CuI was essential for this transformation (entry 2). Then, several copper salts were tested. CuCl and CuI showed almost similar reactivities, whereas the use of the others resulted in either decreased yields (entries 3–7) or only traces of products (entries 8 and 9). Other metallic catalysts were also tested, but no desired product was obtained (entries 10–12). Based on these results, CuCl was selected as the optimal catalyst. The reaction hardly occurred when KSCN was replaced by NH4SCN (entry 13). Then, a series of oxidants were screened (entries 14–17), but none of them matched the efficiency of K2S2O8 except Na2S2O8 (entry 18). Increasing the loading of CuCl to 0.5 equiv. led to a slightly low yield (54%, entry 19), which can be explained by the generation of C5-chlorinated byproduct.10a,c,d The addition of 10 mol% TBAI (tetrabutylammonium iodide) improved the yield to 73% (entry 20). While TBAI was replaced with I2, the yield decreased to 47% (entry 21). Both base and acid additives were found to be detrimental to this reaction (entries 22–26). The solvent and temperature screenings reveal that the use of DCE and 120 °C comprised the optimal conditions (see ESI†).
| Entry | Catalyst | Oxidant | Additive | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.4 mmol), catalyst (20 mol%), oxidant (0.4 mmol), additive (0.4 mmol), DCE (2 mL) at 120 °C for 24 h. b Isolated yield. c 2.0 equiv. of NH4SCN instead of KSCN. d Under O2 atmosphere. e Copper catalyst (0.5 equiv.). f Additive (10 mol%). | ||||
| 1 | CuI | K2S2O8 | — | 62 |
| 2 | — | K2S2O8 | — | 0 |
| 3 | CuBr | K2S2O8 | — | 54 |
| 4 | CuCl | K2S2O8 | — | 65 |
| 5 | CuSCN | K2S2O8 | — | 25 |
| 6 | CuCl2 | K2S2O8 | — | 45 |
| 7 | CuBr2 | K2S2O8 | — | 35 |
| 8 | Cu(OAc)2 | K2S2O8 | — | Trace |
| 9 | Cu(OTf)2 | K2S2O8 | — | Trace |
| 10 | Pd(OAc)2 | K2S2O8 | — | 0 |
| 11 | Co(OAc)2 | K2S2O8 | — | 0 |
| 12 | NiCl2 | K2S2O8 | — | 0 |
| 13c | CuCl | K2S2O8 | — | Trace |
| 14 | CuCl | (NH4)2S2O8 | — | Trace |
| 15 | CuCl | Ag2CO3 | — | 0 |
| 16 | CuCl | PhI(OAc)2 | — | 37 |
| 17d | CuCl | O2 | — | Trace |
| 18 | CuCl | Na2S2O8 | — | 61 |
| 19e | CuCl | K2S2O8 | — | 54 |
| 20 | CuCl | K 2 S 2 O 8 | TBAI | 73 |
| 21f | CuCl | K2S2O8 | I2 | 47 |
| 22 | CuCl | K2S2O8 | K2CO3 | 0 |
| 23 | CuCl | K2S2O8 | NaOAc | 0 |
| 24 | CuCl | K2S2O8 | KH2PO4 | 58 |
| 25 | CuCl | K2S2O8 | AcOH | 34 |
| 26 | CuCl | K2S2O8 | TsOH | Trace |
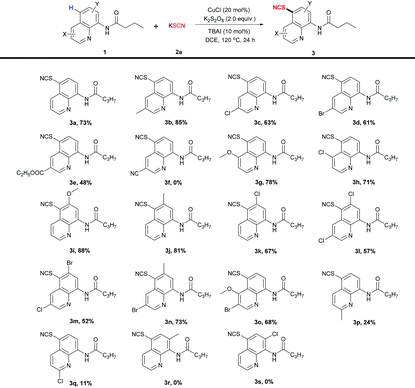
