 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Assessment of certain ionic liquids for separation of binary mixtures based on gamma infinity data measurements†
M. Karpińskaa,
M. Wlazłoa,
D. Ramjugernathb,
P. Naidoob and
U. Domańska*ab
aDepartment of Physical Chemistry, Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664 Warsaw, Poland. E-mail: ula@ch.pw.edu.pl; Fax: +48 22 6282741; Tel: +48 22 6213115
bThermodynamic Research Unit, School of Chemical Engineering, University of KwaZulu-Natal, Howard College Campus, King George V Avenue, Durban 4001, South Africa
First published on 20th January 2017
Abstract
The effect of interactions between organic solvents or water on the interfacial and bulk properties of 1-benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] and 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, [BzMIM][NTf2] were determined via measurement of activity coefficients γ∞13 at infinite dilution for 64 solutes. The data were obtained using the gas–liquid chromatography technique. Measurements were undertaken at six temperatures, in 10 K intervals, in the range of 318.15 to 368.15 K. The solutes studied included both non-polar and polar compounds, such as alkanes, alkenes, and alkynes, as well as aromatic hydrocarbons, alcohols, water, ethers, ketones, acetonitrile, pyridine, 1-nitropropane, thiophene, and esters. Density, ρ, and viscosity η, measurements for a range of temperatures, T for the chosen ionic liquids (ILs), [BzMIM][DCA] and [BzMIM][NTf2] were also undertaken at pressure, p = 101 kPa. The gas–liquid partition coefficients, KL at infinite dilution, and the fundamental thermodynamic functions, partial molar excess Gibbs energy, enthalpy and entropy at infinite dilution were calculated from the experimental data measurements. The values of selectivity and capacity for three separation cases, viz. hexane/hex-1-ene, cyclohexane/cyclohexene, and ethylbenzene/styrene were calculated from γ∞13 values and compared to literature for imidazolium-based or dicyanamide-based, or bis{(trifluoromethyl)sulfonyl}imide-based ionic liquids (ILs). The results from the study indicate that [BzMIM][DCA] has large selectivity values for all three of the separation cases studied.
Introduction
Ionic liquids (ILs) are nonvolatile salts with some rather unusual properties, such as high thermal stability, non-flammability, and high solvation properties. They have been widely studied, and have been proposed as potential replacement solvents for conventional organic solvents1–5 in separation processes. Examples of studies considering ILs for separation include extraction of aromatic hydrocarbons from alkanes,5 desulphurization and denitrification of diesel oil,6–8 and extraction of butan-1-ol from water.9Among the many potential separation applications, one of the most relevant is the employment of ILs as extractants for separation of alkenes from alkanes. Alkenes are particularly useful as substrates for the production of polymers and petrochemicals. The conventional separation technique for the alkane/alkene system is by distillation. Distillation processes can be extremely energy-intensive and technologically complex. Other separation methods, such as membranes technology or adsorption processes generally achieve a poor separation selectivity. Over the last decade, the adsorption of alkenes onto carbon, or zeolite A, or zeolite imidazolate framework ZIF-7, or metal–organic frameworks (MOFs) instant zeolites have been proposed as feasible, economical, and green alternatives to distillation.10–12
Preliminary information about suitable solvents for separation can be obtained from activity coefficients measurements at infinite dilution (γ∞13) by the gas–liquid chromatography (GLC) technique. Solvent suitability can also be determined from liquid–liquid phase equilibrium measurements in ternary systems. The information obtained from infinite dilution activity coefficients and liquid–liquid equilibrium measurement is generally the first step in the engineering design process to determine the most suitable extraction and separation processes. The use of predictive models, such as Mo UNIFAC, PC SAFT, or COSMO RS are to date not accurate and reliable enough for the design of separation processes and restricted to the description of simple systems only. Thus, the importance and need for experimental data. In this study we propose to estimate the solvent separation ability for some ILs by an analysis of their selectivities and capacities, calculated from γ∞13 values.
The present work is focused on new attractive ILs, namely – 1-benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] and 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, [BzMIM][NTf2]. In recent years ILs have been widely promoted as solvents for alkane/alkene separations.13–16 The study of alkane/alkene separations using ILs reported in the literature deal mostly with short chain hydrocarbons. The imidazolium-based ILs were proposed for the propane/prop-1-ene13 and the hexane/hex-1-ene separations.14 Usually, an increase of the substituent size leads to a decrease of the separation factor and to an increase in the capacity.13,14
Recently, γ∞13 data were measured by us for 1-butyl-3-methylimidazolium dicyanamide, [BMIM][DCA],15 and 1-allyl-3-methylimidazolium dicyanamide, [AMIM][DCA].16 These measurements revealed a very promising selectivity and capacity for the hexane/hex-1-ene, cyclohexane/cyclohexene, and ethylbenzene/styrene seperation processes.
The comparison of different ILs, applied for the alkane/alkene separation may be performed from the data reported in the review of Domańska et al.15 Interestingly, high selectivity for the hexane/hex-1-ene separation were obtained for the ILs containing CN– groups substituted on the cation or anion. High selectivitities whereas observed for 1-ethyl-3-methylimidazolium dicyanamide, [EMIM][DCA],17 1-(3-cyano-propyl)-3-methyl-imidazolium bis{(trifluoromethyl)sulfonyl}imide, [CN–C3MIM][NTf2],18 and 1-(3-cyano-propyl)2,4-dimethyl-imidazolium dicyanamide, [CN–C3MIM][DCA].18 The ILs with dicyanamide, or thricyanomethanide anions were also found to be very good for extraction of thiophene, or pyridine from aliphatic hydrocarbons.6,8,19 As an example, [AMIM][NTf2] has a selectivity (S∞12 = γ∞13/γ∞23 = 1.94),20 and [AMIM][DCA] has a selectivity (S∞12 = γ∞13/γ∞23 = 2.51)16 at T = 328.15 K in the hexane/hex-1-ene separation process. The bis{(trifluoromethyl)sulfonyl}imide, [NTf2]-based ILs are appropriate alternatives for apolar molecular liquids and are widely proposed as new entrainers for many processes.21
In this work we continue our investigations on the measurements of γ∞13 and an analysis of the obtained selectivities and capacities for hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene seperation processes. This work proposes to study a particular type of ILs, 1-benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] and 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, [BzMIM][NTf2] as a function of temperature at ambient pressure to compare the results with data previously measured by us for [AMIM][DCA] and [AMIM][NTf2]. According to many sources in literature, the high interaction of aromatic compounds, here of ethylbenzene with the IL is caused by π–π interactions between the cation with the benzyl group and aromatics. Because of this, we have selected cations with the benzyl group. In this paper we report the activity coefficients γ∞13 at infinite dilution, the gas–liquid partition coefficients KL and thermodynamic functions at infinite dilution for all measured solutes. The thermodynamic properties obtained are analyzed with regard to intermolecular interactions. A physico-chemical characterization of the ILs was also performed by measuring their thermal properties with DSC, as well as densities and viscosities as a function of temperature.
Materials and methods
Materials
The ILs were purchased from Io-Li-Tec (Ionic Liquids Technologies, GmbH, Heilbronn, Germany), 1-benzyl-3-methylimidazolium dicyanamide, purity > 0.98 mass fraction; CAS: 958445-60-8, and 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, purity > 0.99 mass fraction; CAS: 433337-24-7. The names, abbreviation of names, structures, molar masses, glass transition temperatures (Tg) and heat capacity, (Cp(g)) values at glass transition are listed in Table 1. The different solutes, purchased from Aldrich or Fluka, had purities better than 0.99 mass fraction and were used without further purification due to the fact that the GLC technique separates any impurities on the column. The specification and purity is shown in Table 1S in the ESI.†| a Standard uncertainties u are as follows; u(Tg) = ±0.1 K; u(ΔCp(g)) = ±5 J mol−1 K−1, u(p) = ±1 kPa.b A midpoint Tg/K = 195.4 with ΔCp(g)/J mol−1 K−1 = 214 in ref. 22.c A midpoint Tg/K = 217 with ΔCp(g)/J mol−1 K−1 = 86.1 in ref. 23.d A midpoint Tg/K = 215.8 in ref. 24; Tg/K= 215.8 K in ref. 25; Tg/K= 210.8 K with jump ΔCp(g)/J mol−1 K−1 = 197 in ref. 22. | ||
|---|---|---|
| Name, abbreviation | 1-Benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] | 1-Benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, [BzMIM][NTf2] |
| Structure | 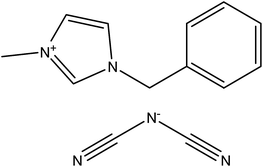 |
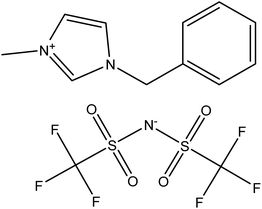 |
| M/g mol−1 | 239.28 | 453.38 |
| Tg/K | 217.0b | 216.9c,d |
| ΔCp(g)/J mol−1 K−1 | 169.9b | 199.5c,d |
Water content
The water content of the solvents was analyzed by the Karl-Fischer titration technique (method TitroLine KF). The sample of IL, or solvent was dissolved in methanol and titrated in steps of 0.0025 cm3. The uncertainty on the water content was u(w.c.) = 10 ppm for the 3 cm3 sample of IL injected. The water content in ILs was 200 ppm and 320 ppm for [BzMIM][DCA] and [BzMIM][NTf2], respectively.Differential scanning calorimetry, DSC
