Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
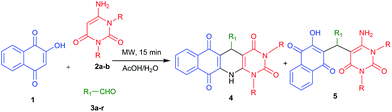
Molecular diversity from the three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes and 6-aminouracils: a reaction condition dependent MCR†
Ruchi Bhartia,
Pooja Kumari a,
Tasneem Parvin
a,
Tasneem Parvin *a and
Lokman H. Choudhury
*a and
Lokman H. Choudhury *b
*b
aDepartment of Chemistry, National Institute of Technology Patna, Ashok Rajpath, Patna-800 005, Bihar, India. E-mail: tasneem@nitp.ac.in
bDepartment of Chemistry, Indian Institute of Technology Patna, Bihta, Patna-801103, Bihar, India. E-mail: lokman@iitp.ac.in
First published on 16th January 2017
Abstract
The three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes, and 6-aminouracil derivatives in acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; v/v) under microwave heating conditions provides 1,4-dihydropyridines fused with naphthaquinone and pyrimidines. On the other hand the same reaction combinations under conventional reflux conditions provide acyclic trisubstituted methane derivatives. Using these tuneable reaction conditions a series of polycyclic fused N-heterocycles has been synthesized. The notable features of this methodology are a simple metal-free one-pot operation, easy purification process, use of the green solvent water, short reaction time and good to moderate yields of the products.
1; v/v) under microwave heating conditions provides 1,4-dihydropyridines fused with naphthaquinone and pyrimidines. On the other hand the same reaction combinations under conventional reflux conditions provide acyclic trisubstituted methane derivatives. Using these tuneable reaction conditions a series of polycyclic fused N-heterocycles has been synthesized. The notable features of this methodology are a simple metal-free one-pot operation, easy purification process, use of the green solvent water, short reaction time and good to moderate yields of the products.
Introduction
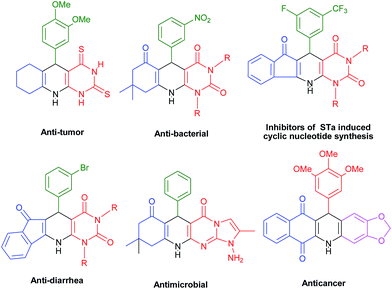
One-pot multicomponent reactions (MCRs) have emerged as an efficient tool for benign synthesis of functionalized heterocycles by virtue of their convergence, productivity, facile execution, and generation of highly diverse and complex products from easily available starting materials in a single operation.1 MCRs are very useful to access “privileged medicinal scaffolds”, especially, for synthesizing various N-heterocyclic compounds which are key constituents of a wide range of both natural and synthetic bioactive compounds.2,3 Microwave assisted multicomponent reactions have drawn remarkable attention from organic and medicinal chemists considering their green features. MW irradiation provides enhanced reaction rates, higher yields of products, better selectivity, rapid optimization of reactions and several ecofriendly advantages.4 Further, in comparison with organic solvents, water is a non-toxic, non-corrosive, non-explosive and is readily available solvent. These properties along with the network of hydrogen bonds, large surface tension, high polarity and high specific heat capacity make it both economical and environmentally friendly and thus suitable as a green solvent.5,6 According to the current synthetic requirements and from green perspective, environmentally benign multicomponent procedures employing microwave methodology in aqueous medium are particularly welcome. Polycyclic fused N-heterocycles have attracted much attention due to their presence in biologically active natural products and pharmaceuticals. They display a wide range of biological activities such as antifungal, antibacterial, antineoplastic, anticancer, antiplasmodial, and as DNA intercalators.7 The presence of several functional groups in one molecule often proves useful to find better bioactivities of compounds. Further, literature survey shows that fused polycyclic N-heterocycles containing naphthaquinone,8–10 1,4-dihydro pyridine11–13 and pyrimidine14 moieties are important in discovering