With the optimized reaction conditions (entry 20), diverse 8-aminoquinoline scaffolds were first examined (Table 2). The results demonstrate that all substrates with electron-donating groups such as methyl (3b, 3j) or methoxy (3g, 3i) at C3-, C4- or C6-position of quinoline were well tolerated, and the desired products were obtained in 78–88% yields. Comparatively, the substrates with electron-withdrawing groups such as chlorine (3c, 3h, 3k), bromine (3d) or ester (3e) showed a lower reactivity, resulting in 48–71% yields. Even when the substrate substituted with the strong electron-withdrawing cyanide group (3f), no product was obtained. These results indicate that the reaction was sensitive to the electron density on the quinoline ring and the electron-rich substrates had better reactivity than the corresponding electron-deficient ones. Moreover, several disubstituted 8-aminoquinolines also smoothly proceeded the reaction (3l–o, 52–73% yields). The substrates with C2 substituents gave 3p and 3q in 24% and 11% yields, respectively. The reaction could not occur if C7-substituted substrates were used (3r and 3s). These results indicate that C2 or C7 substituents affected the coordination process of the Cu catalyst with the substrates because of the steric hindrance, which may be one of the proofs of our proposed mechanism (Scheme 4).
| a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), CuCl (20 mol%), K2S2O8 (0.4 mmol), TBAI (10 mol%), DCE (2 mL) at 120 °C for 24 h. Isolated yields. |
|---|
|
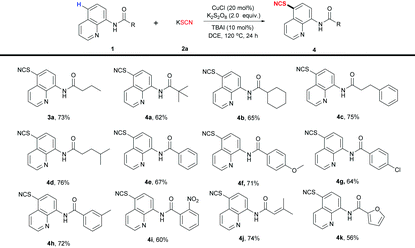
We subsequently explored the substrate scope with respect to the acyl motifs of the aminoquinoline amides (Table 3). This protocol was amenable to both linear (3a) and branched (4a–d) aliphatic-acid-derived substrates. All N-(quinolin-8-yl)benzamides substituted by various electron-withdrawing or electron-donating groups were well tolerated in this reaction system (4e–i). The substrates derived from 3-methyl-2-butenoic acid and 2-furoic acid were also feasible for this reaction (4j and 4k).
| a Reaction conditions: 1 (0.2 mmol), 2a (0.4 mmol), CuCl (20 mol%), K2S2O8 (0.4 mmol), TBAI (10 mol%), DCE (2 mL) at 120 °C for 24 h. Isolated yields. |
|---|
|
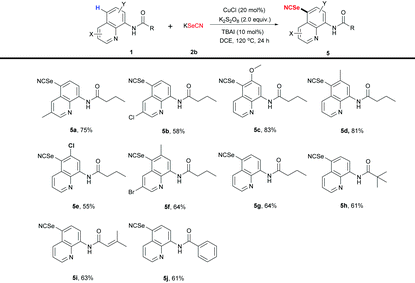
Organic selenocyanates are also important precursors for the synthesis of many seleno-containing compounds and have been discovered with various bioactivities such as antioxidative, antitumor activities, etc.11 Encouraged by the excellent performance of the current catalytic system, we explored the corresponding selenocyanation of 8-aminoquinoline derivatives using KSeCN as the selenocyanation reagent. To our delight, the desired selenocyanated products were obtained in moderate to good yields (Table 4). For C3- or C6-substituted 8-aminoquinolines, the substrates with electron-donating groups showed better reactivity than the corresponding ones with electron-withdrawing groups (e.g., 5avs.5b, 75% vs. 58%), which is similar to the thiocyanation process. The disubstituted substrates also showed good tolerance in this reaction system (5f, 68%). Typical linear or branched alkyl, olefinic or aromatic amide substitution all worked well under the standard reaction conditions (5g–5j).