The basic thermal characteristics of ILs, i.e. temperature of glass transition and heat capacity at glass transition temperature (Tg, Cp(g)) were measured using a differential scanning microcalorimetry technique (DSC). The applied scan rate was 5 K min−1, with power and recorder sensitivities of 16 mJ s−1 and 5 mV, respectively. The apparatus (DSC 1 STAReSystem from Mettler Toledo with Liquid Nitrogen Cooling System) was calibrated with a 0.999999 mol fraction purity indium sample. The repeatability of the glass transition temperature value was ±0.1 K. The uncertainty of the glass temperature was u(Tg) = 0.1 K, and that of the heat capacity at glass transition u(Cp(g)) = 5 J mol−1 K−1. The DSC diagrams of the ILs are shown as Fig. 1Sa and b in the ESI† and the final results together with the literature data are listed in Table 1.22–25Density measurements
The density of the ILs was measured using an Anton Paar GmbH 4500 vibrating-tube densimeter (Graz, Austria), thermostated over a temperature range of (298.15–368.15) K. Two integrated Pt-100 platinum thermometers provided good precision in temperature control internally (T ±0.01 K). The densimeter has an automatic correction for the viscosity of the sample. The apparatus is precise to within 1 × 10−5 g cm−3, and the uncertainty of the measurements was estimated to be u(ρ) = ±1.1 × 10−3 g cm−3. The densimeter's calibration was performed at atmospheric pressure using doubly distilled and degassed water (PURE LAB Option Q Elga Water System), specially purified benzene (CHEMIPAN, Poland 0.999), and dried air. The densities of ILs are listed in Table 2.23–29| T/K | [BzMIM][DCA] | [BzMIM][NTf2] | ||
|---|---|---|---|---|
| ρ/g cm−3 | η/mPa s | ρ/g cm−3 | η/mPa s | |
| a Standard uncertainties u are u(T) = ±0.1 K, u(ρ) = ±1.1 × 10−3 g cm−3, u(η) = ±0.15%, u(p) = ±1 kPa.b ρ/g cm−3 = 1.491 in ref. 23; 1.489 in ref. 24; 1.504 in ref. 25; 1.42 in ref. 26; at T = 293.15 K; 1.151 at T = 293.15 K in ref. 27; 1.491 (room temperature, 25 °C) in ref. 28; 1.441 at T = 295 K in ref. 29.c η/mPa s = 113 in ref. 23, 125 in ref. 24; 61 in ref. 26; 190 at T = 293.15 K; 160 at T = 293.2 K in ref. 27; 150.8 (room temperature, 25 °C) in ref. 28; 40 (room temperature) in ref. 22.d ρ/g cm−3 = 1.458 in ref. 23. | ||||
| 298.15 | 1.15814 | 102 | 1.49098b | 133c |
| 303.15 | 1.15480 | — | 1.48609 | — |
| 308.15 | 1.15147 | 56.1 | 1.48124 | 74.9 |
| 313.15 | 1.14816 | — | 1.47644 | — |
| 318.15 | 1.14488 | 34.5 | 1.47167 | 46.2 |
| 323.15 | 1.14163 | — | 1.46693d | — |
| 328.15 | 1.13839 | 23.2 | 1.46222 | 30.9 |
| 333.15 | 1.13517 | — | 1.45756 | — |
| 338.15 | 1.13183 | 16.5 | 1.45295 | 21.9 |
| 343.15 | 1.12869 | — | 1.44837 | — |
| 348.15 | 1.12528 | 12.3 | 1.44381 | 16.2 |
| 353.15 | 1.12201 | — | 1.43917 | — |
| 358.15 | 1.11876 | 9.56 | 1.43470 | 12.4 |
| 363.15 | 1.11544 | — | 1.43013 | — |
| 368.15 | 1.11211 | 7.61 | 1.42554 | 9.74 |
Viscosity measurements
Viscosity measurements were carried out on an Anton Paar GmbH AMVn (Graz, Austria) programmable rheometer, with a nominal uncertainty of ±0.15% and reproducibility < 0.05% for viscosities from 7.6 mPa s to 140 mPa s. Temperature was controlled internally with a precision of ± 0.01 K in a range from (298.15 to 368.15) K. The diameter of the capillary was 1.8 mm and 3 mm for viscosities from (2.5 to 70) mPa s, and (20 to 230) mPa s, respectively. The diameter of the balls were 1.5 mm and 2.5 mm for the above viscosity ranges. The data obtained for the ILs are listed in Table 2.22–24,26–28Apparatus and experimental procedure
Experiments were performed using a Perkin Elmer Clarus 500 gas chromatograph equipped with a thermal conductivity detector (TCD). The data were collected and processed using the TotalChrom Workstation software. The column preparation and the packing method used in this work have been described in detail in our previous work.19,20 Glass columns of length 1 m, with a 4 mm internal diameter were used. The solid support Chromosorb W/AW-DCMS 100/120 mesh was supplied by Sigma-Aldrich. Coating of the solid support material with the IL was performed by dispersing a certain portion of the IL in methanol, followed by evaporation of the solvent using a rotary evaporator. The masses of the stationary phase and of the solid support were weighed with a precision ±0.0001 g, achieving an uncertainty in the IL loading on the column in the order of 2 × 10−4 mmol. The solvent loading on the column for 1-benzyl-3-methylimidazolium dicyanamide was 45.1% and 49.7% mass percent, and for 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide it was 45.0% and 50.7% mass percent. The large loading prevents possible residual adsorption of solute onto the column packing. Care was taken to ensure that the methanol was completely evaporated from the IL-coated solid support prior to column fabrication. Prior to each experiment, the column was conditioned by blowing hot carrier gas through it at a high flow rate (∼2.0 cm3 s−1) at 370 K for about 8 h. The pressure drop (Pi − Po) was varied between 60 and 80 kPa depending on the flow rate of the carrier gas. The inlet pressure, Pi, was measured by a pressure gauge installed on the gas chromatograph with an uncertainty of ±0.1 kPa and the outlet pressure, Po, was measured using an Agilent Precision Gas Flow Meter having an uncertainty of ±0.07 kPa. The mean column pressure,![[p with combining macron]](https://www.rsc.org/images/entities/i_char_0070_0304.gif) , inlet column pressure, Pi, outlet column pressure, Po and standard state of solutes at given temperatures and st. state are listed in Table 2S in the ESI.†
, inlet column pressure, Pi, outlet column pressure, Po and standard state of solutes at given temperatures and st. state are listed in Table 2S in the ESI.†
The carrier gas used was helium. The flow rate of carrier gas was determined using an Agilent precision gas flow meter which was placed at the outlet after the detector and had an uncertainty of ±0.1 ml min−1. The flow rate was set for a series of runs and was allowed to stabilize for at least 15 min before any γ∞13 determinations were made. Solute injections ranged from 0.01 to 0.3 μl and can be considered to be at “infinite dilution” on the column.
Temperature-dependent experiments were carried out in 10 K steps from (318.15 to 368.15) K. The temperature of the column was maintained constant to within ±0.02 K. At a given temperature, each experiment was repeated two to three times to establish reproducibility. Retention times were generally reproducible to within 10−3 to 10−2 min depending upon the temperature and the individual solute. At each temperature, values of the dead time, tG, equivalent to the retention time of a completely non-retained component were also measured. While our GC was equipped with a TCD detector, air was used as a non-retainable component. The estimated overall error in γ∞13 was less than 3%, taking into account the possible errors in determining the column loading, the retention times, and solute vapor pressure. The resultant activity coefficient values as a function of temperature are summarized in Tables 3 and 4 for [BzMIM][DCA] and [BzMIM][NTf2], respectively.
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 318.15 | 328.15 | 338.15 | 348.15 | 358.15 | 368.15 | |
| a Standard uncertainties u are u(γ∞13) = 3%, u(T) = 0.02 K. | ||||||
| Pentane | 71.4 | 65.0 | 59.5 | 54.7 | 50.5 | |
| Hexane | 105 | 95.4 | 87.8 | 81.0 | 75.1 | 70.2 |
| 3-Methylpentane | 95.0 | 86.1 | 78.6 | 72.2 | 66.5 | 61.7 |
| 2,2-Dimethylbutane | 115 | 101 | 88.8 | 78.9 | 70.4 | |
| Heptane | 159 | 144 | 132 | 121 | 111 | 103 |
| Octane | 245 | 221 | 201 | 184 | 169 | 156 |
| 2,2,4-Trimethylpentane | 216 | 193 | 176 | 160 | 147 | 135 |
| Nonane | 372 | 332 | 299 | 271 | 247 | 227 |
| Decane | 575 | 509 | 455 | 409 | 369 | 336 |
| Cyclopentane | 22.2 | 21.0 | 20.0 | 19.0 | 18.2 | 17.4 |
| Cyclohexane | 36.0 | 33.5 | 31.4 | 29.6 | 27.9 | 26.4 |
| Methylcyclohexane | 58.7 | 54.2 | 50.3 | 46.9 | 43.9 | 41.3 |
| Cycloheptane | 44.1 | 41.0 | 38.4 | 36.0 | 34.0 | 32.2 |
| Cyclooctane | 58.3 | 53.9 | 50.2 | 46.9 | 44.0 | 41.5 |
| Pent-1-ene | 25.8 | 24.7 | 23.7 | 22.9 | 22.1 | 21.4 |
| Hex-1-ene | 40.1 | 38.1 | 36.4 | 34.7 | 33.3 | 32.1 |
| Cyclohexene | 13.9 | 13.6 | 13.3 | 13.0 | 12.8 | 12.5 |
| Hept-1-ene | 62.1 | 58.8 | 56.0 | 53.3 | 51.0 | 49.0 |
| Oct-1-ene | 98.3 | 92.2 | 87.0 | 82.5 | 78.5 | 74.9 |
| Dec-1-ene | 238 | 220 | 205 | 191 | 179 | 168 |
| Pent-1-yne | 5.20 | 5.36 | 5.50 | 5.65 | 5.79 | 5.93 |
| Hex-1-yne | 7.97 | 8.12 | 8.28 | 8.40 | 8.56 | 8.69 |
| Hept-1-yne | 12.7 | 12.8 | 12.9 | 13.0 | 13.1 | 13.2 |
| Oct-1-yne | 20.3 | 20.2 | 20.2 | 20.1 | 20.1 | 20.0 |
| Benzene | 2.36 | 2.44 | 2.53 | 2.61 | 2.70 | 2.78 |
| Toluene | 3.91 | 4.05 | 4.19 | 4.31 | 4.44 | 4.56 |
| Ethylbenzene | 6.56 | 6.71 | 6.85 | 6.97 | 7.10 | 7.22 |
| o-Xylene | 5.35 | 5.51 | 5.65 | 5.80 | 5.94 | 6.06 |
| m-Xylene | 6.77 | 6.95 | 7.12 | 7.27 | 7.42 | 7.57 |
| p-Xylene | 6.60 | 6.76 | 6.93 | 7.08 | 7.25 | 7.39 |
| n-Propylbenzene | 10.9 | 11.0 | 11.2 | 11.3 | 11.4 | 11.6 |
| Iso-propylbenzene | 10.6 | 10.9 | 11.1 | 11.3 | 11.5 | 11.7 |
| Styrene | 2.97 | 3.11 | 3.23 | 3.36 | 3.49 | 3.62 |
| α-Methylstyrene | 4.77 | 5.03 | 5.29 | 5.54 | 5.80 | 6.06 |
| Thiophene | 1.37 | 1.44 | 1.51 | 1.58 | 1.64 | 1.71 |
| Pyridine | 0.989 | 1.03 | 1.08 | 1.12 | 1.16 | 1.21 |
| Methanol | 0.503 | 0.511 | 0.518 | 0.525 | 0.533 | 0.539 |
| Ethanol | 0.874 | 0.872 | 0.87 | 0.869 | 0.868 | 0.867 |
| Propan-1-ol | 1.17 | 1.16 | 1.16 | 1.15 | 1.15 | 1.15 |
| Propan-2-ol | 1.37 | 1.35 | 1.33 | 1.31 | 1.29 | 1.27 |
| Butan-1-ol | 1.68 | 1.66 | 1.64 | 1.62 | 1.60 | 1.59 |
| Butan-2-ol | 1.76 | 1.75 | 1.74 | 1.73 | 1.72 | 1.71 |
| 2-Methyl-1-propanol | 1.72 | 1.68 | 1.65 | 1.62 | 1.60 | 1.57 |
| tert-Butanol | 1.81 | 1.80 | 1.78 | 1.77 | 1.76 | 1.75 |
| 1-Pentanol | 2.26 | 2.24 | 2.23 | 2.21 | 2.20 | 2.19 |
| Water | 0.378 | 0.387 | 0.396 | 0.404 | 0.412 | 0.42 |
| Methyl acetate | 1.85 | 1.94 | 2.03 | 2.11 | 2.19 | 2.28 |
| Methyl propanoate | 2.77 | 2.90 | 3.03 | 3.14 | 3.26 | 3.38 |
| Methyl butanoate | 4.40 | 4.55 | 4.68 | 4.81 | 4.94 | 5.07 |
| Ethyl acetate | 3.11 | 3.22 | 3.34 | 3.45 | 3.56 | 3.67 |
