Open Access Article
Open Access ArticleStructure effects of amphiphilic and non-amphiphilic quaternary ammonium salts on photodegradation of Alizarin Red-S catalyzed by titanium dioxide†
Paola Anastasio a,
Tiziana Del Giacco
a,
Tiziana Del Giacco *ab,
Raimondo Germani
*ab,
Raimondo Germani *ab,
Nicoletta Spreti
*ab,
Nicoletta Spreti c and
Matteo Tiecco
c and
Matteo Tiecco a
a
aDipartimento di Chimica, Biologia e Biotecnologie, Università di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy. E-mail: tiziana.delgiacco@unipg.it
bCentro di Eccellenza Materiali Innovativi Nanostrutturati (CEMIN), Università di Perugia, Via Elce di Sotto 8, 06123 Perugia, Italy
cDipartimento di Scienze Fisiche e Chimiche, Università dell'Aquila, Via Vetoio, 67100 L'Aquila, Italy
First published on 23rd December 2016
Abstract
The role of surfactants such as single- and double-tailed tetraalkylammonium bromide and various non-amphiphilic tetraalkylammonium salts was investigated on the TiO2 photocatalyzed degradation of 3,4-dihydroxy-9,10-dioxo-2-anthracenesulfonic acid sodium salt (Alizarin Red-S, ARS) in an air-equilibrated alkaline medium under UV light irradiation. Absorption spectral analysis showed that the photodegradation efficiency of the dye was significantly enhanced by the addition of cationic surfactants. Two interesting findings emerged from this study: ARS was almost completely degraded at surfactant concentrations close to 1 mM (values well above the cmc in the experimental conditions); moreover, on increasing the surfactant concentration, the photocatalytic reaction became less and less efficient and significantly dependent on the surfactant headgroup size. The presence of a maximum of efficiency depending on the surfactant concentration was due to the combination of catalytic and inhibiting processes. The first one likely depended on the ability of the surfactant to improve the ARS approach to the semiconductor through the formation of cationic bilayers on the TiO2 particles; this effect made the ARS more easily oxidized by TiO2. This catalytic action of the surfactant was opposed by the increase of the micellar aggregate number in the aqueous bulk, which competes with the TiO2 sites in associating to the dye. This was supported by the results obtained with the non-amphiphilic alkylammonium bromide. In this case a higher amount of salt must be added to reach the same maximum efficiency of ARS photooxidation. This is due to the lower capability of neutralization of these salts for both the ARS and the TiO2 surface; the inhibiting effect was not evidenced anymore.
Introduction
The release of dyes and their degradation products in waste waters by textile industries is one of the main causes which determine environmental damage in the water flows and therefore may cause disorders to human health.1 To reduce the risk of environmental pollution from these species, in recent years great efforts have been made by developing new chemical methods to improve the oxidative degradation of organic compounds dissolved or dispersed in aqueous media.2–6 Among the oxidative photodegradation methods, the use of titanium dioxide has found wide use, because of its photostability, non toxicity, cheapness and strong oxidizing power. Heterogeneous photocatalysis has proved to be an efficient destructive technology, leading to the complete mineralization of most organic pollutants.7–9 During the past decade successful improvements in TiO2 photoactivity have been made on the oxidation process: nanostructurated composite materials, as noble metal nanoparticles (Ag, Au and Cu NPs), increase the process efficiency when immobilized on semiconductor surface; doped-TiO2 with metallic and not metallic species also showed interesting results in this field.10 Surfactants play a key-role in this topic,11 generally favoring the adsorption of the dye onto the semiconductor surface due to their unique chemical properties.Previous works reported that TiO2 is a good photocatalyst for ARS (a water-soluble widely used textile dye) and its removal from the aqueous phase.7 The efficiency of the dye photodegradation is reported to be influenced by pH, one of the most important factor controlling both the adsorption of dye on the semiconductor and the reactivity of the process. In most cases, the photodegradation was performed with UV-illuminated titanium dioxide, but in some works visible radiation by photosensitization was used, certainly finalized to the use of the free and inexhaustible sunlight, of which 40% is visible light and less than ca. 5% is UV. Even though there is a basic difference in the primary steps of photodegradation depending on the excitation wavelength (visible or UV irradiation), the photoassisted degradation leads to the dye radical cation formation as an intermediate, which is the main responsible of the degradation of the dye itself.12–15
Our long-standing interest in the mechanistic investigation of TiO2 sensitized photooxidation of various organic compound has recently led us to focus our attention on the effect of the surfactant structure;16–18 the photodegradation efficiency of ARS catalyzed by TiO2 was studied in air-equilibrated basic aqueous dispersion under UV light irradiation using surfactants differing in their counterions.19 A series of surfactants such as cetyltrimethylammonium salts (CTAX, with X = Br−, Cl−, SO42−, CH3SO3−, 4-CH3C6H4SO3−) were used. From these studies emerged that the photocatalytic efficiency was significantly dependent both on the surfactant concentration and on the nature of the counterion X−.