| a Reaction conditions: 1 (0.2 mmol), 2b (0.4 mmol), CuCl (20 mol%), K2S2O8 (0.4 mmol), TBAI (10 mol%), DCE (2 mL) at 120 °C for 24 h. Isolated yields. |
|---|
|
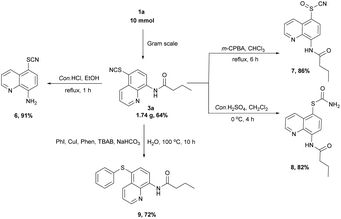
To demonstrate the synthetic utility of the protocol, we conducted the reaction on a 10 mmol gram scale, and the desired product was obtained in 64% yield (Scheme 2). The amide bond can be easily broken without damaging the thiocyanate group of 3a to produce compound 6 in 91% yield.10f The thiocyanate 3a can be oxidized to sulfinyl cyanide 7 in 86% yield2h or transformed to thiocarbamate 8 in 82% yield.2g In addition, the coupling of 3a with iodobenzene can produce the thioether 9 in 72% yield according to a modified method.2e
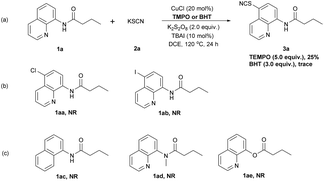
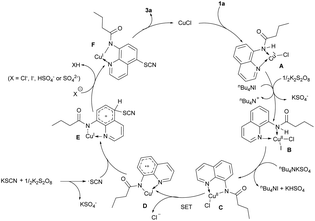
Furthermore, a series of control experiments were performed to explore the reaction mechanism as shown in Scheme 3. When the radical quencher TEMPO (2,2,6,6-tetramethylpiperidine-N-oxide) or BHT (2,6-di-tert-butyl-4-methylphenol) was added to the reaction system, the yield sharply decreased (Scheme 3a, 25% or trace). This empirical evidence reveals that a single-electron transfer (SET) process may be a key step in the catalytic system. The addition of substrates 1aa or 1ab instead of 1a to the reaction system led to the recovery of the corresponding starting materials (Scheme 3b). This result indicates that the thiocyanation was a direct functionalization process without the formation of chlorinated or iodinated intermediates.10a,c,d A bidentate coordination model was obviously preferred based on the comparison of substrate 1a with 1ac, 1ad, 1ae (Scheme 3c).
Based on the above results and previous reports,10 we proposed a plausible reaction mechanism as shown in Scheme 4. First, a Cu(I)-quinoline complex A with bidentate nitrogen atom chelation was formed. With the assistance of TBAI, complex A was oxidized to a Cu(II) intermediate B by K2S2O8. The deprotonation of B produced the amidate-Cu(II) intermediate C, which might experience an intramolecular SET process to produce a cationic radical D.10h,n,o A radical coupling reaction of D and the SCN radical that was generated by the oxidation of KSCN in the presence of K2S2O8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5d afforded the cationic intermediate E. Further deprotonation and ligand-exchange process produced the desired product 3a and regenerated the Cu(I) catalyst.
5d afforded the cationic intermediate E. Further deprotonation and ligand-exchange process produced the desired product 3a and regenerated the Cu(I) catalyst.
In summary, an efficient method of copper-catalyzed direct C5-selective thio/selenocyanation of quinolines was developed. The protocol featured the use of commercially available and inexpensive KSCN/SeCN as the thio/selenocyanation reagent, which had good tolerance toward various aliphatic or aromatic 8-aminoquinoline derivatives. Besides, a direct thiocyanation was proposed. It should be noted that the thiocyanation product could be easily transformed to some useful compounds such as sulfinyl cyanide, thiocarbamate and thioether, which provides an alternative method to synthesize 5-thio-8-aminoquinoline derivatives.
Financial support from the National Natural Science Foundation (No. 81373280, 81673306), the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMZZCX201404), and the Innovation project of Jiangsu Province (No. KYLX15_0633) is gratefully acknowledged.