| Tetrahydrofuran | 1.71 | 1.78 | 1.86 | 1.93 | 2.00 | 2.08 |
| 1,4-Dioxane | 1.09 | 1.16 | 1.23 | 1.30 | 1.37 | 1.45 |
| tert-Butyl methyl ether | 11.4 | 11.5 | 11.7 | 11.8 | 12.0 | 12.1 |
| tert-Butyl ethyl ether | 30.9 | 30.6 | 30.5 | 30.3 | 30.1 | 30.0 |
| tert-Amyl methyl ether | 16.9 | 17.0 | 17.1 | 17.2 | 17.3 | 17.3 |
| Diethyl ether | 9.88 | 9.94 | 9.99 | 10.0 | 10.1 | 10.1 |
| Di-n-propyl ether | 32.3 | 31.6 | 31.0 | 30.4 | 29.9 | 29.4 |
| Di-iso-propyl ether | 39.0 | 37.8 | 36.8 | 35.9 | 35.1 | 34.3 |
| Di-n-butyl ether | 81.2 | 77.2 | 73.8 | 70.8 | 68.0 | 65.5 |
| Acetone | 1.12 | 1.17 | 1.23 | 1.28 | 1.33 | 1.39 |
| Pentan-2-one | 2.50 | 2.59 | 2.70 | 2.79 | 2.89 | 2.98 |
| Pentan-3-one | 2.52 | 2.62 | 2.73 | 2.83 | 2.93 | 3.02 |
| Butanal | 1.96 | 2.04 | 2.13 | 2.21 | 2.29 | 2.36 |
| Acetonitrile | 0.879 | 0.903 | 0.927 | 0.952 | 0.974 | 0.996 |
| 1-Nitropropane | 1.53 | 1.58 | 1.63 | 1.68 | 1.73 | 1.77 |
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 318.15 | 328.15 | 338.15 | 348.15 | 358.15 | 368.15 | |
| a Standard uncertainties u are u(γ∞13) = 3%, u(T) = 0.02 K. | ||||||
| Pentane | 13.5 | 12.9 | 12.3 | 11.8 | 11.4 | 11.0 |
| Hexane | 18.4 | 17.6 | 16.8 | 16.1 | 15.5 | 14.9 |
| 3-Methylpentane | 16.1 | 15.4 | 14.9 | 14.4 | 13.9 | 13.5 |
| 2,2-Dimethylbutane | 15.8 | 15.1 | 14.6 | 14.1 | 13.6 | 13.2 |
| Heptane | 26.2 | 24.8 | 23.5 | 22.4 | 21.4 | 20.4 |
| Octane | 37.9 | 35.3 | 33.0 | 31.0 | 29.2 | 27.5 |
| 2,2,4-Trimethylpentane | 26.1 | 24.9 | 23.8 | 22.8 | 21.9 | 21.1 |
| Nonane | 53.2 | 49.3 | 45.9 | 42.9 | 40.2 | 37.8 |
| Decane | 77.3 | 70.7 | 65.0 | 60.1 | 55.7 | 51.9 |
| Cyclopentane | 6.82 | 6.61 | 6.43 | 6.28 | 6.11 | 5.98 |
| Cyclohexane | 9.85 | 9.39 | 9.01 | 8.64 | 8.33 | 8.01 |
| Methylcyclohexane | 13.6 | 13.0 | 12.5 | 12.0 | 11.6 | 11.2 |
| Cycloheptane | 12.7 | 12.1 | 11.6 | 11.1 | 10.7 | 10.3 |
| Cyclooctane | 16.5 | 15.6 | 14.8 | 14.0 | 13.3 | 12.7 |
| Pent-1-ene | 6.66 | 6.61 | 6.56 | 6.52 | 6.47 | 6.43 |
| Hex-1-ene | 9.53 | 9.27 | 9.05 | 8.85 | 8.65 | 8.49 |
| Cyclohexene | 5.40 | 5.31 | 5.25 | 5.18 | 5.11 | 5.05 |
| Hept-1-ene | 13.2 | 12.9 | 12.6 | 12.4 | 12.1 | 11.9 |
| Oct-1-ene | 19.3 | 18.6 | 17.9 | 17.2 | 16.7 | 16.2 |
| Dec-1-ene | 38.8 | 36.8 | 35.1 | 33.5 | 32.1 | 30.8 |
| Pent-1-yne | 2.38 | 2.46 | 2.53 | 2.60 | 2.67 | 2.74 |
| Hex-1-yne | 3.32 | 3.40 | 3.47 | 3.55 | 3.62 | 3.68 |
| Hept-1-yne | 4.69 | 4.76 | 4.83 | 4.90 | 4.96 | 5.02 |
| Oct-1-yne | 6.64 | 6.65 | 6.7 | 6.73 | 6.75 | 6.77 |
| Benzene | 0.999 | 1.05 | 1.10 | 1.14 | 1.19 | 1.23 |
| Toluene | 1.49 | 1.55 | 1.60 | 1.66 | 1.72 | 1.77 |
| Ethylbenzene | 2.19 | 2.26 | 2.33 | 2.40 | 2.48 | 2.54 |
| o-Xylene | 1.98 | 2.04 | 2.10 | 2.16 | 2.22 | 2.27 |
| m-Xylene | 2.20 | 2.28 | 2.37 | 2.45 | 2.53 | 2.61 |
| p-Xylene | 2.21 | 2.30 | 2.38 | 2.46 | 2.54 | 2.62 |
| n-Propylbenzene | 3.17 | 3.26 | 3.35 | 3.43 | 3.52 | 3.59 |
| Iso-propylbenzene | 3.11 | 3.19 | 3.27 | 3.35 | 3.43 | 3.51 |
| Styrene | 1.26 | 1.32 | 1.39 | 1.46 | 1.52 | 1.59 |
| α-Methylstyrene | 1.88 | 1.98 | 2.10 | 2.20 | 2.31 | 2.42 |
| Thiophene | 0.860 | 0.900 | 0.938 | 0.974 | 1.01 | 1.05 |
| Pyridine | 0.487 | 0.512 | 0.537 | 0.561 | 0.586 | 0.610 |
| Methanol | 1.34 | 1.28 | 1.22 | 1.16 | 1.12 | 1.07 |
| Ethanol | 1.71 | 1.61 | 1.52 | 1.45 | 1.37 | 1.31 |
| Propan-1-ol | 2.08 | 1.96 | 1.85 | 1.76 | 1.68 | 1.60 |
| Propan-2-ol | 1.96 | 1.82 | 1.69 | 1.58 | 1.49 | 1.40 |
| Butan-1-ol | 2.69 | 2.50 | 2.33 | 2.19 | 2.06 | 1.95 |
| Butan-2-ol | 2.30 | 2.15 | 2.03 | 1.91 | 1.81 | 1.72 |
| 2-Methyl-1-propanol | 2.64 | 2.44 | 2.25 | 2.09 | 1.95 | 1.83 |
| tert-Butanol | 1.91 | 1.81 | 1.73 | 1.65 | 1.59 | 1.53 |
| 1-Pentanol | 3.18 | 2.97 | 2.78 | 2.62 | 2.47 | 2.33 |
| Water | 3.27 | 2.97 | 2.72 | 2.50 | 2.32 | 2.15 |
| Methyl acetate | 0.603 | 0.638 | 0.673 | 0.707 | 0.742 | 0.776 |
| Methyl propanoate | 0.768 | 0.814 | 0.863 | 0.909 | 0.955 | 1.00 |
| Methyl butanoate | 1.09 | 1.14 | 1.19 | 1.24 | 1.29 | 1.34 |
| Ethyl acetate | 0.788 | 0.834 | 0.881 | 0.926 | 0.974 | 1.02 |
| Tetrahydrofuran | 0.610 | 0.648 | 0.684 | 0.721 | 0.758 | 0.794 |
| 1,4-Dioxane | 0.484 | 0.523 | 0.562 | 0.602 | 0.644 | 0.683 |
| tert-Butyl methyl ether | 2.30 | 2.41 | 2.52 | 2.62 | 2.72 | 2.83 |
| tert-Butyl ethyl ether | 4.97 | 5.11 | 5.26 | 5.40 | 5.54 | 5.67 |
| tert-Amyl methyl ether | 3.33 | 3.44 | 3.53 | 3.63 | 3.73 | 3.82 |
| Diethyl ether | 2.30 | 2.39 | 2.47 | 2.56 | 2.64 | 2.72 |
| Di-n-propyl ether | 5.93 | 5.99 | 6.04 | 6.08 | 6.13 | 6.19 |
| Di-iso-propyl ether | 5.68 | 5.79 | 5.88 | 5.98 | 6.08 | 6.18 |
| Di-n-butyl ether | 12.5 | 12.2 | 11.9 | 11.7 | 11.5 | 11.3 |
| Acetone | 0.386 | 0.410 | 0.434 | 0.459 | 0.483 | 0.507 |
| Pentan-2-one | 0.652 | 0.693 | 0.734 | 0.773 | 0.814 | 0.855 |
| Pentan-3-one | 0.651 | 0.697 | 0.741 | 0.788 | 0.833 | 0.879 |
| Butanal | 0.675 | 0.711 | 0.747 | 0.782 | 0.818 | 0.852 |
| Acetonitrile | 0.436 | 0.455 | 0.472 | 0.490 | 0.507 | 0.523 |
| 1-Nitropropane | 0.669 | 0.692 | 0.714 | 0.736 | 0.758 | 0.777 |
Theoretical basis
The equations developed by Everett30 and Cruickshank et al.31 were used in this work to calculate γ∞13 for solutes in ILs:
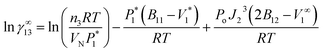 | (1) |
In this expression, n3 is the number of moles of solvent on the column packing, R is the universal gas constant, T is the column temperature, VN denotes the net retention volume of the solute, 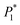 is the saturated vapor pressure of the solute at temperature T, B11 is the second virial coefficient of pure solute,
is the saturated vapor pressure of the solute at temperature T, B11 is the second virial coefficient of pure solute, 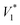 is the molar volume of the solute, Po is the outlet pressure, PoJ23 is the mean column pressure, B12 (where the subscript “2” refers to the carrier gas, in this case helium) is the mixed second virial coefficient of the solute and carrier gas, and V∞1 is the partial molar volume of the solute at infinite dilution in the solvent. The thermophysical properties required in developing the activity coefficients at infinite dilution were calculated using equations and constants known from literature.32 The values of B12 were calculated using the Tsonopolous equation.33 The pressure correction term, J23, is given by:
is the molar volume of the solute, Po is the outlet pressure, PoJ23 is the mean column pressure, B12 (where the subscript “2” refers to the carrier gas, in this case helium) is the mixed second virial coefficient of the solute and carrier gas, and V∞1 is the partial molar volume of the solute at infinite dilution in the solvent. The thermophysical properties required in developing the activity coefficients at infinite dilution were calculated using equations and constants known from literature.32 The values of B12 were calculated using the Tsonopolous equation.33 The pressure correction term, J23, is given by:
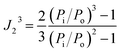 | (2) |
The net retention volume of the solute, VN, is given by:
| VN = (J23)−1Uo(tR − tG) | (3) |
While the activity coefficients at infinite dilution are determined as a function of temperature, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ∞13 can be split to its respective partial molar excess thermodynamic functions:
γ∞13 can be split to its respective partial molar excess thermodynamic functions:
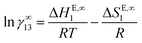 | (4) |
Assuming that the temperature dependence follows a linear van't Hoff plot:
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ∞13 = a/T + b γ∞13 = a/T + b
| (5) |
The gas–liquid partition coefficient KL = (cL1/cG1) for a solute partitioning between a carrier gas and the IL was calculated from the solute retention according to the following equation
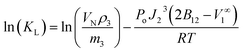 | (6) |
Results and discussion
Thermal and physico-chemical characteristic
The DSC diagrams for [BzMIM][DCA] and [BzMIM][NTf2] are shown in the ESI.† Numerical data are included in Table 1. The temperatures of the glass phase transition (Tg, midpoint) are very close to each other and are 217.0 ± 0.1 K ([BzMIM][DCA]) and 216.9 K ± 0.1 ([BzMIM][NTf2]). The literature values are 195.4 K (ref. 22) for [BzMIM][DCA] and 217 K (ref. 23), 215.8 K (ref. 24), 215.8 K (ref. 25) and 210.8 K (ref. 22) for [BzMIM][NTf2].The values of ΔCp(g) are in a range of 169.9 ± 5 J mol−1 K−1 ([BzMIM][DCA]) to 199.5 ± 5 J mol−1 K−1 [BzMIM][NTf2]. It differs from values in ref. 31 (ΔCp(g)/J mol−1 K−1 = 86.1) and is very close to ref. 30 (ΔCp(g)/J mol−1 K−1 = 197) for [BzMIM][NTf2].
Density and viscosity of [BzMIM][DCA] and [BzMIM][NTf2] were measured in this work as a function of temperature and are listed in Table 2 together with information from literature for the same ILs. The density measurements were carried out in the temperature range from (298.15 to 368.15) K at atmospheric pressure. The value obtained at T = 298.15 K is 1.15814 g cm−3 for [BzMIM][DCA] and 1.49098 g cm−3 for [BzMIM][NTf2]. The latter IL was measured in many laboratories, mostly at T = 298.15 K (ref. 23–26 and 28) and T = 293.15 K.27 The density of [BzMIM][NTf2] in literature differs slightly from our data at higher temperatures.23 The mean percent deviation for six temperatures in a range from 298.15 K to 323.15 K between our values and published in ref. 23 is – 0.32%, in ref. 24 (one point) is – 0.13% and in ref. 26 (one point) it is – 4.76%. The other literature data are at different temperatures, thus it is difficult to compare the results. The viscosity at T = 298.15 K is 102 mPa s for [BzMIM][DCA] and 133 mPa s for [BzMIM][NTf2], which differ from the available literature values for [BzMIM][NTf2]23,24 and is lower than those in other literature.34,36 The mean percent deviation for two temperatures 298.15 K and 308.15 K between our values and published in ref. 23 is +17.5%, in ref. 24 (one point) is +6% and in ref. 26 (one point) it is +54%. Undoubtedly, the purity of the compound in different publications is responsible for such a differences.