new bioactive compounds due to their fascinating molecular structure and remarkable pharmacological efficiency. This class of building blocks are useful for treating Alzheimer's disease15 and also exhibit anti-tumor,16 antimicrobial,17 anti-diarrhea,18 and anti-cancer activities.19 Some of the pharmacologically active fused polycyclic N-heterocycles are shown in Fig. 1.6-Aminouracil is considered as a very popular and useful starting material for the synthesis of heterocyclic scaffolds using multicomponent reaction.20 Recently various research groups have explored 6-amino uracil in multicomponent reactions to construct fused heterocycles.21
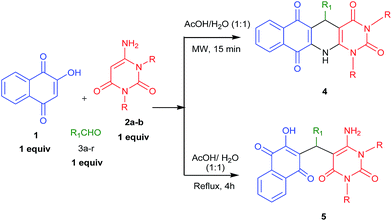
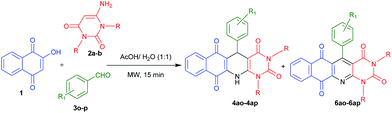
Considering the importance of fused polycyclic N-heterocycles having naphthaquinone, 1,4-DHP and pyrimidine moieties in pharmaceutical and chemical domains, and also as a part of our continuous effort on the synthesis of highly functionalized or fused heterocycles,22–24 we turned our attention to design a thee component reaction of 2-hydroxy-1,4-naphthaquinone (1), 6-aminouracils (2) and aldehydes (3). In acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under reflux conditions we ended with acyclic products 5 (Scheme 1). Interestingly, when we carried out the same reaction under microwaves in acetic acid/water (1
1) under reflux conditions we ended with acyclic products 5 (Scheme 1). Interestingly, when we carried out the same reaction under microwaves in acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
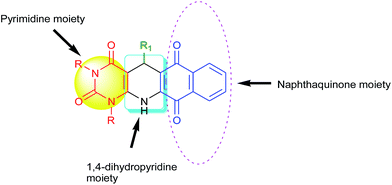
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), we ended with fused polycyclic N-heterocycles 4 (Scheme 1). It consists of three important bioactive moieties naphthaquinone, 1,4-dihydropyridine and pyrimidine (Fig. 2).
1), we ended with fused polycyclic N-heterocycles 4 (Scheme 1). It consists of three important bioactive moieties naphthaquinone, 1,4-dihydropyridine and pyrimidine (Fig. 2).
Results and discussion
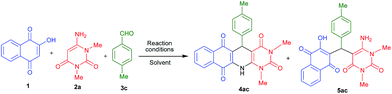
For the preliminary investigation, reaction of 2-hydroxy-1,4-naphthaquinone 1, 1,3-dimethyl-6-aminouracil 2a and 4-methyl benzaldehyde 3c was chosen as model reaction. In the presence of acetic acid under the reflux conditions, this combination provided 75% of acyclic product 5ac within 5 h (Table 1, entry 1) and we did not get our desired three component cyclic product 4ac under this reaction conditions. Next, we attempted to get the cyclized product by varying various parameters of the reaction, such as using microwave heating, solvent etc. Interestingly, the same model reaction provided 77% yield of the corresponding fused polycyclic N-heterocyclic product 4ac and 12% of acyclic product 5ac, after microwave heating at 130 °C for 15 minutes in acetic acid medium (Table 1, entry 2). The product 4ac was fully characterized by recording IR, 1H & 13C NMR as well as HRMS. Encouraged by this positive result, the same set of reaction was performed in various solvents under microwave heating and the results are summarized in Table 1 (entries 3–6). Interestingly, it has been observed that when the reaction was performed in the presence of protic solvents like AcOH, H2O, and EtOH it gives cyclic product 4ac as major and acyclic product 5ac as minor product (Table 1 entries 2–4). But when the reaction was performed in DMSO and PEG-400, only acyclic product 5ac was observed (Table 1, entries 5 and 6). To further investigate, we also performed the same model reaction using mixed solvent like acetic acid and other solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under reflux as well as microwave reaction conditions (Table 1, entries 7–12). Among them, acetic acid/water (1
1) under reflux as well as microwave reaction conditions (Table 1, entries 7–12). Among them, acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under microwave heating was found as optimum reaction conditions in terms of yield obtained (Table 1, entry 9).