Notes and references
- For selected examples, see: (a) Y. Yasmana, R. A. Edrada, V. Wray and P. Proksch, J. Nat. Prod., 2003, 66, 1512 CrossRef PubMed; (b) S. Dutta, H. Abe, S. Aoyagi, C. Kibayashi and K. S. Gates, J. Am. Chem. Soc., 2005, 127, 15004 CrossRef CAS PubMed; (c) I. C. Piña, J. T. Gautschi, G. Y. Wang, M. L. Sanders, F. J. Schmitz, D. France, S. Cornell-Kennon, L. C. Sambucetti, S. W. Remiszewski, L. B. Perez, K. W. Bair and P. Crews, J. Org. Chem., 2003, 68, 3866 CrossRef PubMed.
- For reviews, see: (a) T. Castanheiro, J. Suffert, M. Donnard and M. Gulea, Chem. Soc. Rev., 2016, 45, 494 RSC; (b) A. W. Erian and S. M. Sherif, Tetrahedron, 1999, 55, 7957 CrossRef CAS. Selected examples: (c) X. Lu, H. Wang, R. Gao, D. Sun and X. Bi, RSC Adv., 2014, 4, 28794 RSC; (d) L. Melzig, C. B. Rauhut, N. Naredi-Rainer and P. Knochel, Chem. – Eur. J., 2011, 17, 5362 CrossRef CAS PubMed; (e) F. Ke, Y. Y. Qu, Z. Q. Jiang, Z. K. Li, D. Wu and X. G. Zhou, Org. Lett., 2011, 13, 454 CrossRef CAS PubMed; (f) A. R. Hajipour, R. Pourkaveh and H. Karimi, Appl. Organomet. Chem., 2014, 28, 879 CrossRef CAS; (g) R. Riemschneider, F. Wojahn and G. Orlick, J. Am. Chem. Soc., 1951, 73, 5905 CrossRef; (h) M. A. Boerma-Markerink, J. C. Jagt, H. Meyer, J. Wildeman and A. M. Van Leusen, Synth. Commun., 1975, 5, 147 CrossRef CAS.
- For selected examples, see: (a) K. Takagi, H. Takachi and K. Sasaki, J. Org. Chem., 1995, 60, 6552 CrossRef CAS; (b) M. Barbero, I. Degani, N. Diulgheroff, S. Dughera and R. Fochi, Synthesis, 2005, 961 Search PubMed; (c) M. Hosseini-Sarvari and M. Tavakolian, J. Chem. Res., 2008, 6, 318 CrossRef; (d) A. Batool, A. R. Pourali and M. Rahmani, Synth. Commun., 2012, 42, 1184 CrossRef; (e) N. Sun, H. Zhang, W. Mo, B. Hu, Z. Shen and X. Hu, Synlett, 2013, 1443 CAS.
- For selected examples, see: (a) K. Yamaguchi, K. Sakagami, Y. Miyamoto, X. Jin and N. Mizuno, Org. Biomol. Chem., 2014, 12, 9200 RSC; (b) T. Castanheiro, M. Gulea, M. Donnard and J. Suffert, Eur. J. Org. Chem., 2014, 7814 CrossRef CAS; (c) F. Teng, J. T. Yu, H. Yang, Y. Jiang and J. Cheng, Chem. Commun., 2014, 50, 12139 RSC; (d) D. Zhu, D. Chang and L. Shi, Chem. Commun., 2015, 51, 7180 RSC.
- For selected examples, see: (a) W. G. Fan, Q. Yang, F. S. Xu and P. X. Li, J. Org. Chem., 2014, 79, 10588 CrossRef CAS PubMed; (b) L. Wang, C. C. Wang, W. J. Liu, Q. Chen and M. Y. He, Tetrahedron Lett., 2016, 57, 1771 CrossRef CAS; (c) S. Mitra, M. Ghosh, S. Mishra and A. Hajra, J. Org. Chem., 2015, 80, 8275 CrossRef CAS PubMed; (d) D. Yang, K. Yan, W. Wei, G. Q. Li, S. L. Lu, C. X. Zhao, L. J. Tian and H. Wang, J. Org. Chem., 2015, 80, 11073 CrossRef CAS PubMed.