The average values of γ∞13 for the measured solutes in [BzMIM][DCA] and [BzMIM][NTf2], determined at six temperatures in a range from (318.15 to 368.15) K at atmospheric pressure are listed in Tables 3 and 4, respectively. Most of the virial coefficient values B11 and B12 and critical parameters for solutes used in this work were presented in our previous study.34 An analysis of the values of γ∞13 shows large differences in possible interaction between a solute and the IL at infinite dilution. The data for [BzMIM][NTf2] shows almost five times lower values of γ∞13 for the non-polar solutes in comparison with [BzMIM][DCA], which means much larger interaction between the [NTf2]− anion with hydrocarbons than those of the [DCA]− anion. Aromatic hydrocarbons show much smaller differences; the values of γ∞13 for benzene, toluene and others are only two times smaller for the [NTf2]− anion. For polar substances, such as alcohols, the interaction between solute and the IL is similar and for water it is much lower for the [NTf2]− anion. This confirms the common information about hydrophobicity of [NTf2]− – based ILs which is the most important for consideration for the three separation cases, viz. hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene. The values for the mentioned solutes at T = 328.15 K: for hexane γ∞13 = 95.4 or γ∞13 = 17.6 in [BzMIM][DCA] and [BzMIM][NTf2], respectively; for hex-1-ene γ∞13 = 38.1 or γ∞13 = 9.27 in [BzMIM][DCA] and [BzMIM][NTf2], respectively; for cyclohexane γ∞13 = 33.5 or γ∞13 = 9.39 in [BzMIM][DCA] and [BzMIM][NTf2], respectively; for cyclohexene γ∞13 = 13.6 or γ∞13 = 5.31 in [BzMIM][DCA] and [BzMIM][NTf2], respectively; for ethylbenzene γ∞13 = 6.71 or γ∞13 = 2.26 in [BzMIM][DCA] and [BzMIM][NTf2], respectively; and for styrene γ∞13 = 3.11 or γ∞13 = 1.32 in [BzMIM][DCA] and [BzMIM][NTf2], respectively. For all these solutes, the interaction with the IL is almost three times larger for [BzMIM][NTf2]. Thus, the selectivities for separation may be expected to not be very different from each other for the two ILs.
The global analysis drawn from Tables 3 and 4 shows that the γ∞13 values increase with the alkyl chain length in a series of alkanes, cycloalkanes, alkenes, alkynes, aromatic hydrocarbons (increasing radicals), alcohols, ethers, and ketones, which is an indication of the decrease of interactions between the solute and the IL at infinite dilution. Furthermore, despite the lower number of alkyl chains, the higher measured retention time is an evident observation of the decrease of the γ∞13 values, and of an increase in the interactions between the IL and solute. The lowest values of γ∞13 at all temperatures (the strongest interaction) in [BzMIM][DCA] are observed for water (γ∞13 = 0.387), methanol (γ∞13 = 0.511), ethanol (γ∞13 = 0.872), acetonitrile (γ∞13 = 0.903), and pyridine (γ∞13 = 1.03) at T = 328.15 K. The lowest values of γ∞13 at all temperatures (the largest interaction) in [BzMIM][NTf2] are observed for acetone (γ∞13 = 0.410), acetonitrile (γ∞13 = 0.455), 1,4-dioxane (γ∞13 = 0.523), tetrahydrofuran (γ∞13 = 0.648), 1-nitropropane (γ∞13 = 0.692), and pentan-2-one (γ∞13 = 0.693) at T = 328.15 K. As can be seen, the strongest interactions for the two different anions is observed for different solutes. Only acetonitrile is common for the two ILs, with the value two times larger in [BzMIM][DCA] than that in [BzMIM][NTf2].
The largest values of γ∞13 provide the initial information about the lower interactions. For [BzMIM][DCA] it may be observed for decane (γ∞13 = 509), dec-1-ene (γ∞13 = 220) and di-n-butyl ether (γ∞13 = 77.2) at T = 328.15 K. The largest values of γ∞13 for [BzMIM][NTf2] are observed for decane (γ∞13 = 70.7) and dec-1-ene (γ∞13 = 36.8) at T = 328.15 K. These latter values are appreciably lower than those for [BzMIM][DCA].
An interesting feature worth noting is a comparison to two ILs, [AMIM][DCA]16 and [AMIM][NTf2]20 which show the same relations in the interactions for the measured solutes. Strong interactions for [AMIM][DCA] were observed with water and methanol (γ∞13 = 0.341, γ∞13 = 0.505 at T = 328.15 K), all alcohols as well as with thiophene, pyridine, 1,4-dioxane, acetone, acetonitrile, and 1-nitropropane with γ∞13 < 2 at T = 328.15 K.16 The γ∞13 data for the [NTf2]−-based ILs show much smaller interactions with water and alcohols. Nonetheless, the lower values of γ∞13 for polar solutes such as benzene, thiophene, pyridine, and 1-nitropropane for the [NTf2]−-based ILs suggest the high potential for the extraction of these compounds from alkanes, which is important in petrochemical processes, e.g. desulphurization and denitrification of fuels. The hydrocarbons, such as alkanes, alkenes and alkynes reveal much lower values of γ∞13 in [AMIM][NTf2]20 than those observed earlier for [AMIM][DCA]16 (γ∞13 < 100 at T = 328.15 K). The γ∞13 values in [AMIM][NTf2] were γ∞13 = 20.8 and γ∞13 = 10.7 at T = 328.15 K for hexane and hex-1-ene, respectively.
The experimental γ∞13 values exhibit diverse temperature dependences corresponding on both endothermic and exothermic effects accompanying the interactions of solutes with the IL. The γ∞13 values decrease with an increasing temperature for alkanes, alkenes, cycloalkanes and cycloalkenes (see Fig. 2S and 3S in ESI†). The opposite influence of temperature is presented for alkynes (see Fig. 3S in ESI†) and for aromatic hydrocarbons (see Fig. 4S in ESI†). Only for the case of alcohols and water in [BzMIM][NTf2] do the γ∞13 values decrease with an increasing temperature (see Fig. 5Sb in ESI†). For alcohols in [BzMIM][DCA] (see Fig. 5Sa in ESI†) and most of the ethers (see Fig. 6S in ESI†), esters, tetrahydrofuran, 1,4-dioxane, thiophene (see Fig. 7S in ESI†) and ketones, as well as 1-nitropropane (see Fig. 8S in ESI†) there is an increase in γ∞13 values with an increasing temperature. The diagrams mentioned above show the plot of the natural logarithm of the γ∞13 as a function of the inverse absolute temperature for all investigated solutes.
The gas–liquid partition coefficient, KL, calculated from eqn (6) is an important property of the IL. This property shows the suitability of the IL for particular application in extraction. The data for [BzMIM][DCA] and [BzMIM][NTf2] are listed in Tables 5 and 6. By inspection of these tables one can see that the lowest values are observed for alkanes (KL = 2.19 at T = 328.15 K in [BzMIM][DCA]), cycloalkanes, cylcloalkenes, alkenes, alkynes and ethers, especially for diethyl ether in [BzMIM][DCA] (KL = 7.05 at T = 328.15 K). The large values of KL are observed for 1-pentanol (KL = 2290 at T = 328.15 K in [BzMIM][DCA]), water (KL = 2139 at T = 328.15 K in [BzMIM][DCA]) and α-methylstyrene (KL = 1358 at T = 328.15 K in [BzMIM][DCA] and KL = 2336 at T = 328.15 K in [BzMIM][NTf2]) as well as for 1-nitropropane (KL = 2007 at T = 328.15 K in [BzMIM][NTf2]). These values are much lower than those observed for [AMIM][DCA]16 and similar to those observed for [AMIM][NTf2].20 The high KL value corresponds to a large affinity of the solute to the liquid phase. The KL value increases with a decrease of temperature and with an increase of the alkane chain length for alkanes, alkenes, alkynes, cycloalkanes, alcohols, esters and ethers. The KL values increase with an increase of the radicals in the aromatic compounds.