1) under microwave heating was found as optimum reaction conditions in terms of yield obtained (Table 1, entry 9).
| Entry | Solvent | Reaction conditions | Yieldb (%) 4ac/5ac | Time (h/min) |
|---|---|---|---|---|
a Reactions were carried out in 1.0 mmol scale with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methyl benzaldehyde in 2 ml solvent.b Isolated yields. 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methyl benzaldehyde in 2 ml solvent.b Isolated yields. |
||||
| 1 | AcOH | Reflux | 0/75 | 5 h |
| 2 | AcOH | MW | 77/12 | 15 min |
| 3 | H2O | MW | 66/15 | 15 min |
| 4 | EtOH | MW | 51/12 | 15 min |
| 5 | DMSO | MW | 0/53 | 15 min |
| 6 | PEG-400 | MW | 0/72 | 15 min |
| 7 | AcOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reflux | 0/80 | 5 h |
| 8 | AcOH/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MW | 76/15 | 15 min |
| 9 | AcOH/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MW | 90/8 | 15 min |
| 10 | AcOH/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MW | 56/21 | 15 min |
| 11 | AcOH/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MW | 68/14 | 15 min |
| 12 | AcOH/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MW | 48/40 | 15 min |
In order to explore the scope of this multicomponent reaction, a wide variety of aldehydes 3a–r were reacted with 2-hydroxy-1,4-naphthaquinone 1 and aminouracil derivatives 2a–b under the optimized reaction conditions and the results are summarized in Table 2. It is notable that the characteristics of 2a–b and 3a–r had an important influence on the final products. In most of the cases when 2a was employed, cyclic product 4 was obtained as major and acyclic product 5 as minor product (Table 2, entries 1–7 and 9). However, in the cases of 2-chlorobenzaldehyde 3h, naphthaldehyde 3j and 2-methoxybenzaldehyde 3k, we obtained exclusively cyclic product and no acyclic product (Table 2, entries 8, 10 and 11) was observed. Further, when 6-aminouracil 2b was tested with 1 and different substituted aldehydes 3(a, d–e, g–i, k–o) only cyclic products were observed (Table 2, entries 12–22). Next we examined some aliphatic aldehydes such as cyclohexyl carboxaldehyde 3q and butyraldehyde 3r. Unfortunately, they provided acyclic product only (Table 2, entries 23 and 24). However, when 4-cyanobenzaldehyde 3o and 3-nitrobenzaldehyde 3p were used, we obtained 6ao and 6ap along with 4ao and 4ap (Table 3, entries 1 and 2) respectively.
| Entry | R | R1 | % yieldb of 4 | % yieldb of 5c | ||
|---|---|---|---|---|---|---|
a Reactions were carried out in 1.0 mmol scale with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methylbenzaldehyde in acetic acid/water (1 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methylbenzaldehyde in acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in microwave.b Isolated yields.c For full characterization of compounds 5, please see ref. 22. 1) in microwave.b Isolated yields.c For full characterization of compounds 5, please see ref. 22. |
||||||
| 1 | CH3 | C6H5 | 4aa | 79 | 5aa | 13 |
| 2 | CH3 | 4-ClC6H4 | 4ab | 87 | 5ab | 10 |
| 3 | CH3 | 4-CH3C6H4 | 4ac | 90 | 5ac | 8 |
| 4 | CH3 | 4-OCH3C6H4 | 4ad | 85 | 5ad | 10 |
| 5 | CH3 | 4-CH(CH3)2C6H4 | 4ae | 82 | 5ae | 12 |
| 6 | CH3 | 4-NO2C6H4 | 4af | 64 | 5af | 32 |
| 7 | CH3 | 4-FC6H4 | 4ag | 71 | 5ag | 25 |
| 8 | CH3 | 2-ClC6H4 | 4ah | 90 | 5ah | — |