- For reviews, see: (a) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (b) K. Kaur, M. Jain, R. P. Reddy and R. Jain, Eur. J. Med. Chem., 2010, 45, 3245 CrossRef CAS PubMed. Selected examples: (c) C. C. Hughes, J. B. MacMillan, S. P. Gaudencio, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2009, 48, 725 CrossRef CAS PubMed; (d) E. Pan, N. W. Oswald, A. G. Legako, J. M. Life, B. A. Posner and J. B. MacMillan, Chem. Sci., 2013, 4, 482 RSC; (e) C. C. Hughes and W. Fenical, J. Am. Chem. Soc., 2010, 132, 2528 CrossRef CAS PubMed; (f) Y. Takayama, T. Yamada, S. Tatekabe and K. Nagasawa, Chem. Commun., 2013, 49, 6519 RSC; (g) J. Colomb, G. Becker, S. Fieux, L. Zimmer and T. Billard, J. Med. Chem., 2014, 57, 3884 CrossRef CAS PubMed.
- For reviews, see: (a) M. Corbet and F. De Campo, Angew. Chem., Int. Ed., 2013, 52, 9896 CrossRef CAS PubMed; (b) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed. For selected examples, see: (c) S. Hong, Y. M. Lee, K. B. Cho, K. Sundaravel, J. Cho, M. J. Kim, W. Shin and W. Nam, J. Am. Chem. Soc., 2011, 133, 11876 CrossRef CAS PubMed; (d) X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2014, 136, 1789 CrossRef CAS PubMed; (e) T. Matsubara, S. Asako, L. Ilies and E. Nakamura, J. Am. Chem. Soc., 2014, 136, 646 CrossRef CAS PubMed; (f) Q. H. Zhu, D. Z. Ji, T. T. Liang, X. Y. Wang and Y. G. Xun, Org. Lett., 2015, 17, 3798 CrossRef CAS PubMed.
- For selected examples, see: (a) X. M. Meng, S. X. Wang, Y. M. Li, M. Z. Zhu and Q. X. Guo, Chem. Commun., 2012, 48, 4196 RSC; (b) J. Huang, Y. Xu and X. Qian, Dalton Trans., 2014, 43, 5983 RSC; (c) S. S. Zhu, W. Y. Lin and L. Yuan, Dyes Pigm., 2013, 99, 465 CrossRef CAS; (d) J. W. Meeusen, H. Tomasiewicz, A. Nowakowski and D. H. Petering, Inorg. Chem., 2011, 50, 7563 CrossRef CAS PubMed; (e) S. Mukherjee and S. Talukder, J. Lumin., 2016, 177, 40 CrossRef CAS.
- For selected examples, see: (a) I. B. Seiple, S. Su, R. A. Rodriguez, R. Gianatassio, Y. Fujiwara, A. L. Sobel and P. S. Baran, J. Am. Chem. Soc., 2010, 132, 13194 CrossRef CAS PubMed; (b) M. Chen, S. Ichikawa and S. L. Buchwald, Angew. Chem., Int. Ed., 2015, 54, 263 CrossRef CAS PubMed; (c) Q. Chen, X. M. du Jourdin and P. Knochel, J. Am. Chem. Soc., 2013, 135, 4958 CrossRef CAS PubMed; (d) M. Nagase, Y. Kuninobu and M. Kanai, J. Am. Chem. Soc., 2016, 138, 6103 CAS; (e) H. Hwang, J. Kim, J. Jeong and S. Chang, J. Am. Chem. Soc., 2014, 136, 10770 CrossRef CAS PubMed; (f) J. Kwak, M. Kim and S. Chang, J. Am. Chem. Soc., 2011, 133, 3780 CrossRef CAS PubMed.