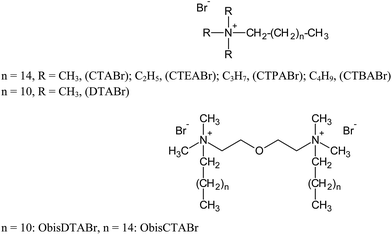
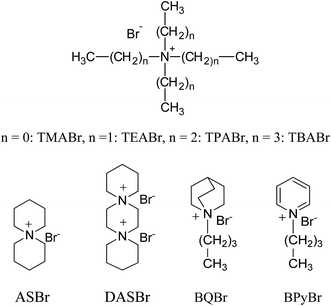
Changes in the molecular structures of surfactants lead to significant changes in their properties and in the properties of their micellar aggregates. For these reasons the use of synthetic novel amphiphiles represents a step ahead in the chemistry of these molecules, and it can lead to important advantages.20,21 The topics where these molecules find an effective role are in fact wide, that is because small chemical modifications of the structures can provoke dramatic changes on their effect.22,23 In this work we extended the analysis of the structural effects of tetraalkylammonium bromide surfactants on the TiO2-mediated photodegradation of ARS, considering other surfactants structures, varied in their hydrophobic chains portions and in their headgroup size. Thus we have investigated single-chain surfactants, such as alkylammonium bromide, with different headgroup size (cetyltrimethylammonium, CTA, cetyltriethylammonium, CTEA, cetyltripropylammonium, CTPA, and cetyltributylammonium, CTBA) and variable length tail (dodecyltrimethyl, DTA), and gemini surfactants, with varying tail and two quaternary ammonium headgroups connected by a diethylene oxide spacer (2-[2-(dimethylalkylazanium)ethoxy]ethyl-dimethylalkylazanium, Obis-alkyl-TA). The comparison between single-chain and gemini systems has given also information on the role played by positive charge number of the surfactant headgroups. All the surfactant structures are shown in Scheme 1. The use of non-amphiphilic ammonium salts in TiO2-catalyzed degradation processes has not received the same attention of amphiphilic salts so far;24–26 however this could help the understanding of the role of the headgroup portions of the surfactants in this process. Therefore in this work we have also compared the ability of the various surfactants in assisting the ARS degradation with that of not amphiphilic alkylammonium salts (structures in Scheme 2), such tetraalkylammonium bromide (TRABr), 6-azoniaspiro[5.5]undecane bromide (ASBr), 6,9-diazoniaspiro[5.2.3.2.]hexadecane dibromide (DASBr), butylquinuclidinium bromide (BQBr) and butylpyridinium bromide (BPyBr). This comparison has shown that the hydrophobic degree of the salt strongly influences the reactivity of the oxidation process of the dye.
Experimental
Materials
Alizarin Red S (Carlo Erba) and TiO2 (P25, Degussa, 99.5%, dried in vacuo) were analytical grade reagents. Milli-Q water (18 MΩ, pH 5.9) was used for all photodegradation experiments. CTABr and DTABr surfactants were purchased from Sigma-Aldrich and recrystallized from acetone and methanol before use. CTEABr, CTPABr, CTBABr, ObisCTABr and ObisDTABr surfactants were synthesized by quaternization reaction of the correspondent tertiary amines with the appropriate alkyl halide and purified by subsequent crystallizations from acetone or ethyl acetate with different amounts of methanol.27 Quaternary ammonium salts TMABr, TEABr, TPABr and TBABr (Sigma-Aldrich and Carlo Erba, purity > 98%) were dried in vacuo over P2O5 before use. Spiro cyclic quaternary ammonium salts ASBr and DASBr were synthesized as reported in literature.28 BQBr and BPyBr were synthesized by quaternization reaction of the correspondent tertiary amines with butyl bromide and purified by subsequent crystallizations from acetone/methanol mixture.Photodegradation experiments
An identical procedure was followed both with surfactants and with non-amphiphilic salts. The degradation experiments were performed by an Applied Photophysics multilamp apparatus with 12 phosphor-coated fluorescent lamps (15 W each) emitting at 355 nm (Δλ1/2 = 20 nm), radiation adsorbed mainly by the semiconductor, as the dye absorbs more weakly at these wavelengths.29TiO2 suspensions were prepared by adding P25 TiO2 powder (0.4 g l−1) to 50 ml of an aqueous solution containing ARS (0.20 mM) and various concentrations of ammonium salt. The degradations were carried out at 20 °C. The starting pH of the irradiated suspensions was adjusted to 12 using NaOH. Before irradiation, the suspensions were magnetically stirred in the dark for 30 min to reach equilibrated adsorption between TiO2, dye and atmospheric oxygen. The irradiated dispersion was kept under constant air-equilibrated and stirred condition.