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 318.15 | 328.15 | 338.15 | 348.15 | 358.15 | 368.15 | |
| Pentane | 1.38 | 1.16 | 0.989 | 0.855 | 0.749 | |
| Hexane | 2.76 | 2.19 | 1.77 | 1.45 | 1.22 | 1.03 |
| 3-Methylpentane | 2.51 | 2.03 | 1.67 | 1.39 | 1.18 | 1.02 |
| 2,2-Dimethylbutane | 1.33 | 1.15 | 1.01 | 0.892 | 0.802 | |
| Heptane | 5.29 | 3.99 | 3.07 | 2.41 | 1.94 | 1.58 |
| Octane | 9.85 | 7.07 | 5.18 | 3.92 | 3.02 | 2.37 |
| 2,2,4-Trimethylpentane | 3.73 | 2.88 | 2.26 | 1.82 | 1.49 | 1.23 |
| Nonane | 18.4 | 12.6 | 8.89 | 6.45 | 4.80 | 3.65 |
| Decane | 33.8 | 22.1 | 15.0 | 10.5 | 7.53 | 5.52 |
| Cyclopentane | 6.70 | 5.29 | 4.24 | 3.46 | 2.87 | 2.41 |
| Cyclohexane | 12.0 | 9.12 | 7.10 | 5.63 | 4.54 | 3.72 |
| Methylcyclohexane | 14.4 | 10.8 | 8.26 | 6.45 | 5.14 | 4.16 |
| Cycloheptane | 37.8 | 27.1 | 19.9 | 15.0 | 11.5 | 9.05 |
| Cyclooctane | 97.1 | 66.3 | 46.4 | 33.4 | 24.6 | 18.5 |
| Pent-1-ene | 3.16 | 2.55 | 2.08 | 1.73 | 1.46 | 1.25 |
| Hex-1-ene | 6.10 | 4.65 | 3.61 | 2.88 | 2.33 | 1.91 |
| Cyclohexene | 33.3 | 24.2 | 17.9 | 13.6 | 10.5 | 8.33 |
| Hept-1-ene | 11.2 | 8.19 | 6.11 | 4.69 | 3.66 | 2.91 |
| Oct-1-ene | 20.4 | 14.2 | 10.2 | 7.45 | 5.60 | 4.30 |
| Dec-1-ene | 64.4 | 41.5 | 27.5 | 18.8 | 13.3 | 9.53 |
| Pent-1-yne | 21.4 | 15.6 | 11.7 | 8.92 | 6.95 | 5.50 |
| Hex-1-yne | 40.2 | 28.2 | 20.3 | 15.0 | 11.3 | 8.65 |
| Hept-1-yne | 71.6 | 48.2 | 33.4 | 23.7 | 17.3 | 12.9 |
| Oct-1-yne | 126 | 81.3 | 54.1 | 37.2 | 26.2 | 19.0 |
| Benzene | 183 | 125 | 86.9 | 62.4 | 45.7 | 34.2 |
| Toluene | 331 | 215 | 144 | 99.1 | 70.1 | 50.8 |
| Ethylbenzene | 525 | 329 | 213 | 143 | 98.3 | 69.4 |
| o-Xylene | 896 | 548 | 349 | 228 | 154 | 107 |
| m-Xylene | 575 | 357 | 230 | 153 | 104 | 73.2 |
| p-Xylene | 568 | 354 | 228 | 152 | 104 | 72.8 |
| n-Propylbenzene | 790 | 480 | 301 | 196 | 131 | 90.1 |
| Iso-propylbenzene | 628 | 379 | 238 | 155 | 104 | 71.4 |
| Styrene | 1710 | 1023 | 637 | 409 | 272 | 185 |
| α-Methylstyrene | 2347 | 1358 | 818 | 509 | 327 | 215 |
| Thiophene | 369 | 244 | 167 | 117 | 84.5 | 62.3 |
| Pyridine | 1691 | 1058 | 685 | 458 | 315 | 222 |
| Methanol | 579 | 380 | 258 | 179 | 128 | 93.4 |
| Ethanol | 636 | 407 | 269 | 183 | 128 | 91.5 |
| Propan-1-ol | 1181 | 719 | 455 | 298 | 201 | 140 |
| Propan-2-ol | 512 | 322 | 211 | 142 | 99.5 | 71.4 |
| Butan-1-ol | 2241 | 1298 | 785 | 495 | 323 | 218 |
| Butan-2-ol | 889 | 533 | 333 | 217 | 146 | 102 |
| 2-Methyl-1-propanol | 1422 | 836 | 515 | 329 | 219 | 150 |
| tert-Butanol | 391 | 243 | 157 | 106 | 73.9 | 53.2 |
| 1-Pentanol | 4132 | 2290 | 1328 | 804 | 506 | 330 |
| Water | 3504 | 2139 | 1351 | 880 | 592 | 406 |
| Methyl acetate | 108 | 74.2 | 52.3 | 37.9 | 28.1 | 21.3 |
| Methyl propanoate | 165 | 108 | 73.8 | 51.8 | 37.3 | 27.5 |
| Methyl butanoate | 250 | 160 | 106 | 72.5 | 51.0 | 36.7 |
| Ethyl acetate | 136 | 90.4 | 62.0 | 43.8 | 31.7 | 23.6 |
| Tetrahydrofuran | 155 | 107 | 75.1 | 54.4 | 40.3 | 30.4 |
| 1,4-Dioxane | 907 | 576 | 376 | 253 | 175 | 124 |
| tert-Butyl methyl ether | 16.1 | 11.7 | 8.71 | 6.64 | 5.17 | 4.10 |
| tert-Butyl ethyl ether | 10.9 | 7.80 | 5.74 | 4.34 | 3.34 | 2.63 |
| tert-Amyl methyl ether | 31.8 | 22.1 | 15.8 | 11.6 | 8.70 | 6.67 |
| Diethyl ether | 9.36 | 7.05 | 5.44 | 4.28 | 3.43 | 2.80 |
| Di-n-propyl ether | 19.5 | 13.7 | 9.95 | 7.38 | 5.60 | 4.34 |
| Di-iso-propyl ether | 7.51 | 5.55 | 4.20 | 3.24 | 2.55 | 2.05 |
| Di-n-butyl ether | 60.2 | 39.2 | 26.4 | 18.2 | 13.0 | 9.45 |
| Acetone | 173 | 119 | 84.7 | 61.7 | 45.8 | 34.8 |
| Pentan-2-one | 412 | 266 | 177 | 121 | 85.1 | 61.3 |
| Pentan-3-one | 400 | 259 | 173 | 118 | 83.2 | 59.9 |
| Butanal | 188 | 128 | 89.1 | 63.9 | 46.8 | 35.2 |
| Acetonitrile | 536 | 369 | 260 | 188 | 139 | 105 |
| 1-Nitropropane | 2102 | 1293 | 823 | 542 | 367 | 255 |
| Solute | T/K | |||||
|---|---|---|---|---|---|---|
| 318.15 | 328.15 | 338.15 | 348.15 | 358.15 | 368.15 | |
| Pentane | 4.95 | 3.96 | 3.23 | 2.67 | 2.25 | 1.91 |
| Hexane | 10.6 | 8.05 | 6.25 | 4.95 | 4.00 | 3.28 |
| 3-Methylpentane | 10.1 | 7.66 | 5.96 | 4.73 | 3.83 | 3.15 |
| 2,2-Dimethylbutane | 6.59 | 5.18 | 4.15 | 3.39 | 2.81 | 2.36 |
| Heptane | 21.8 | 15.8 | 11.7 | 8.85 | 6.85 | 5.41 |
| Octane | 43.1 | 29.9 | 21.5 | 15.7 | 11.8 | 9.09 |
| 2,2,4-Trimethylpentane | 20.9 | 15.2 | 11.3 | 8.64 | 6.73 | 5.34 |
| Nonane | 87.2 | 57.5 | 39.3 | 27.6 | 20.0 | 14.8 |
| Decane | 170 | 108 | 71.0 | 48.2 | 33.7 | 24.2 |
| Cyclopentane | 14.8 | 11.4 | 8.91 | 7.10 | 5.77 | 4.75 |
| Cyclohexane | 29.7 | 22.1 | 16.8 | 13.0 | 10.3 | 8.31 |
| Methylcyclohexane | 42.3 | 30.5 | 22.6 | 17.1 | 13.2 | 10.4 |
| Cycloheptane | 88.8 | 62.1 | 44.7 | 32.9 | 24.9 | 19.1 |
| Cyclooctane | 233 | 156 | 107 | 75.8 | 55.1 | 40.9 |
| Pent-1-ene | 8.31 | 6.45 | 5.10 | 4.12 | 3.38 | 2.82 |
| Hex-1-ene | 17.4 | 13.0 | 9.85 | 7.65 | 6.07 | 4.89 |
| Cyclohexene | 58.3 | 41.8 | 30.8 | 23.2 | 17.8 | 14.0 |
| Hept-1-ene | 35.8 | 25.3 | 18.4 | 13.7 | 10.4 | 8.10 |
| Oct-1-ene | 70.5 | 47.9 | 33.5 | 24.2 | 17.8 | 13.5 |
| Dec-1-ene | 268 | 168 | 109 | 72.7 | 49.9 | 35.2 |
| Pent-1-yne | 31.7 | 23.1 | 17.2 | 13.1 | 10.2 | 8.06 |
| Hex-1-yne | 65.5 | 45.7 | 32.8 | 24.0 | 18.0 | 13.8 |
| Hept-1-yne | 131 | 87.8 | 60.4 | 42.7 | 31.0 | 23.0 |
| Oct-1-yne | 261 | 167 | 111 | 75.4 | 52.9 | 38.0 |
| Benzene | 294 | 197 | 136 | 96.5 | 70.2 | 52.2 |
| Toluene | 590 | 381 | 254 | 174 | 123 | 88.3 |
| Ethylbenzene | 1067 | 661 | 424 | 281 | 191 | 133 |
| o-Xylene | 1645 | 1003 | 634 | 415 | 279 | 193 |
| m-Xylene | 1204 | 739 | 469 | 307 | 207 | 144 |
| p-Xylene | 1150 | 706 | 450 | 296 | 200 | 139 |
| n-Propylbenzene | 1845 | 1102 | 682 | 438 | 288 | 196 |
| Iso-propylbenzene | 1455 | 877 | 548 | 354 | 236 | 161 |
| Styrene | 2741 | 1627 | 1003 | 640 | 421 | 285 |
| α-Methylstyrene | 4036 | 2336 | 1398 | 867 | 555 | 365 |
| Thiophene | 398 | 265 | 182 | 128 | 92.9 | 68.7 |
| Pyridine | 2330 | 1451 | 935 | 620 | 424 | 297 |
| Methanol | 147 | 103 | 74.4 | 54.8 | 41.3 | 31.8 |
| Ethanol | 221 | 149 | 104 | 74.3 | 54.6 | 40.8 |
| Propan-1-ol | 450 | 289 | 193 | 132 | 93.5 | 68.1 |
| Propan-2-ol | 242 | 162 | 112 | 79.7 | 58.2 | 43.9 |
| Butan-1-ol | 949 | 584 | 374 | 248 | 170 | 120 |
| Butan-2-ol | 463 | 294 | 194 | 133 | 94.1 | 68.5 |
| 2-Methyl-1-propanol | 626 | 391 | 256 | 173 | 121 | 87.3 |
| tert-Butanol | 251 | 163 | 110 | 76.9 | 55.4 | 41.2 |
| 1-Pentanol | 1989 | 1172 | 722 | 461 | 306 | 209 |
| Water | 275 | 189 | 133 | 96.3 | 71.1 | 53.8 |
| Methyl acetate | 226 | 153 | 106 | 76.5 | 56.1 | 42.2 |
| Methyl propanoate | 404 | 262 | 175 | 121 | 86.1 | 62.5 |
| Methyl butanoate | 687 | 434 | 283 | 191 | 132 | 94.2 |
| Ethyl acetate | 363 | 237 | 159 | 111 | 78.6 | 57.4 |
| Tetrahydrofuran | 295 | 199 | 138 | 98.7 | 72.1 | 53.8 |
| 1,4-Dioxane | 1383 | 865 | 558 | 371 | 253 | 177 |
| tert-Butyl methyl ether | 54.2 | 38.0 | 27.4 | 20.3 | 15.4 | 11.9 |
| tert-Butyl ethyl ether | 45.8 | 31.7 | 22.5 | 16.5 | 12.3 | 9.40 |
| tert-Amyl methyl ether | 109 | 74.1 | 51.8 | 37.2 | 27.3 | 20.5 |
| Diethyl ether | 27.3 | 19.9 | 14.9 | 11.4 | 8.87 | 7.04 |
| Di-n-propyl ether | 72.0 | 49.1 | 34.5 | 25.0 | 18.5 | 14.0 |
| Di-iso-propyl ether | 34.9 | 24.6 | 17.8 | 13.2 | 9.96 | 7.67 |
| Di-n-butyl ether | 266 | 168 | 110 | 74.6 | 51.9 | 37.0 |
| Acetone | 340 | 232 | 162 | 117 | 85.7 | 64.3 |
| Pentan-2-one | 1072 | 675 | 440 | 296 | 204 | 145 |
| Pentan-3-one | 1049 | 661 | 430 | 288 | 198 | 139 |
| Butanal | 371 | 249 | 172 | 122 | 88.8 | 66.1 |
| Acetonitrile | 732 | 497 | 346 | 247 | 181 | 135 |
| 1-Nitropropane | 3265 | 2007 | 1276 | 838 | 566 | 394 |
Table 7 lists the partial molar excess Gibbs energies, ΔGE,∞1, at infinite dilution, the molar excess enthalpies, ΔHE,∞1, at infinite dilution, and the partial molar excess entropies at infinite dilution, TrefΔSE,∞1, for all the solutes studied in [BzMIM][DCA] and [BzMIM][NTf2], respectively, at a reference temperature T = 328.15 K. These thermodynamic functions specify the interaction between solute and the IL and are an important consideration in determining the suitability of the IL for extraction. The ΔGE,∞1 was calculated from the temperature dependence of γ∞13. The values of ΔGE,∞1 are positive for all solutes except methanol, ethanol, water and acetonitrile in [BzMIM][DCA], as well as thiophene, pyridine, esters, tetrahydrofuran, 1,4-dioxane, ketones, acetonitrile and 1-nitropropane in [BzMIM][NTf2]. This is similar to the earlier measured data for [AMIM][DCA]16 and [AMIM][NTf2].20 The infinite dilution activity coefficient values are lower than one for these solutes, i.e. γ∞13 < 1, which corresponds to the (IL + solute) binary system with negative deviations from Raoult's law. The largest negative value of ΔGE,∞1 was observed for water in [BzMIM][DCA] (−2.59 kJ mol−1) and for acetone in [BzMIM][NTf2] (−2.43 kJ mol−1). For the remaining solutes, including hexane (ΔGE,∞1 = 12.48 kJ mol−1 in [BzMIM][DCA]) and hex-1-ene (ΔGE,∞1 = 9.93 kJ mol−1 in [BzMIM][DCA]), positive deviations from ideality are obtained. The large positive values are observed for long-chain alkanes in [BzMIM][DCA], which indicates that the solute–IL interaction is not strong and limited miscibility is possible in the liquid phase.