- (a) A. M. Suess, M. Z. Ertem, C. J. Cramer and S. S. Stahl, J. Am. Chem. Soc., 2013, 135, 9797 CrossRef CAS PubMed; (b) X. F. Cong and X. F. Zeng, Org. Lett., 2014, 16, 3716 CrossRef CAS PubMed; (c) J. Xu, X. L. Zhu, G. B. Zhou, B. B. Ying, P. P. Ye, Y. Y. Su, C. Shen and P. F. Zhang, Org. Biomol. Chem., 2016, 14, 3016 RSC; (d) H. L. Guo, M. M. Chen, P. Jiang, J. Chen, L. X. Pan, M. Wang, C. S. Xie and Y. H. Zhang, Tetrahedron, 2015, 71, 70 CrossRef CAS; (e) L. Z. Zhu, R. H. Qiu, X. Cao, S. Xiao, X. H. Xu, C. T. Au and S. F. Yin, Org. Lett., 2015, 17, 5528 CrossRef CAS PubMed; (f) H. W. Liang, K. Jiang, W. Ding, Y. Yuan, Li Shuai, Y. C. Chen and Y. Wei, Chem. Commun., 2015, 51, 16928 RSC; (g) H. J. Qiao, S. Y. Sun, F. Yang, Y. Zhu, W. G. Zhu, Y. X. Dong, Y. S. Wu, X. T. Kong, L. Jiang and Y. J. Wu, Org. Lett., 2015, 17, 6086 CrossRef CAS PubMed; (h) J. Xu, C. Shen, X. L. Zhu, P. Zhang, M. J. Ajitha, K. W. Huang, Z. F. An and X. G. Liu, Chem. – Asian J., 2015, 11, 882 CrossRef PubMed; (i) J. Wei, J. J. Jiang, X. S. Xiao, D. G. Lin, Y. F. Ke, H. F. Jiang and W. Zeng, J. Org. Chem., 2016, 81, 946 CrossRef CAS PubMed; (j) C. C. Xia, K. Wang, J. Xu, Z. J. Wei, C. Shen, G. D. Duan, Q. Zhu and P. F. Zahng, RSC Adv., 2016, 6, 37173 RSC; (k) S. Liang and G. Manolikakes, Adv. Synth. Catal., 2016, 358, 2371 CrossRef CAS; (l) H. Sahoo, M. K. Reddy, I. Ramakrishna and M. Baidya, Chem. – Eur. J., 2016, 22, 1592 CrossRef CAS PubMed; (m) Y. Kuninobu, M. Nishi and M. Kanai, Org. Biomol. Chem., 2016, 14, 8092 RSC; (n) H. Sahoo, A. Mandal, J. Selvakumar and M. Baidya, Eur. J. Org. Chem., 2016, 4321 CrossRef CAS; (o) C. J. Whiteoak, O. Planas, A. Company and X. Ribas, Adv. Synth. Catal., 2016, 358, 1679 CrossRef CAS; (p) H. Chen, P. H. Li, M. Lin and L. Wang, Org. Lett., 2016, 18, 4794 CrossRef CAS PubMed.
- For review, see: (a) J. C. Guillemin, Curr. Org. Chem., 2011, 15, 1670 CrossRef CAS. Selected examples: (b) N. B. Rashid Baig, R. N. Chandrakala, V. Sai Sudhir and S. Chandrasekaran, J. Org. Chem., 2010, 75, 2910 CrossRef CAS PubMed; (c) J. Thomas, W. Maes, K. Robeyns, M. Ovaere, L. V. Meervelt, M. Smet and W. Dehaen, Org. Lett., 2009, 11, 3040 CrossRef CAS PubMed; (d) R. K. Das, S. K. U. Hossain and S. Bhattacharya, Cancer Lett., 2005, 230, 90 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, characterization of new compounds, and X-ray analysis. CCDC 1483507. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00590j |
| This journal is © the Partner Organisations 2017 |