| 9 | CH3 | 3-BrC6H4 | 4ai | 78 | 5ai | 18 |
| 10 | CH3 | Naphthyl | 4aj | 85 | 5aj | — |
| 11 | CH3 | 2-OCH3C6H4 | 4ak | 92 | 5ak | — |
| 12 | H | C6H5 | 4ba | 89 | 5ba | — |
| 13 | H | 4-OCH3C6H4 | 4bd | 91 | 5bd | — |
| 14 | H | 4-CH(CH3)2C6H4 | 4be | 87 | 5be | — |
| 15 | H | 4-FC6H4 | 4bg | 89 | 5bg | — |
| 16 | H | 2-ClC6H4 | 4bh | 87 | 5bh | — |
| 17 | H | 3-BrC6H4 | 4bi | 92 | 5bi | — |
| 18 | H | 2-OCH3C6H4 | 4bk | 96 | 5bk | — |
| 19 | H | 2-CH3C6H4 | 4bl | 93 | 5bl | — |
| 20 | H | 2,6-DiCH3C6H3 | 4bm | 87 | 5am | — |
| 21 | H | 4-BrC6H4 | 4bn | 87 | 5bn | — |
| 22 | H | 4-CNC6H4 | 4bo | 78 | 5bo | — |
| 23 | CH3 | Cyclohexyl | 4aq | — | 5aq | 81 |
| 24 | CH3 | Butyl | 4ar | — | 5ar | 79 |
| Entry | R | R1 | % yieldb of 4 | % yieldb of 6 | ||
|---|---|---|---|---|---|---|
a Reactions were carried out with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methylbenzaldehyde in acetic acid/water (1 1 ratio of 2-hydroxy-1,4-naphthaquinone, 1,3-dimethyl-6-aminouracil and 4-methylbenzaldehyde in acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under microwave irradiation.b Isolated yields. 1) under microwave irradiation.b Isolated yields. |
||||||
| 1 | CH3 | 4-CNC6H4 | 4ao | 38 | 6ao | 52 |
| 2 | CH3 | 3-NO2C6H4 | 4ap | 35 | 6ap | 58 |
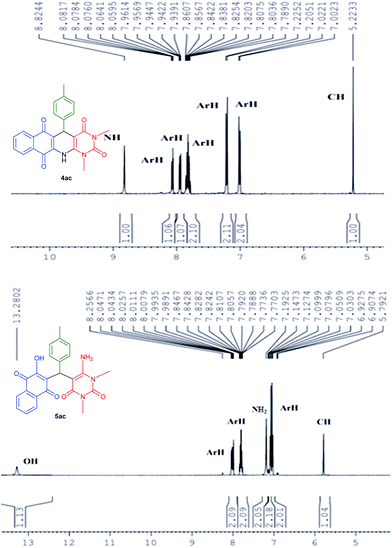
All the products were fully characterized by IR, 1H NMR, 13C NMR spectroscopy as well as HRMS. The formation of 4 and 5 were ascertained from NMR spectroscopy with compound 4ac and 5ac (Fig. 3). As a representative case, 1H NMR of 4ac was interpreted by the presence of a singlet at 8.82 for –NH proton, 8.08–7.00 ppm for eight Ar-H protons, and singlet at 5.22 for CH proton. While, 1H NMR of 5ac was characterized by the presence of a singlet at 13.28 for –OH proton, 8.26–7.77 and 7.15–6.90 ppm for eight Ar-H proton and two singlets at 7.19 and 5.79 for –NH2 and –CH protons respectively. From this it is clear that the product 4ac is the fused polycyclic N-heterocycle whereas the acyclic product is 5ac where free –NH2 and –OH groups are present.
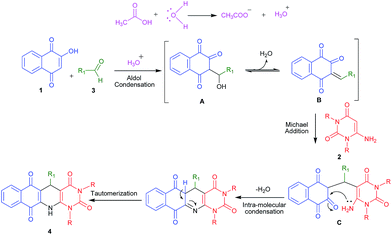
The formation of product 4, can be explained by the proposed mechanism, as shown in Scheme 2. The reaction is initiated by a acetic acid assisted aldol condensation to provide A which transforms to B after elimination of water molecule. Then B reacts with 6-aminouracil derivatives 2a–b in a Michael-type fashion and gave C which undergoes intramolecular condensation followed by tautomerization to give the corresponding product (4).
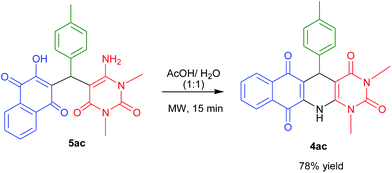
Next, we attempted to convert acyclic product which we obtained under reflux conditions to the corresponding cyclic products. For that we have treated the acyclic product 5ac in acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) under MW heating for 15 min and the corresponding cyclic product 4ac was obtained in 78% yield (Scheme 3).
1) under MW heating for 15 min and the corresponding cyclic product 4ac was obtained in 78% yield (Scheme 3).
Finally, we explored cinnamaldehyde an α,β-unsaturated aldehyde in this three component reaction under the similar reaction conditions. To our surprise, this aldehyde did not provide expected acyclic product 5as or cyclic product 4as, instead of these a novel unexpected cyclic product 7as was obtained as shown in Scheme 4.