| Solute | [BzMIM][DCA] | [BzMIM][NTf2] | ||||
|---|---|---|---|---|---|---|
| ΔGE,∞1/kJ mol−1 | ΔHE,∞1/kJ mol−1 | TrefΔSE,∞1/kJ mol−1 | ΔGE,∞1/kJ mol−1 | ΔHE,∞1/kJ mol−1 | TrefΔSE,∞1/kJ mol−1 | |
| Pentane | 11.4 | 8.20 | −3.19 | 6.98 | 4.03 | −2.95 |
| Hexane | 12.4 | 7.77 | −4.67 | 7.82 | 4.15 | −3.67 |
| 3-Methylpentane | 12.2 | 8.41 | −3.75 | 7.46 | 3.39 | −4.07 |
| 2,2-Dimethylbutane | 12.6 | 11.69 | −0.90 | 7.41 | 3.50 | −3.90 |
| Heptane | 13.6 | 8.40 | −5.16 | 8.76 | 4.80 | −3.96 |
| Octane | 14.7 | 8.74 | −5.98 | 9.72 | 6.21 | −3.52 |
| 2,2,4-Trimethylpentane | 14.4 | 9.10 | −5.26 | 8.77 | 4.14 | −4.63 |
| Nonane | 15.8 | 9.67 | −6.17 | 10.6 | 6.63 | −4.00 |
| Decane | 17.0 | 10.45 | −6.55 | 11.6 | 7.77 | −3.85 |
| Cyclopentane | 8.31 | 4.75 | −3.56 | 5.15 | 2.55 | −2.60 |
| Cyclohexane | 9.58 | 5.99 | −3.59 | 6.11 | 3.98 | −2.13 |
| Methylcyclohexane | 10.9 | 6.87 | −4.02 | 7.00 | 3.82 | −3.17 |
| Cycloheptane | 10.1 | 6.12 | −4.01 | 6.80 | 4.11 | −2.69 |
| Cyclooctane | 10.9 | 6.61 | −4.27 | 7.50 | 5.07 | −2.43 |
| Pent-1-ene | 8.75 | 3.63 | −5.12 | 5.15 | 0.70 | −4.45 |
| Hex-1-ene | 9.93 | 4.36 | −5.57 | 6.08 | 2.25 | −3.83 |
| Cyclohexene | 7.12 | 2.03 | −5.10 | 4.56 | 1.29 | −3.27 |
| Hept-1-ene | 11.1 | 4.61 | −6.50 | 6.98 | 2.03 | −4.95 |
| Oct-1-ene | 12.3 | 5.28 | −7.07 | 7.98 | 3.50 | −4.47 |
| Dec-1-ene | 14.7 | 6.79 | −7.93 | 9.84 | 4.47 | −5.37 |
| Pent-1-yne | 4.58 | −2.55 | −7.13 | 2.46 | −2.72 | −5.18 |
| Hex-1-yne | 5.71 | −1.69 | −7.40 | 3.34 | −2.04 | −5.38 |
| Hept-1-yne | 6.96 | −0.85 | −7.81 | 4.26 | −1.32 | −5.58 |
| Oct-1-yne | 8.20 | 0.22 | −7.98 | 5.17 | −0.41 | −5.58 |
| Benzene | 2.43 | −3.21 | −5.64 | 0.13 | −4.13 | −4.26 |
| Toluene | 3.82 | −2.96 | −6.78 | 1.20 | −3.42 | −4.61 |
| Ethylbenzene | 5.19 | −1.85 | −7.04 | 2.22 | −2.91 | −5.13 |
| o-Xylene | 4.66 | −2.44 | −7.10 | 1.95 | −2.73 | −4.67 |
| m-Xylene | 5.29 | −2.16 | −7.45 | 2.25 | −3.39 | −5.64 |
| p-Xylene | 5.21 | −2.23 | −7.44 | 2.27 | −3.32 | −5.60 |
| n-Propylbenzene | 6.54 | −1.13 | −7.68 | 3.22 | −2.45 | −5.67 |
| Iso-propylbenzene | 6.52 | −1.93 | −8.44 | 3.16 | −2.37 | −5.54 |
| Styrene | 3.10 | −3.80 | −6.90 | 0.76 | −4.53 | −5.29 |
| α-Methylstyrene | 4.41 | −4.65 | −9.06 | 1.86 | −4.93 | −6.79 |
| Thiophene | 0.99 | −4.33 | −5.32 | −0.29 | −3.82 | −3.53 |
| Pyridine | 0.081 | −3.87 | −3.96 | −1.83 | −4.39 | −2.56 |
| Methanol | −1.83 | −1.36 | 0.48 | 0.67 | 4.37 | 3.70 |
| Ethanol | −0.37 | 0.14 | 0.52 | 1.30 | 5.14 | 3.84 |
| Propan-1-ol | 0.40 | 0.40 | 0.00 | 1.84 | 5.14 | 3.30 |
| Propan-2-ol | 0.82 | 1.42 | 0.60 | 1.63 | 6.57 | 4.94 |
| Butan-1-ol | 1.38 | 1.06 | −0.32 | 2.50 | 6.27 | 3.77 |
| Butan-2-ol | 1.53 | 0.55 | −0.97 | 2.09 | 5.61 | 3.52 |
| 2-Methyl-1-propanol | 1.42 | 1.71 | 0.29 | 2.43 | 7.20 | 4.76 |
| tert-Butanol | 1.60 | 0.62 | −0.99 | 1.62 | 4.33 | 2.71 |
| 1-Pentanol | 2.20 | 0.59 | −1.61 | 2.97 | 6.04 | 3.07 |
| Water | −2.59 | −2.06 | 0.53 | 2.97 | 8.16 | 5.19 |
| Methyl acetate | 1.81 | −3.98 | −5.79 | −1.23 | −4.91 | −3.68 |
| Methyl propanoate | 2.90 | −3.82 | −6.72 | −0.56 | −5.20 | −4.64 |
| Methyl butanoate | 4.13 | −2.74 | −6.87 | 0.36 | −4.03 | −4.39 |
| Ethyl acetate | 3.19 | −3.26 | −6.45 | −0.50 | −5.03 | −4.53 |
| Tetrahydrofuran | 1.57 | −3.78 | −5.36 | −1.18 | −5.13 | −3.95 |
| 1,4-Dioxane | 0.40 | −5.56 | −5.96 | −1.77 | −6.72 | −4.95 |
| tert-Butyl methyl ether | 6.66 | −1.21 | −7.87 | 2.40 | −4.03 | −6.43 |
| tert-Butyl ethyl ether | 9.33 | 0.57 | −8.76 | 4.45 | −2.59 | −7.04 |
| tert-Amyl methyl ether | 7.73 | −0.53 | −8.26 | 3.37 | −2.67 | −6.04 |
| Diethyl ether | 6.27 | −0.49 | −6.75 | 2.38 | −3.30 | −5.68 |
| Di-n-propyl ether | 9.42 | 1.78 | −7.65 | 4.88 | −0.80 | −5.68 |
| Di-iso-propyl ether | 9.91 | 2.49 | −7.42 | 4.79 | −1.62 | −6.41 |
| Di-n-butyl ether | 11.9 | 4.17 | −7.69 | 6.82 | 1.91 | −4.91 |
| Acetone | 0.43 | −4.15 | −4.58 | −2.43 | −5.33 | −2.89 |
| Pentan-2-one | 2.60 | −3.45 | −6.04 | −1.00 | −5.25 | −4.25 |
| Pentan-3-one | 2.63 | −3.59 | −6.22 | −0.98 | −5.86 | −4.87 |
| Butanal | 1.95 | −3.63 | −5.57 | −0.93 | −4.53 | −3.60 |
| Acetonitrile | −0.28 | −2.45 | −2.17 | −2.15 | −3.53 | −1.39 |
| 1-Nitropropane | 1.25 | −2.84 | −4.09 | −1.00 | −2.93 | −1.92 |
The partial excess molar enthalpies at infinite dilution, ΔHE,∞1, determined from the Gibbs–Helmholtz equation exhibit negative values for alkynes, aromatic hydrocarbons, esters some ethers, ketones and 1-nitropropane for both ILs. For these solutes relatively strong energetic solute–solvent interactions are observed. Additionally, there are negative ΔHE,∞1 values for [BzMIM][DCA] with water and methanol. As expected, the [NTf2]−, the hydrophobic anion of the IL used in this work, exhibits possible π–π, or n–π interactions with most of the solutes, which leads to negative values of ΔHE,∞1. For the other solutes, no particular affinity between the IL and solute molecule is expected. Thus for aliphatic hydrocarbons, the endothermic interaction, resulting from the energetic weakness of their interaction with the IL is observed.
The partial excess molar entropies at infinite dilution, TrefΔSE,∞1, are small and negative for all solutes studied, excluding alcohols and water in [BzMIM][NTf2], and excluding a few alcohols and water in [BzMIM][DCA]. The solution of the majority of the solutes in both ILs is accompanied by entropy losses, which may suggest that the solute molecule arranges itself in the IL structure. The most positive value of the TrefΔSE,∞1 term was observed for water, 0.53 kJ mol−1 and 5.19 kJ mol−1 for [BzMIM][DCA] and [BzMIM][NTf2], respectively, whilst for the previously measured [AMIM][DCA]16 it was 11.79 kJ mol−1.