Analytical procedures
The degradation process was followed by UV-Vis spectrophotometric analysis. Mixture samples (2 ml) were collected at different irradiation time. To recover quantitatively the dye adsorbed on TiO2, an equal volume of ethanol was added to the sample, together with 0.2 ml of HCl 1.2 M; in this condition ARS was all protonated. The suspension was then shaken by vortex mixer and centrifuged at 10 °C for 15 min at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm to remove TiO2. The supernatant (2 ml) was carefully transferred from the centrifugation tube into a 4 ml quartz cuvette for UV/VIS analysis. Absorption spectra were recorded on a Shimadzu UV-2401 PC spectrophotometer connected to a CPS-controller thermostat kept at 25.0 ± 0.1 °C.
000 rpm to remove TiO2. The supernatant (2 ml) was carefully transferred from the centrifugation tube into a 4 ml quartz cuvette for UV/VIS analysis. Absorption spectra were recorded on a Shimadzu UV-2401 PC spectrophotometer connected to a CPS-controller thermostat kept at 25.0 ± 0.1 °C.
The kinetic experiments were performed using 6–12 phosphor-coated fluorescent lamps for irradiating the samples. Rate constants for ARS degradation were obtained by monitoring the change of absorbance at the maximum wavelength (423 nm) as a function of the irradiation time at 25 °C. The k values were the average of at least three determinations. The average error estimated on the rate constants was ±10%.
Product analysis was carried out as follows. An aqueous dispersion of TiO2 (40 mg in 100 ml) was irradiated in the presence of 1.0 mM CTABr for 30 min. Subsequently, the semiconductor particles were removed by centrifugation after adding of ethanol and HCl (see above). The supernatant was carefully transferred from the centrifugation tubes into a separating funnel and water was added to the mixture. The aqueous layer was extracted three times with chloroform. The residue, obtained after removal of the solvent under reduced pressures from the combined organic extracts, was diluted with methanol. A LCMS-ESI analysis was performed on the diluted samples by an Agilent 6540 UHD accurate mass Q-TOF LC/MSMS instrument. The analysis of the irradiated samples was always compared with that of the corresponding not-irradiated samples, prepared following the same experimental procedure. The chromatogram highlighted a main peak at 0.484 min acquisition time, which was assigned to phthalic acid by MS spectrum (negative ion acquisition): calcd for C8H5O4 [M − H]−: 165.1266, found: 165.0199 (data in ESI†).