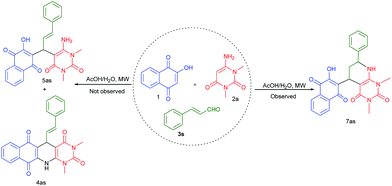 | ||
| Scheme 4 Synthesis of unexpected product 7as from the reaction of cinnamaldehyde, 2-hydroxy-1,4-naphthaquinone and 2a. | ||
Conclusions
In conclusion, we have demonstrated the effects of reaction conditions on the formation of two types of products from the three-component reaction of 2-hydroxy-1,4-naphthaquinone, aldehydes and 6-aminouracils. This method is a green tool for the synthesis of fused polycyclic N-heterocycles in acetic acid/water under microwave heating. The main advantages of this method are (i) easy purification process of the products avoiding column chromatographic purification, (ii) high atom economy of the reaction (iii) use of water as solvent, (iv) short reaction time, (v) good yields of the products and (vi) environmentally benign procedures. Considering the presence of naphthaquinone and pyrimidine moiety fused with 1,4-DHPs, it is expected that these products will exhibit promising bioactivities.Acknowledgements
We are grateful to NIT Patna and IIT Patna for providing general research facilities to carryout this work. T. P. is thankful to SERB-DST, India for financial support with Sanction No. EMR/2016/000960. The authors are also thankful to SAIF-Panjab University and SAIF-IIT Patna for providing the analytical facilities for characterization of products.Notes and references
- (a) I. Ugi, Pure Appl. Chem., 2001, 73, 187 CrossRef CAS; (b) D. J. Ramon and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602 CrossRef CAS PubMed; (c) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed; (d) E. Ruijter and R. V. A. Orru, in Multicomponent Reactions in Organic Synthesis, ed. J. Zhu, Q. Wang and M.-X. Wang, Wiley-VCH, Weinheim, 2014, ch. 2, p. 13 Search PubMed; (e) V. Nair, C. Rajesh, A. Vinod, U. S. Bindu, A. R. Streekenth, J. S. Mathen and L. Balagopal, Acc. Chem. Res., 2003, 36, 899 CrossRef CAS PubMed.
- (a) J. Gerencser, G. Dorman and F. Darvas, QSAR Comb. Sci., 2006, 25, 439 CrossRef CAS; (b) R. V. A. Orru and M. de Greef, Synthesis, 2003, 10, 1471 CrossRef; (c) C. Hulme and V. Gore, Curr. Med. Chem., 2003, 10, 51 CrossRef CAS PubMed; (d) B. E. Evans, K. E. Rittle, M. G. Bock, R. M. DiPardo, R. M Freidinger, W. L. Whitter, G. F. Lundell, D. F. Veber, P. S. Anderson, R. S. L. Chang, V. J. Lotti, D. J. Cerino, T. B. Chen, P. J. Kling, K. A. Kunkel, J. P. Springer and J. Hirshfield, J. Med. Chem., 1988, 31, 2235 CrossRef CAS PubMed.
- D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435 RSC.
- (a) S. Caddick and R. Fitzmaurice, Tetrahedron, 2009, 65, 3325 CrossRef CAS; (b) C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250 CrossRef CAS PubMed; (c) D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563 CrossRef CAS PubMed; (d) W. S. Chow and T. H. Chan, Tetrahedron Lett., 2009, 50, 1286 CrossRef CAS.
- (a) P. A. Grieco, Organic Synthesis In Water, Thomson Science, London, 1998, pp. 1–278 Search PubMed; (b) R. Breslow and U. Maitra, Tetrahedron Lett., 1984, 25, 1239 CrossRef CAS; (c) D. C. Rideout and R. Breslow, J. Am. Chem. Soc., 1980, 102, 7816 CrossRef CAS; (d) S. Narayan, J. Muldoon, M. G. Finn, V. V. Fokin, H. C. Kolb and K. B. Sharpless, Angew. Chem., Int. Ed., 2005, 44, 3275 CrossRef CAS PubMed.
- B. Sharifzadeh, N. O. Mahmoodi, M. Mamaghani, K. Tabatabaeian, A. S. Chirani and I. Nikokar, Bioorg. Med. Chem. Lett., 2013, 23, 548 CrossRef CAS PubMed.