Separation of hexane/hex-1-ene, cyclohexane/cyclohexene and ethylbenzene/styrene
To analyze the performance of the [BzMIM][DCA] and [BzMIM][NTf2] ILs as extraction solvents for the chosen separation processes, the characteristic parameters for the separation, the selectivity (S∞12 = γ∞1/γ∞2) and the capacity (k∞2 = 1/γ∞2) were calculated from the experimental activity coefficients at infinite dilution values. The results are listed in Table 8 for both ILs for the three separation cases: hexane (1)/hex-1-ene (2), cyclohexane (1)/cyclohexene (2), and ethylbenzene (1)/styrene (2) at T = 328.15 K, along with literature data for ILs with the same anions, [DCA]− and [NTf2]−. To study the effect of the anion and cation structure on the extractive properties, the selectivity of 65 ILs have been compared to [BzMIM][DCA] and [BzMIM][NTf2] at the same temperature. In addition, to evaluate the potential of the ILs to be used at industrial scale, a comparison of capacity has also been performed.| Ionic liquid abbreviation | S∞12 | k∞12 | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Hexane/hexene | Cyclohexane/cyclohexene | Ethylbenzene/styrene | Hexene | Cyclohexene | Styrene | ||
| a Pe-pentyl. | |||||||
| [BzMIM][DCA] | 2.50 | 2.46 | 2.16 | 0.03 | 0.07 | 0.32 | This work |
| [BzMIM][NTf2] | 1.90 | 1.77 | 1.71 | 0.11 | 0.19 | 0.76 | This work |
| [EMIM][DCA] | 2.46 | — | — | 0.02 | — | — | 17 |
| [AMIM][DCA] | 2.51 | 2.51 | 2.24 | 0.02 | 0.05 | 0.25 | 16 |
| [BMIM][DCA] | 2.29 | 2.19 | 2.14 | 0.04 | 0.09 | 0.44 | 15 |
| [BMPY][DCA] | 2.34 | 2.32 | 2.31 | 0.05 | 0.12 | 0.72 | 35 |
| [CPMIM][DCA] | 2.30 | — | — | 0.02 | — | — | 18 |
| [CPMMIM][DCA] | 2.16 | — | — | 0.04 | — | — | 18 |
| [N–C3OHMIM][DCA] | 2.79 | — | — | 0.01 | — | — | 36 |
| [MMIM][NTf2] | 2.10 | — | — | 0.07 | — | — | 37 |
| [EMIM][NTf2] | 2.02 | 1.81 | — | 0.09 | 0.14 | — | 37 |
| [EMIM][NTf2] | 3.02 | — | — | 0.13 | — | — | 38 |
| [EMIM][NTf2] | — | 1.86 | 1.68 | — | 1.86 | 1.68 | 39 |
| [EMMIM][NTf2] | — | 1.86 | 1.83 | — | 0.14 | 0.66 | 39 |
| [AMIM][NTf2] | 1.94 | 1.79 | 1.75 | 0.09 | 0.15 | 0.63 | 20 |
| [BMIM][NTf2] | 1.80 | — | — | 0.15 | — | — | 37 |
| [BMIM][NTf2] | 2.13 | — | — | 0.08 | — | — | 18 |
| [BMIM][NTf2] | 1.82 | 1.65 | — | 0.15 | 0.22 | — | 40 |
| [HMIM][NTf2] | 1.67 | — | — | 0.23 | — | — | 41 |
| [HMIM][NTf2] | 1.72 | 1.57 | — | 0.24 | 0.33 | — | 42 |
| [HMIM][NTf2] | 1.53 | 1.56 | — | 0.23 | 0.64 | — | 43 |
| [OMIM][NTf2] | 1.53 | 3.51 | — | 0.31 | 1.52 | — | 43 |
| [D2MIM][NTf2] | 1.31 | — | — | 0.72 | — | — | 44 |
| [C6H13OCH2MIM][NTf2] | 1.63 | — | — | 0.22 | — | — | 45 |
| [(C6H13OCH2)2IM][NTf2] | 1.41 | — | — | 0.47 | — | — | 45 |
| [C2OHMIM][NTf2] | 1.99 | — | — | 0.05 | — | — | 36 |
| [COC2MIM][NTf2] | 1.89 | — | — | 0.10 | — | — | 36 |
| [(CO)2IM][NTf2] | 2.13 | — | — | 0.05 | — | — | 36 |
| [CPMIM][NTf2] | 2.41 | — | — | 0.02 | — | — | 18 |
| [CPMMIM][NTf2] | 2.16 | — | — | 0.04 | — | — | 19 |
| [COC2MMOR][NTf2] | 2.13 | 1.97 | 1.82 | 0.07 | 0.12 | 0.57 | 46 |
| [N–C3OHMMOR][NTf2] | 2.37 | 2.58 | 2.07 | 0.02 | 0.05 | 0.28 | 47 |
| [PMPIP][NTf2] | 1.99 | — | — | 0.11 | — | — | 48 |
| [BMPIP][NTf2] | 1.75 | — | — | 0.17 | — | — | 49 |
| [PeMPIP][NTf2]a | 1.73 | 1.60 | — | 0.19 | 0.28 | — | 50 |
| [HMPIP][NTf2] | 1.63 | 1.56 | — | 0.23 | 0.34 | — | 50 |
| [COC2MPIP][NTf2] | 1.90 | 1.77 | 1.75 | 0.12 | 0.19 | 1.75 | 51 |
| [BCN4PY][NTf2] | 2.13 | 2.07 | 1.85 | 0.07 | 0.14 | 0.69 | 52 |
| [1,4BMPY][NTf2] | 1.93 | — | — | 0.15 | — | — | 53 |
| [EPY][NTf2] | 2.20 | 1.95 | — | 0.08 | 0.13 | — | 54 |
| [EPY][NTf2] | 1.08 | — | — | 0.14 | — | — | 55 |
| [BPY][NTf2] | 1.37 | — | — | 0.16 | — | — | 55 |
| [PePY][NTf2]a | 1.46 | — | — | 0.23 | — | — | 55 |
| [N–C3OHPy][NTf2] | 2.15 | 2.04 | — | 0.05 | 0.10 | — | 56 |
| [PMPYR][NTf2] | 2.00 | — | — | 0.13 | — | — | 21 |
| [BMPYR][NTf2] | 1.72 | — | — | 0.15 | — | — | 43 |
| [BMPYR][NTf2] | 1.86 | — | — | 0.14 | — | — | 21 |
| [PeMPYR][NTf2]a | 1.86 | — | — | 0.17 | — | — | 21 |
| [HMPYR][NTf2] | 1.53 | — | — | 0.22 | — | — | 57 |
| [HMPYR][NTf2] | 1.65 | — | — | 0.22 | — | — | 58 |
| [OMPYR][NTf2] | 1.52 | — | — | 0.31 | — | — | 57 |
| [OMPYR][NTf2] | 1.58 | — | — | 0.33 | — | — | 58 |
| [DMPYR][NTf2] | 1.46 | — | — | 0.38 | — | — | 58 |
| [COC2MPYR][NTf2] | 1.91 | 1.78 | 1.7 | 0.11 | 0.17 | 1.70 | 59 |
| [C8iQuin][NTf2] | 1.28 | — | — | 0.32 | — | — | 60 |
| [Et3S][NTf2] | 2.09 | — | — | 0.09 | — | — | 61 |
| [N1,1,1,2OH][NTf2] | 2.22 | 2.03 | 0.97 | 0.04 | 0.06 | 0.24 | 62 |
| [N1,1,1,4][NTf2] | 1.84 | 0.57 | — | 0.11 | 0.16 | — | 63 |
| [N1,1,1,6][NTf2] | 0.88 | — | — | 0.84 | — | — | 17 |
| [N1,1,1,8][NTf2] | 1.54 | — | — | 0.26 | — | — | 64 |
| [N1,1,1,10][NTf2] | 1.50 | — | — | 0.36 | — | — | 64 |
| [N1,4,4,4][NTf2] | 1.55 | — | — | 0.23 | — | — | 64 |
| [N1,8,8,8][NTf2] | 1.27 | — | — | 1.16 | — | — | 65 |
| [N2,2,2,8][NTf2] | 1.54 | 1.48 | 1.65 | 0.28 | 0.40 | 1.39 | 66 |
| [N8,8,8,8][NTf2] | 1.23 | — | — | 0.95 | — | — | 64 |
| [P6,6,6,1,4][NTf2] | 1.51 | — | — | 2.04 | — | — | 67 |
| [P6,6,6,1,4][NTf2] | 1.21 | — | — | 1.09 | — | — | 68 |
| [P6,6,6,1,4][NTf2] | 1.19 | — | — | 0.98 | — | — | 69 |
Even though the experimental data present large discrepancies among different literature sources, one can conclude that the selectivities for the hexane (1)/hex-1-ene (2) separation, obtained with benzyl-based ILs is quite high with the best value for the imidazolium-based IL, [AMIM][DCA] (S∞12 = 2.51).16 The selectivities for [BzMIM][DCA] are much larger than those for [BzMIM][NTf2] for all separation cases. However, the capacity is two to three times larger for [BzMIM][NTf2] in comparison with [BzMIM][DCA]. The largest values of selectivity for the hexane (1)/hex-1-ene (2) separation are for [DCA]−-based ILs at T = 328.15 K which are: 1-(3-hydroxypropyl)methylimidazolium dicyanamide, [N–C3OHMIM][DCA] (S∞12 = 2.79)36 > [AMIM][DCA] (S∞12 = 2.51)16 > [BzMIM][DCA] (S∞12 = 2.50). The largest values of selectivity for the cyclohexane (1)/cyclohexene (2) separation are for [DCA]−-based ILs at T = 328.15 K which are: [AMIM][DCA] (S∞12 = 2.51)16 > [BzMIM][DCA] (S∞12 = 2.46). The largest values of selectivity for the ethylbenzene (1)/styrene (2) separation are for [DCA]−-based ILs at T = 328.15 K which are: 1-butyl-4-methylpyridinium dicyanamide, [BMPY][DCA] (S∞12 = 2.31)35 > [AMIM][DCA] (S∞12 = 2.24)16 > [BzMIM][DCA] (S∞12 = 2.16). The largest values of selectivity for the hexane (1)/hex-1-ene (2) separation for [NTf2]−-based ILs at T = 328.15 K are: 1-ethyl-3-methyl imidazoliumbis{(trifluoromethyl)sulfonyl}imide, [EMIM][NTf2] (S∞12 = 3.02)38 > [CPMIM][NTf2] (S∞12 = 2.41)18 > 1-(3-hydroxypropyl)methylmorpholinium bis{(trifluoromethyl)sulfonyl}imide, [N–C3OHMOR][NTf2] (S∞12 = 2.37)47 > [N1,1,1,2OH][NTf2] (S∞12 = 2.22)62 > [EPY][NTf2] (S∞12 = 2.20).54 The largest values of selectivity for the cyclohexane (1)/cyclohexene (2) separation for [NTf2]−-based ILs at T = 328.15 K are: 1-octyl-3-methyl imidazolium bis{(trifluoromethyl)sulfonyl}imide, [OMIM][NTf2] (S∞12 = 3.51)43 > [N–C3OHMOR][NTf2] (S∞12 = 2.58).47 The largest values of selectivity for the ethylbenzene (1)/styrene (2) separation for [NTf2]−-based ILs at T = 328.15 K are: [N–C3OHMOR][NTf2] (S∞12 = 2.07)47 > [BCN4PY][NTf2] (S∞12 = 1.85)52 > [EMMIM][NTf2] (S∞12 = 1.83).39
Summing up, for all three separation cases, the [DCA]−-based ILs reveal larger selectivities than those for [NTf2]−-based ILs (with the exception of [EMIM][NTf2],38 which is probably an experimental error in comparison with other results of the same IL). This fact reveals that the interaction of unsaturated hydrocarbons and aromatic hydrocarbons with [DCA]−-based ILs is larger than that with aliphatic hydrocarbons. Unfortunately, the high selectivity of the above-mentioned ILs are accompanied with low capacity. Among the 67 ILs listed in Table 8, the capacity is in a range (k∞2 is from 0.02 to 2.04) for hex-1-ene, (k∞2 is from 0.05 to 1.86) for cyclohexene, and (k∞2 is from 0.24 to 1.75) for styrene at T = 328.15 K. The highest capacity for the investigated ILs is exhibited by [BzMIM][NTf2] for hexene (k∞2 = 0.11) and for styrene (k∞2 = 0.76). The introduction of a benzyl group in the imidazolium cation has slightly increased the capacity, especially for styrene, with the selectivity on the same level in comparison to [AMIM][DCA]16 and with slightly lower selectivity in comparison with [AMIM][NTf2].20 The replacement of the ethyl group by the allyl group at the cation for [NTf2]−-based ILs has caused the opposite effect on the selectivity in the ethylbenzene (1)/styrene (2) separation, but increases the capacity for cyclohexene (k∞2 = 1.86)39 and for styrene (k∞2 = 1.68).39
Conclusions
In this work, we have studied the potential use of 1-benzyl-3-methylimidazolium dicyanamide, [BzMIM][DCA] and 1-benzyl-3-methylimidazolium bis{(trifluoromethyl)sulfonyl}imide, [BzMIM][NTf2] ILs in three processes for separation, viz. hexane/hex-1-ene, cyclohexane/cyclohexene, and ethylbenzene/styrene. For that purpose, experimental measurements were performed for the activity coefficients at infinite dilution for 64 solutes in both ILs at six temperatures ranging between (318.15 and 368.15) K. In addition, thermal properties, densities, and viscosities of both ILs were also measured as a function of temperature. The interactions of various types of organic solutes and water with two ionic liquids at infinite dilution were discussed and shown with regard to the activity coefficients, the gas–liquid-partition coefficients and the thermodynamic functions. Using the reported experimental data, along with other data from literature, the impact of the ILs cation and anion in the three chosen separation problems was analyzed. The reported results show high values of selectivity and low values of capacity for [BzMIM][DCA] and the opposite trend for [BzMIM][NTf2] ionic liquid as a separation agent. However, [BzMIM][DCA] has exhibited adequate extraction properties for all separation processes with high values of selectivities which are similar to [AMIM][DCA]16 measured by us earlier. Moreover, densities and viscosities of [BzMIM][DCA] were lower than those of [BzMIM][NTf2] and slightly higher than those for [AMIM][DCA].16,27 Therefore, in this context, the conclusion must be made that the presence of the benzyl group in the imidazolium cation has not considerably increased the selectivity in both ILs in comparison with the allyl group, but has increased the capacity, especially for styrene.Acknowledgements
The authors M. K., M. W. and U. D. are grateful to our colleagues from the Thermodynamic Research Unit, School of Chemical Engineering, University of KwaZulu-Natal, for co-operation. This work has been supported by the Ministry of Science and Higher Education in Poland in the years 2015–2017 (Project “Iuventus Plus” No. IP2014051373).References
- N. V. Plechkova and K. S. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC
.
- J. P. Hallet and T. Welton, Chem. Rev., 2011, 111, 3508–3576 CrossRef PubMed
.
- U. Domańska, Ionic Liquids in Chemical Analysis, General Review of Ionic Liquids and Their Properties, CRC Pres, Taylor & Francis Group, Abingdon, UK, 2008, ch. 1 Search PubMed
.