The photostability of the surfactant was checked as follows. An aqueous dispersion of TiO2 (20 mg in 50 ml) was irradiated in the presence of 50 mM CTABr, the surfactant taken as test, for 30 min. Subsequently, the semiconductor particles were removed by centrifugation (see above) after adding of HCl and the supernatant was analyzed directly. The analysis of the irradiated samples was always compared with that of the corresponding not-irradiated samples, prepared following the same experimental procedure. The photostability of the surfactant was monitored by IR spectroscopic methods, using a Perkin Elmer Spectrum RX I FT-IR System instrument. The FTIR spectra were calculated from 16 scans at 1 cm−1 resolution. The intensities of the bands at 2924 and 2854 cm−1 (C–H stretching modes) and at 1470 cm−1 (C–H bending modes), all mainly due to the methylene tails, were evaluated before and after irradiation (data in ESI†).30
Micellar parameter determination by surface tension measurements
The critical micellar concentration (cmc) values of the various cationic surfactants were measured at pH 12 in the absence of TiO2 according to the Du Noüy ring method using a Fisher Surface Tensiometer (manual model). The measurements were performed on solutions (from 1.0 to 0.0050 mM) prepared by adding rates of a surfactant stock solution. The cmc values were obtained by the intersection of the two linear correlations obtained in the graph of the surface tensions in function of −log[surfactant], whereas the ionization degree (α) was determined by the ratio of the slopes of the two correlations. The average value of three measurements at 25 °C was taken as the result. The average error was estimated to be ca. 5%.Results and discussion
TiO2-photocatalyzed degradation of ARS assisted by surfactants
In a previous paper, we reported that the TiO2-photodegradation efficiency of ARS assisted by cetyltrimethylammonium salts improved by increasing the pH value up to 12.19 The pH affected both the amount of hydroxyl radicals produced by oxidation of OH− ions available on the semiconductor surface7 and also the stabilization, due to the electrostatic attraction between the photocatalyst surface (negatively charged at this pH) and the cation radical intermediates. On the basis of these results, all the experiments presented in this work were carried out at pH 12.Blank experiments, performed on irradiated ARS solutions but not containing TiO2, or on dark TiO2/ASR dispersions, showed that no dye degradation occurred along a time shorter than 120 min. It is also well known that the surfactant molecules themselves are photocatalytic degraded over TiO2 powder,32,33 but we checked by FTIR analysis (data in ESI†), that the photooxidation of cetyltrialkylammonium bromide, the surfactant taken as test, by irradiated semiconductor particles is rather slow during the time in which ARS degrades. Indeed, the CTABr disappearance percentage, measured as transmittance change of the surfactant characteristic peaks (see Experimental) before and after the irradiation, is less than 2%.
The effect of single-tailed surfactants has been examined in terms of variation of the headgroup size in the CTRABr series (Scheme 1). For this purpose, the experiments were performed in the presence of surfactants at concentrations ranging between 0.20 and 5.0 mM, keeping constant the ARS and TiO2 concentrations.
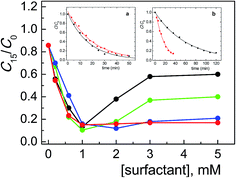
The shape of the absorption spectra recorded after 15 min irradiation in the presence of surfactant were very similar to those recorded without surfactant (as an example, in Fig. S1 and S2† the spectra in the presence of CTABr and CTBABr are shown); this indicates that the surfactant role is mainly to affect the process kinetics. The degradation profiles, shown in Fig. 1, are reported as the changes of concentration of ARS as a function of [CTRAX]; the changes of ARS concentration are expressed as the ratio of residual ARS concentration after 15 min irradiation (C15) on its initial concentration (C0) (both values determined by absorbance measurements, see Experimental). The first observation emerging from the analysis of these profiles is that the photocatalytic efficiency depends weakly on the surfactant structure up to a concentration of about 1 mM, where the maximum efficiency of ARS degradation is reached (about 90%). By comparing the minimum value of the ratio C15/C0 (ca. 0.1) with that determined in the absence of CTRAX (ca. 0.9), it is possible to establish a significant catalytic efficiency by the surfactant of almost 10 times. This concentration is well above the cmc values of all CTRAX examined; indeed, the cmc values ranged from 0.23 to 0.056 mM in alkaline medium (Table 1) increasing the chain length of the alkyl residues of the headgroup. As expected, the cmc values at pH 12 are lower than in neutral water (Table 1), according to the effect due to the addition of ionic species (as Na+OH−). The second interesting evidence is that, for surfactant concentration above 1.0 mM, the photocatalytic degradation of the dye is strongly influenced on the headgroup structure. In particular, the extent of photodegradation does not change with propyl- and butyl-in the headgroup, while with methyl and ethyl it decreases rapidly with the monomer concentration until reaching a plateau value (at ca. 3 mM surfactant), corresponding to 40% and 60% of degraded Alizarin, respectively.
These results are confirmed by kinetic analysis of the degradation obtained in the presence of CTABr or CTBABr, the two surfactants at the extremes of the degradation efficiency. Indeed, the degradation data (C/C0 vs. time), determined on 1.0 mM CTRAX solutions, were well fitted by a pseudo-first order kinetic law (inset a in Fig. 1) with slightly different observed rate constants (0.050 and 0.058 min−1 for CTBABr and CTABr, respectively). In the presence of 5.0 mM surfactant the observed first order rate constant with CTBABr (0.061 min−1) was found similar to that at 1.0 mM and greater than that determined with CTABr (0.016 min−1) by a factor of ca. 4 (inset b in Fig. 1), which is almost the same reactivity ratio determined after 15 minutes of irradiation.