- (a) A. R. Katritzky, D. O. Tymoshenko, D. Monteux, V. Vvedensky, G. Nikonov, C. B. Cooper and M. Deshpande, J. Org. Chem., 2000, 65, 8059 CrossRef CAS PubMed; (b) B. E. Maryanoff, D. F. McComsey, W. Ho, R. P. Shank and B. Dubinsky, Bioorg. Med. Chem. Lett., 1996, 6, 333 CrossRef CAS; (c) A. B. Reitz, D. A. Gauthier, W. Ho and B. E. Maryanoff, Tetrahedron, 2000, 56, 8809 CrossRef CAS; (d) S. M. Rida, F. S. G. Soliman, E. Badawey and T. Kappe, J. Heterocycl. Chem., 1988, 25, 1725 CrossRef CAS; (e) S. M. Rida, F. S. G. Soliman, E. Badawey, E. El-Ghazzawi, O. Kader and T. Kappe, J. Heterocycl. Chem., 1988, 25, 1087 CrossRef CAS; (f) E. Badawey and T. Kappe, Eur. J. Med. Chem., 1995, 30, 327 CrossRef CAS; (g) S. A. M. El-Hawash, E. Badawey and T. Kappe, Pharmazie, 1999, 54, 341 CAS.
- (a) C. Ibis, A. F. Tuyun, H. Bahar, S. Sahinler Ayla, M. V. Stasevych, R. Y. Musyanovych, O. Komarovska-Porokhnyavets and V. P. Novikov, Med. Chem. Res., 2013, 22, 2879 CrossRef CAS; (b) C. Ibis, A. F. Tuyun, H. Bahar, S. Sahinler Ayla, M. V. Stasevych, R. Y. Musyanovych, O. Komarovska-Porokhnyavets and V. P. Novikov, Med. Chem. Res., 2014, 23, 2140 CrossRef CAS.
- (a) Y. M. Kanaan, J. R. Das, O. Bakare, N. M. Enwerem, S. Berhe, D. Beyene, V. Williams, Y. Zhou and R. L. Copeland Jr, Anticancer Res., 2009, 1, 191 Search PubMed; (b) N. M. Rahmoun, Z. Boucherit-Atmani, M. M. Benabdallah, K. Boucherit, D. Villemin and N. Choukchou-Braham, Am. J. Med. Biol. Res., 2013, 1, 16 CrossRef CAS.
- T. Kayashima, M. Mori, H. Yoshida, Y. Mizushina and K. Matsubara, Cancer Lett., 2009, 278, 34 CrossRef CAS PubMed.
- (a) H. Z. Si, T. Wang, K. J. Zhang, Z. D. Hu and B. T. Fan, Bioorg. Med. Chem., 2006, 14, 4834 CrossRef CAS PubMed; (b) S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754 CrossRef CAS; (c) T. Yamamoto, S. Niwa, M. Tokumasu, T. Onishi, S. Ohno, M. Hagihara, H. Koganei, S. Fujita, T. Takeda, Y. Saitou, S. Iwayama, A. Takahara, S. Iwata and M. Shoji, Bioorg. Med. Chem. Lett., 2012, 22, 3639 CrossRef CAS PubMed.
- (a) A. M. Vijesh, A. M. Isloor, S. K. Peethambar, K. N. Shivananda, T. Arulmoli and N. A. Isloor, Eur. J. Med. Chem., 2011, 46, 5591 CrossRef CAS PubMed; (b) G. Sabitha, G. S. K. K. Reddy, C. S. Reddy and J. S. Yadav, Tetrahedron Lett., 2003, 44, 4129 CrossRef CAS; (c) N. Tewari, N. Dwivedi and R. P. Tripathi, Tetrahedron Lett., 2004, 45, 9011 CrossRef CAS; (d) A. R. Trivedi, D. K. Dodiya, B. H. Dholariya, V. B. Kataria, V. R. Bhuva and V. H. Shah, Bioorg. Med. Chem. Lett., 2011, 21, 5181 CrossRef CAS PubMed.
- (a) B. Desai, D. Sureja, Y. Naliapara, A. Shah and A. Saxena, Bioorg. Med. Chem., 2001, 9, 1993 CrossRef CAS PubMed; (b) P. S. Kharkar, B. Desai, H. Gaveria, B. Varu, R. Loriya, Y. Naliapara, A. Shah and V. M. Kulkarni, J. Med. Chem., 2002, 45, 4858 CrossRef CAS PubMed; (c) B. R. Prashantha, M. Kumar, E. Pankaj, A. Karthikeyan, S. Bansal and P. Vijayan, Med. Chem. Res., 2010, 19, 344 CrossRef.