- A. Cole, Ionic Liquids in Separation Technology, Elsevier Science and Technology, Research and Markets, 2014, http://www.researchandmarkets.com/reports/2899469/ionic-liquids-in-separation-technology.htm Search PubMed
.
- G. W. Meindersma, A. R. Hansmeier and A. B. de Haan, Ind. Eng. Chem. Res., 2010, 49, 7530–7540 CrossRef CAS
.
- A. R. Hansmeier, G. W. Meindersma and A. B. de Haan, Green Chem., 2011, 13, 1907–1913 RSC
.
- K. Kędra-Królik, F. Mutelet and J.-N. Joubert, Ind. Eng. Chem. Res., 2011, 50, 2296–2306 CrossRef
.
- U. Domańska and M. Wlazło, Fuel, 2014, 134, 114–125 CrossRef
.
- A. Nann, C. Held and G. Sadowski, Ind. Eng. Chem. Res., 2013, 52, 18472–18481 CrossRef CAS
.
- M. Bülow, C. J. Guo, D. Shen, F. R. Fitch, A. I. Shirley and V. A. Malik, Separation of alkenes and alkanes, US Pat., 6200366, 2001, http://www.google.com/patents/US6200366?utm_source=gb-gplus-sharePatent
.
- J. Bergh, C. Gücüyener, E. A. Pidko, E. J. M. Hensen, J. Gascon and F. Kapteijn, Chem.–Eur. J., 2011, 17, 8832–8840 CrossRef PubMed
.
- D. Peralta, G. Chaplais, A. Simon-Masseron, K. Barthelet, C. Chizallet, A. A. Quoineaud and G. D. Pirngruber, J. Am. Chem. Soc., 2012, 134, 8115–8126 CrossRef CAS PubMed
.
- V. Mokrushin, D. Assenbaum, N. Paape, D. Gerhard, L. Mokrushina, P. Wasserscheid, W. Arlt, H. Kistenmacher, S. Neuendorf and V. Göke, Chem. Eng. Technol., 2010, 33, 63–73 CrossRef CAS
.
- Z. Lei, W. Arlt and P. Wasserscheid, Fluid Phase Equilib., 2006, 241, 290–299 CrossRef CAS
.
- U. Domańska, M. Wlazło and M. Karpińska, Fluid Phase Equilib., 2016, 417, 50–61 CrossRef
.
- M. Wlazło, J. Gawkowska and U. Domańska, Ind. Eng. Chem. Res., 2016, 55, 5054–5056 CrossRef
.
- F. Mutelet, A. L. Revelli, J.-N. Jaubert, L. M. Sprunger, W. E. Acree Jr and G. A. Baker, J. Chem. Eng. Data, 2010, 55, 234–242 CrossRef CAS
.
- J. Zhang, Q. Zhang, B. Qiao and Y. Deng, J. Chem. Eng. Data, 2007, 52, 2277–2283 CrossRef CAS
.
- U. Domańska, E. V. Lukoshko and M. Królikowski, J. Chem. Thermodyn., 2013, 61, 126–131 CrossRef
.
- M. Wlazło, M. Karpińska and U. Domańska, J. Chem. Thermodyn., 2016, 102, 39–47 CrossRef
.
- F. Mutelet, E.-S. R. E. Hassan, T. W. Stephens, W. E. Acree Jr and G. A. Baker, J. Chem. Eng. Data, 2013, 58, 2210–2218 CrossRef CAS
.
- P. S. Kulkarni, L. C. Branco, J. G. Crespo, M. C. Nunes, A. Raymundo and C. A. M. Afonso, Chem.–Eur. J., 2007, 13, 8478–8488 CrossRef CAS PubMed
.
- S. V. Dzyuba and R. A. Bartsch, ChemPhysChem, 2002, 3, 161–166 CrossRef CAS PubMed
.
- T. Mandai, A. Matsumura, M. Imanari and K. Nishikawa, J. Phys. Chem. B, 2012, 116, 2090–2095 CrossRef CAS PubMed
.
- H. Shirota, H. Matsuzaki, S. Ramati and J. F. Wishart, J. Phys. Chem. B, 2015, 119, 9173–9187 CrossRef CAS PubMed
.
- S. M. Mahurin, T. Dai, J. S. Yeary, H. Luo and S. Dai, Ind. Eng. Chem. Res., 2011, 50, 14061–14069 CrossRef CAS
.
- M. Larriba, P. Navarro, M. Gonzalez-Miquel, S. Omar, J. Palomar, J. García and F. Rodríguez, Chem. Eng. Res. Des., 2016, 109, 561–572 CrossRef CAS
.
- B. Gabri, A. Sander, M. C. Bubalo and D. Macut, Sci. World J., 2013, 2013, 512953, DOI:10.1155/2013/512953
.
- C. A. Ohlin, P. J. Dyson and G. Laurenczy, Chem. Commun., 2004, 1070–1071 RSC
.
- D. H. Everett, Trans. Faraday Soc., 1965, 61, 1637–1645 RSC
.
- A. J. B. Cruickshank, B. W. Gainey, C. P. Hicks, T. M. Letcher, R. W. Moody and C. L. Young, Trans. Faraday Soc., 1969, 65, 1014–1031 RSC
.
- Design institute for physical properties, sponsored by AICHE (2005; 2008; 2009; 2010). Dippr project 801-full version. Design institute for physical property research/aiche. Online version available at: http://knovel.com/web/portal/browse/display?_ext_knovel_display_bookid=1187%26verticalid=0.
- B. E. Poling and J. M. Prausnitz, Properties of Gases and Liquids, McGraw-Hill Publishing, 2001, http://lib.myilibrary.com?ID=91317 Search PubMed
.
- U. Domańska, M. Królikowska, W. E. Acree Jr and G. A. Baker, J. Chem. Thermodyn., 2011, 43, 1050–1057 CrossRef
.
- M. Królikowski and M. Królikowska, J. Chem. Thermodyn., 2014, 68, 138–144 CrossRef
.
- A.-L. Revelli, F. Mutelet, J.-N. Jaubert, M. Garcia-Martinez, L. M. Sprunger, W. E. Acree Jr and G. A. Baker, J. Chem. Eng. Data, 2010, 55, 2434–2443 CrossRef CAS
.
- M. Krummen, P. Wasserscheid and J. Gmehling, J. Chem. Eng. Data, 2002, 47, 1411–1417 CrossRef CAS
.
- N. Deenadajalu, T. M. Letcher and P. Reddy, J. Chem. Eng. Data, 2005, 50, 105–108 CrossRef
.
- A. Heintz, D. V. Kulikov and S. P. Verevkin, J. Chem. Eng. Data, 2002, 47, 894–899 CrossRef CAS
.
- A. Heintz, L. M. Casás, I. A. Nesterov, V. N. Emel'yanenko and S. P. Verevkin, J. Chem. Eng. Data, 2005, 50, 1510–1514 CrossRef CAS
.
- T. M. Letcher, A. Marciniak, M. Marciniak and U. Domańska, J. Chem. Thermodyn., 2005, 37, 1327–1331 CrossRef CAS
.
- A. Heintz and S. P. Verevkin, J. Chem. Eng. Data, 2006, 51, 434–437 CrossRef CAS
.
- R. Kato and J. Gmehling, J. Chem. Thermodyn., 2005, 37, 603–619 CrossRef CAS
.
- J.-C. Moise, F. Mutelet, J. N. Jaubert, L. M. Grubbs, W. E. Acree Jr and G. A. Baker, J. Chem. Eng. Data, 2011, 56, 3106–3114 CrossRef CAS
.
- U. Domańska and A. Marciniak, Fluid Phase Equilib., 2009, 286, 154–161 CrossRef
.
- A. Marciniak and M. Wlazło, J. Chem.
Thermodyn., 2012, 47, 382–388 CrossRef CAS
.
- M. Wlazło, A. Marciniak, M. Zawadzki and B. Dudkiewicz, J. Chem. Thermodyn., 2015, 86, 154–161 CrossRef
.
- U. Domańska and K. Paduszyński, J. Chem. Thermodyn., 2010, 42, 1361–1366 CrossRef
.
- K. Paduszyński and U. Domańska, J. Phys. Chem. B, 2011, 115, 8207–8215 CrossRef PubMed
.
- K. Paduszyński and U. Domańska, J. Chem. Thermodyn., 2013, 60, 169–173 CrossRef
.
- A. Marciniak and M. Wlazło, J. Chem. Thermodyn., 2012, 49, 137–145 CrossRef CAS
.
- M. Wlazło, M. Karpińska and U. Domańska, J. Chem. Thermodyn., 2016, 97, 253–260 CrossRef
.
- U. Domańska and A. Marciniak, J. Chem. Thermodyn., 2009, 41, 1350–1355 CrossRef
.
- R. Kato and J. Gmehling, Fluid Phase Equilib., 2004, 226, 37–44 CrossRef CAS
.
- P.-F. Yan, Q.-S. Liu, M. Yang, X.-M. Liu, Z.-C. Tan and U. Welz-Biermann, J. Chem. Thermodyn., 2010, 42, 1415–1422 CrossRef CAS
.
- A. Marciniak, J. Chem. Thermodyn., 2011, 43, 1446–1452 CrossRef CAS
.
- S. Nebig, V. Liebert and J. Gmehling, Fluid Phase Equilib., 2009, 277, 61–67 CrossRef CAS
.
- W. E. Acree Jr, G. A. Baker, A.-L. Revelli, J.-Ch. Moise and F. Mutelet, J. Chem. Eng. Data, 2012, 57, 3510–3518 CrossRef
.
- A. Marciniak and M. Wlazło, J. Chem. Thermodyn., 2012, 54, 90–96 CrossRef CAS
.
- U. Domańska, M. Zawadzki, M. Królikowska, M. M. Tshibangu, D. Ramjugernath and T. M. Letcher, J. Chem. Thermodyn., 2011, 43, 499–504 CrossRef
.
- U. Domańska and A. Marciniak, J. Chem. Thermodyn., 2009, 41, 754–758 CrossRef
.
- U. Domańska, P. Papis and J. Szydłowski, J. Chem. Thermodyn., 2014, 77, 63–70 CrossRef
.
- A. Heintz, T. V. Vasitsova, J. Safarov, E. Bich and S. P. Verevkin, J. Chem. Eng. Data, 2006, 51, 648–655 CrossRef CAS
.
- W. E. Acree Jr, G. A. Baker, F. Mutelet and J.-C. Moise, J. Chem. Eng. Data, 2011, 56, 3688–3697 CrossRef
.
- N. V. Gwala, N. Deenadayalu, K. Tumba and D. Ramjugernath, J. Chem. Thermodyn., 2010, 42, 256–261 CrossRef CAS
.
- M. Wlazło and U. Domańska, Sep. Purif. Technol., 2016, 162, 162–170 CrossRef
.
- T. Banerjee and A. Khanna, J. Chem. Eng. Data, 2006, 51, 2170–2177 CrossRef CAS
.
- K. Tumba, T. M. Letcher, P. Naidoo and D. Ramjugernath, J. Chem. Thermodyn., 2013, 65, 159–167 CrossRef CAS
.
- T. M. Letcher, D. Ramjugernath, M. Laskowska, M. Królikowski, P. Naidoo and U. Domańska, J. Chem. Eng. Data, 2008, 53, 2044–2049 CrossRef CAS
.
Footnote |
† Electronic supplementary information (ESI) available: The sources and mass fraction purities of materials. Mean column pressure, ![[p with combining macron]](https://www.rsc.org/images/entities/i_char_0070_0304.gif) , inlet column pressure, pi, outlet column pressure, po and standard state of solutes at given temperatures, at standard state. Plots of ln(γ∞13) versus 1/T for the organic solute in ILs. See DOI: 10.1039/c6ra25208g , inlet column pressure, pi, outlet column pressure, po and standard state of solutes at given temperatures, at standard state. Plots of ln(γ∞13) versus 1/T for the organic solute in ILs. See DOI: 10.1039/c6ra25208g |
| This journal is © The Royal Society of Chemistry 2017 |