The graphics reported in Fig. 1 are very similar to that already reported for the same system, ARS/TiO2/pH 12, but in the presence of cetyltrimethylammonium salt with different counterions (CTAX),19 at least as regards the trend of the degradation efficiency up to 1.0 mM surfactant concentration, which corresponds to the ARS degradation maximum. Also in this case the formation of positively charged close-packed bilayers on the TiO2 surface is supposed,11,30 which has the role of approaching the dye to the semiconductor, otherwise impossible being both negatively charged. Interestingly, in this range of surfactant concentrations, the ARS degradation efficiency is very little affected both by the steric hindrance of the headgroup alkyl chains and by the cmc values (Table 1); this probably can be due to a combination of hydrophobic and electrostatic interactions.
At monomer concentrations higher than 1.0 mM, the dye competed between two association sites, the TiO2 surface and the micellar aggregates of CTABr and CTEABr, while with larger headgroup size (CTPABr and CTBABr) the degradation efficiency did not undergo any variation. In particular, the dye oxidation efficiency decreased in the order of alkyl chain length methyl < ethyl < propyl ≅ butyl, i.e. by decreasing the micellar dissociation degree α (Table 1), a parameter correlated with the positive charge density on the micellar surface.34 In the case of CTAX surfactants, an opposite behavior was instead observed: micelles more ionized (with greater α value) were able to bind more efficiently the negatively charged dye, subtracting it from the photodegradation process. The association degree of ARS to the micellar aggregates evidently does not depends only on the charge density, but also on the hydrophobic nature of the micelle surface. Previous studies showed that an increase in the headgroup size not only lowered the cmc value and increased the α parameter, but also reduced the water content and the hydrophobicity of the interface.27 In particular, the alkyl chains of CTPABr and CTBABr did not extend into the water, but were oriented along the micellar surface to reduce water-alkyl group contact. Therefore, the reduced inhibition of the photodegradation with increasing of the headgroup size can be interpreted in terms of increased difficulty of ARS to intercalate into the micelle because of steric effect due to the alkyl chains. We observed this effect also with other aromatic molecules (sodium trans-orthomethoxycinnamate) not intercalating in CTBABr micelles due to steric hindrance of the headgroups in other previous studies.34
In Fig. 2 the degradation profiles of the concentration change of ARS, expressed as C15/C0 ratio as a function of surfactant concentration, for ObisCTABr and CTABr are compared. Two most significant aspects emerged from the analysis of these profiles. The first one was that the maximum photocatalytic efficiency for ObisCTABr, ca. 65% of degraded ARS, was at a concentration (0.2 mM) 5 times lower than that for CTABr (1 mM). Both values are higher than the corresponding cmc values (0.049 and 2.3 mM at pH 12, Table 1). The other relevant aspect concerns the shape of the profiles, namely the curve appeared less broaden going from CTABr to ObisCTABr, reaching a plateau value from ca. 60% to 82% of residual ARS.
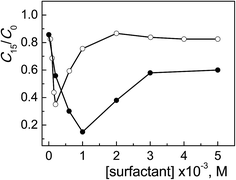 | ||
| Fig. 2 Photodegradation of ARS (0.20 mM) with TiO2 (0.4 g l−1) at pH 12 after 15 min of irradiation as a function of ObisCTABr (○) and CTABr (●) concentration. | ||
The highest inhibition effect on the dye degradation process observed with ObisCTABr is in line with its much lower cmc value and higher α parameter (cmc: 0.049 and 0.23 mM, α: 0.36 and 0.25 for ObisCTABr and CTABr, respectively, Table 1). Indeed, the lower cmc value implied that, in the presence of the same amount of surfactant, the gemini system provided a greater number of micellar aggregates able to associate ARS and thus to subtract it from the TiO2 catalytic action. Furthermore, the ObisCTABr micelle aggregates, being more ionized because less associated with the counterions, interacted more effectively with the negatively charged ARS.