- (a) N. Fujiwara, T. Nakajima, Y. Ueda, H. K. Fujita and H. Awakami, Bioorg. Med. Chem., 2008, 16, 9804 CrossRef CAS PubMed; (b) L. Ballell, R. A. Field, G. A. C. Chung and R. J. Young, Bioorg. Med. Chem. Lett., 2007, 17, 1736 CrossRef CAS PubMed; (c) E. Wagner, K. Al-Kadasi, M. Zimecki and W. Sawka-Dobrowolska, Eur. J. Med. Chem., 2008, 43, 2498 CrossRef CAS PubMed.
- (a) R. Leon, C. Rios, J. Maro-Contelles, O. Huertas, X. Barril, F. J. Luque, M. G. Lopez, A. G. Garcia and M. Villarroya, Bioorg. Med. Chem., 2008, 16, 7759 CrossRef CAS PubMed; (b) J. Marco-Contelles, R. Leon, C. Rios, A. Samadi, V. Andrisano, O. Huertas, X. Barril, F. J. Luque, M. I. Rodriguez-Franco, B. Lopez, M. G. Lopez, A. G. Garcia, M. C. Carreiras and M. Villarroya, J. Med. Chem., 2009, 52, 2724 CrossRef CAS PubMed.
- M. B. El-Ashmawy, M. A. El-Sherbeny and N. S. El-Gohary, Med. Chem. Res., 2013, 22, 2724 CrossRef CAS.
- H.-A. S. Abbas, H. N. Hafez and A.-R. B. A. El-Gazzar, Eur. J. Med. Chem., 2011, 46, 21 CrossRef CAS PubMed.
- (a) A. Y. Kots, B.-K. Choi, M. E. Estrella-Jimenez, C. A. Warren, S. R. Gilbertson, R. L. Guerrant and F. Murad, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 8440 CrossRef CAS PubMed; (b) E. A. Tanifum, A. Y. Kots, B.-K. Choi, F. Murad and S. R. Gibertson, Bioorg. Med. Chem. Lett., 2009, 19, 3067 CrossRef CAS PubMed; (c) A. A. Toropov, A. P. Toropova, E. Benfenati, G. Gini, D. Leszczynska, J. Leszczynski and G. De Nucci, Biochem. Biophys. Res. Commun., 2013, 432, 214 CrossRef CAS PubMed.
- (a) V. K. Tandon and S. Kumar, Expert Opin. Ther. Pat., 2013, 23, 1087 CrossRef CAS PubMed; (b) S. G. McNaughton, B. A. Estevez, A. S. Jimenez, R. A. D. Gutierrez, P. L. Fernandez, C. Diaz and N. Bonifacio, WO 2014016314 A1 20140130, PCT Int. Appl. 2014.
- G. M. Ziarani, N. H. Nasab and N. Lashgari, RSC Adv., 2016, 6, 38827 RSC.
- (a) I. R. Siddiqui, P. Rai, Rahila, H. Sagir and P. Sing, RSC Adv., 2015, 5, 27603 RSC; (b) J. Azizian, A. S. Delbari and K. Yadollahzadeh, Synth. Commun., 2014, 44, 3277 CrossRef CAS; (c) B.-X. Du, B. Zhao, G. Cai, Y.-L. Li and X.-S. Wang, J. Chem. Res., 2012, 36, 453 CrossRef CAS.
- R. Bharti and T. Parvin, RSC Adv., 2015, 5, 66833 RSC.
- (a) R. Bharti and T. Parvin, Mol. Diversity, 2016, 20, 867 CrossRef CAS PubMed; (b) R. Bharti and T. Parvin, Synth. Commun., 2015, 45, 1442 CrossRef CAS; (c) R. Bharti and T. Parvin, J. Heterocycl. Chem., 2015, 52, 1806 CrossRef CAS.
- (a) S. Karamthulla, S. Pal, T. Parvin and L. H. Choudhury, RSC Adv., 2014, 4, 15319 RSC; (b) S. Pal, T. Parvin and L. H. Choudhury, Mol. Diversity, 2012, 16, 129 CrossRef CAS PubMed; (c) A. T. Khan, T. Parvin and L. H. Choudhury, J. Org. Chem., 2008, 73, 8398 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedure, characterization data, with copies of 1H, 13C NMR spectrum. See DOI: 10.1039/c6ra18828a |
| This journal is © The Royal Society of Chemistry 2017 |