The comparison of the profiles C15/C0 against the surfactant concentration obtained with ObisDTABr and DTABr (Fig. S3 in ESI†) pointed out smaller differences between the two systems. The maximum efficiency of degradation with ObisDTABr (ca. 85%) was obtained at 0.5 mM, a concentration that is half of the value found for the corresponding single-tailed DTABr (maximum efficiency ca. 80%); the C15/C0 values at the plateau in this case were more similar (0.7 and 0.63 for ObisDTABr and DTABr, respectively). These results were in line with the similar cmc and α values for the two systems, as observed from the data collected in Table 1 (cmc: 1.0 and 1.6, α: 0.26 and 0.28 for ObisDTABr and DTABr, respectively). In this case the favorable adsorption by electrostatic and hydrophobic interaction of ObisRTABr surfactants on the TiO2, due to the double charge and double chain in its structure, seemed to play a role only in anticipating, in terms of concentration, the maximum photodegradation point of ARS.
TiO2-photocatalyzed degradation of ARS assisted by tetraalkyl quaternary ammonium salts
In order to investigate the role of the amphiphilic character of the quaternary ammonium salts in assisting the TiO2 photocatalyzed degradation of Alizarin, we examined numerous bromide salts, with variously structured ammonium groups, not able to form micelles in the same experimental conditions described above.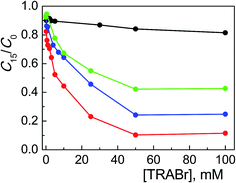 | ||
Fig. 3 Photodegradation of ARS (0.20 mM) with TiO2 (0.4 g l−1) at pH 12 after 15 min of irradiation as a function of TMABr (●), TEABr ( ), TPABr ( ), TPABr ( ) and TBABr ( ) and TBABr ( ) concentration. ) concentration. | ||
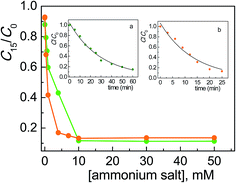
Comparing these results with those collected with the corresponding surfactants (CTABr, CTEABr, CTPABr and CTBABr), the main evidences were: (i) the maximum catalytic effect was still very high, of the same entity (90%) but reached at much higher concentration of salt; (ii) the inhibition effect due to the competition with the micelles was obviously missing; (iii) the chain length effect of the alkyl residue on the efficiency of the degradation process was roughly kept, i.e., butyl > propyl > ethyl > methyl. Indeed tetrabutylammonium, being the softest cation, neutralized better the negative charge of the dye, which is also soft since the charge is delocalized on the aromatic rings. This neutralization could allow a more effective approach of the dye to the semiconductor, a necessary condition to facilitate kinetically the electron transfer from the dye to the positive hole photogenerated on the TiO2 surface. At the same time, the tetrabutylammonium group could create a coating on the catalyst surface not only by electrostatic interactions, but also by hydrophobic interactions between the butyl chains, reducing in this way their steric hindrance. The trimethylammonium, being the hardest of the four salts examined, could prefer to interact electrostatically by the positive charged nitrogen with the surface hard TiO− groups, rather than with the dye.7 So, the less Alizarin molecules were in proximity of the semiconductor, the less efficient was their photodegradation. TEABr and TPABr clearly followed the trend due to the hydrophobicity of the system.
The concentration of 50 mM, in correspondence of which the photodegradation reached its maximum value, corresponds to the situation of maximum adsorbed amount of Alizarin on the semiconductor in the presence of different salts. It is plausible that the formation of positive charged close-packed bilayers of surfactant molecules adsorbed on the TiO2 surface, which are known to assist the ARS degradation in micellar media,19 was achieved at lower surfactant concentrations because of the importance of non-electrostatic hydrocarbon tail–tail interactions.
From the C15/C0 ratio as a function of salt concentration profiles shown in Fig. 4, it is observable that the catalytic efficiency decreased going from the structure with a higher degree of conformational freedom, such that of TPABr, to the more rigid molecular structures of BQBr and ASBr. To explain this trend, the hydrophobicity of alkyl residues can not be invoked, since the number of the carbon atoms is similar. Indeed, in the case of BQBr and ASBr, the ammonium positive charge should have been more available for the interaction both with the dye and with the negatively charged catalyst surface, compared with the higher steric hindrance of TPABr.
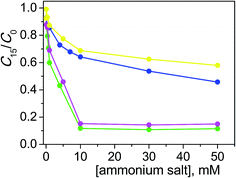 | ||
Fig. 4 Photodegradation of ARS (0.20 mM) with TiO2 (0.4 g l−1) at pH 12 after 15 min of irradiation as a function of TPABr ( ), BPyBr ( ), BPyBr ( ), ASBr ( ), ASBr ( ), BQBr ( ), BQBr ( ). ). | ||
The analogous behavior of BQBr and ASBr suggests that the ammonium charge availability is similar with the two salts. Evidently, in the BQBr structure, the conformational freedom of the butyl residue is balanced by the rigidity of quinuclidinium portion to the same extent of the two rigid chair conformation of ASBr.
The weaker effect of BPyBr on the degradation reaction may seem unexpected. Also in this case the hydrophobicity can not be influent, since its carbon atom number similar to that of the others (9, 12, 11 and 10 for BPyBr, TPABr, BQBr and ASBr, respectively). The rigidity of the BPyBr should have determined a better accessibility of the ammonium, but two main factors could hinder the neutralization: the first is electronic cloud of the aromatic ring that repulses the negatively charged dye and then the approach of the dye to the semiconductor; the second factor could be the different hybridization of the nitrogen atom (sp2 in pyridinium, sp3 in the other cases) that determines a lower positive charge density on nitrogen.
Conclusions
The addition of CTRABr surfactants significantly enhanced ARS decomposition kinetics on the TiO2 surface in air-equilibrated alkaline aqueous dispersions under UV light irradiation. This effect could be mainly explained by the increased approach of ARS molecules to the solid surface of the semiconductor through hemimicelle/admicelle structures adsorbed on the TiO2 particles. This facilitated the reaction of the dye with the photo-yielded oxidative species formed on the semiconductor surface. The ARS photodegradation rate reached a maximum value when the surfactant concentration is about 1 mM, a value lower than the cmc of all examined surfactants. Above this value, the ARS degradation rate is reduced because of the competition of the micellar aggregates in adsorbing the dye. These results were also compared with that obtained with analogous surfactant in which structure length and number of the tails have been varied.The assistance of non-amphiphilic quaternary ammonium salts to the TiO2 catalyzed photodegradation of ARS was realized by reducing the electrostatic repulsion between the anionic dye and the negatively charged semiconductor. The results collected in this study highlighted that the salt action is accomplished by its hydrophobic character, the availability of the positive charge on the ammonium group and the number of charged nitrogen atoms.
The advantages of using non-amphiphilic salts were that the dye photodegradation process did not undergo inhibition, although the lack of hydrophobic tails required greater salt concentrations to achieve values of degradation efficiency comparable to those obtained with the surfactants. This effect was however offset by lower environmental impact of non-amphiphilic alkylammonium salts with respect to the corresponding surfactants.
Acknowledgements
Thanks are due to MIUR (Ministero dell'Istruzione, dell'Università e della Ricerca Scientifica), Italy (PRIN 2010–2011, grant number 2010FM738P) and to the University of Perugia (Fondo Ricerca di Base 2015) for financial support.References
- P. B. S. Ratna, Int. J. Environ. Sci., 2012, 3, 940–955 Search PubMed.
- B. R. Babu, A. Parande, S. Raghu and T. P. Kumar, J. Cotton Sci., 2007, 11, 141–153 CAS.
- O. J. Hao, H. Kim and P.-C. Chiang, Crit. Rev. Environ. Sci. Technol., 2000, 30, 449–505 CrossRef CAS.
- U. Wiesmann, I. S. Choi and E.-M. Dombrowski, Fundamentals of biological wastewater treatment, Wiley-VCH Verlag GmbH & Co. KGaA, 2007 Search PubMed.
- Y. Anjaneyulu, N. S. Chary and D. S. S. Raj, Rev. Environ. Sci. Bio/Technol., 2005, 4, 245–273 CrossRef CAS.
- C. Zaharia, D. Suteu, A. Muresan, R. Muresan and A. Popescu, Environ. Eng. Manage. J., 2009, 8, 1359–1369 CAS.
- U. Akpan and B. Hameed, J. Hazard. Mater., 2009, 170, 520–529 CrossRef CAS PubMed.
- G. A. Epling and C. Lin, Chemosphere, 2002, 46, 561–570 CrossRef CAS PubMed.
- I. K. Konstantinou and T. A. Albanis, Appl. Catal., B, 2004, 49, 1–14 CrossRef CAS.
- T. Tachikawa, M. Fujitsuka and T. Majima, J. Phys. Chem. C, 2007, 111, 5259–5275 CAS.
- R. Zhang and P. Somasundaran, Adv. Colloid Interface Sci., 2006, 123, 213–229 CrossRef PubMed.
- T. H. Talib, M. A. AlDamen and R. R. Alani, Am. Chem. Sci. J., 2014, 4, 918–933 CrossRef CAS.
- K. Joshi and V. Shrivastava, Int. J. Environ. Sci., 2011, 2, 8–21 CAS.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- M. L. de Souza and P. Corio, Appl. Catal., B, 2013, 136, 325–333 CrossRef.
- M. Bettoni, T. Del Giacco, C. Rol and G. V. Sebastiani, J. Photochem. Photobiol., A, 2007, 190, 34–40 CrossRef CAS.
- M. Bettoni, T. Del Giacco, F. Elisei, C. Rol and G. V. Sebastiani, Phys. Chem. Chem. Phys., 2010, 12, 5425–5430 RSC.
- M. Bettoni, L. Brinchi, T. Del Giacco, R. Germani, S. Meniconi, C. Rol and G. V. Sebastiani, J. Photochem. Photobiol., A, 2012, 229, 53–59 CrossRef CAS.
- T. Del Giacco, R. Germani, F. Saracino and M. Stradiotto, J. Photochem. Photobiol., A, 2017, 332, 546–553 CrossRef CAS.
- M. Tiecco, L. Roscini, L. Corte, C. Colabella, R. Germani and G. Cardinali, Langmuir, 2016, 32, 1101–1110 CrossRef CAS PubMed.
- L. Corte, M. Tiecco, L. Roscini, R. Germani and G. Cardinali, Colloids Surf., B, 2014, 116, 761–771 CrossRef CAS PubMed.
- M. Tiecco, P. Di Profio, R. Germani and G. Savelli, Nucleosides, Nucleotides Nucleic Acids, 2009, 28, 911–923 CAS.
- M. J. Rosen and J. T. Kunjappu, Surfactants and interfacial phenomena, John Wiley & Sons, 2012 Search PubMed.
- Y. Zhang, H. Wu, J. Zhang, H. Wang and W. Lu, J. Hazard. Mater., 2012, 221, 92–99 CrossRef PubMed.
- D. Fabbri, A. Crime, M. Davezza, C. Medana, C. Baiocchi, A. B. Prevot and E. Pramauro, Appl. Catal., B, 2009, 92, 318–325 CrossRef CAS.
- M. Davezza, D. Fabbri, E. Pramauro and A. B. Prevot, Environ. Sci. Pollut. Res., 2013, 20, 3224–3231 CrossRef CAS PubMed.
- R. Germani, P. P. Ponti, T. Romeo, G. Savelli, N. Spreti, G. Cerichelli, L. Luchetti, G. Mancini and C. A. Bunton, J. Phys. Org. Chem., 1989, 2, 553–558 CrossRef CAS.
- R. A. Aitken, E. F. Philp, F. G. Riddell and M. H. Smith, ARKIVOC, 2002, 3, 63–70 Search PubMed.
- T. Tachikawa, S. Tojo, M. Fujitsuka and T. Majima, Chem.–Eur. J., 2006, 12, 3124–3131 CrossRef CAS PubMed.
- H. Li and C. P. Tripp, Langmuir, 2002, 18, 9441–9446 CrossRef CAS.
- J. Zhao, T. Wu, K. Wu, K. Oikawa, H. Hidaka and N. Serpone, Environ. Sci. Technol., 1998, 32, 2394–2400 CrossRef CAS.
- B. Singhal, A. Porwal, A. Sharma, R. Ameta and S. C. Ameta, J. Photochem. Photobiol., A, 1997, 108, 85–88 CrossRef CAS.
- R. Zhang, L. Gao and Q. Zhang, Chemosphere, 2004, 54, 405–411 CrossRef CAS PubMed.
- P. Baglioni, E. Braccalenti, E. Carretti, R. Germani, L. Goracci, G. Savelli and M. Tiecco, Langmuir, 2009, 25, 5467–5475 CrossRef CAS PubMed.
- S. Manne, T. Schäffer, Q. Huo, P. Hansma, D. Morse, G. Stucky and I. Aksay, Langmuir, 1997, 13, 6382–6387 CrossRef CAS.
- S. Ardizzone, C. Bianchi, P. Quagliotto and G. Viscardi, Surf. Interface Anal., 2002, 34, 652–656 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables of the numerical values of the figures. Photodegradation of ARS with TiO2 as a function of ObisDTABr and DTABr concentration. LCMS-ESI analysis of the ARS photodegradation with TiO2 in the presence of CTABr. FTIR analysis of the CTABr photostability with TiO2. See DOI: 10.1039/c6ra25421g |
| This journal is © The Royal Society of Chemistry 2017 |